Abstract
In order to reduce side effects in the course of allergen specific immunotherapy hypoallergenic allergen derivatives with reduced IgE reactivity have been made by genetic engineering. In contrast to other recombinant hypoallergenic allergen derivatives which showed reduced IgE reactivity, a recombinant trimer of the major birch pollen allergen Bet v 1 showed reduced allergenic activity despite preserved IgE reactivity. We studied rBet v 1 trimer by SDS-PAGE, mass spectrometry, circular dichroism and gel filtration. Furthermore we investigated IgE and IgG reactivity of the rBet v 1 trimer in solid and liquid phase assays and compared its allergenic activity with that of rBet v 1 wildtype using basophil activation assays. In solid phase immunoassays rBet v 1 trimer exhibited even stronger IgE reactivity than the rBet v 1 wildtype, whereas both proteins were equally well recognized by Bet v 1-specific IgG antibody probes. In fluid phase IgE experiments rBet v 1 trimer inhibited IgE reactivity to rBet v 1 wildtype but showed a more than 10-fold reduced allergenic activity compared to the rBet v 1 monomer. By analytical gel filtration it was demonstrated that, despite its monomeric appearance in SDS-PAGE the trimer occurred in fluid phase in the form of defined high molecular weight (>600 kDa) aggregates whereas rBet v 1 wildtype strictly appeared as monomeric protein. The results indicate that the hypoallergenic nature of the rBet v 1 trimer is due to formation of defined high molecular weight aggregates which may be responsible for an altered presentation of IgE epitopes in a form with reduced capacity to crosslink effector-cell bound IgE. We thus provide evidence for a novel mechanism for hypoallergenic activity.
Keywords: Immunotherapy, Protein aggregation, Recombinant hypoallergenic molecules, rBet v 1
1. Introduction
Allergen-specific immunotherapy (SIT) represents the only antigen-specific and disease-modifying treatment approach for IgE-mediated allergy (Bousquet et al., 1998; Durham and Till, 1998; Larché et al., 2006). However, the administration of allergens in the course of immunotherapy can induce local and systemic inflammation and in the worst case severe systemic and life-threatening side effects (Winther et al., 2006; Bernstein et al., 2004).
In order to overcome the most severe type of side effects, i.e., systemic life-threatening anaphylaxis, which is caused by IgE-mediated mast cell and basophil degranulation, technologies have been developed for the reduction of IgE reactivity of allergy vaccines (Valenta et al., 2010). Already 40 years ago David Marsh and colleagues developed chemically modified allergen extracts which exhibited reduced IgE reactivity but at the same time could induce allergen-specific IgG antibodies and retained allergenspecific T cell epitopes (Marsh et al., 1970). These chemically modified allergen preparations have been designated “allergoids” and until today these or similar preparations are the active ingredients of many routinely used allergy vaccines. Based on allergen-encoding sequences, several research groups have started to develop hypoallergenic allergen derivatives based on recombinant DNA technology (Valenta et al., 2010).
All hypoallergenic allergen derivatives exhibited reduced IgE reactivity and the reduced allergenic activity thus resulted from this reduction of IgE reactivity.
One exception to this rule is a recombinant trimer of the major birch pollen allergen, Bet v 1, which has been obtained by the expression of three copies of the cDNA coding for Bet v 1 (Vrtala et al., 2001). The recombinant Bet v 1 trimer exhibited a strongly reduced ability to induce IgE-mediated basophil activation, showed reduced induction of immediate type skin reactions and nasal reactivity and has been used in high doses for immunotherapy of birch pollen allergic patients (van Hage-Hamsten et al., 1999; Niederberger et al., 2004).
Surprisingly, despite its reduced allergenic activity, rBet v 1 trimer was found to exhibit IgE reactivity compared to the rBet v 1 wildtype allergen (Vrtala et al., 2001).
In this study we conducted a series of protein–chemical, structural and immunological experiments to re-investigate the unusual reduction of allergenic activity of the rBet v 1 trimer.
2. Materials and methods
2.1. Plasmids, patients’ sera and antibodies
The plasmids expressing rBet v 1 and rBet v 1 trimer have been described (Hoffmann-Sommergruber et al., 1997; Vrtala et al., 2001).
Birch pollen allergic patients (n = 11) were characterized by case history and skin prick testing. Specific IgE levels to birch pollen extract and rBet v 1 were determined by immuno CAP measurements (Phadia, Uppsala, Sweden). Control serum was taken from a non-allergic volunteer with no history of birch pollen allergy, lack of skin reactivity and birch pollen-specific IgE. IgE reactivity testing and basophil activation experiments were done with serum samples and cells obtained from the same birch pollen allergic patients. Specific polyclonal rabbit Abs against the purified rBet v 1, rBet v 1 trimer and against two rBet v 1 fragments (F1 and F2) are described (Vrtala et al., 2000, 2001). Monoclonal mouse IgG Abs against peptide 2 (mAb#2) comprising amino acids 30–59 of Bet v 1 and against peptide 6 (mAb#12) comprising amino acids 74–104 of Bet v 1 were obtained by immunization of mice using KLH-coupled synthetic peptides (peptide 2: LFPKVAPQAISSVENIEGNGGPPTIKKISF; peptide 6: EDVHTNFKYNYSVIEGGPIGDTLEKISNEIK). Monoclonal Bet v 1-specific antibody, Bip 1, is described (Laffer et al., 1996). The mouse monoclonal antibody 4A6 was raised against purified recombinant birch pollen profilin (Wiedemann et al., 1996). Anti-IgE mAb E-124.2.8 was obtained from Immunotech (Marseille, France). Chimeric Bip 1, an IgE monoclonal antibody with specificity for Bet v 1, was generated and purified as described (Laffer et al., 2001).
2.2. Expression and purification of recombinant allergens
Recombinant Bet v 1 and grass pollen allergen, Phl p 5, were obtained from Biomay (Vienna, Austria).
Recombinant Bet v 1 trimer was expressed in E. coli BL 21 (DE3) (Stratagene, La Jolla, CA). Batch fermentation of E. coli BL 21 (DE3)/pET-17b-Bet v 1 trimer was carried out in a 10 L Bioflow 3000 fermenter (New Brunswick Scientific, NJ) in LB medium with the addition of 0.05% (v/v) glycerol, 0.25% (w/v) MgSO4·7H2O, and 0.18% Na2HPO4·2H2O for 8 h at 37 °C, until a cell density (OD600nm) of 7 was reached. As soon as OD600nm reached 1, expression of Bet v 1 trimer and formation of inclusion bodies was induced by adding isopropyl-β-thiogalactopyranoside (IPTG) (Calbiochem, Merck KgaA, Darmstadt, Germany) to a final concentration of 0.5 mM. Inclusion body fractions containing rBet v 1 trimer were isolated by an enzymatic treatment using lysozyme (0.1 mg/g cells) (Sigma–Aldrich, St. Louis, MO) and benzonase (6 U/g cells) (Merck KgaA, Darmstadt, Germany), followed by repetitive freezing and thawing in a buffer containing 50 mM Trisbase pH 8.0, 1 mM EDTA and 0.1% v/v Triton X-100 (5 ml/g cells). After the freezing and thawing, NaCl and EDTA were added to a final concentration of 200 mM and 2 mM, respectively, and the suspension was centrifuged (10,000 × g for 30 min at 4 °C) leaving Bet v 1 trimer containing inclusion bodies in the pellet. After washing the pellet (3 times with 1% v/v Triton, 2 mM EDTA, 2 mM β-mercaptoethanol, 20 mM Tris/HCl pH 8.0 and 2 times with 50% ethanol, 20 mM Tris/HCl pH 8.0), inclusion bodies were suspended and stirred for 15 min in buffer A (6 M urea, 10 mM Tris, 1 mM EDTA, pH 8.0). After centrifugation (10,000 × g for 30 min at 4 °C), the protein was applied to a DEAE sepharose column (Amersham Biosciences, Uppsala, Sweden) and equilibrated with buffer A. The protein was eluted with a linear gradient from 0 to 500 mM NaCl in buffer A. Fractions containing Bet v 1 trimer as the major component were identified by SDS-PAGE, pooled, and dialyzed against buffer B (6 M urea, 10 mM NaH2PO4, 1 mM EDTA, pH 4.8).
