Abstract
While TRAIL is a promising anticancer agent due to its ability to selectively induce apoptosis in neoplastic cells, many tumors, including pancreatic ductal adenocarcinoma (PDA), display intrinsic resistance, highlighting the need for TRAIL-sensitizing agents. Here we report that TRAIL-induced apoptosis in PDA cell lines is enhanced by pharmacological inhibition of glycogen synthase kinase-3 (GSK-3) or by shRNA-mediated depletion of either GSK-3α or GSK-3β. In contrast, depletion of GSK-3β, but not GSK-3α, sensitized PDA cell lines to TNFα-induced cell death. Further experiments demonstrated that TNFα-stimulated IκBα phosphorylation and degradation as well as p65 nuclear translocation were normal in GSK-3β-deficient MEFs. Nonetheless, inhibition of GSK-3β function in MEFs or PDA cell lines impaired the expression of the NF-κB target genes Bcl-xL and cIAP2, but not IκBα. Significantly, the expression of Bcl-xL and cIAP2 could be reestablished by expression of GSK-3β targeted to the nucleus but not GSK-3β targeted to the cytoplasm, suggesting that GSK-3β regulates NF-κB function within the nucleus. Consistent with this notion, chromatin immunoprecipitation demonstrated that GSK-3 inhibition resulted in either decreased p65 binding to the promoter of BIR3, which encodes cIAP2, or increased p50 binding as well as recruitment of SIRT1 and HDAC3 to the promoter of BCL2L1, which encodes Bcl-xL. Importantly, depletion of Bcl-xL but not cIAP2, mimicked the sensitizing effect of GSK-3 inhibition on TRAIL-induced apoptosis, whereas Bcl-xL overexpression ameliorated the sensitization by GSK-3 inhibition. These results not only suggest that GSK-3β overexpression and nuclear localization contribute to TNFα and TRAIL resistance via anti-apoptotic NF-κB genes such as Bcl-xL, but also provide a rationale for further exploration of GSK-3 inhibitors combined with TRAIL for the treatment of PDA.
Keywords: apoptosis, GSK-3, NF-κB, pancreatic cancer, TNFα, TRAIL
Pancreatic ductal adenocarcinoma (PDA) is one of the most lethal human malignancies and is highly resistant to conventional chemotherapy due, in part, to its ability to resist induction of apoptosis.1 There has been substantial interest in death receptor (DR)-initiated apoptosis as a potential means of circumventing chemotherapy resistance;2, 3, 4 and the death ligand TRAIL has undergone extensive preclinical and clinical testing due to its selective killing of cancer cells.3, 5, 6, 7, 8 Binding of TRAIL to DR4 and DR5 initiates assembly of the death-inducing signaling complex (DISC), which directly triggers caspase activation and, in some cells, activates the mitochondrial apoptotic pathway.5, 6, 7, 8, 9 Unfortunately, a potential limitation of TRAIL-based therapy is that many cancer cells display TRAIL resistance, which can arise at any step of the apoptotic process from impairment of DISC formation to constitutive upregulation of anti-apoptotic proteins of both the extrinsic and mitochondrial pathways.1, 7, 10, 11, 12
The outcome of TRAIL binding to cancer cells is highly context dependent. In addition to activating caspase-dependent cell death, DR4 and DR5 ligation can initiate signaling that results in increased survival, invasion and metastasis.12, 13, 14, 15 In particular, TRAIL has also been reported to activate the transcription factor NF-κB, which transactivates the genes encoding several key anti-apoptotic proteins, including cFlip, Bcl-xL, Mcl-1 and IAPs, each of which has been implicated in TRAIL resistance.10, 12, 13, 16, 17 Novel strategies that overcome this survival signaling could potentially enhance the antitumor effects of TRAIL.
The two glycogen synthase kinase-3 (GSK-3) isoforms, GSK-3α and GSK-3β, were initially described as key enzymes involved in glycogen metabolism, but have subsequently been shown to regulate a diverse range of cellular functions, including apoptosis.18, 19 In particular, GSK-3β phosphorylates and regulates the stability of a number of proteins that are critical for proliferation and survival.20 Importantly, GSK-3β deficient mouse embryonic fibroblasts (MEFs) exhibit defective NF-κB activation in response to TNFα, although the reason for this defect remains unclear.21 Conversely, constitutive NF-κB activation in PDA contributes to cell proliferation and survival, as well as resistance to TNFα- and TRAIL-induced apoptosis.7, 16, 17, 22
We and others have shown that GSK-3β is progressively overexpressed during progression from pancreatic intraepithelial neoplasia to advanced PDA and is localized to the nucleus in most moderately and poorly differentiated tumors.23, 24, 25, 26 Additionally, it has been observed that GSK-3β overexpression contributes to PDA cell proliferation and survival, whereas GSK-3 inhibition reduces pancreatic cancer cell viability in vitro and suppresses tumor xenograft growth in vivo, at least partly, via downregulation of NF-κB activity.23, 24, 25, 26, 27, 28 In view of the impact of NF-κB target genes on TRAIL-induced apoptosis, we have assessed the impact of GSK-3 inhibition or downregulation on TRAIL-induced killing and have examined the mechanism of GSK-3 inhibitor (GSK-3i)-mediated TRAIL sensitization in PDA cells. Our results demonstrate for the first time that GSK-3β, acting within the nucleus, regulates the action of NF-κB at a subset of its target genes to modulate TRAIL-induced apoptosis.
