Key Points
Radiation is appropriate primary therapy in approximately 25% of cases.
Radiation provides long-term remissions in more than 50% of patients.
Abstract
POEMS syndrome is a rare plasma cell dyscrasia presenting with polyneuropathy and other systemic findings. Patients with 1 to 3 bone lesions and negative bone marrows are often treated with involved field radiation therapy as the initial and potentially definitive therapy. Long-term outcomes of patients treated with this approach have not been systematically studied. Of the 146 patients with POEMS syndrome seen at the Mayo Clinic between January 1999 and September 2011, 38 (26%) were given targeted radiation as their initial primary therapy and are the ones studied here. The median number of bone lesions was 1 (range: 1-6). The median dose of radiation administered was 45 Gy (range: 35-54 Gy). Complete or partial hematologic, vascular endothelial growth factor, fluorodeoxyglucose–positron emission tomography, and clinical responses were documented in 31%, 14%, 22%, and 47%, respectively. With median follow-up of 43 months, the 4-year overall survival is 97% and event-free survival is 52%. Risk factors for needing salvage therapy included reduced pulmonary diffusing capacity of carbon monoxide and increased urinary total protein. The presence of 3 lesions compared with 1 or 2 did not increase risk for treatment failure. Among selected patients with POEMS syndrome, radiation produces durable, meaningful responses.
Introduction
POEMS syndrome is a rare paraneoplastic syndrome driven by neoplastic plasma cells. POEMS is an acronym that refers to 5 of the 11 symptoms and signs that predominate: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasmaproliferative disorder, and skin changes.1 Due to the rarity of the disease, prospective therapeutic clinical trials are rare.2,3 Based on experience with patients with solitary plasmacytomas of bone (SPB), the approach for patients with POEMS, negative bone marrows, and limited bone lesions has been targeted irradiation for patients with 1 to 3 isolated bone lesions.4 Anecdotal reports have shown benefit,5-10 but there are no published data on long-term results or time to treatment failure with this approach.
Using our database of patients with POEMS syndrome, we studied the outcomes among patients with newly diagnosed POEMS syndrome who were treated with targeted radiation as their primary therapy.
Patients and methods
Patients
One hundred and forty-six patients with POEMS syndrome were seen at the Mayo Clinic in Rochester, MN, between January 1999 and September 2011. Records of patients with research authorization were reviewed with Institutional Review Board approval. Thirty-eight patients (26%) satisfied the eligibility criteria and make up the study population. The other 108 either never received radiation or had received an autologous hematopoietic stem cell transplant (ASCT) before any salvage radiation had been given.
Eligibility into this retrospective study included a new diagnosis of POEMS syndrome and use of radiation as the initial definitive therapy. POEMS syndrome was defined as previously reported in Dispenzieri1 as (1) the presence of both a polyradiculoneuropathy and a monoclonal plasma cell proliferative disorder; (2) the existence of 1 of the following other 3 major criteria: Castleman disease, sclerotic bone lesions, or vascular endothelial growth factor (VEGF) elevation; and (3) 1 of 6 possible minor criteria: organomegaly, extravascular volume overload, endocrinopathy, skin changes (hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, and white nails), papilledema, and either thrombocytosis or polycythemia.
To be considered a bone lesion, a sclerotic or lytic lesion must have been visualized on computed tomography (CT), which is very useful in picking up small lesions,11 and been at least 0.8 cm in the longest dimension. The presence of a POEMS-related bone marrow clone on random iliac crest bone marrow analysis was an ineligibility factor.
“Initial definitive therapy for POEMS syndrome” was defined as the first treatment given for the diagnosis of POEMS (and not for another incorrect presumptive diagnosis such as chronic inflammatory polyneuropathy [CIDP]). There was 1 exception to this rule, that is, a patient who received 3 cycles of cyclophosphamide as a temporizer because primary radiation therapy was delayed due to insurance issues. Another 3 patients who received chemotherapy that may have had an impact on the plasma cell clone before radiation were included in this series because these therapies were not given to treat the diagnosis of POEMS syndrome (Table 1): (1) a single dose of bortezomib and doxorubicin given for an incorrect diagnosis of multiple myeloma; (2) 4 cycles of cyclophosphamide for the diagnosis of refractory CIDP 5 months before the correct diagnosis of POEMS syndrome and radiation; and (3) a single dose of cyclophosphamide given for stem cell mobilization. One patient, who was included in this series, received 2 doses of melphalan with radiation. Three of the above patients also received concomitant corticosteroids. In total, 5 patients received plasmacytoma-altering therapies with or before radiation.
Table 1.