The Bet v 1 trimer was rechromatographed on an SP sepharose (Amersham Biosciences) and equilibrated with buffer B. Protein was eluted with a linear gradient from 0 to 500 mM NaCl in buffer B. Fractions containing pure Bet v 1 trimer as the major component were identified by SDS-PAGE, pooled, and dialyzed stepwise against 5 mM sodium phosphate buffer pH 7.4 containing decreasing concentrations (6–0 M) of urea. Finally, the protein was subjected to 0.2 μm filtration.
The purity of the protein preparations was checked by SDS-PAGE and Coomassie Blue staining and the presence of endotoxins was assessed by endotoxin testing (QCL-1000® Chromogenic LAL Endpoint Assay, Bio-Whittaker, Walkersville, MD). The concentration of rBet v 1 and rBet v 1 trimer was checked by colorimetric detection and quantification of total protein using bicinchoninic acid (BCA) as the detection reagent for Cu1+ (Micro BCA™ Protein Assay Kit, Thermo Scientific, Pierce, Rockford, IL). Tests were performed in triplicates. Diluted protein samples (0.5–20 μg/ml) were incubated for 1 h at 60 °C and the absorbance was measured at 562 nm according to the assay protocol. Determinations of protein concentrations were performed for all of the proteins in parallel in order to ensure that the concentrations of the different proteins can be directly compared. Determinations of the protein concentrations were also repeated for each of the proteins shortly before experiments were performed (e.g., IgE antibody reactivity testing, IgE inhibition assays, CD analysis, basophil activation experiments, gel filtration), in order to exclude that results are influenced by alterations in the protein concentrations.
2.3. SDS-PAGE and immunoblotting
Purified rBet v 1 wildtype and rBet v 1 trimer (5 μg protein/slot) were separated by 12.5% SDS-PAGE in the presence or absence of 2-mercaptoethanol (Fling and Gregerson, 1986). Proteins were visualized by staining with Coomassie Brilliant Blue.
IgG and IgE-binding capacity of purified rBet v 1 wildtype and trimer were also tested by Western blotting. For Western blotting, 5 μg of each protein/slot was separated by SDS-PAGE (18) and blotted onto nitrocellulose. Nitrocelluloses membrane containing blotted proteins were incubated either with a 1:2000 dilution of a rabbit anti-rBet v 1 fragment 1 or fragment 2 specific antiserum, or with 1:10 dilutions of sera from eleven birch pollen allergic individuals or serum from one non-allergic individual. Bound IgG and IgE antibodies were detected with a 1:1000 dilution of 125I-labeled donkey anti-rabbit antibodies and a 1:20 dilution of 125I-labeled anti-human IgE antibodies (RAST RIA, Demeditec Diagnostics, Germany), respectively. Bound 125I-labeled antibodies were visualized by autoradiography (Vrtala et al., 2001).
2.4. Mass spectrometry and circular dichroism
Matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectra were acquired in a linear mode on a Microflex mass spectrometer (Bruker, Billerica, MA) equipped with a 337 nm ultraviolet laser (150 μJ at 337 nm). Hundred single laser shots were averaged for each mass spectrum. Samples were dissolved in 10% acetonitrile (0.1% trifluoroacetic acid), and dihydroxybenzoic acid (dissolved in 60% acetonitrile, 0.1% trifluoroacetic acid) was used as a matrix. For sample preparation a 1:1 mixture of protein and matrix solution was deposited onto the target and air-dried.
Circular dichroism (CD) measurements were done using a JASCO (Tokyo, Japan) J-810 spectropolarimeter. The CD spectra of purified rBet v 1 wildtype and trimer were measured at room temperature at concentration of 0.1 mg/ml using a rectangular quartz cuvette with 0.2-cm path length. Far ultraviolet (UV) spectra were recorded in the wavelength ranges between 190 and 260 nm with a resolution of 0.5 nm at a scan speed of 50 nm/min. Data of three measurements were averaged. The final spectra were baseline-corrected and results were expressed as the mean residue ellipticity (Θ) at a given wavelength. The secondary structure content of rBet v 1 and rBet v 1 trimer was calculated using the secondary structure estimation program CDSSTR (Whitmore and Wallace, 2004).
2.5. IgE and IgG reactivity of rBet v 1 and rBet v 1 trimer
Purified rBet v 1 wildtype and trimer were tested for IgE and IgG reactivity in dot blot assays, ELISA and ELISA inhibition.
For dot blot experiments, 2 μL aliquots containing 1 μg of purified rBet v 1 wildtype, rBet v 1 trimer, bovine serum albumin (BSA) and human serum albumin (HSA) (negative controls) (Roth, Karlsruhe, Germany) were dotted on nitrocellulose membranes. Nitrocellulose membranes containing dot-blotted proteins were incubated with a 1:10 dilution, in PBS, of sera from eleven birch pollen allergic individuals, one non-allergic individual or buffer without addition of serum. Bound IgE antibodies were detected with a 1:20 dilution of 125I-labeled anti-human IgE antibodies (RAST RIA) and visualized by autoradiography.
ELISA plates (Greiner, Kremsmünster, Austria) were coated with rBet v 1, rBet v 1 trimer or BSA (5 μg in100 μl/well diluted in PBS) at 4 °C overnight. Plates were blocked with 2% w/v bovine serum albumin (BSA) (Roth) in PBS-T (PBS + 0.05% v/v Tween 20) for 6 h, and incubated either with rabbit anti-Bet v 1, rabbit anti-Bet v 1 fragment 1, rabbit anti-Bet v 1 fragment 2, rabbit anti-Bet v 1 trimer, or pre-immune sera in four different dilutions (1:1000, 1:5000, 1:10,000 and 1:50,000), in PBS 0.5% w/v BSA/0.05% v/v Tween, at 4 °C overnight. Bound rabbit IgG antibodies were detected with a 1:1000 diluted anti-rabbit IgG, Horseradish Peroxidase-linked whole antibody from donkey (GE Healthcare, UK Limited) for 1 h at 37 °C and for 1 h at 4 °C.
Detection of rBet v 1 trimer and rBet v 1 with mouse monoclonal antibodies was performed as follows. Plates were coated with rBet v 1, rBet v 1 trimer or BSA (5 μg in100 μl/well, diluted in PBS) at 4 °C overnight, and then incubated either with mouse monoclonal IgG antibodies against peptide 2 (mAb#2) (aa 30–59) or peptide 6 (mAb#12) (aa 74–104) from Bet v 1, with mouse monoclonal antibody (4A6), with Bip 1 or with mouse monoclonal IgG1 (murine anti-IgE) in four different dilutions (1:1000, 1:5000, 1:10,000 and 1:50,000), in PBS 0.5% BSA/0.05% Tween, at 4 °C overnight. Bound mouse monoclonal IgG antibodies were detected with a 1:1000 diluted anti-mouse IgG, Horseradish Peroxidase-linked whole antibody from sheep (GE Healthcare, UK Limited) for 1 h at 37 °C and 4 °C.
Plates were washed with PBS-T (PBS + 0.05% Tween 20) between incubation steps and color development was performed by addition of staining solution ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) diammonium salt; Sigma–Aldrich) (100 μl/well). The optical density was measured in an ELISA Reader (Dynatech, Denkendorf, Germany) at 405 nm. Results represent means of duplicate determination with a variation of ≤ 10%.
For IgE ELISA inhibition assay, ELISA plates (Greiner) were coated with rBet v 1, rBet v 1 trimer or BSA (5 μg in 100 μl/well, diluted in PBS) for 6 h at room temperature. Plates were blocked with 2% bovine serum albumin (BSA) (Roth) in PBS-T (PBS 0.05% Tween 20) at 4°C overnight. Sera from eleven birch allergic individuals (1:4 diluted in PBS) were preincubated overnight at 4°C with increasing concentrations (0.5 ng–5 μg) of rBet v 1, rBet v 1 trimer or BSA before they were applied to the plates (100 μl/well). Bound human IgE was detected using a 1:2500 diluted AP-conjugated (alkaline phosphatase) mouse monoclonal anti-human IgE antibody (BD Pharmingen, San Diego, CA). Plates were washed with PBS-T (PBS + 0.05% Tween 20) between incubation steps and color development was performed by addition of staining solution ABTS (2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid)diammonium salt; Sigma–Aldrich) (100 μl/well). The optical density was measured in an ELISA Reader (Dynatech) at 405 nm.