Results
Isoform-specific GSK-3 depletion sensitizes PDA cells differentially to TNFα- and TRAIL-induced apoptosis
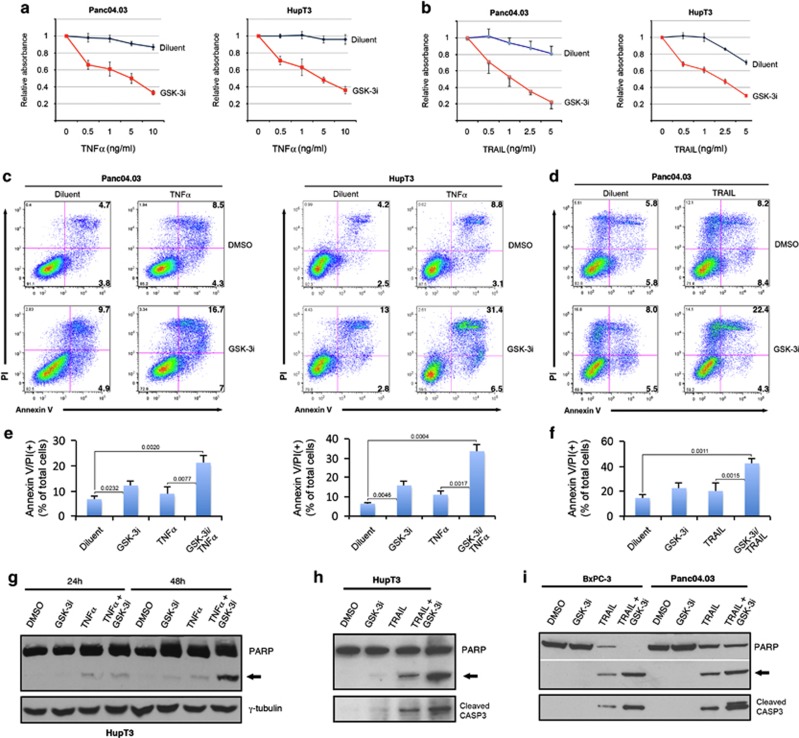
To provide a baseline for subsequent combination studies, we examined the effect of a potent and specific GSK-3i (LY2064827) on pancreatic cancer cell growth. We observed growth inhibition in all tested cell lines (Supplementary Figures S1a–e) with IC50s ranging from 50 nM to 5 μM after a 72-hour treatment (Supplementary Figure S1f). We next tested the effect of GSK-3i on TNFα and TRAIL-induced apoptosis (Figure 1). At concentrations that had little impact by themselves, GSK-3i enhanced TNFα-induced cell death as assessed by MTS assay, DNA fragmentation, annexin V binding and PARP cleavage (Figures 1a, c, e and g). Likewise, GSK-3i enhanced TRAIL-induced apoptosis in all PDA cell lines tested as demonstrated by DNA fragmentation, annexin V binding and PARP as well as CASP3 cleavage (Figures 1b, d, f, h and I and Supplementary Figure S2). This sensitization occurred with minimal effect on DR4 or DR5 mRNA expression and little if any change in cell surface levels of these receptors (Supplementary Figure S3).
Figure 1.
GSK-3 inhibition enhances both TNFα- and TRAIL-induced apoptosis in pancreatic cancer cells. (a, b) Cell proliferation determined by MTS assay. Pancreatic cancer cells were pretreated for 30 min with 0.2 μM of GSK-3i (LY2064827) or diluent (0.1% DMSO) followed by co-treatment with the indicated concentrations of TNFα or TRAIL for 24 h. (c–f) Cells were treated with diluent, GSK-3i and TNFα or TRAIL for 24 h as in (a) and dually stained with annexin V-FITC and PI for flow cytometry. (e, f) Results from three independent experiments were quantified and shown as mean±S.D. with indicated P-values (Student's t-test). (g) HupT3 cells were treated with GSK-3i and TNFα alone or in combination for 24 and 48 h, respectively. Whole-cell extracts were prepared for immunoblot analysis for PARP1. (h, i) Indicated pancreatic cancer cells were treated with GSK-3i (0.2 μM) and TRAIL (2 ng/ml) alone or in combination for 24 h. Whole-cell extracts were immunoblotted with antibodies specific for PARP1 and cleaved CASP3. Arrows indicate cleaved PARP1 (g–i)
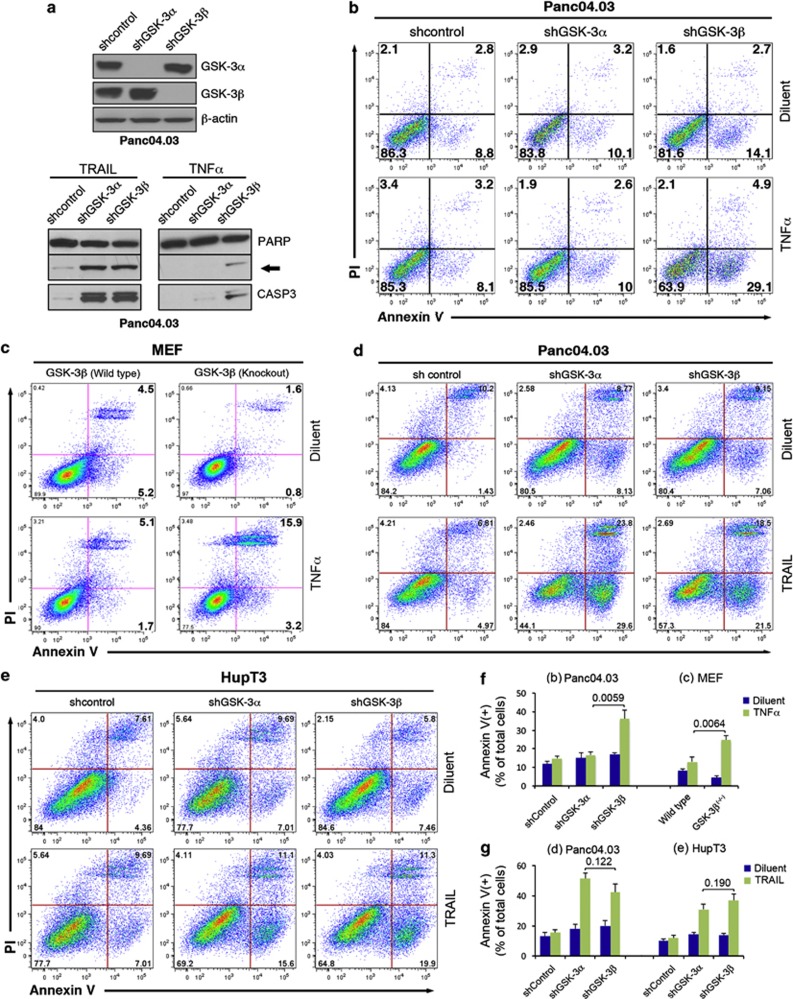
Because GSK-3 inhibitors, including LY2064827, do not discriminate between the two GSK-3 isoforms, we knocked down each GSK-3 isoform selectively using multiple lentiviral shRNA constructs (Figure 2a and Supplementary Figure S4a). Interestingly, GSK-3β, but not GSK-3α supression, led to increased TNFα-induced apoptosis, as manifested by increased PARP and CASP3 cleavage as well as annexin V binding (Figures 2a, b and f and Supplementary Figure S4b). Consistent with this observation, more apoptosis was observed in GSK-3β-null mouse embryonic fibroblasts (MEFs) than in wild-type (WT) MEFS after TNFα treatment (Figures 2c and f). In contrast, suppression of either GSK-3α or GSK-3α sensitized similarly to TRAIL-induced apoptosis in Panc04.03 and HupT3 cells (Figures 2d, e and g). Similar enhancement of TRAIL-induced apoptosis was observed using two additional GSK-3α or GSK-3β lentiviral shRNA construct (Supplementary Figures S4b and c). These results point to a unique role for GSK-3β in suppressing TNFα-induced apoptosis, whereas both GSK-3α and GSK-3β contribute to TRAIL resistance in pancreatic cancer cells.