Baseline characteristics
| Characteristic | N | % | Median (range) |
|---|---|---|---|
| Age, y | 38 | 50 (19-82) | |
| Gender, male | 29 | 76 | |
| Patients with prior therapies directed at CIDP or MPD, number patients treated | 22 | 58 | |
| Eastern Cooperative Oncology Group PS 2/3 | 12/11 | 31/29 | |
| Polyneuropathy | 38 | 100 | |
| Monoclonal plasma cell disorder | 38 | 100 | |
| VEGF, pg/mL | 19 | 375 (<32-3764) | |
| Castleman disease | 3 | 8 | |
| Organomegaly | 22 | 58 | |
| Lymphadenopathy | 16 | 42 | |
| Hepatomegaly | 6 | 16 | |
| Splenomegaly | 12 | 32 | |
| Endocrinopathy | 33 | 87 | |
| Serum immunoglobulin isotypes | NA | ||
| IgG/IgA | 27/7 | 71/18 | |
| κ/λ | 2/32 | 5/84 | |
| Nonsecretory | 4 | 11 | |
| Bone marrow | 38 | 100 | |
| Plasma cells, % | NA | 1 (1-20*) | |
| Polyclonal/monoclonal | 38/0* | 100/0* | |
| Skin involvement | 30 | 79 | |
| Papilledema | 5 | 13 | |
| DLCO, % predicted | 15 | 67 (34-108) | |
| RSVP, mm water | 11 | 34 (20-62) | |
| Thrombocytosis or erythrocytosis | 22 | 58 | |
| Extravascular volume overload | 31 | 82 | |
| Lower extremity edema | 28 | 74 | |
| Pericardial effusion | 3 | 8 | |
| Pleural effusion | 5 | 13 | |
| Ascites | 7 | 18 | |
| Bone lesions per patient | NA | 1 (1-6) | |
| Bone lesions with sclerosis | 35 | 92 | |
| Number of bone lesions in total† | 66 | ||
| Spine‡ | 19 | 29 | |
| Pelvis | 31 | 47 | |
| Long bone | 5 | 8 | |
| Rib, scapula, clavicle, or sternum | 11 | 17 |
MPD, myeloproliferative disorder; RSVP, right ventricular systolic pressure; NA, not applicable/available.
There was 1 biclonal patient with iliac crest bone marrow containing clonal plasma cells not related to POEMS syndrome that instead related to concomitant smoldering multiple myeloma.
N refers to the number of lesions, not patients, and the percentage is calculated from total lesions.
Spine only includes cervical, thoracic, and lumbar regions.
Twenty-two patients who were included in the current series received therapies directed at presumptive (but incorrect) diagnoses of CIDP or essential thrombocytosis before their POEMS diagnoses. Most of these therapies were given within a year before radiation: corticosteroids (n = 18, excluding 1 patient who got corticosteroids in close proximity to irradiation); intravenous gamma globulin (n = 13); plasmapheresis (n = 10); rituximab (n = 2); hydroxyurea (n = 3); or cyclosporine, azathioprine, or mycophenolate (n = 4). None of these, with the exception of corticosteroids, has an impact on the plasma cell dyscrasia.
Study end points
The primary end points of the study were response and event-free survival. Both death and the reinstitution of corticosteroids, further radiation, or additional chemotherapy were considered events. Time to progression was not specifically emphasized because follow-up was not standardized. Responses of patents were categorized in accordance with the definitions previously published in D’Souza et al12 and Kyle and Rajkumar.13 Four types of response were considered: VEGF, fluorodeoxyglucose–positron emission tomography (FDG-PET) avidity, hematologic response, and clinical response. If there was no abnormality or an insufficient abnormality in any of the categories, these patients were considered not assessable for that category. Disease relapse was defined as initial improvement with subsequent worsening.
Hematologic responses.
Hematologic responses were modified from uniform response criteria for multiple myeloma13,14 and included complete response (CRH), negative bone marrow and negative immunofixation of serum and urine; very good partial response (VGPRH), 90% reduction in M-protein or immunofixation-positive as long as the baseline was at least 0.5 g/dL; partial response (PRH), 50% reduction in M-protein or immunofixation-positive as long as the baseline M-protein was at least 1.0 g/dL; and no response (NRH), not fulfilling the above criteria. To qualify as a CRH, patients were not required to have a repeat bone marrow aspirate if the baseline bone marrow was negative.
VEGF responses.
A complete response for plasma VEGF (CRV) was normalization of VEGF (less than 87 pg/mL). A partial response (PRV) had a decrease of ≥50% (baseline must be ≥200 pg/mL). No plasma VEGF level response (NRV) was defined as failure to meet the previous criteria.
FDG-PET responses.
A complete FDG-PET response (CRR) was defined as the disappearance of all initial spots of FDG avidity. A partial FDG-PET response (PRR) was defined as ≥50% improvement in Standardized Uptake Units of all initial spots of FDG avidity. No FDG-PET response (NRR) was defined as failure to meet the previous criteria.
Clinical response.
There were 4 clinical response categories: clinical improvement (IC), clinical progression (PC), mixed clinical response (MC), and clinical stability (SC). The symptoms and signs eligible for clinical response included peripheral neuropathy, organomegaly, papilledema, erythrocytosis, thrombocytosis, endocrinopathy, extravascular fluid overload (ascites/effusions/edema), and abnormal pulmonary function tests. Peripheral neuropathy was not further quantified in a standardized scale as only a handful of the patients had electromyograms or highly detailed functional statuses both before and after therapy, and therefore we must rely upon qualitative responses to achieve a semblance of uniformity. Category MC was added to our prior categorization schema to account for heterogeneous responses, and category Sc was added to denote stability.