2.6. Basophil activation by flow cytometry: CD203c assay
Peripheral blood samples were obtained from ten out of the eleven birch pollen allergic patients used for IgE reactivity testing. Blood was collected in heparinized tubes after informed consent was given. Blood aliquots (100 μl) were incubated (unique) with serial dilutions of rBet v 1 wildtype (0.05 pM to 0.5 nM), rBet v 1 trimer (0.05 pM to 0.5 nM) or anti-IgE mAb E-124.2.8 (Immunotech) (1 μg/ml), or PBS for 15 min at 37 °C, and CD203c expression was measured (Hauswirth et al., 2002).
2.7. Gel filtration
For gel filtration 300 μl aliquots of the proteins (rBet v 1 trimer: c = 1.9 mg/ml; rBet v 1 wildtype: c = 1 mg/ml) were loaded onto a Superdex 200 10/300 GL column (GE Healthcare, Uppsala, Sweden) at 4 °C, equilibrated with 10 mM phosphate buffer pH 7.5 containing 150 mM NaCl. The flow rate was 0.5 ml/min and fractions of 0.5 ml were collected. The molecular masses (MMs) of the fractions were calculated based on the gel filtration of standard proteins performed under identical conditions (BioRad: Thyroglobulin, 670 kDa; Bovine gamma globulin, 158 kDa; Chicken ovalbumin, 44 kDa; Equine myoglobin, 17 kDa; vitamin B12, 1.35 kDa; vitamin B12). The peak fractions were concentrated using a CENTRIVAP (Labconco Corp., Kansas City, MO) vacuum concentrator without heating. Concentrated fractions were subjected to immunological investigations or for 5 days at 4 °C and reapplied to gel filtration with the Superdex 200 10/300 GL column to check the stability of elution profile. In addition analytical gel filtration was performed on a Superdex 200 5/150 GL column (GE Healthcare, Uppsala, Sweden) at 4 °C, equilibrated with the identical buffer (10 mM sodium phosphate, pH 7.5, 150 mM NaCl) with a flow rate of 0.3 mL/min, where 20 μl aliquots of rBet v 1 trimer at a concentration of 3.4 mg/ml were loaded.
2.8. Rat basophil leukemia cells mediator-release assay
Rat basophil leukemia cells (RBL-2H3) transfected with the human high affinity IgE receptor FcεRI (clone RBL-703/21), kindly provided by Vogel et al. (2005), were loaded with different dilutions of the chimeric Bip 1 antibody (10 μg/ml, 1 μg/ml, 100 ng/ml, 10 ng/ml, 1 ng/ml, 100 pg/ml, 10 pg/ml and 1 pg/ml), washed 3 times with Tyrode’s buffer (Sigma–Aldrich) and then exposed to different concentrations (10 μg/ml, 1 μg/ml, 100 ng/ml, 10 ng/ml, 1 ng/ml, 100 pg/ml) of rBet v 1 wildtype, rBet v 1 trimer or a non-cross-reactive grass pollen allergen, Phl p 5. The release of β-hexosaminidase was measured as described (Vogel et al., 2005).
2.9. Detection of basophil-bound Bet v 1 and Bet v 1 trimer by flow cytometry
Heparinized peripheral blood samples from two birch pollen allergic patients (50 μl aliquots) were incubated in duplicates with rBet v 1 wildtype or rBet v 1 trimer in different concentrations (0.05–125 pM) at 4 °C for 15 min. Then cells were washed with ice-cold PBS and exposed either to the Bet v 1-specific mouse monoclonal antibodies, Bip 1 or mAb#12, or with a mouse IgG1 isotype control antibody (mouse IgG1, clone 107.3; BD Biosciences, San Jose, CA). After washing with ice-cold PBS, cells were stained with PE-labeled goat anti-mouse-Ig (Southern Biotech, Birmingham, AL). For identification of basophils, cells were first washed as described above and then stained with an APC-labeled anti-CD123 antibody (Miltenyi Biotec, Bergisch Gladbach, Germany). Incubation and staining steps were performed at 4 °C for 15 min.
Thereafter, erythrocytes lysis was performed (FACS Lysing solution) (BD Biosciences) and cells were subjected to flow cytometry using a FACSCalibur (BD Biosciences) and analyzed with FlowJo (Treestar, Ashland, OR). Binding of the monoclonal antibodies to rBet v 1 or rBet v 1 trimer was calculated from the mean of the mean fluorescence intensities (MFIs) of the duplicates (less than 5% variation) and were expressed as staining index (SI = MFIstim/MFIcontrol), where MFIstim is the mean fluorescence intensity obtained with the anti-Bet v 1 antibodies, and MFIcontrol the mean fluorescence intensity obtained with the isotype control.
2.10. Stability of Bet v 1 trimer aggregates in different buffers studied by gel filtration analysis
Purified rBet v 1 trimer (5 mM Na2PO4, pH 7.4) was exposed to three buffers known to disrupt hydrophobic interactions (buffer A: 5 mM Na2PO4, 0.15% SDS, pH 7.4; buffer B: 5 mM Na2PO4, 0.15% CHAPS, pH 7.4; buffer C: 5 mM Na2PO4, 0.5% Octyl-β-Glucose, pH 7.4), and to three buffers known to disrupt salt bridges (buffer D: 5 mM Na2PO4, 1.5 M NaCl, pH 7.4; buffer E: 5 mM Na2PO4, 500 mM Na-acetate, pH 7.4; buffer F: 5 mM Na2PO4, 500 mM NH4-sulfate, pH 7.4). Each of the buffers was added to rBet v 1 trimer dissolved in 5 mM Na2PO4, pH 7.4 as concentrate to reach the final composition given above. After thorough mixing samples were stored for four days either at 4 °C or −20 °C and then subjected to gel filtration analysis.
3. Results
3.1. Expression, purification and physicochemical properties of rBet v 1 wildtype and rBet v 1 trimer
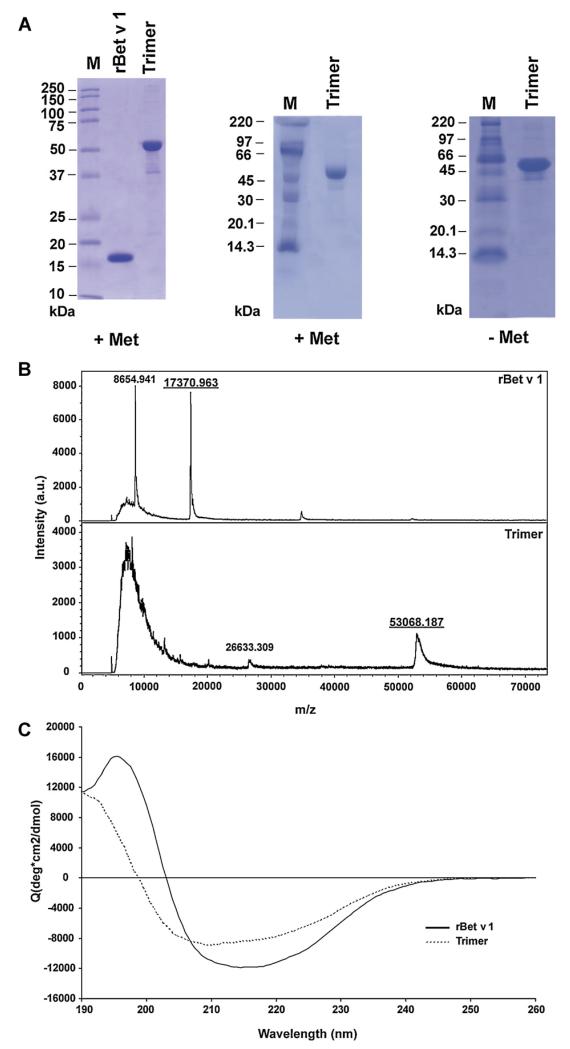
The Coomassie Blue-stained SDS-PAGE shows that recombinant Bet v 1 trimer appears in SDS-PAGE under reducing as well as under non-reducing conditions mainly as a monomeric protein of approximately 52 kDa (Fig. 1A, +Met, and −Met). Few very weak bands of lower and higher molecular weight than 52 kDa were observed in the recombinant trimer preparation. Recombinant Bet v 1 wildtype migrated as a single protein band at approximately 17 kDa (Fig. 1A, left).