Figure 2.
Isoform-specific role of GSK-3 in TRAIL- and TNFα-induced apoptosis. (a) Whole-cell extracts prepared from cells stably transduced with isoform-specific lentiviral shRNAs or scrambled control shRNA were subjected to immunoblotting to demonstrate specific and efficient knockdown of endogenous GSK-3 proteins (upper panel). Lentiviral shRNA transduced cells were treated with TRAIL or TNFα for 24 h. Whole-cell extracts were subjected to immunoblot against PARP1 and cleaved CASP3 (lower panel). Arrow indicates cleaved PARP1. (b, c, f) Panc04.03 stably transduced with lentiviral shRNA (b) or MEFs (c) were treated with TNFα(20 and 2 ng/ml, respectively) for 18 h and stained with annexin V-FITC and PI for flow cytometry. (d, e, g) Cells stably transduced with the indicated shRNA were treated with TRAIL (5 ng/ml) or diluent for 18 h, stained with annexin V-FITC/PI and examined by flow cytometry. (f, g) Results from three independent experiments were quantified and shown as mean±S.D. with indicated P-values (Student's t-test). Note that only GSK-3β knockdown or deletion enhanced TNFα-induced apoptosis in Panc04.03 or MEF cells (f), whereas knockdown of either GSK-3α or GSK-3β sensitized the cells equally to TRAIL (g)
GSK-3β is not essential for TNFα-induced p65 nuclear translocation
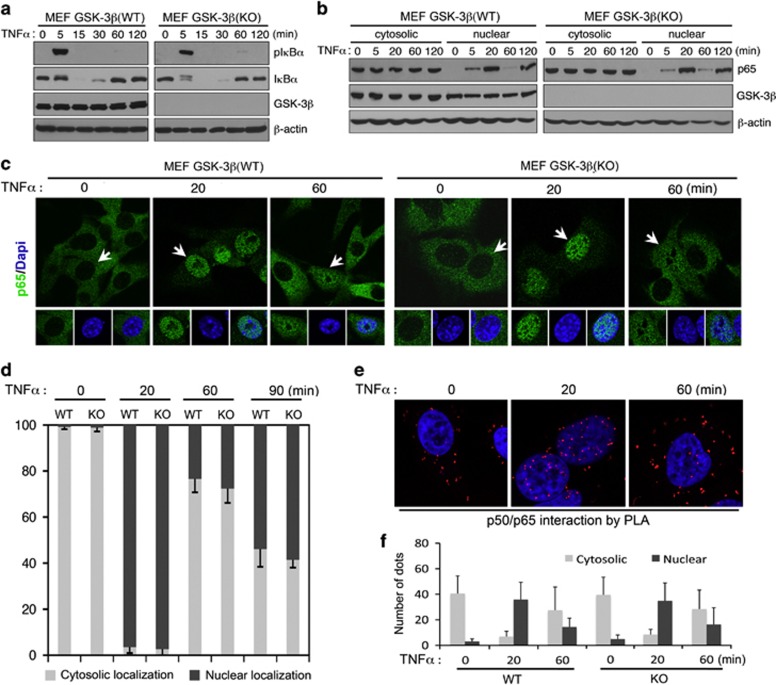
To determine how GSK-3 modulation affects death ligand signaling, we started by evaluating the effect of GSK-3β deletion on key steps of NF-κB activation. TNFα-treatment of either WT or GSK-3β-null MEFs resulted in robust IκBα phosphorylation by 5 min and loss of total IκBα protein by 15 min (Figure 3a), with robust accumulation of p65 in the nucleus by 20 min in both cell lines (Figure 3b). The pattern and extent of nuclear p65 oscillations were also similar at extended periods following TNFαtreatment (Figures 3c and d). Taken together, these results suggest that GSK-3β is not essential for either the initial wave of IκBα degradation leading to p65 nuclear entry or the subsequent IκBα gene expression that drives the kinetics of the p65 nuclear oscillations.
Figure 3.
GSK-3β is not essential for TNFα-induced IKBα degradation or p65 nuclear translocation. (a, b) Western blot analysis of whole-cell extracts (a) or cytosolic and nuclear extracts (b) from MEFs treated with 10 ng/ml TNFα for the indicated periods of time. Note that there is no discernible difference in TNFα- induced IκBα phosphorylation/degradation (a) or p65 nuclear translocation (b) between WT and GSK-3β-null MEF cells. (c) Immunofluorescence staining and confocal microscopic imaging showing p65 expression/localization in MEF cells following TNFα stimulation (10 ng/ml) for the indicated periods of time. (d) Quantification of images (c) for percentage of p65 with predominant cytoplasmic or nuclear localization in wild-type (WT) and Gsk-3β−/− (KO) MEF cells at the indicated time points. Arrows point to the cells shown in inset with p65 predominantly localized to the cytoplasm (0 min) or nucleus (20 and 60 min). (e) Confocal microscopic imaging showing in situ p50 and p65 interaction detected by PLA following TNFα stimulation. (f) Quantification of dots corresponding to sites of interaction as shown in (e) as mean±S.D. as described in Materials and Methods
Because the predominant form of NF-κB in the cytosol in resting cells and in the nucleus upon NF-κB activation is a p50/p65 heterodimer, we further investigated the effect of GSK-3β on p50/p65 interactions using a proximity ligation assay (PLA), a highly sensitive technique to detect protein–protein interactions in situ. As predicted, the p50/p65 interactions were observed almost exclusively in the cytosolic compartment in resting cells, mainly in the nucleus 20 min after TNFα exposure, and largely in the cytosol again at 60 min (Figures 3e and f). There was no significant difference in the number or the sites of p50/p65 interactions following TNFα exposure in WT versus GSK-3β null MEFs (Figures 3e and f). Accordingly, GSK-3β deficiency does not appear to impact TNFα-induced canonical NF-κB activation up to the point of p50/p65 nuclear entry.