Statistical analyses
Statistical analyses were performed using JMP statistical software (SAS, Carey, NC). Fisher exact and Kruskal-Wallis tests were used to define differences among categorical and continuous variables, respectively. Survival and event-free survival were calculated from the start of radiation and were estimated using the method of Kaplan-Meier. Two patients had a very short follow-up of less than 2 months after radiation and thus have been excluded from all analyses, except those referring to baseline characteristics and time to radiation from diagnosis. Cox modeling was used to identify baseline risk factors for treatment failure.
Results
The median age of the 38 patients was 50 years (range: 19-82) (Table 1). Seventy-six percent were male, and 82% were Caucasian. Thirty-two percent had an Eastern Cooperative Oncology Group performance status (PS) of 2, and 29% had a PS of 3. All had a polyneuropathy and a monoclonal plasma cell proliferative disorder. Ninety-two percent had sclerotic bone lesions (the rest having only lytic lesions); 74% of those tested had VEGF elevations (median 375 pg/mL, with a normal range of 31-86 pg/mL), and 8% had Castleman disease. Four patients, who had a normal VEGF at the time of diagnosis of POEMS syndrome, had received at least 6 months of corticosteroid therapy for their presumptive diagnosis of CIDP.
The immunoglobulin heavy chain isotypes were IgG in 27 patients (71%) and IgA in 7 (18%), and the light chain isotypes were λ in 32 (84%) and κ in 2 (5%). The 4 patients with nonsecretory disease had λ clonality documented by biopsy of a bone plasmacytoma. Iliac crest bone marrow aspirates with biopsy were performed on all patients. The median bone marrow plasmacytosis on iliac crest aspirate/biopsy was 1% (range: 1%-20%). One patient had concomitant smoldering myeloma (20% κ restricted plasma cells) and POEMS syndrome due to a λ restricted plasmacytoma; his marrow had no evidence of λ restricted plasma cells. Excluding this unusual patient’s bone marrow result, the bone marrow plasmacytosis ranged up to 10%. The median number of bone lesions was 1 (range: 1-6); only 3 patients had more than 3 lesions. The sizes of the lesions were typically 2 to 6 cm in the largest dimension.
The median time from symptom onset to diagnosis was 19 months (range: 1.1-70 months) (Table 2). Median time from diagnosis of POEMS syndrome to radiation was 0.7 months (range: 0-9.4 months). In total, 54 lesions of the possible 66 were irradiated. Of the 12 lesions that were not irradiated, most were small equivocal spinal lesions. The decision to not treat all possible lesions was made at the discretion of the treating physician due to the lesions being considered unrelated or incidental. The median dose of radiation was 45 Gy (range: 35-54 Gy). Eighty-four percent had all lesions irradiated. Of the 54 lesions that were irradiated, 22% were in the spine, 52% were in the pelvis, 9% were in the long bones, and 17% were in the other bones including ribs, scapula, clavicle, and sternum.
Table 2.
Radiation therapy
| Characteristic | N | Median | Range |
|---|---|---|---|
| Time from symptom onset to diagnosis, m | 37* | 19 | 1.1-70 |
| Time from diagnosis to radiation, m | 38 | 0.7 | 0-9.4 |
| Number of lesions irradiated | 54 | NA | |
| Patients with all lesions irradiated | 32 | NA | |
| Site irradiated | |||
| Spine | 12 | NA | |
| Pelvis | 28 | NA | |
| Long bone | 5 | NA | |
| Rib, scapula, clavicle, sternum | 9 | NA | |
| Dose, cGy | 32† | 4500 | 3500-5400 |
| Fractions | 32† | 25 | 15-30 |
NA, not applicable/available.
One patient had poor documentation of onset and was excluded.
All patients received irradiation, but specifics were not always available.
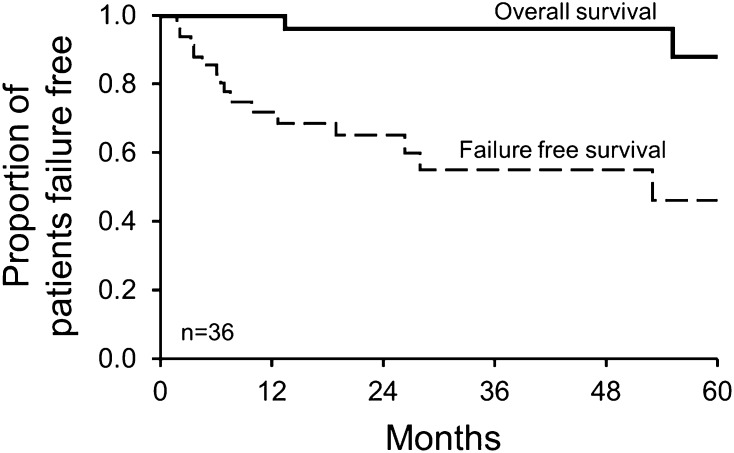
The median follow-up of patients after radiation was 43 months, and the 4-year overall survival was 97% (Figure 1). Eight patients had a hematologic CRH, 2 had a VGPRH, 1 had a PRH, 15 had NRH, and the remaining were nonsecretory (n = 4) or not calculable (n = 6) (Table 3 and Figure 2). Of the 14 patients that had VEGF elevations at baseline, 4 had a CRV, 1 had a PRV, 5 had NRV, and the rest were not documented. Of the 16 patients that had FDG-PETs performed, 4 had a CRR, 4 had a PRR, 5 had NRR, and the remaining did not have postradiation scans to analyze. In terms of clinical responses, 47% had an IC, 28% had a MC, 6% a SC, and 19% had a PC. Patients who had improvement of their neuropathy typically had a marked reduction of their disability although they often were left with minor disability, for example, a patient who had been unable to walk could now do so but with the help with a cane or ankle braces. Concerning the pulmonary response rates of the 5 patients with abnormal total lung capacity, 2 improved, 1 worsened, and 2 were not evaluated afterward. Of the 11 patients with abnormal diffusing capacity of the lung (DLCO), 6 improved, 1 worsened, and 4 were not evaluated afterward.