Fig. 1. Physical–chemical characterization of rBet v 1 wildtype and rBet v 1 trimer by SDS-PAGE.
(A) Comparison of purified recombinant Bet v 1 and rBet v 1 trimer (left). rBet v 1 trimer run in the presence (+Met) or absence (−Met) of 2-mercaptoethanol. M, molecular mass markers (kDa). (B) Mass spectrometric analysis of rBet v 1 and rBet v 1 trimer. The mass/charge ratios are shown in the x-axes, and the signal intensities are displayed in the y-axis as absolute value in the investigated mass range. (C) Circular dichroism analysis of rBet v 1 and rBet v 1 trimer. The mean residue ellipticities (y-axes) are shown for rBet v 1 trimer and for rBet v 1 at different wavelengths (x-axes).
MALDI-ToF analysis of purified rBet v 1 resulted in two prominent mass peaks of 17370.9 Da, corresponding to the monomeric form without methionine and another of 8654.9 Da, corresponding to the double charged molecule (Fig. 1B). MALDI-ToF analysis of rBet v 1 trimer resulted in a peak of 53068.1 Da. The mass determined by mass spectrometry for the trimer was slightly greater than the predicted mass which could be due to binding of urea (+43 Da), Na (+22 Da), K (+38 Da), oxidation of methionine (131 Da) and combinations of these modifications (Fig. 1B). A peak corresponding to half of the molecular weight of the trimer molecule (double charged 26633.3 Da) was also observed. For measurement of the trimer a higher laser intensity than for wildtype had to be used (trimer: 69%; wildtype: 52%), which resulted in a prominent matrix peak between 6000 to 14,000 Da.
The Bet v 1 CD spectrum exhibits a shape typical for a mixed α/β-fold, with a broad minimum at 215 nm and a maximum at 195 nm (Fig. 1C). In contrast, the CD spectrum of Bet v 1 trimer displays a shift of the minimum as well as the maximum towards shorter wavelengths, indicating a higher content of random-coil elements contributing to the overall structure (Fig. 1C). For rBet v 1 wildtype an α-helix content of 27% and a β-sheet content of 30% were calculated and for the trimer the values were 16% and 28%, respectively, indicating a loss of mainly α-helical secondary structure elements in the trimer as compared with the monomer structure.
3.2. Recombinant Bet v 1 trimer shows even stronger IgE reactivity than rBet v 1 wildtype
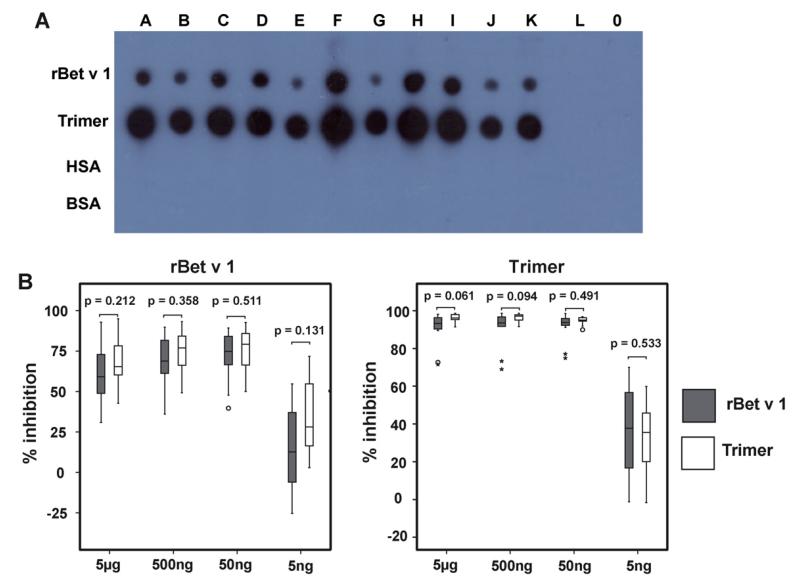
Fig. 2A shows the IgE reactivity from 11 birch pollen allergic individuals, one non-allergic person and buffer to rBet v 1 wildtype and trimer. Each of the birch allergic patients sera displayed IgE reactivity to trimer and to rBet v 1 wildtype (Fig. 2A, A–K).
Fig. 2. IgE and IgG reactivity of rBet v 1 wildtype and rBet v 1 trimer.
(A) IgE reactivity of dot-blotted rBet v 1 and rBet v 1 trimer. IgE reactivity of nitrocellulose-dotted rBet v 1, rBet v 1 trimer, human serum albumin (HSA) and bovine serum albumin (BSA) after incubation with sera from 11 birch pollen allergic patients (A–K), serum from one non-allergic person (L) or buffer (0). (B) Inhibition of IgE binding to solid phase-bound rBet v 1 or rBet v 1 trimer by rBet v 1 or Bet v 1 trimer in fluid phase. The percentages of inhibition of IgE binding to solid phase-bound rBet v 1 (left) and rBet v 1 trimer (right) are shown on the (y-axes) after pre-incubation of sera from 11 birch pollen allergic patients with different concentrations (x-axes) of rBet v 1 or rBet v 1 trimer. Results are displayed as box plots with median values in the boxes, standard deviations and extremes (asterisks) and outliers (circle).
Interestingly, rBet v 1 trimer showed always more intensive IgE reactivity than rBet v 1 wildtype. No IgE reactivity was observed with serum from the non-allergic individual and with the buffer control (Fig. 2A, L and 0). Similar results were obtained in a Western blot assay. Each of the allergic patients showed stronger IgE reactivity to trimer than to rBet v 1 wildtype (data not shown).
Also by ELISA we found that rBet v 1 trimer exhibited a much stronger IgE reactivity than rBet v 1 wildtype (Table 1).
Table 1. IgE reactivity of ELISA plate-bound rBet v 1 wildtype and rBet v 1 Trimer.
IgE reactivities (OD values) of sera from 11 birch pollen allergic patients (A–K) to rBet v 1 and rBet v 1 trimer.
| A | B | C | D | E | F | G | H | I | J | K | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| rBet v 1 | 0.389 | 0.065 | 0.191 | 0.094 | 0.134 | 0.51 | 0.327 | 0.268 | 0.328 | 0.182 | 0.908 |
| rBet v 1 Trimer | 2.47 | 0.661 | 1.958 | 0.628 | 0.978 | 3.608 | 3.114 | 2.244 | 3.148 | 1.367 | 3.567 |
3.3. Similar recognition of solid-phase-bound rBet v 1 trimer and rBet v 1 wildtype by IgG antibodies
We then tested the binding of polyclonal rabbit antisera which had been raised against purified rBet v 1, rBet v 1 trimer and against two rBet v 1 fragments (F1 and F2) as well as mouse monoclonal IgG antibodies raised against peptide 2 (mAb#2) comprising amino acids 30–59 of Bet v 1 and against peptide 6 (mAb#12) comprising amino acids 75–104 of Bet v 1 or the Bip 1 mouse monoclonal antibody raised against natural, pollen-derived Bet v 1 to rBet v 1 and rBet v 1 trimer. Unlike allergic patients IgE, each of the IgG antibodies reacted equally well with the two proteins at each of the tested dilutions (data not shown).
3.4. rBet v 1 trimer and rBet v 1 wildtype inhibit allergic patients IgE reactivity to each other equally well in fluid phase
Next we investigated the extent to which each of the two proteins can inhibit IgE reactivity to the other protein, by IgE ELISA competition assays. The same sera from the same 11 Bet v 1 allergic patients which had been tested for IgE reactivity before, were pre-incubated with different concentrations of either rBet v 1 trimer, rBet v 1 or BSA as control protein and then allowed to bind to ELISA plate-coupled rBet v 1 trimer or rBet v 1 (Fig. 2B). We found that trimer and rBet v 1 wildtype inhibited patients IgE reactivity to rBet v 1 in a comparable manner at each of the four inhibitor concentrations (5 ng–5 μg/ml) (Fig. 2B, left). Similar results were obtained when rBet v 1 trimer and rBet v 1 were tested for the inhibition of allergic patients IgE binding to rBet v 1 trimer (Fig. 2B, right).