Isoform-specific regulation of BCL2L1 and BIRC3 by GSK-3 in pancreatic cancer cells
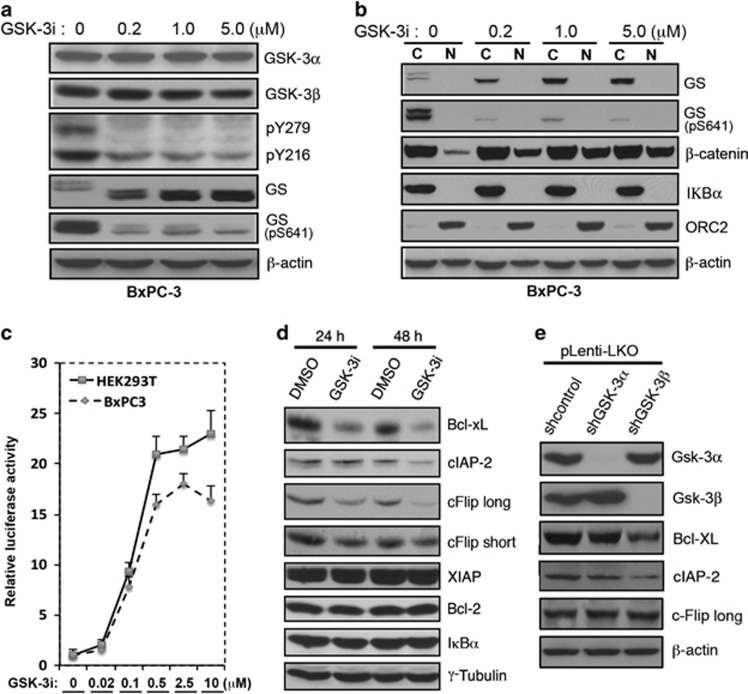
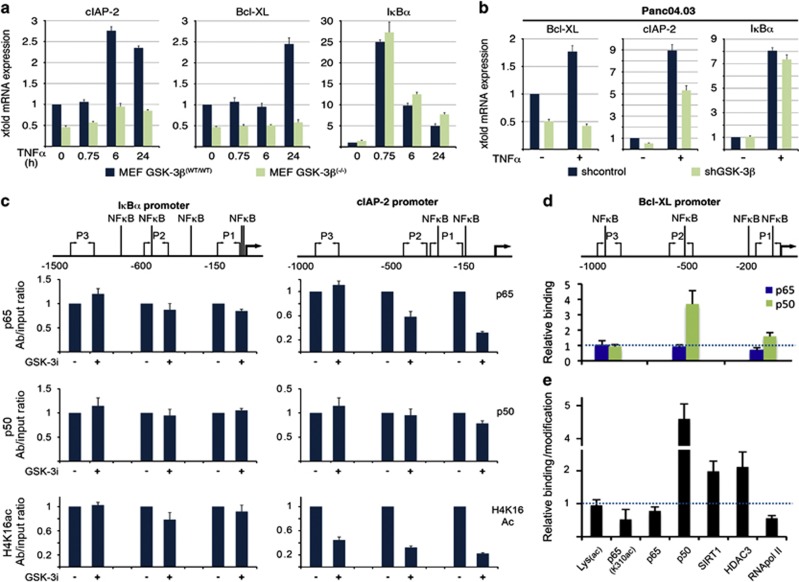
In further experiments, we examined the effects of GSK-3i and isoform-specific shRNA on NF-κB target genes that affect the DR pathway. Dose-response experiments demonstrated that concentrations of 200–1000 nM GSK-3i decreased phosphorylation of GSK-3α/β Y276/Y216 and glycogen synthase S641 (Figure 4a), increased nuclear accumulation of β-catenin (Figure 4b), and increased activity of pLenti-TopFlash Luciferase reporter (Figure 4c), which reflects β-catenin nuclear accumulation. The same 500 nM GSK-3i concentration that produced near-maximal pLenti-TopFlash Luciferase activation (Figure 4c) decreased expression of Bcl-xL, cIAP2 and cFlip (both long and short isoforms), while IκBα and Bcl-2, and XIAP were not affected (Figure 4d). Interestingly, in cells expressing GSK-3 isoform-specific shRNA, Bcl-xL and cIAP2 were decreased with GSK-3β, but not withGSK-3α suppression (Figure 4e and data not shown). cFlip, however, was not reduced by either GSK-3α or GSK-3β shRNA, suggesting that both kinases might need to be inhibited to affect this protein.
Figure 4.
GSK-3 inhibition and GSK-3β suppression regulate a subset of anti-apoptotic NF-κB target genes. (a, b) Western blot analysis of whole-cell extracts (a) or cytosolic and nuclear fractions (b) with indicated antibodies. BxPC-3 cells were treated for 24 h with the indicated concentrations of GSK-3i before preparation of protein extracts. (c) Luciferase reporter assay in BxPC-3 and HEK293T cells stably transduced with TopFlash and treated with the indicated concentration of GSK-3i for 6 h. The relative firefly luciferase activities were normalized to renilla luciferase activity and plotted as a histogram. (d) After Panc04.03 cells were treated with 0.5 μM of GSK-3i for 24 and 48 h, respectively, whole-cell extracts were prepared and immunoblotted with the indicated antibodies. (e) Whole-cell extracts from Panc04.03 cells stably transduced with GSK-3α or GSK-3β lentiviral shRNA were probed with the indicated antibodies
GSK-3β modulates NF-κB binding and cofactor recruitment at the BCL2L1 and BIRC3 promoters
The preceding results suggest that GSK-3β, but not GSK-3α modulates a subset of NF-κB target genes. This possibility was further evaluated by qRT-PCR and chromatin immunoprecipitation (Figure 5). qRT-PCR revealed that IκBα mRNA increased more than 20-fold following a 45-min TNFα treatment in both WT and GSK-3β null MEF cells (Figure 5a). In contrast, Bcl-xL and cIAP2 mRNA levels increased in WT MEFs but not GSK-3β-null MEFs (Figure 5a). These alterations in transcript levels largely correlated to protein expression changes as determined by immunoblotting (Supplementary Figure S5). Depletion of GSK-3β in Panc04.03 cells similarly impaired TNFα-induced expression of Bcl-xL and cIAP2 but spared IκBα (Figure 5b). These results indicate that GSK-3β loss does not globally impact NF-κB transcriptional activity, but instead impairs the expression of a specific set of NF-κB target genes.
Figure 5.
