Abstract
Rothia aeria caused a necrotic lymphadenitis and neck abscess in a patient with CGD. This infection was aggressive, crossed tissue planes, required two surgeries, as well as prolonged antibiotics for complete resolution. Rothia aeria is a rare pathogen that can be added to the spectrum of agents causing disease in CGD, a finding that further reinforces the importance of microbiologic identification of infections in this patient population.
Keywords: Chronic granulomatous disease, Rothia aeria, neck abscess, lymphadenitis
Introduction
Chronic granulomatous disease (CGD) is a phagocyte defect characterized by recurrent life-threatening bacterial and fungal infections dueto defective NADPH oxidase activityleading to impaired superoxide and downstream hydrogen peroxide (H2O2) production. This defect can be inherited as an X-linked trait, affecting the NADPH oxidase gp91phox subunit (CYBB [cytochrome b-245, alpha polypeptide]), or as an autosomal recessive trait, affecting the NADPH oxidase subunits (p22phox (CYBA [cytochrome b-245, beta polypeptide]), p47phox (NCF1 [neutrophil cytosolic factor 1]), p67phox (NCF2 [neutrophil cytosolic factor 2]), and p40phox (NCF4 [neutrophil cytosolic factor 4]) [1]. These recurrent infections primarily affect the lung, skin, lymph nodes and liver, and are typically due to a rather narrow spectrum of bacteria and fungi, including Staphylococcus aureus, Serratia marcescens, Burkoldheria cepacia complex, Nocardia species and Aspergillus species. The mechanisms behind these pathogen susceptibility patterns remain to be fully elucidated and are likely complex [2]. Emerging pathogens (e.g. Granulibacter bethesdensis) [3], previously misidentified pathogens (e.g. Neosartorya udagawae) [4] and common pathogens (e.g. streptococci) [5] have also been identified in CGD.
We report necrotic cervical lymphadenitis due to Rothia aeria in a patient with CGD. This infection was aggressive, crossed tissue planes, and required two surgical interventions, as well as a prolonged course of antibiotics for complete resolution.
Case Presentation
A 19 year-old Caucasian man from Ohio presented with an eight-week history of a right-sided neck mass while being maintained on prophylactic trimethoprim-sulfamethoxazole, moxifloxacin and posaconazole. The mass began as a small bump that waxed and waned. After 2 weeks, it became fluctuant, erythematous, and tender measuring approximately eight centimeters in diameter. A prednisone taper was without clinical response. He denied any fevers, chills or sweats, but did have a ventriculoperitoneal shunt catheter tracking posterior to the mass.
The patient was diagnosed with X-linked CGD at birth due to a largeintragenicdeletion in CYBB but sparing Xk based on a positive family history. At 5 months of age, a ventriculoperitoneal shunt was placed for hydrocephalus. This was complicated by fungal meningitis, resulting in its removal and subsequent replacement at 16 months of age. At 11 years he had sepsis of unclear etiology and later developed Aspergillus fumigatus pneumonia. By 15 years he developed a lower gastro-intestinal bleed, which was diagnosed as CGD proctitis, treated with pramoxine-hydrocortisonefoam for 1 year. He had no history of infectious contacts, foreign travel, recent dental or surgical procedures and had been clinically well for 3 years prior to his current presentation with the exception of one episode of left neck lymphadenitis of unknown etiology.
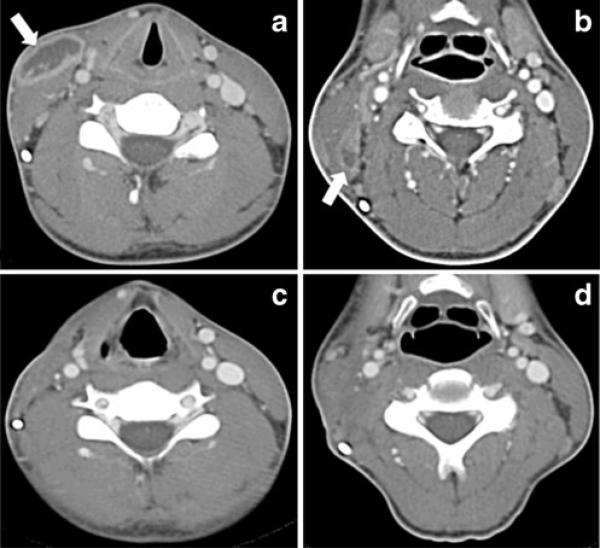
He was afebrile with an 8 cm fluctuant mass overlying the right sternocleidomastoid muscle. Oro-pharynx was clear with no evidence of poor dentition or dental abscesses. His C-reactive protein was 24 mg/L and his erythrocyte sedimentation rate 7 mm/h. Complete blood count was normal. Computed axial tomography (CT) of the neck with intravenous contrast showed a 6×3×1.5 cm fluid density with enhancing periphery that was partially ventral to the right sternocleidomastoid muscle compatible with a subcutaneous soft tissue abscess and two subcentimeter foci deep to the sternocleidomastoid muscle (Fig. 1a). Ultrasound confirmed the mass surrounding the ventriculoperitoneal shunt catheter.