3.5. Reduced allergenic activity of rBet v 1-trimer compared to rBet v 1 wildtype as determined by CD203c up-regulation on patients’ basophils
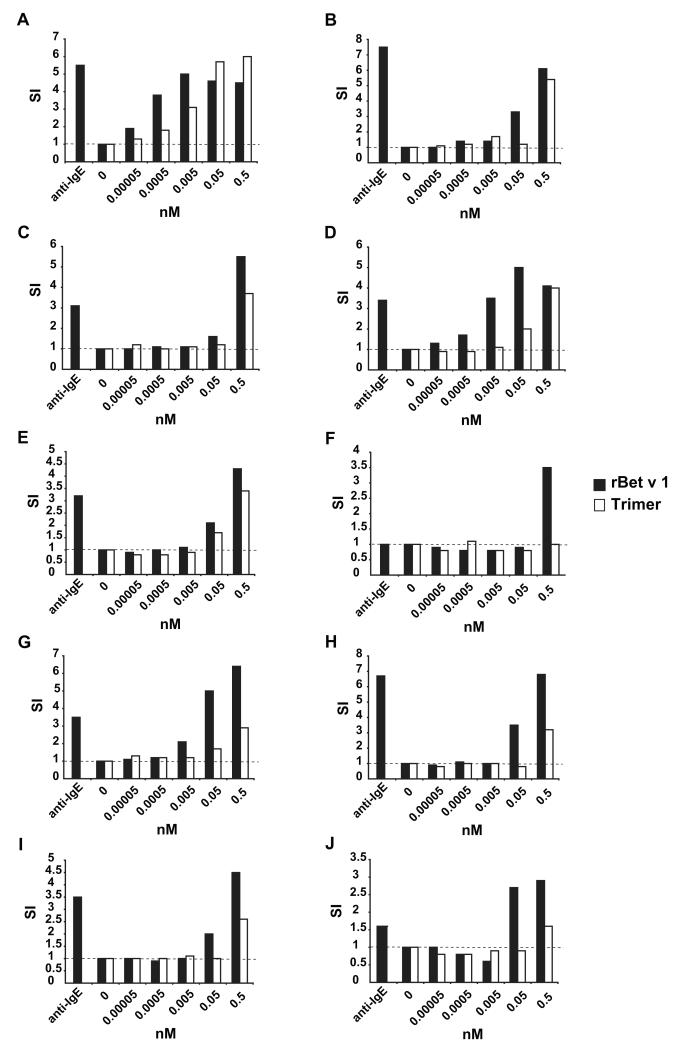
Heparinized blood samples from ten of the eleven allergic patients tested for IgE reactivity were incubated with different concentrations of rBet v 1 trimer or rBet v 1 (Fig. 3). For these experiments we used equimolar amounts of the proteins so that the amount of rBet v 1 equivalents in the trimer preparation were approximately threefold as high as in the rBet v 1 wildtype samples. Nevertheless, we found that trimer exhibited a 10- to 100-fold reduced allergenic activity compared to rBet v 1 wildtype in almost each of the tested patients (Fig. 3).
Fig. 3. In vitro allergenic activity of rBet v 1 wildtype and rBet v 1 trimer as determined by CD203c up-regulation on allergic patients basophils.
Blood samples from birch pollen allergic individuals were exposed to different concentrations of rBet v 1 (black bars) and rBet v 1 trimer (open bars), anti-IgE or buffer (0) (x-axes). The upregulation of CD203c expression is displayed in the form of stimulation indices (SI) (y-axis) for each of the 10 patients (A–J).
3.6. In solution rBet v 1 trimer occurs in the form of aggregates whereas rBet v 1 wildtype is monomeric
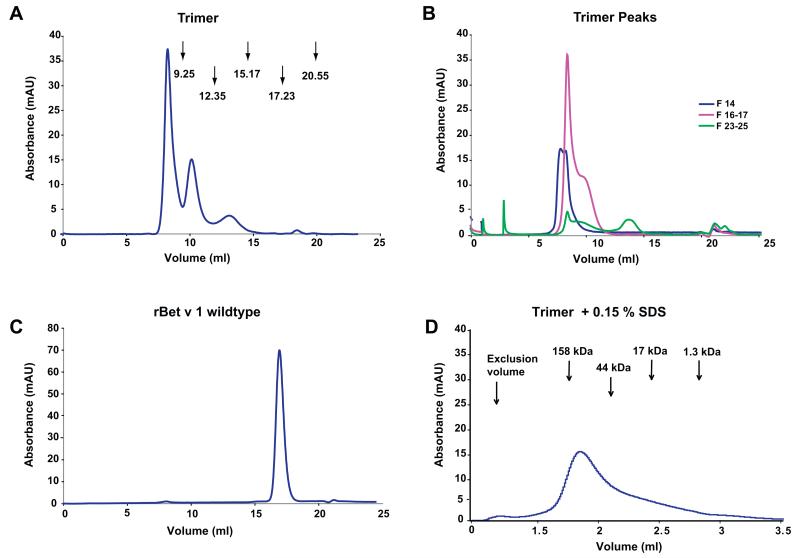
Analytical gel filtration showed that the rBet v 1 trimer appeared in three distinct peaks in liquid phase. The first and most prominent peak eluted at 7.5 ml close to the exclusion volume containing aggregates with an apparent molecular mass larger than 1000 kDa. The second peak of about half of the height, appeared at a molecular mass of 350 kDa. The third peak eluted at a molecular mass of 90 kDa corresponding to approximately the twofold of the trimer molecule (Fig. 4A). When the peaks of the trimer were re-run on a gel filtration column after storage at 4 °C (Fig. 4B) a similar profile was obtained. Most of the trimer appeared in form of the aggregates with high molecular mass (Fig. 4B). When rBet v 1 was applied to analytical gel filtration, the protein eluted exclusively as a single peak at 16.9 ml with apparent molecular mass of 15 kDa corresponding to the monomeric form of the protein with a molecular mass of 17 kDa (Fig. 4C). The stability the trimer aggregates was analyzed using different buffers known to disrupt hydrophobic interactions or to affect salt bridges. We found that only the addition of a strong detergent (i.e., 0.15% SDS) dissolved rBet v 1 trimer aggregates which then appeared as a monomer, eluting as a single peak at 1.85 ml from the analytical gel- filtration column, corresponding to an apparent molecular mass of 100 kDa (Fig. 4D). The addition of other weaker detergents had no effects and addition of salts even promoted aggregation (data not shown).
Fig. 4. Aggregate formation of rBet v 1 trimer in solution.
Gel filtrations run on a Superdex 200 10/300 GL column (A–C) or on a Superdex 200 5/150 GL column (D). (A) Demonstration by gel filtration that rBet v 1 trimer occurs in fluid phase in the form of defined aggregates. Profile of rBet v 1 trimer eluted from a gel filtration column (x-axis: elution volumes in ml; y-axis: absorbance at 280 nm). Arrows indicate peaks of a protein standard consisting of thyroglobulin 670 kDa (9.25 ml), bovine gamma globulin 158 kDa (12.35 ml), chicken ovalbumin 44 kDa (15.17 ml), equine myoglobin 17 kDa (17.23 ml), vitamin B12 1.35 kDa (20.55 ml). (B) Gel filtration re-run of the trimer peaks (blue: sample 14, ~8 ml; pink: samples 16–17, ~9–9.5 ml; green: samples 24–26, ~12–13.5 ml) after 5 days of incubation at 4 °C. (C) Elution profile of rBet v 1 (16.9 ml). (D) Gel filtration profile of rBet v 1 trimer in SDS-containing buffer A on Superdex 200 5/150 GL column. The protein elutes at 1.83 ml.