Figure 1.
Overall survival and failure-free survival. Four-year overall survival was 97%, and 4-year failure-free survival was 52%. Failure is defined as death, for overall survival, and as time of next therapy, for failure-free survival.
Table 3.
Tabulated responses (N = 36 patients)
| Type of response | N | % |
|---|---|---|
| Hematologic response | ||
| Complete response | 8 | 22 |
| Very good partial response | 2 | 6 |
| Partial response | 1 | 3 |
| No response | 15 | 42 |
| Not assessed or assessable | 10 | 28 |
| VEGF response | ||
| Complete response | 4 | 11 |
| Partial response | 1 | 3 |
| No response | 5 | 14 |
| Not assessed or assessable | 26 | 72 |
| FDG-PET response | ||
| Complete response | 4 | 11 |
| Partial response | 4 | 11 |
| No response | 5 | 14 |
| Not assessed or assessable | 23 | 64 |
| Clinical response | ||
| Improved | 17 | 47 |
| Mixed | 10 | 28 |
| Stable | 2 | 6 |
| Progressed | 7 | 19 |
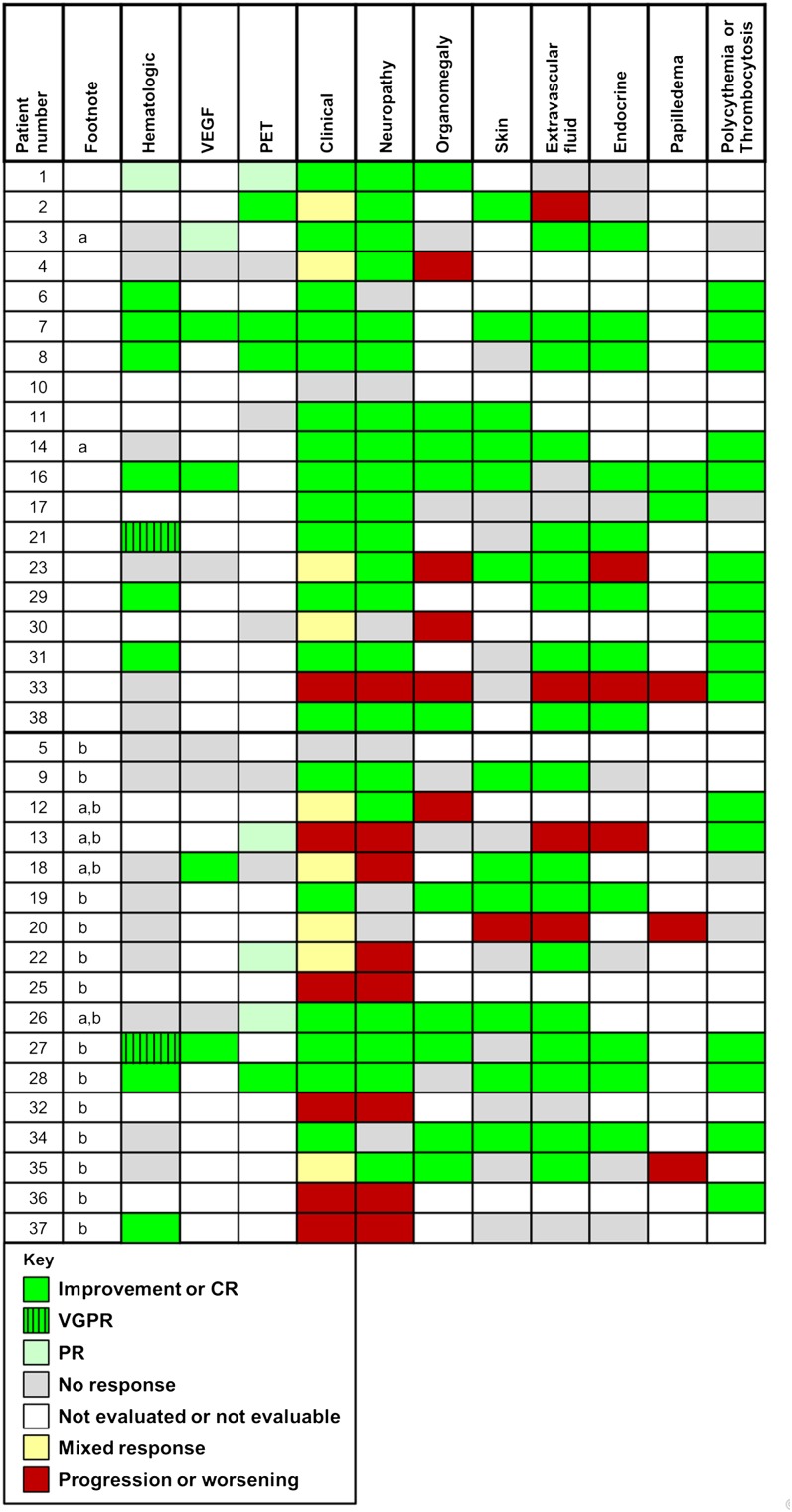
Figure 2.