Fig. 1.
A CT scan of the neck at presentation. Irregular 6× 3× 1.5 cm fluid density with enhancing periphery. b CT scan of the neck during second admission, prior to neck dissection. Interval resolution of the abscess overlying the right sternocleidomastoid with interval development of a cluster of necrotic nodes (level II B) deep to the right sternocleidomastoid muscle. c and d CT scan of the neck 2 months after neck dissection. Evidence of previous right neck surgery with loss of the fascial fat planes. The right sternocleidomastoid muscle is diminished with right vocal cord paralysis. No pathologic lymphadenopathy or neck masses are seen
Incision and drainage released frank pus; a drain was placed. Gram stain showed few neutrophils and no organisms. Cultures in liquid media only grew a branching gram-positive rod identified as Rothia species by MALDI-TOF MS (see below). The patient received amoxicillin-clavulanate for 14 days. Two months later he returned with 2 days of right neck swelling and tenderness with a pustule at his previous incision. CTscan of the neck showed interval resolution of the previous abscess overlying the right sternocleidomastoid. However, necrotic nodes deep to the sternocleidomastoid muscle (Fig. 1b) led to a right neck dissection of a granulomatous lymphadenitis and intramuscular sternocleidomastoid abscess. Gram stain again showed few neutrophils without organisms. Culture of the abscess fluid grew a gram-positive organism. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry(MALDI-TOF MS) using a MALDI-TOF MicroFlex LT mass spectrometer (Bruker Daltonics, Billerica, MA) identified it as Rothia species with best match to R. aeria (highest score 0 2.0). Full 16S rRNA gene sequencing (~1,500 bp) confirmed the species level identification with 99.8 % match to R. aeria type strain. Antibiotic susceptibility testing performed by the Microscan microdilution method using the Streptococcus panel showed the following minimal inhibitory concentrations [MIC] in μg/ml: penicillin (MIC00.06), clindamycin (MIC00.5), azithromycin (MIC0<0.25), levofloxacin (MIC>4) and meropenem (MIC>0.5).
Meropenem was switched to high dose penicillin based on in vitro sensitivities. He was discharged on amoxicillin 1 g and probenicid 500 mg, each orally three times daily. After 2 weeks a right neck hematoma was drained. The patient continued to improve clinically. His inflammatory markers returned to normal, and follow up CT scan 2 months later showed no lymphadenopathy, masses or abscesses (Fig. 1c and d).
Discussion
R. aeria belongs to the genus Rothia, family Micrococcaceae. The genus Rothia was proposed in 1967 with R. dentocariosa as the type species (first isolated from dental caries). Only in 2000 was the second species, R. nasimurium, added, followed by R. mucilaginosa, and R. amarae. R. aeria was first identified in 2004, and like most Rothia species, is an oral colonizer. However, it owes its name to its first isolation from air and condensation water samples from the Russian space laboratory, Mir. Rothia species are aerobic, Gram-positive, mostly catalase-positive, non-acid-fast, non-spore-forming, non-hemolytic, and non-motile bacteria that may appear coccoid, cocco-bacillary or filamentous [6].
In the general population, infections with Rothia species are rare. Most reported cases are due to infections with R. dentocariosa, followed byinfections with R. mucilaginosa. R. dentocariosa has been associated with severediseases such as endocarditis [7] and bacteremia [8], peritonitis (especially in the peritoneal dialysis population)[9], pneumonia [10], septic arthritis [11], and ocular infections such as corneal ulcers [12] and endopthalmitis [13]. R. mucilaginosa is less commonly reported, but has been associated with bacteremia [14], peritonitis [15], meningitis [16], pneumonia [17], and septic arthritis [18]. Only four infections due to R. aeria have been reported including sepsis, septic arthritis, acute bronchitis and pneumonia (Table I). In three out of four cases, the patients were on immunosuppressive medications, and two cases were associated with dental pathology. All patients survived antibiotic therapy.
Table I.