3.7. rBet v 1 trimer but not rBet v 1 wildtype induces β-hexosaminidase release from basophils loaded with monoclonal Bet v 1-specific IgE
RBL cells which had been transfected with human FcεRI were loaded with a rBet v 1-specific human monoclonal IgE antibody and subsequently exposed to different concentrations of rBet v 1 and rBet v 1 trimer. rBet v 1 trimer induced a dose-dependent release of β-hexosaminidase, starting at a concentration of 10 ng/ml with 14.86% of total release and increased to 69.22% when the RBL were exposed to the highest concentration of allergen tested (10 μg/ml) (Table 1). rBet v 1 failed to induce mediator release in sensitized RBL cells due to its monomeric nature (Table 2).
Table 2. Induction of β-hexosaminidase release.
RBL cells that had been transfected with human FcεRI were sensitized with different concentrations of human IgE mAb α Bet v 1 (Bip 1) and then stimulated with increasing concentrations of rBet v 1 and rBet v 1 trimer. The release of β-hexosaminidase is shown as percentage of the total β-hexosaminidase contents of the cells.
| Bip 1 dilution | rBet v 1 |
Trimer |
||||||
|---|---|---|---|---|---|---|---|---|
| 10 μg/ml | 1 μg/ml | 100 ng/ml | 10 ng/ml | 10 μg/ml | 1 μg/ml | 100 ng/ml | 10 ng/ml | |
| 10 μg/ml | 4.0 | 2.8 | 2.1 | 1.4 | 69.2 | 65.3 | 14.9 | 0.3 |
| 1 μg/ml | 1.8 | 1.5 | 1.2 | 0.8 | 69.1 | 58.5 | 9.6 | 0.1 |
| 100 ng/ml | 0.4 | 0.3 | 0.3 | 0.3 | 50 | 41.8 | 1.8 | 0.5 |
3.8. mAb#12 reacts with basophil-bound rBet v 1 trimer but not with rBet v 1 wildtype
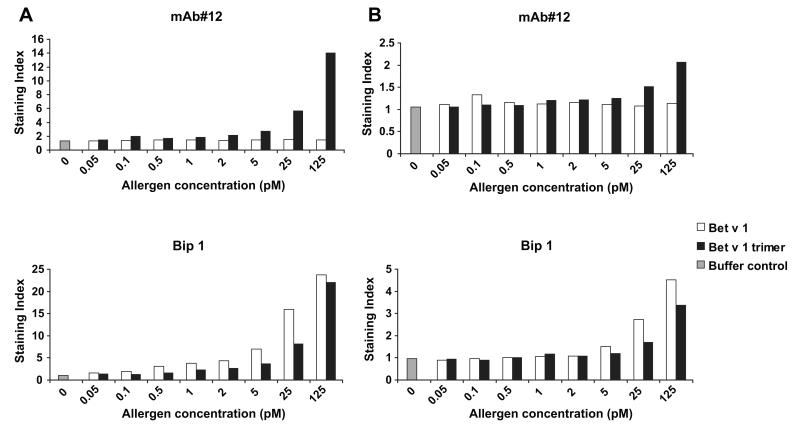
If basophil-bound rBet v 1 trimer contains IgE epitopes which are not engaged with basophil-bound IgE as predicted in Fig. 5, we assumed that it should be possible to detect basophil-bound trimer with an antibody such as mAb#12 which competes with patients IgE. We therefore incubated peripheral blood samples from two birch pollen allergic patients (Fig. 6A and B) with rBet v 1 wildtype or trimer and then exposed the cells to Bet v 1-specific antibodies, mAb#12 or Bip 1. Using flow cytometry analysis it was demonstrated that mAb#12 recognized basophil-bound trimer but not to the Bet v 1 wildtype (Fig. 6A and B mAb#12). The binding of rBet v 1 wildtype and trimer to the basophils was demonstrated with the Bip 1 antibody, which reacts with epitopes on Bet v 1 that are distinct from the IgE binding sites (Fig. 6A and B Bip 1).
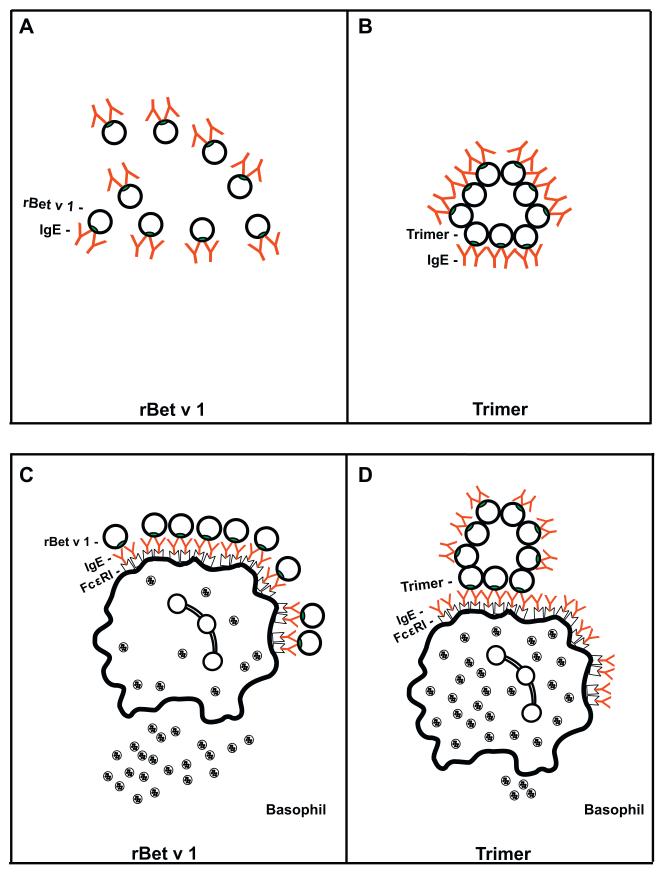
Fig. 5. Model for the hypoallergenic behavior of rBet v 1 trimer.
Bet v 1 molecules in their monomeric form (A: rBet v 1) or as aggregated trimer (B: Trimer) can bind a comparable number of IgE antibodies in solution. However, monomeric rBet v 1 molecules may be more efficient in cross-linking of effector cell-bound IgE (C) than the aggregated trimer (D). IgE epitopes on monomeric forms of Bet v 1 can be well accessed by effector cell-bound IgE whereas only a certain portion of the IgE epitopes on the aggregated trimers comes into an optimal position for cross-linking of effector cell-bound IgE.
Fig. 6. Flow cytometric analysis of Bet v 1 wildtype and trimer binding to basophils.
Peripheral whole blood samples from two birch pollen allergic patients (A, B) were exposed to different concentrations of rBet v 1 wildtype (open bars), rBet v 1 trimer (black bars), or buffer control (gray bars) (x-axes) and incubated with mAb#12 or Bip 1. Binding of the Bet v 1-specific monoclonal antibodies to basophil-bound rBet v 1 wildtype or trimer is expressed as staining index (y-axis).
4. Discussion
In the last 10 years an increasing number of recombinant or synthetic hypoallergenic allergen-derivatives has been engineered which are also characterized by a reduced reactivity with IgE antibodies (Valenta et al., 2010). Here we have investigated an unusual hypoallergenic allergen derivative, a recombinant trimeric form of the major birch pollen allergen, Bet v 1, which despite retained IgE reactivity was shown to exhibit reduced allergenic activity (Vrtala et al., 2001; Niederberger et al., 2004).
Using SDS-PAGE performed under reducing and non-reducing conditions as well as mass spectrometry, rBet v 1 trimer appeared as a monomeric, soluble protein with a molecular weight corresponding to the molecular weight which was predicted based on its sequence. The comparison of the fold of the rBet v 1 trimer with that of the Bet v 1 wildtype demonstrated that the trimer exhibited a similar fold as rBet v 1 with a reduction of alpha helical elements in its secondary structure. It has been shown for other hypoallergenic Bet v 1 derivatives such as recombinant fragments (Vrtala et al., 1997), a folding variant of Bet v 1 obtained by exposing rBet v 1 to NaOH (Kahlert et al., 2008) and for certain isoforms/mutants (Ferreira et al., 1996, 1998; Spangfort et al., 2003) that a loss of fold or altered fold is associated with reduced IgE reactivity. However, despite the altered fold, rBet v 1 trimer reacted with patients IgE antibodies as well as with several different IgG antibody probes recognizing sequential or conformational epitopes of Bet v 1 or certain Bet v 1 portions in a similar manner as rBet v 1 wildtype. The IgE reactivity of rBet v 1 trimer was even higher than that of rBet v 1 wildtype in two different solid phase assays and comparable in fluid phase competition assays. The higher IgE reactivity in the solid phase assays may be explained by the fact that the immobilization procedure occupies more IgE epitopes on a small molecule (i.e., Bet v 1) as compared to the bigger trimer. Yet, both molecules showed identical IgE reactivity in fluid phase inhibition assays using different concentrations of each molecule for the inhibition of IgE reactivity.