GSK-3β differentially affects the binding of NF-κB p65 and p50 to the BCL2L1 and BIRC3 promoters. (a, b) qRT-PCR analysis of MEF and Panc04.03 cells following TNFα-stimulation. Note that Bcl-xL and cIAP2 transcripts, but not IKBα, were reduced in Gsk-3β-null MEFs compared with WT control (a). Similarly, Bcl-xL and cIAP2, but not IKBα mRNA expression was reduced in TNFα-treated Panc04.03 cells transduced with lentiviral GSK-3β shRNA (b). (c–e). ChIP analysis of Panc04.03 cells treated with GSK-3i (0.5 μM LY2064827) or diluent (0.1% DMSO) for 16 h. The relative amount of DNA precipitated with the specified antibodies was accessed by qPCR using three sets of primers targeting promoter regions containing the depicted NF-κB sites. The qPCR was performed in triplicate; and the result is normalized to diluent control
As we found no gross defect in GSK-3β-null cells up to p65 nuclear entry, we next examined whether GSK-3 may affect binding of p65 and p50 to the promoters. For the IκBα promoter, we found no significant change in either p65 or p50 loading or histone 4 lysine 16 acetylation (a marker of gene activation) following GSK3i treatment (Figure 5c). In contrast, at the promoter of the BIRC3 gene, which encodes cIAP2, p65 binding and H4K16ac modification were significantly reduced, with only a marginal decrease in p50 binding (Figure 5c). Unexpectedly, we detected increased p50 binding at the promoter of the BCL2L1 gene, which encodes Bcl-xL (Figure 5d), while p65 binding was only marginally decreased (Figure 5e). Further analysis of the BCL2L1 promoter indicated that GSK-3i treatment was also associated with increased SIRT1 and HDAC3 loading along with reduced binding of lysine 310-acetylated p65 and RNA pol II, indicating that the GSK-3i induced the formation of repressive chromatin. These results suggest that GSK-3i may differentially impact p65 and/or p50 binding and unloading from chromatin depending on the target gene promoter.
Nuclear GSK-3β contributes to Bcl-xL and cIAP2 expression
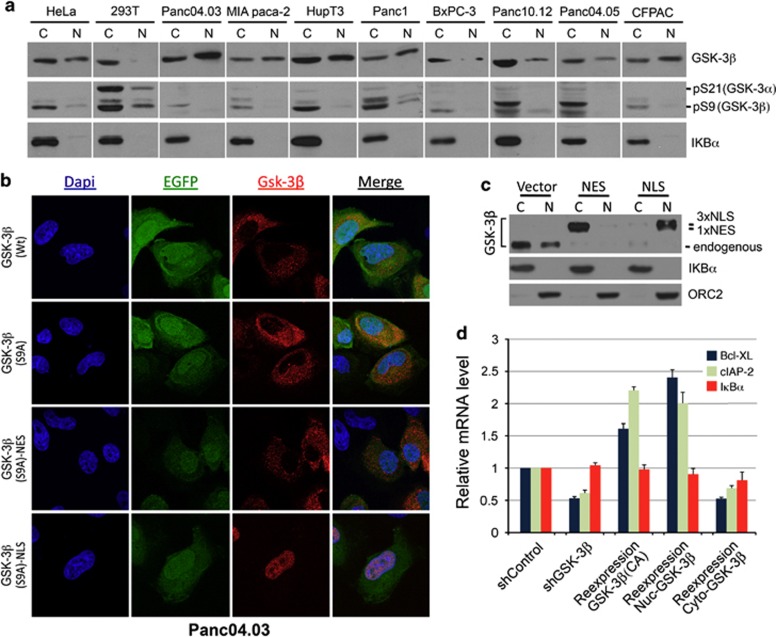
The preceding results not only show that GSK-3β modulates the effects of NF-κB on a specific subset of its target genes, but also suggest that these effects occur after NF-κB enters the nucleus. Consistent with these results, we detected abundant nuclear GSK-3β in all pancreatic cancer cell lines examined (Figure 6a). Moreover, nuclear GSK-3β exhibited minimal S9 phosphorylation, an auto-inhibitory modification, suggesting that the nuclear pool of GSK-3β is highly active in these cells (Figure 6a). These results prompted us to determine the role of nuclear GSK-3β in the regulation of these NF-κB target genes. For this purpose, we generated a set of mammalian suppression/re-expression plasmids that knocked down endogenous GSK-3β and expressed modified GSK-3β that contained either a nuclear export signal (NES) for cytoplasmic targeting or a nuclear localization signal (NLS) for nuclear targeting. Immunofluorescence and cellular fractionation followed by immunoblotting confirmed that GSK-3β(S9A).NES.F and GSK-3β(S9A).NLS.F localized mainly to the cytoplasm and nucleus, respectively (Figures 6b and c). Using this reconstituted system, we investigated the effect of GSK-3β subcellular localization on the expression of IκBα, Bcl-xL and cIAP2. Consistent with our previous data, neither depletion of GSK-3β nor re-expression of a cytosolic or nuclear form of GSK-3β affected IκBα gene expression (Figure 6d). Interestingly, expression of cytosolic GSK-3β resulted in reduced expression of both Bcl-xL and cIAP2 similar to that of GSK-3β depletion (Figure 6d). In contrast, expression of either the S9A mutant or the nuclear-localized GSK-3β resulted in increased expression of both Bcl-xL and cIAP2 (Figure 6d). Taken together, these data indicate that active nuclear GSK-3β has the potential to regulate NF-κB target genes involved in cell survival.
Figure 6.