Reported cases of Rothia aeria infection
| Reference | Age/Gender | Risk Factor | Presentation | Treatment | Outcome |
|---|---|---|---|---|---|
| Present case | 18 years/male | X-linked CGD | Neck abscess with necrotic lymphadenitis | Penicillin G followed by amoxicillin and probenecid | Survived |
| [19] | 88 years/female | Dental abscesses, methotrexate and prednisone for rheumatoid arthritis | Septic shoulder | Penicillin G, 14 day course | Survived |
| [20] | 66 years/male | Etanercept for rheumatoid arthritis, diabetes | Acute bronchitis | Amoxicillin and cefpodoxime, 3 week course followed by amoxicillin-clavulanate and moxifloxacin, 1 week course | Survived |
| [21] | 53 years/female | Azathioprine and steroids for neurosarcoidosis | Cavitary pneumonia | Benzylpenicillin, 3 month course followed by amoxicillin, 5 month course | Survived |
| [22] | Neonate/female | Mother underwent extraction of decayed tooth 4 days prior to delivery without antibiotic prophylaxis | Sepsis | Ampicillin and cefotaxime, 11 day course | Survived |
Patients with CGD are generally prone to infections by catalase-producing organisms, which are thought to degrade both microbial and host-derived H2O2. R. aeria is a catalase-positive organism and an oral colonizer. Our patient's risk factors for infection with R. aeria included his underlying immunodeficiency with CGD and low dose intermittent steroids for CGD colitis. He did not, however, have poor dentition. R. aeria is typically resistant to both levofloxacin and meropenem.
This unusual and fastidious pathogen caused recurrent, extensive, persistent disease in a patient with CGD, a rare genetic phagocyte disorder. R. aeria should be suspected and pursued diagnostically in the CGD population, especially when conventional microbiological methods are unrevealing. Rothia aeria is a rare pathogen that can be added to the spectrum of agents causing disease in CGD, a finding that further reinforces the importance of microbiologic identification and susceptibility testing in infections in this patient population. This is the first reported case of necrotizing cervical lymphadenitis due to R. aeria in a patient with CGD, and to date the only soft tissue infection caused by this organism reported in the literature.
Acknowledgments
Funding This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD 20892. The views expressed in this article are those of the authors and do not reflect the official policy of the U.S. Government.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Contributor Information
E. Liana Falcone, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, CRC, Rm B3 4141, MSC 1684, Bethesda, MD 20892-1684, USA.
Adrian M. Zelazny, Microbiology Service, Department of Laboratory Medicine, Clinical Center, National Institutes of Health, Bethesda, MD, USA
Steven M. Holland, Laboratory of Clinical Infectious Diseases, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 10 Center Drive, CRC, Rm B3 4141, MSC 1684, Bethesda, MD 20892-1684, USA
References
- 1.Kuhns DB, Alvord WG, Heller T, Feld JJ, Pike KM, Marciano BE, et al. Residual NADPH oxidase and survival in chronic granulomatous disease. N Engl J Med. 2010;363:2600–10. doi: 10.1056/NEJMoa1007097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towbin AJ, Chaves I. Chronic granulomatous disease. Pediatr Radiol. 2010;40:657–68. doi: 10.1007/s00247-009-1503-3. quiz 792-653. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg DE, Shoffner AR, Zelazny AM, Fenster ME, Zarember KA, Stock F, et al. Recurrent Granulibacter bethesdensis infections and chronic granulomatous disease. Emerg Infect Dis. 2010;16:1341–8. doi: 10.3201/eid1609.091800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugui JA, Vinh DC, Nardone G, Shea YR, Chang YC, Zelazny AM, et al. Neosartorya udagawae (Aspergillus udagawae), an emerging agent of aspergillosis: how different is it from Aspergillus fumigatus? J Clin Microbiol. 2010;48:220–8. doi: 10.1128/JCM.01556-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falcone EL, Hanses S, Stock F, Holland SM, Zelazny AM, Uzel G. Streptococcal infections in patients with chronic granulomatous disease: case report and review of the literature. J Clin Immunol. 2012 doi: 10.1007/s10875-012-9661-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Y, Kawamura Y, Fujiwara N, Naka T, Liu H, Huang X, et al. Rothia aeria sp. nov., Rhodococcus baikonurensis sp. nov. and Arthrobacter russicus sp. nov., isolated from air in the Russian space laboratory Mir. Int J Syst Evol Microbiol.;2004;54:827–35. doi: 10.1099/ijs.0.02828-0. [DOI] [PubMed] [Google Scholar]