Individual patient responses. These patients (footnote a) did not have all of their lesions irradiated. These patients (footnote b) received additional therapy after radiation.
Overall, 17 patients (48%) received salvage therapy. Median time to therapy for those receiving salvage was 7.6 months (range: 2-73 months) after irradiation. Event-free survival was 52% at 4 years (Figure 1 and Table 4). The most common indications for additional therapy after radiation were neurologic worsening in 8, insufficient neurologic improvement in 3, radiographic worsening in 4, both new bone lesions and neurologic worsening in 1, and new papilledema in 1. The most common secondary therapies received at progression or relapse were ASCT in 10 and additional radiation in 3 patients.
Table 4.
Patients who received further therapy
| Patient | Time to next therapy, m | Reason for next therapy | Next therapy |
|---|---|---|---|
| 36 | A) 1.6 | Worsening PN | A) Doxorubicin, corticosteroids |
| B) 4.1 | B) ASCT | ||
| 37 | 2.2 | Worsening PN | Bevacizumab, thalidomide |
| 18* | A) 3.3 | A) Worsening PN but improved skin findings and extravasation | A) CTX, corticosteroids |
| B) 32 | B) New bone lesion | B) Irradiation | |
| 13* | 3.5 | Worsening PN, lower extremity edema, endocrinopathy | ASCT |
| 25 | 4.6 | Worsening PN | ASCT |
| 32 | 6.0 | Worsening PN | ASCT |
| 9† | 6.5 | Return of fatigue and edema, worsening PN despite initial improvement (PN, skin, volume overload, and PET/CT) | ASCT |
| 5 | 6.9 | Inadequate PN improvement and worsening VEGF | ASCT |
| 20 | 7.6 | Inadequate PN improvement with worsening skin findings, volume overload, and papilledema | ASCT |
| 22 | 10.0 | Worsening PN and persistent M-spike | ASCT |
| 19 | 12.6 | Inadequate PN improvement | Lenalidomide, corticosteroids |
| 35† | A) 19 | A) New papilledema | A) Corticosteroids |
| B) 71 | B) New calvarial lesion | B) Irradiation | |
| 26*,† | 26.3 | Worsening VEGF and new bone lesions | ASCT |
| 12* | A) 28 | A) New bone lesions and adenopathy | A) Corticosteroids |
| B) 35 | B) Worsening PN | B) CTX, corticosteroids | |
| C) 43 | C) Worsening PN | C) CTX, corticosteroids | |
| 34† | 53 | Worsening VEGF, new bone lesions (20 m), new bone marrow involvement (27 m), and worsening skin (49 m) | ASCT |
| 27† | 66 | Worsening erythrocytosis and bone lesions by PET/CT | Lenalidomide, corticosteroids |
| 28† | 73 | Reemergence of M-spike and new bone lesion | Irradiation and CyberKnife concurrently |
CTX, cyclophosphamide; PN, peripheral neuropathy.
In these patients not all bone lesions initially were irradiated.
This patient met the criteria for relapsed disease, that is, initial improvement but then worsening.
Six of the patients receiving salvage therapy had early response with eventual relapse. Among these relapsing patients, the median time to the next therapy was 40 months (range: 6.5-73 months) (Table 4). The 3 patients that had their first relapse after 36 months were asymptomatic and were found by surveillance. Only 2 patients developed symptomatic relapse: one developed reemergence of all of his prior clinical symptoms including edema, neuropathy, and adenopathy; and the other developed new papilledema.