Nuclear GSK-3β is active and contributes to regulation of Bcl-xL and cIAP2. (a) Western blot analysis of cytosolic and nuclear fractions from the indicated cell lines. Note the abundant presence of nuclear GSK-3β and minimal S9 phosphorylation in all pancreatic cancer cells. (b) Immunofluorescence staining and confocal microscopic imaging showing the expression/localization of GSK-3β in Panc04.03. Cells were transfected for 48 h with pCMS4-eGFP-H1P suppression/re-expression vectors for the expression of C-terminal Flag-tagged GSK-3β including WT, S9A mutant, and S9A with NLS and NES signals. The plasmid also expresses EGFP driven by an independent promoter, making it feasible to identify individual transfected cells. (c) Cytosolic and nuclear protein samples from above transfected cells were subjected to immunoblotting with indicated antibodies. ORC2 is used as a marker for nuclear protein. (d) qRT-PCR showing effect of reconstituted GSK-3β expression on Bcl-xL, cIAP2 and IκBα expression. Results of one representative experiment are shown as mean of triplicates ±S.D. and normalized to RPLP0 expression
Bcl-xL and XIAP, but not cIAP2, contribute to GSK-3i-mediated sensitization to TRAIL- and TNFα-induced apoptosis
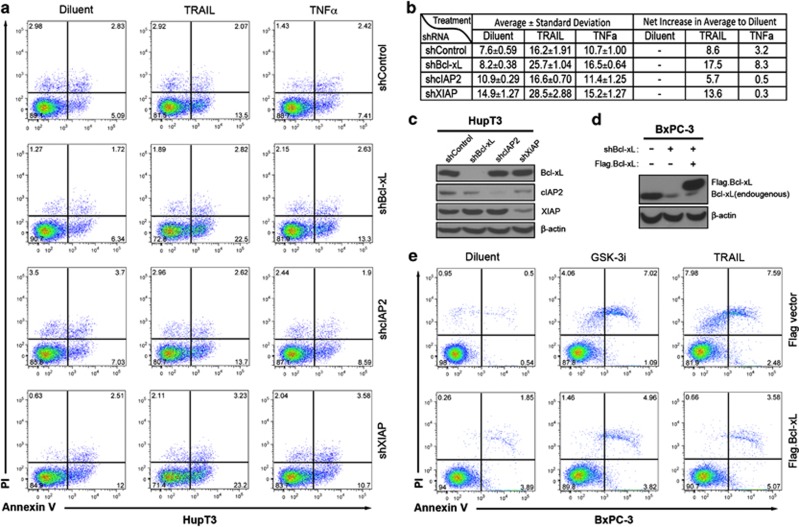
Bcl-xL, cIAP2 and XIAP are all important apoptotic regulators. To assess their roles in TRAIL- and TNFα-induced apoptosis in PDA cells, we used lentiviral shRNA to generate HupT3 cells with stable knockdown of each gene and determined their response to TRAIL and TNFα (Figures 7a–c). Suppression of XIAP as well as cIAP2 increased basal levels of apoptosis (shXIAP=14.9%±1.27 and shcIAP2=10.9%±0.29 versus shcontrol=7.6%±0.59). At the concentrations studied, TRAIL induced more apoptosis than TNFα in scramble control. Importantly, suppression of either Bcl-xL or XIAP markedly enhanced TRAIL-induced apoptosis (25.7%±1.0 and 28.5%±2.9, respectively, versus 16.2%±1.9 in scrambled control). However, only shBcl-xL cells displayed significantly increased sensitivity to TNFα (16.5%±0.6 versus 8.2%±0.4 in diluent control), whereas shXIAP cells exhibited higher spontaneous apoptosis, but no significant enhancement of TNFα activity (15.2%±1.3 versus 14.9%±1.3 in diluent control). On the other hand, cIAP2 knockdown did not affect either TRAIL (16.6%±0.7 versus 16.2%±1.9 in shcontrol) or TNFα-induced apoptosis (11.4%±1.2 versus 10.7%±1.0 in shcontrol). These results highlight potentially distinct roles for these anti-apoptotic proteins when apoptosis is initiated by different stimuli and also indicate that only Bcl-xL is both regulated by GSK-3β suppression or GSK-3i and contributes to TRAIL resistance. To further examine if Bcl-xL actively participates in GSK-3i-mediated TRAIL sensitization, we expressed Bcl-xL off an NF-κB- and GSK-3i-independent promoter in BxPC-3 cells (Figure 7d) and measured apoptosis following treatment with either GSK-3i or TRAIL (Figure 7e). Similar to HupT3 cells, Bcl-xL knockdown BxPC-3 cells transduced with empty vector showed markedly increased apoptotic induction following GSK-3i or TRAIL exposure (7.1 and 9.0% increase relative to diluent control (Figure 7e upper panel). Importantly, Bcl-xL knockdown cells reconstituted with pLenti-Flag.Bcl-xL, although yielding more spontaneous apoptosis (5.7% versus flag vectsor 1.1%), partially reversed GSK-3i and TRAIL-induced apoptosis (net increase of apoptosis down from 10 to 4.7% and 16.1 to 3.3%, respectively) under the same treatment conditions (Figure 7e, lower panel). These results confirm that Bcl-xL downregulation contributes to GSK-3i-induced sensitization to TRAIL.
Figure 7.
Bcl-xL and XIAP participate in GSK-3i-induced sensitization to TRAIL/ and TNFα. (a) Representative flow cytometric profile of annexin V-FITC and PI stained HupT3 cells to evaluate apoptosis induction. HupT3 cells with stable expression of lentiviral shRNA for Bcl-xL, cIAP2 or XIAP (or scrambled control) were treated with diluent, TNFα or TRAIL as indicated for 18 h before harvesting and staining. (b) Summarized results from three independent analyses as percentage of annexin V-FITC and/or PI cells to total gated cells were shown as average±S.D. (c, d) Whole-cell extracts from above HupT3 cells (c) and BxPC-3 cells stably re-expressing Flag.Bcl-xL (d) were subjected to immunoblotting to confirm efficient suppression of endogenous gene expression and rescued Bcl-xL expression. (e) BxPC-3 cells with stable lentiviral-mediated expression of Flag.Bcl-xL were treated and stained as in (a) for apoptosis analysis
Discussion
In this work, we examined the hypothesis that GSK-3 inhibition can overcome key anti-apoptotic mechanism(s) associated with TRAIL resistance. The rationale behind this combination was based on the premise that GSK-3i treatment downregulates the expression of key anti-apoptotic proteins that mediate TRAIL resistance. A connection between GSK-3β and TRAIL-induced apoptosis was first described in prostate cancer cells, in which GSK-3 inhibition or GSK-3β suppression eliminated TRAIL resistance.29 A more recent study reported a similar caspase-dependent TRAIL sensitization involving the mitochondrial apoptotic pathway in both prostate and pancreatic cancer cells.30 The present work not only confirmed that GSK-3 inhibition enhanced TRAIL-induced apoptosis in various pancreatic cell lines, but also uncovered differential effects of GSK-3 isoforms on TRAIL- and TNFα-induced cell death. Furthermore, our results demonstrated a unique mechanism by which nuclear GSK-3 regulates the NF-κB target genes BCL2L1 and BIRC3.
Most published work on GSK-3 has focused on GSK-3β, while much less is known regarding the relative contribution of GSK-3α despite its widespread tissue distribution.31, 32 Research using genetically engineered mouse models revealed that GSK-3α and GSK-3β largely compensate for each other during embryogenesis, as demonstrated for β-catenin via the Wnt pathway.33 Nevertheless, isoform-specific phenotypes have been reported, including the observation that GSK-3β loss causes a defect in NF-κB activation and craniofacial anomalies,21, 34 while GSK-3α loss results in premature death and acceleration of age-related pathologies due to activation of mTORC1 and associated suppression of autophagy markers.35 A recent study has identified a distinct role for GSK-3α in noncanonical NF-κB signaling via stabilization of TAK1-TAB downstream of oncogenic K-Ras.27, 32 These reports point to GSK-3 isoform-specific effects on the regulation of NF-κB activity.