Despite the fact that rBet v 1 trimer and rBet v 1 wildtype inhibited IgE binding to each other in a similar manner, we found that the trimeric form exhibited an a 10–100-fold reduction of allergenic activity when exposed to basophils from allergic patients. This surprising reduction of allergenic activity cannot be explained by a variability of protein concentrations in the experiments because extra care was taken to determine the concentrations of trimer and rBet v 1 before each of the critical experiments in parallel and all experiments were performed using protein concentrations calculated to contain the same number of Bet v 1 molecules for IgE binding assays. For the basophil activation studies we even used equimolar amounts of trimer and rBet v 1. Thus the trimer preparation contained a threefold excess of Bet v 1 molecules compared to rBet v 1 but still the allergenic activity was 10–100-fold reduced. Furthermore, serum samples and cells were obtained from the very same patients for IgE reactivity testing and for the assessment of allergenic activity to assure that the results are not caused by a variability regarding patients materials.
Based on the first set of experiments we therefore had to conclude that the trimer despite maintained IgE reactivity exhibited a strongly reduced allergenic activity, presumably due to a less efficient cross-linking of basophil-bound IgE compared to the rBet v 1 wildtype.
Gel filtration/size exclusion experiments showed that the trimer despite its overall monomeric appearance in stained gels, occurred in the form of high molecular weight aggregates in solution. Only treatment with a strong detergent could disrupt the aggregates indicating that they are due to hydrophobic interaction (Fig. 4D). By contrast, rBet v 1 wildtype behaved strictly as monomeric protein in the gel filtration experiments. The monomeric appearance of rBet v 1 wildtype is in contrast to earlier published work which claimed that Bet v 1 and allergens in general exhibit their allergenic activity (i.e., cross-linking of effector cell bound IgE) due to oligomerization (Schöll et al., 2005). We therefore performed another type of experiment in which we loaded rat basophil leukemia cells which had been transfected with the human FcεRI with Bet v 1-specific human monoclonal IgE antibodies and subsequently exposed them to the trimer and rBet v 1 wildtype allergen. The results of these experiments confirmed that rBet v 1 wildtype behaves as monomeric protein in solution because it did not cross-link monoclonal IgE whereas the trimer induced cross-linking, albeit at relatively high concentrations which is in accordance with its low allergenic activity and in good agreement with earlier data which had been obtained with purified human mast cells (Sellge et al., 2005).
Based on the obtained results we propose the following explanation for the hypoallergenic nature of the rBet v 1 trimer as shown in Fig. 5. According to structural studies and site directed mutagenesis experiments there is evidence that Bet v 1 contains a major IgE epitope-containing region (Ferreira et al., 1998; Spangfort et al., 2003) which is obviously accessible to IgE antibodies on the monomeric as well as on the trimeric form of Bet v 1 although trimer forms high molecular weight aggregates. Since Bet v 1 does not contain cysteins we assume that the formation of aggregates is due to an association of hydrophobic portions of the trimer within the aggregate leaving hydrophilic, IgE-reactive areas available outside. In this scenario both, the aggregated trimer and the monomers can bind a comparable number of IgE antibodies in solution (Fig. 5A and B), an assumption which is supported by the results of the IgE inhibition experiments. However, when the molecules are exposed to cell-bound IgE antibodies only the monomeric Bet v 1 molecules seem to be able to cause efficient cross-linking of IgE antibodies due to the fact that each of these molecules can present the IgE epitope containing areas well to cell-bound IgE (Fig. 5C and D). For the aggregated trimer we assume that only a certain percentage of the IgE epitopes which are available on the surface of this molecule assume a spatial arrangement which allows cross-linking of cell-bound IgE whereas a considerable number of the IgE epitopes is less well accessible to the cell-bound IgE antibodies (Fig. 5D). This assumption is in fact supported by the finding that a monoclonal Bet v 1-specific antibody which competes with IgE binding to Bet v 1 reacted with basophil-bound trimer but not Bet v 1 wildtype (Fig. 6).
Our hypothesis is in agreement with data obtained for other allergens indicating that the spatial arrangement of IgE epitopes on a given protein may have an important influence on the allergenic activity of this protein in terms of its capacity to induce allergic inflammation via cross-link of effector cell-bound IgE (Fedorov et al., 1997; Flicker et al., 2000, 2006). It may thus be possible to convert highly allergenic proteins into low allergenic proteins for therapeutic purposes by a hitherto unknown mechanism, i.e., a reorientation of IgE epitopes.
Acknowledgements
This study was supported by grant F1815, F1803, F1804, F1809 of the Austrian Science Fund (FWF), by the Christian Doppler Research Association Vienna, Austria and by a research grant from Biomay, Vienna, Austria.
References
- Bernstein DI, Wanner M, Orish L, Liss GM, Immunotherapy Committee, American Academy of Allergy, Asthma and Immunology. Twelve-year survey of fatal reactions to allergen injections and skin testing: 1990-2001. J. Allergy Clin. Immunol. 2004;113:1129–1136. doi: 10.1016/j.jaci.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Bousquet J, Lockey R, Malling HJ. Allergen immunotherapy: therapeutic vaccines for allergic diseases. A WHO position paper. J. Allergy Clin. Immunol. 1998;102:558–562. doi: 10.1016/s0091-6749(98)70271-4. [DOI] [PubMed] [Google Scholar]
- Durham SR, Till SJ. Immunologic changes associated with allergen immunotherapy. J. Allergy Clin. Immunol. 1998;102:157–164. doi: 10.1016/s0091-6749(98)70079-x. [DOI] [PubMed] [Google Scholar]
- Fedorov AA, Ball T, Mahoney NM, Valenta R, Almo SC. The molecular basis for allergen cross-reactivity: crystal structure and IgE-epitope mapping of birch pollen profilin. Structure. 1997;5:33–45. doi: 10.1016/s0969-2126(97)00164-0. [DOI] [PubMed] [Google Scholar]
- Ferreira F, Hirtenlehner K, Jilek A, Godnik-Cvar J, Breiteneder H, Grimm R, Hoffmann-Sommergruber K, Scheiner O, Kraft D, Breitenbach M, Rheinberger HJ, Ebner C. Dissection of immunoglobulin E and T lymphocyte reactivity of isoforms of the major birch pollen allergen Bet v 1: potential use of hypoallergenic isoforms for immunotherapy. J. Exp. Med. 1996;183:599–609. doi: 10.1084/jem.183.2.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, Grimm R, Jahn-Schmid B, Breiteneder H, Kraft D, Breitenbach M, Rheinberger HJ, Scheiner O. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998;12:231–242. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- Flicker S, Vrtala S, Steinberger P, Vangelista L, Bufe A, Petersen A, Ghannadan M, sperr WR, Valent P, Norderhaug L, Bohle B, Stockinger H, Suphioglu C, Ong EK, Kraft D, Valenta R. A human monoclonal IgE antibody defines a highly allergenic fragment of the major timothy grass pollen allergen, Phl p 5: molecular, immunological, and structural characterization of the epitope-containing domain. J. Immunol. 2000;165:3849–3859. doi: 10.4049/jimmunol.165.7.3849. [DOI] [PubMed] [Google Scholar]