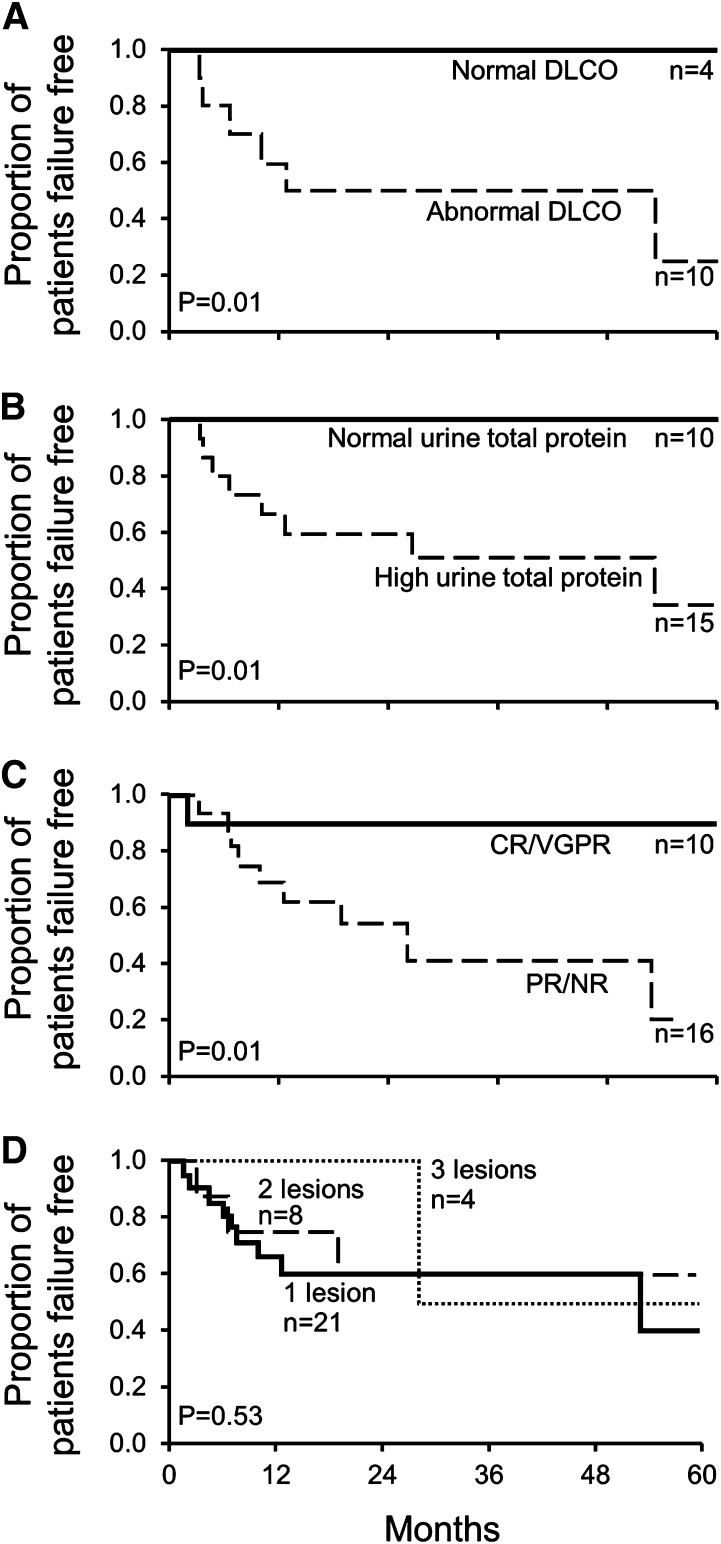
Two baseline factors were identified that predicted for treatment failure (Figure 3A-B): DLCO <75%, P = .01, and elevated urine total protein (≥0.093 g per 24 hours), P = .01. Looking at the period after irradiation, patients that achieved a CRH or a VGPRH had significantly less risk of treatment failure compared with patients who had only a partial or no hematologic response, P = .014 (Figure 3C). The treatment failure curve for patients with nonevaluable responses was similar to the curve of the patients with NRH and PRH (data not shown). Only 3 of 10 patients with a CRH or VGPRH failed treatment: 2 developed new lesions after 5 years, and 1 failed treatment at 3 months due to progressive peripheral neuropathy symptoms alone but in the absence of any evidence of hematologic relapse. Among the 33 patients with baseline bone lesions ranging between 1 to 3, increasing numbers of lesions did not predict for treatment failure (Figure 3D). For patients who did not have all possible (including the equivocal) lesions treated, there was not an increased risk for treatment failure.
Figure 3.
Baseline risk factors predicting for further therapy. (A) Abnormal baseline DLCO (<75% predicted) increases risk. (B) High urine total protein (≥0.093 g per 24 hours) increases risk. (C) Lack of CR or VGPR increases risk. (D) Increasing bone lesions does not increase risk. This figure does not include 3 patients who had more than 3 lesions.
Discussion
We have demonstrated that approximately 26% of patients with POEMS syndrome are candidates for definitive therapy with radiation and that these same patients treated with radiation therapy have a 4-year progression-free survival of 52% and a 4-year overall survival rate of 97%. Irradiating 1 to 3 lesions in patients with POEMS syndrome with negative bone marrows has the potential to improve peripheral neuropathy, anasarca, organomegaly, papilledema, skin changes, serum M-spikes, and plasma VEGF levels. The fact that nearly half of our patients required additional therapy illustrates that radiation is not a panacea, but that it is a simple and effective option for a subset of patients with the disease. It is also striking that despite the fact that nearly 40% of the POEMS syndrome cohort had more than 1 bone lesion, rates of progression and overall survival were excellent without any clear difference in risk of relapse in patients with 1 or 3 lesions.