Because GSK-3α and GSK-3β share a highly homologous kinase domain, the available GSK-3 kinase inhibitors, including LY2064827, do not distinguish between the two isoforms.36 We used isoform-specific lentiviral shRNA vectors to evaluate the role of each GSK-3 isoform in TRAIL-induced apoptosis. Contrary to the reported minor effect of GSK-3α,30 we showed that both isoforms similarly contributed to TRAIL resistance in PDA cells. This discrepancy likely arises from the efficiency of GSK-3α suppression. Our analysis utilized multiple shRNAs, each of which achieved nearly complete suppression of GSK-3α at both mRNA and protein levels and caused similar TRAIL sensitization. Our data are also consistent with a previous report by Sun et al.37, which identified GSK-3 (both GSK-3α and 3β) as a component of an anti-apoptotic protein complex with DDX3 and cIAP1 associated with DR5 in breast cancer cells.37 Although the precise mechanism and substrate(s) of GSK-3 action within this membrane complex remain to be defined, GSK-3 inhibition was found to overcome TRAIL resistance by promoting the initial step of DISC formation leading to caspase-8 and caspase-3 activation.37 Our results, which focused on another aspect of TRAIL killing, also point to an effect of both GSK-3β isoforms in modulating TRAIL sensitivity.
In contrast to TRAIL, only GSK-3β suppression led to sensitization of TNFα-induced cell death in PDA, an effect recapitulated in GSK-3β-null MEFs. As the first report on genetic ablation of the murine GSK-3β gene leading to a defect in NF-κB activation,21 the involvement of GSK-3β in NF-κB signaling has been documented in many cell types. However, conflicting results have been reported regarding the role of GSK-3β loss on NF-κB activation, ranging from major defects in TNFα-induced IκBα phosphorylation/degradation, to minimal or no effect on the cytosolic signaling and p65 nuclear translocation.28, 38, 39, 40 Our group previously proposed that GSK-3β exerts its effect on NF-κB at a point distal to activation of the IKK complex in PDA cells, whereas a role of GSK-3β in maintaining constitutive NF-κB signaling via IKK was also reported.25, 28 Here we provide evidence that GSK-3β is basically dispensable during TNFα-induced canonical NF-κB activation up to p65/p50 nuclear entry, and is only involved in the regulation of a subset of genes such as BCL2L1 and BIRC3, but not IκBα and several other NF-κB target genes (Figures 4 and 5 and data not shown).
Our observation that GSK-3β affects only a subset of NF-κB target genes, raised the possibility that GSK-3β contributes to NF-κB target gene expression in a promoter- or chromatin context-dependent manner. In fact, GSK-3 inhibition in Panc04.03 cells affected NF-κB binding to the BCL2L1 and BIRC3 promoters, but not the IκBα promoter. Where present, the effects of GSK-3 inhibition were characterized by either decreased p65 or increased p50 binding along with increased HDAC3 and SIRT1 recruitment. The underlying mechanism is not presently clear, but may involve posttranslational modification of NF-κB proteins, as GSK-3β has been shown to phosphorylate both p65 and p50.24, 41, 42 GSK-3β has also been reported to phosphorylate and regulate the processing/degradation of NF-κB1 (p105), the precursor of p50 and, therefore, could alter levels of p50 in cells.43 Although detailed mechanism(s) underlying GSK-3β regulation of NF-κB hetero- and homodimer formation and chromatin binding remain to be further characterized, the difference in DNA binding specificity/affinity as well as in transcriptional activity of NF-κB dimer forms may constitute a mechanism for selective activation/repression of a subset of NF-κB target genes.44, 45, 46 In light of our direct experimental evidence that nuclear-localized GSK-3β can efficiently drive the expression of the NF-κB target genes BCL2L1 and BIRC3 in pancreatic cancer cells, it is of interest that many GSK-3β substrates are nuclear transcription factors.20 As GSK-3β is overexpressed and localized to nuclei in the majority of moderately and poorly differentiated PDAs, it is tempting to speculate that, in addition to driving the expression of select NF-κB target genes such as BCL2L1 and BIRC3, nuclear GSK-3β might affect the action of other transcription factors as well.
Among the key NF-κB-target anti-apoptotic proteins linked to TRAIL resistance, cFLIP functions at the apex of death receptor initiated signaling by preventing the recruitment and activation of caspase-8.47 A recent report has identified cFLIP (both long and short isoforms) as a crucial negative regulator of death receptor-induced apoptosis in pancreatic carcinoma cells.48 We initially found that the long isoform of cFLlP is reduced in GSK-3i treated cells, but this was not observed in either GSK-3α or GSK-3β knockdown cells, suggesting a potential redundant role of GSK-3α or GSK-3β in its regulation. Interestingly, suppression of Bcl-xL or XIAP, but not cIAP2, sensitized PDA cells to TRAIL-induced apoptosis. Moreover, expression of Bcl-xL off a GSK-3i-independent promoter could partially reverse the GSK-3i-induced TRAIL sensitization. Our data, which are consistent with previous work showing that both Bcl-xL and XIAP are important in mediating TRAIL resistance,7, 11, 49, 50, 51, 52 demonstrate that downregulation of Bcl-xL by GSK-3i or GSK-3β suppression accounts for part of the GSK-3i-induced death ligand sensitization in PDA cells.
The signaling cascades activating NF-κB pathways are attractive targets for cancer therapy.7, 10, 22 However, the complex mechanisms underlying constitutive NF-κB activation, including both canonical and noncanonical pathways in PDA, require a combination of inhibitors in order to widely block NF-κB activities to achieve therapeutic benefit. Results presented here demonstrate that combining GSK-3i with TRAIL dramatically enhances TRAIL cytotoxicity in pancreatic cancer cells. Our further studies show that GSK-3β in the nucleus of pancreatic cancer cells contributes to TRAIL resistance by impacting binding of NF-κB dimers to a subset of NF-κB-responsive promoters, ultimately modulating the expression of specific anti-apoptotic NF-κB target genes such as BCL2L1. The present findings, together with published work, identify GSK-3-mediated mechanisms of TRAIL resistance at the plasma membrane and in the nucleus. Taken together, these observations provide a strong rationale as well as a molecular basis for further study of TRAIL in combination with GSK-3i in order to potentially improve PDA therapy.