- Flicker S, Steinberger P, Ball T, Krauth MT, Verdino P, Valent P, Almo S, Valenta R. Spatial clustering of the IgE epitopes on the major timothy grass pollen allergen Phl p 1: importance for allergenic activity. J. Allergy Clin. Immunol. 2006;117:1336–1343. doi: 10.1016/j.jaci.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Fling SP, Gregerson DS. Peptide and protein molecular weight determination by electrophoresis using a high-molarity tris buffer system without urea. Anal. Biochem. 1986;155:83–88. doi: 10.1016/0003-2697(86)90228-9. [DOI] [PubMed] [Google Scholar]
- Hauswirth AW, Natter S, Ghannadan M, Majlesi Y, Schernthaner GH, Sperr WR, Bühring HJ, Valenta R, Valent P. Recombinant allergens promote expression of CD203c on basophils in sensitized individuals. J. Allergy Clin. Immunol. 2002;110:102–109. doi: 10.1067/mai.2002.125257. [DOI] [PubMed] [Google Scholar]
- Hoffmann-Sommergruber K, Susani M, Ferreira F, Jertschin P, Ahorn H, Steiner R, Kraft D, Scheiner O, Breiteneder H. High-level expression and purification of the major birch pollen allergen, Bet v 1. Protein Expr. Purif. 1997;9:33–39. doi: 10.1006/prep.1996.0671. [DOI] [PubMed] [Google Scholar]
- Kahlert H, Suck R, Weber B, Nandy A, Wald M, Keller W, Cromwell O, Fiebig H. Characterization of hypoallergenic recombinant Bet v 1 variant as a candidate of allergen-specific immunotherapy. Int. Arch. Allergy Immunol. 2008;145:193–206. doi: 10.1159/000109288. [DOI] [PubMed] [Google Scholar]
- Laffer S, Vangelista L, Steinberger P, Kraft D, Pastore A, Valenta R. Molecular characterization of Bip 1, a monoclonal antibody that modulates IgE binding to birch pollen allergen, Bet v 1. J. Immunol. 1996;157:4953–4962. [PubMed] [Google Scholar]
- Laffer S, Hogbom E, Roux KH, Sperr WR, Valent P, Bankl HC, Vangelista L, Kricek F, Kraft D, Grönlind H, Valenta R. A molecular model of type I allergy: Identification and characterization of a nonanaphylactic anti-human IgE antibody fragment that blocks the IgE–FcεRI interaction and reacts with receptor-bound IgE. J. Allergy Clin. Immunol. 2001;108:409–416. doi: 10.1067/mai.2001.117593. [DOI] [PubMed] [Google Scholar]
- Larché M, Akdis CA, Valenta R. Immunological mechanisms of allergenspecific immunotherapy. Nat. Rev. Immunol. 2006;6:761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- Marsh DG, Lichtenstein LM, Campbell DH. Studies on ‘Allergoids’ prepared from naturally occurring allergens. I. Assay of allergenicity and antigenicity of formalinized rye group I component. Immunology. 1970;18:705–722. [PMC free article] [PubMed] [Google Scholar]
- Niederberger V, Horak F, Vrtala S, Spitzauer S, Krauth MT, Valent P, Reisinger J, Pelzmann M, Hayek B, Kronqvist M, Gafvelin G, Grönlund H, Purohit A, Suck R, Fiebig H, Cromwell O, Pauli G, van Hage-Hamsten M, Valenta R. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. U.S.A. 2004;101:14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schöll I, Kalkura N, Shedziankova Y, Bergmann A, Verdino P, Knittelfelder R, Kopp T, Hantusch B, Betzel C, Dierks K, Scheiner O, Boltz-Nitulescu G, Keller W, Jensen-Jarolim E. Dimerization of the major birch pollen allergen Bet v 1 is important for its in vivo IgE-cross-linking potential in mice. J. Immunol. 2005;175:6645–6650. doi: 10.4049/jimmunol.175.10.6645. [DOI] [PubMed] [Google Scholar]
- Sellge G, Laffer S, Mierke C, Vrtala S, Hoffmann MW, Klempnauer J, Manns MP, Valenta R, Bischoff SC. Development of an in vitro system for the study of allergens and allergen-specific immunoglobulin E and immunoglobulin G: Fcε receptor I supercross-linking is a possible new mechanism of immunoglobulin G-dependent enhancement of type I allergic reactions. Clin. Exp. Allergy. 2005;35:774–781. doi: 10.1111/j.1365-2222.2005.02248.x. [DOI] [PubMed] [Google Scholar]
- Spangfort MD, Mirza O, Ipsen H, van Neerven RJ, Gajhede M, Larsen JN. Dominating IgE-binding epitope of Bet v 1, the major allergen of birch pollen, characterized by X-ray crystallography and site-directed mutagenesis. J. Immunol. 2003;171:3084–3090. doi: 10.4049/jimmunol.171.6.3084. [DOI] [PubMed] [Google Scholar]
- Valenta R, Ferreira F, Focke-Tejkl M, Linhart B, Niederberger V, Swoboda I, Vrtala S. From allergen genes to allergy vaccines. Annu. Rev. Immunol. 2010;28:211–241. doi: 10.1146/annurev-immunol-030409-101218. [DOI] [PubMed] [Google Scholar]
- van Hage-Hamsten M, Kronqvist M, Zetterström O, Johansson E, Niederberger V, Vrtala S, Grönlund H, Grönneberg R, Valenta R. Skin test evaluation of genetically engineered hypoallergenic derivatives of the major birch pollen allergen, Bet v 1: results obtained with a mix of two recombinant Bet v 1 fragments and recombinant Bet v 1 trimer in a Swedish population before the birch pollen season. J. Allergy Clin. Immunol. 1999;104:969–977. doi: 10.1016/s0091-6749(99)70077-1. [DOI] [PubMed] [Google Scholar]
- Vrtala S, Hirtenlehner K, Vangelista L, Pastore A, Eichler HG, Sperr WR, Valent P, Ebner C, Kraft D, Valenta R. Conversion of the major birch pollen allergen, Bet v 1, into two nonanaphylactic T cell epitope-containing fragments: candidates for a novel form of specific immunotherapy. J. Clin. Invest. 1997;99:1673–1681. doi: 10.1172/JCI119330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtala S, Akdis CA, Budak F, Akdis M, Blaser K, Kraft D, Valenta R. The cell epitope-containing hypoallergenic recombinant fragments of the major birch pollen allergen, Bet v 1, induce blocking antibodies. J. Immunol. 2000;165:6653–6659. doi: 10.4049/jimmunol.165.11.6653. [DOI] [PubMed] [Google Scholar]
- Vrtala S, Hirtenlehner K, Susani M, Akdis M, Kussebi F, Akdis CA, Blase K, Hufnagl P, Binder BR, Politou A, Pastore A, Vangelista L, Sperr WR, Semper H, Valent P, Ebner C, Kraft D, Valenta R. Genetic engineering of a hypoallergenic trimer of the major birch pollen allergen Bet v 1. FASEB J. 2001;15:2045–2057. doi: 10.1096/fj.00-0767fje. [DOI] [PubMed] [Google Scholar]
- Vogel L, Lüttkopf D, Hatahet L, Haustein D, Vieths S. Development of a functional in vitro assay as a novel tool for the standardization of allergen extracts in the human system. Allergy. 2005;60:1021–1028. doi: 10.1111/j.1398-9995.2005.00803.x. [DOI] [PubMed] [Google Scholar]
- Whitmore L, Wallace BA. DICHROWEB, an online server for protein secondary structure analyses from circular dichroism spectroscopic data. Nucleic Acids Res. 2004;32:W668–673. doi: 10.1093/nar/gkh371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann P, Giehl K, Almo SC, Fedorov AA, Girvin M, Steinberger P, Rüdiger M, Ortner M, Sippl M, Dolecek C, Kraft D, Jockusch B, Valenta R. Molecular and structural analysis of a continuous birch profilin epitope defined by a monoclonal antibody. J. Biol. Chem. 1996;271:29915–29921. doi: 10.1074/jbc.271.47.29915. [DOI] [PubMed] [Google Scholar]
- Winther L, Arnved J, Malling HJ, Nolte H, Mosbech H. Side-effects of allergen-specific immunotherapy: a prospective multi-centre study. Clin. Exp. Allergy. 2006;36:254–260. doi: 10.1111/j.1365-2222.2006.02340.x. [DOI] [PubMed] [Google Scholar]