These data are important not only in terms of clarifying expectations for patients with POEMS syndrome and their physicians, but also in terms of emphasizing the biological differences between the malignant plasma cells in patients with POEMS as compared with those with SPB. This study surprisingly shows that, when compared with SPB, there were major differences in outcomes. The event-free rates of our patients were similar to or slightly better than those expected among patients with SBP who have 5-year progression-free survival rates of 36% to 60%. Our patients had 5-year overall survival rates that compared favorably to the 74% to 87% reported for SPB patients.5,15-20 Based on present data and the published literature,5,15-17 fundamental differences between SPB and POEMS syndrome include the types of lesions (lytic in the former and sclerotic/mixed in the latter), the clonal preference (κ in former and λ in the latter), the presenting symptoms (bone pain in the former and polyneuropathy in the latter), types of progression (to myeloma in the former and to progressive POEMS syndrome in the latter), and overall survival. Shared characteristics include age (midfifties), high rates of local control, and rates of disappearance of monoclonal protein after radiation (20%-50% in both). Reported factors predictive of relapse among patients with SPB are osteopenia, low levels of uninvolved immunoglobulins, persistence of monoclonal protein after radiation, nonsecretory disease,5 abnormal serum immunoglobulin free light chain ratio at baseline,21 tumor size greater than 5 cm, a nonvertebral presentation,22 and inadequate staging.23 In contrast, in patients with POEMS syndrome undergoing radiation, risks for relapse/progression were abnormal baseline DLCO and elevated urinary total protein.
Because most of the bone lesions in patients with POEMS syndrome are at least in part sclerotic, it can be difficult to decide whether a small area of sclerosis is part of the disease or a benign bone island, fibrous dysplasia, a nonossifying fibroma, or an aneurysmal bone cyst. The mostly densely sclerotic bone lesions in POEMS syndrome do not take up FDG, making that test unreliable in differentiating between a benign lesion and a lesion due to underlying plasma cells. Several such equivocal lesions progressed in the current series, while others have not, and since there was not a significant increase in the risk of progression in patients with untreated lesions, the decision to treat all questionable lesions will still have to be left to the individual patient and physician.
Although the FDG-PET/CT scan is very useful for tracking the response of the disease and in finding new sites of activity, it still misses very small and very sclerotic lesions. We recommend that these scans still be used before and after but should be used in combination with complete blood counts, VEGFs, serum protein electrophoresis with immunofixation, and 24-hour urine protein electrophoresis with immunofixation. Markers of endocrinopathy should only be tracked if, at baseline, they were abnormal, as no one who progressed was discovered to have progressed based on these labs alone.
A major limitation of the present study is that it is a small (albeit the largest of its kind) retrospective series without uniform decision criteria used to designate when and if salvage therapy should have been instituted. Because neuropathy typically takes approximately 3 months to stabilize and 6 months to begin to improve, with maximal improvements seen 2 to 3 years after definitive therapy, patients and their physicians can become impatient, potentially instituting unnecessary additional treatment. The radiation approach appears to have inferior event-free survival compared with ASCT,24 but overall survival rates are comparable, demonstrating that these patients are easily salvaged if radiation does not suffice. Moreover, the need for salvage therapy may therefore be overestimated in this series. Finally, this study does not address the role radiation plays in patients with disseminated bone marrow involvement because all of our patients had random iliac crest bone marrow biopsies without evidence of the POEMS plasma cell clone.
Despite these limitations, the following conclusions can be drawn. First, only half the patients failed treatment at 4 years, and therefore radiation is shown to be sufficient to produce durable meaningful responses. Second, misdiagnosis is common, resulting in two-thirds of patients having received prior therapies like corticosteroids, intravenous immunoglobulins, and plasmapheresis. Finally, salvage with other therapies is possible among patients progressing through or relapsing after primary radiation therapy as evidenced by the excellent overall survival rates in the present study. The hope is that with heightened awareness of this syndrome, more physicians will be aware of this rare disease, which could translate into earlier diagnosis, less morbidity, and potentially durable complete remissions.
Acknowledgments
The authors thank Kate Johanns for maintaining the Dysproteinemia Database.
This work was supported by the JABBS Foundation, the Predolin Foundation, the Robert A. Kyle Hematologic Malignancies Fund, and the Andrew and Lillian A. Posey Foundation.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: M.S.H. abstracted the data, performed statistical analysis, and wrote the manuscript; M.A.G., M.Q.L., R.A.K., S.R.H., S.K.K., J.A.L., T.E.W., F.K.B., S.V.R., S.R.Z., S.J.R., D.D., Y.L., P.K., and N.L. assisted in manuscript preparation; A.D. designed the concept, analyzed and interpreted the data, and wrote the manuscript; and all authors reviewed the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Angela Dispenzieri, Mayo Clinic, 200 First St SW, Rochester, MN 55905; e-mail: dispenzieri.angela@mayo.edu.