- 7.Boudewijns M, Magerman K, Verhaegen J, Debrock G, Peetermans WE, Donkersloot P, et al. Rothia dentocariosa, endocarditis and mycotic aneurysms: case report and review of the literature. Clin Microbiol Infection: Off Publ Eur Soc Clin Microbiol Infectious Dis. 2003;9:222–9. doi: 10.1046/j.1469-0691.2003.00503.x. [DOI] [PubMed] [Google Scholar]
- 8.Salamon SA, Prag J. Three cases of Rothia dentocariosa bacteraemia: frequency in Denmark and a review. Scand J Infect Dis. 2002;34:153–7. doi: 10.1080/00365540110076877. [DOI] [PubMed] [Google Scholar]
- 9.Ergin C, Sezer MT, Agalar C, Katirci S, Demirdal T, Yayli G. A case of peritonitis due to Rothia dentocariosa in a CAPD patient. Peritoneal Dialysis Int: J Int Soc Peritoneal Dialysis. 2000;20:242–3. [PubMed] [Google Scholar]
- 10.Bousquet A, Soler C, Martinaud C, Join-Lambert O, Malfuson JV. Pneumonia and Rothia dentocariosa. Med Mal Infect. 2011;41:621–2. doi: 10.1016/j.medmal.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Favero M, Raffeiner B, Cecchin D, Schiavon F. Septic arthritis caused by Rothia dentocariosa in a patient with rheumatoid arthritis receiving etanercept therapy. J Rheumatol. 2009;36:2846–7. doi: 10.3899/jrheum.090276. [DOI] [PubMed] [Google Scholar]
- 12.Morley AM, Tuft SJ. Rothia dentocariosa isolated from a corneal ulcer. Cornea. 2006;25:1128–9. doi: 10.1097/01.ico.0000226362.11431.81. [DOI] [PubMed] [Google Scholar]
- 13.MacKinnon MM, Amezaga MR, MacKinnon JR. A case of Rothia dentocariosa endophthalmitis. Eur J Clin Microb Infectious Dis: Off Publ Eur Soc Clin Microbiol. 2001;20:756–7. doi: 10.1007/s100960100589. [DOI] [PubMed] [Google Scholar]
- 14.Morgan EA, Henrich TJ, Jarell AD, Shieh WJ, Zaki SR, Marty FM, et al. Infectious granulomatous dermatitis associated with Rothia mucilaginosa bacteremia: A case report. Am J Dermatopathol. 2010;32:175–9. doi: 10.1097/DAD.0b013e3181b1c5ad. [DOI] [PubMed] [Google Scholar]
- 15.Hodzic E, Snyder S. A case of peritonitis dueto Rothia mucilaginosa. Peritoneal Dialysis Int: J Int Soc Peritoneal Dialysis. 2010;30:379–80. doi: 10.3747/pdi.2009.00146. [DOI] [PubMed] [Google Scholar]
- 16.Lee AB, Harker-Murray P, Ferrieri P, Schleiss MR, Tolar J. Bacterial meningitis from Rothia mucilaginosa in patients with malignancy or undergoing hematopoietic stem cell transplantation. Pediatric Blood Cancer. 2008;50:673–6. doi: 10.1002/pbc.21286. [DOI] [PubMed] [Google Scholar]
- 17.Fusconi M, Conti C, De Virgilio A, de Vincentiis M. Pauci-symptomatic pneumonia due to Rothia mucilaginosa: case report and literature review. Le Infezioni Med: Riv Period di Eziol, Epidemiol, Diagnostica, Clin Terapia Patologie Infettive. 2009;17:100–4. [PubMed] [Google Scholar]
- 18.Kaasch AJ, Saxler G, Seifert H. Septic arthritis due to Rothia mucilaginosa. Infection. 2011;39:81–2. doi: 10.1007/s15010-010-0065-5. [DOI] [PubMed] [Google Scholar]
- 19.Verrall AJ, Robinson PC, Tan CE, Mackie WG, Blackmore TK. Rothia aeria as a cause of sepsis in a native joint. J Clin Microbiol. 2010;48:2648–50. doi: 10.1128/JCM.02217-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michon J, Jeulin D, Lang JM, Cattoir V. Rothia aeria acute bronchitis: the first reported case. Infection. 2010;38:335–7. doi: 10.1007/s15010-010-0012-5. [DOI] [PubMed] [Google Scholar]
- 21.Hiyamuta H, Tsuruta N, Matsuyama T, Satake M, Ohkusu K, Higuchi K. [First case report of respiratory infection with Rothia aeria]. Nihon Kokyuki Gakkai Zasshi 0 J Jpn Respir Soc. 2010;48:219–23. [PubMed] [Google Scholar]
- 22.Monju A, Shimizu N, Yamamoto M, Oda K, Kawamoto Y, Ohkusu K. First case report of sepsis due to Rothia aeria in a neonate. J Clin Microbiol. 2009;47:1605–6. doi: 10.1128/JCM.02337-08. [DOI] [PMC free article] [PubMed] [Google Scholar]