References
- 1.Dispenzieri A. POEMS syndrome: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86(7):591–601. doi: 10.1002/ajh.22050. [DOI] [PubMed] [Google Scholar]
- 2.Royer B, Merlusca L, Abraham J, et al. Efficacy of lenalidomide in POEMS syndrome: a retrospective study of 20 patients. Am J Hematol. 2013;88(3):207–212. doi: 10.1002/ajh.23374. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zhang W, Jiao L, et al. Combination of melphalan and dexamethasone for patients with newly diagnosed POEMS syndrome [published online ahead of print March 12, 2011]. Blood. 2011;117(24):6445–6449. doi: 10.1182/blood-2010-12-328112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soutar R, Lucraft H, Jackson G, Reece A, Bird J, Low E, Samson D Working Group of the UK Myeloma Forum; British Committee for Standards in Haematology; British Society for Haematology. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin Oncol (R Coll Radiol) 2004;16(6):405–413. doi: 10.1016/j.clon.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 5.Liebross RH, Ha CS, Cox JD, Weber D, Delasalle K, Alexanian R. Solitary bone plasmacytoma: outcome and prognostic factors following radiotherapy. Int J Radiat Oncol Biol Phys. 1998;41(5):1063–1067. doi: 10.1016/s0360-3016(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 6.Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome[published online ahead of print November 29, 2002]. Blood. 2003;101(7):2496–2506. doi: 10.1182/blood-2002-07-2299. [DOI] [PubMed] [Google Scholar]
- 7.Morley JB, Schwieger AC. The relation between chronic polyneuropathy and osteosclerotic myeloma. J Neurol Neurosurg Psychiatry. 1967;30(5):432–442. doi: 10.1136/jnnp.30.5.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis LE, Drachman DB. Myeloma neuropathy. Successful treatment of two patients and review of cases. Arch Neurol. 1972;27(6):507–511. doi: 10.1001/archneur.1972.00490180043010. [DOI] [PubMed] [Google Scholar]
- 9.Reitan JB, Pape E, Fosså SD, Julsrud OJ, Slettnes ON, Solheim OP. Osteosclerotic myeloma with polyneuropathy. Acta Med Scand. 1980;208(1-2):137–144. doi: 10.1111/j.0954-6820.1980.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwashita H, Ohnishi A, Asada M, Kanazawa Y, Kuroiwa Y. Polyneuropathy, skin hyperpigmentation, edema, and hypertrichosis in localized osteosclerotic myeloma. Neurology. 1977;27(7):675–681. doi: 10.1212/wnl.27.7.675. [DOI] [PubMed] [Google Scholar]
- 11.Shibuya K, Misawa S, Horikoshi T, et al. Detection of bone lesions by CT in POEMS syndrome. Intern Med. 2011;50(13):1393–1396. doi: 10.2169/internalmedicine.50.5263. [DOI] [PubMed] [Google Scholar]
- 12.D’Souza A, Lacy M, Gertz M, et al. Long-term outcomes after autologous stem cell transplantation for patients with POEMS syndrome (osteosclerotic myeloma): a single-center experience [published online ahead of print May 23, 2012]. Blood. 2012;120(1):56–62. doi: 10.1182/blood-2012-04-423178. [DOI] [PubMed] [Google Scholar]
- 13.Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009;23(1):3–9. doi: 10.1038/leu.2008.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. Leukemia. 2006;20(9):1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 15.Dimopoulos MA, Moulopoulos LA, Maniatis A, Alexanian R. Solitary plasmacytoma of bone and asymptomatic multiple myeloma . Blood. 2000;96(6):2037–2044. [PubMed] [Google Scholar]
- 16.Mill WB. Radiation therapy in multiple myeloma. Radiology. 1975;115(1):175–178. doi: 10.1148/115.1.175. [DOI] [PubMed] [Google Scholar]
- 17.Frassica DA, Frassica FJ, Schray MF, Sim FH, Kyle RA. Solitary plasmacytoma of bone: Mayo Clinic experience. Int J Radiat Oncol Biol. 1989;16(1):43-48. [DOI] [PubMed]
- 18.Suh YG, Suh CO, Kim JS, Kim SJ, Pyun HO, Cho J. Radiotherapy for solitary plasmacytoma of bone and soft tissue: outcomes and prognostic factors. Ann Hematol. 2012;91(11):1785–1793. doi: 10.1007/s00277-012-1510-6. [DOI] [PubMed] [Google Scholar]
- 19.Ozsahin M, Tsang RW, Poortmans P, et al. Outcomes and patterns of failure in solitary plasmacytoma: a multicenter Rare Cancer Network study of 258 patients. Int J Radiat Oncol Biol Phys. 2006;64(1):210–217. doi: 10.1016/j.ijrobp.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 20.Bolek TW, Marcus RB, Jr, Mendenhall NP. Solitary plasmacytoma of bone and soft tissue. Int J Radiat Oncol Biol Phys. 1996;36(2):329–333. doi: 10.1016/s0360-3016(96)00334-3. [DOI] [PubMed] [Google Scholar]
- 21.Dingli D, Kyle RA, Rajkumar SV, et al. Immunoglobulin free light chains and solitary plasmacytoma of bone [published online ahead of print June 3, 2006]. Blood. 2006;108(6):1979-1983. [DOI] [PMC free article] [PubMed]
- 22.Knobel D, Zouhair A, Tsang RW, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006;6:118. [DOI] [PMC free article] [PubMed]
- 23.Warsame R, Gertz MA, Lacy MQ, et al. Trends and outcomes of modern staging of solitary plasmacytoma of bone. Am J Hematol. 2012;87(7):647–651. doi: 10.1002/ajh.23201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Imai N, Taguchi J, Yagi N, Konishi T, Serizawa M, Kobari M. Relapse of polyneuropathy, organomegaly, endocrinopathy, M-protein, and skin changes (POEMS) syndrome without increased level of vascular endothelial growth factor following successful autologous peripheral blood stem cell transplantation. Neuromuscul Disord. 2009;19(5):363–365. doi: 10.1016/j.nmd.2009.02.004. [DOI] [PubMed] [Google Scholar]