Materials and Methods
Regents and antibodies
GSK-3i (LY2064827) was obtained from Eli Lilly (Indianapolis, IN, USA). Recombinant human TRAIL, human and mouse TNFα were purchased from R&D Systems (Minneapolis, MN, USA). Fluorescein isothiocyanate (FITC)-conjugated annexin V was from Invitrogen (Carlsbad, CA, USA). Blasticidin and puromycin were obtained from InvivoGen (San Diego, CA, USA). Antibodies from Cell Signaling Technologies (Beverly, MA, USA) include rabbit monoclonal anti-GSK-3α/β (D75D3), Bcl-xL (54H6), phospho-IKBαSer32/36) (5A5), p65 (D14E12), cFLIP and Cleaved Caspase-3 (Asp175). Antibodies from Epitomics (Burlingame, CA, USA) include rabbit monoclonal anti-GS, phospho-GS (pS641) and rabbit polyclonal anti-cIAP2. Antibodies from BD PharMingen (San Diego, CA, USA) include mouse monoclonal anti-Bcl-2, XIAP, GSK-3β, β-catenin, ORC2 and PARP. Antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA) include rabbit polyclonal anti-p65, p50, IκBα and mouse monoclonal anti-p50. Mouse monoclonal β-actin, γ-tubulin, anti-FLAG M2 and M2 agarose were from Sigma-Aldrich (St. Louis, MO, USA).
Plasmid construction
For knockdown of endogenous gene expression, including GSK-3α, GSK-3β, Bcl-xL, cIAP2 and XIAP, we used lentivirus-mediated short hairpin RNA (shRNA) expression system (Sigma, St. Louis, MO, USA).53 The plasmids were either constructed in pLKO.1 vector or obtained from the Mayo Clinic RNA Interference Shared Resource (RISR). At least two distinct shRNA target sequences for each gene with confirmed suppression (over 70% decrease at mRNA level relative to scrambled control) were used (Supplementary Table S1). For stable reconstituted expression of Bcl-xL, we cloned its full-length coding region with an N-terminal Flag-tag in pLenti6.3 vector (Invitrogen). To generate GSK-3β mammalian suppression/re-expression constructs, cDNA sequence (5′-CACTGGTCACGTTTGGAAAGA-3′) derived from human GSK-3β 3′ UTR region was first cloned as a hairpin into pCMS4-eGFP-H1P vector.53 Human GSK-3β protein coding region (both WT and constitutively active S9A mutant) were subcloned into this pCMS4-eGFP-H1P-shGSK-3β vector at MluI/NotI sites. For suppression/re-expression of cytosolic and nuclear-localized GSK-3β, the stop codon was mutated and replaced with a coding sequence for either NES from the human protein kinase inhibitor α or 3 tandem copies of the NLS sequence from SV40 large T-antigen, which is followed by a C-terminal Flag-tag. All cDNA and shRNA expression plasmids were verified by direct sequencing at the Mayo Molecular Biology Core Facility.
Lentiviral packaging, transduction and selection of stable cells
Lentivirus packaging, cell infection and selection of pLKO-shRNA stable pancreatic cancer cells with puromycin were performed as previously described following institutional biosafety regulations.53 For stable overexpression of BCL-XL in BxPC-3 cells, pLenti6.3-Flag.BCL-XL or pLenti6.3-Flag.vector viral infected cells were selected in culture media supplemented with blasticidin (5 μg/ml) for two passages (about 6 days) and pooled blasticidin-resistant cells were used as stable overexpression cells. To generate pLenti-TopFlash luciferase vector, we used pLenti6.3-Topo vector (Invitrogen) as a backbone and replaced the ClaI and MluI fragment (containing the CMV promoter, Topo cloning site the V5 tag coding sequence) with seven tandem copies of TCF/LEF binding site followed by the firefly luciferase ORF amplified from pGL3E vector (Promega, Madison, WI, USA). The resulting pLenti-7xTopFlash luciferase vector was packaged into lentiviral particles and used for infection of 293T and BxPC-3 cells, respectively. Blasticidin-selected stable cell clones were used in dual luciferase reporter assays.
Cell culture/transfection, protein extraction and immunoblotting
GSK-3β-null mouse embryonic fibroblasts (MEFs) and matching WT MEFs were a kind gift from Dr. Jim Woodgett (Ontario Cancer Institute, Toronto, ON, Canada).21 HEK293T, HeLa and all pancreatic cancer cell lines were obtained from ATCC, maintained under recommended culture conditions and transfected as previously described.53, 54, 55 Whole cell or nuclear/cytosolic fractionated protein extracts were prepared, quantified and subject to SDS-PAGE and immunoblotting.
Cell proliferation and apoptosis analysis
Proliferation of MEFs and pancreatic cancer cells was measured by MTS assay (Promega) as described.53 To assess DNA fragmentation, pancreatic cancer cells were incubated in buffer containing 0.1% (w/v) Triton X-100, 50 mg/ml PI and 0.1% (w/v) sodium citrate overnight at 4 °C and analyzed by flow cytometry for subdiploid events. For annexin V5 and PI staining, MEFs or pancreatic cancer cells were detached by trypsinization and stained with annexin V labeled with FITC (eBioscience, San Diego, CA, USA) and propidium iodide (PI, 1 mg/ml, Sigma) for 20 min. Cells (20 000 per condition) were then analyzed on a Becton Dickinson FACS Caliber flow cytometer (Franklin Lakes, NJ, USA) as described.56 The fraction of cells positive for annexin V and/or PI was calculated using FlowJo software (Tree Star, Ashland, OR, USA).54
Immunofluorescent staining, proximity ligation assay (PLA) and confocal microscopy
For localization of endogenous p65 in MEFs, WT and GSK-3β-null MEFs were grown on coverslips for 24 h and stimulated with 2 μg/ml of TNFα for the indicated periods of time. Cells were fixed, permeabilized, stained with rabbit monoclonal anti-p65 (1 : 300 dilution) and analyzed via confocal microscopy as previously described.53 To detect subcellular distribution of GSK-3β, Panc04.03 cells were transfected with in pCMS4-H1P-eGFP- GSK-3β vectors by electroporation and grown on coverslips for 48 h before staining with mouse monoclonal anti-flag M2 antibody (1 : 800). For PLA detection of p50/p65 interaction in situ, we used the Duolink in situ PLA kit from Olink Bioscience according to the supplier's instructions. Mouse mAb against p50 (SC8414, Santa Cruz Biotechnology) and rabbit mAb against p65 (#8242, Cell Signaling Technology) were used as primary antibodies at dilutions of 1 : 50 and 1 : 300, respectively. The anti-rabbit plus and anti-mouse minus secondary antibodies were used as PLA probes and Texas red as detection regent (Supplementary information).
Luciferase reporter assay
Luciferase reporter assays were performed as previously described.57 Firefly luciferase activity was normalized to Renilla luciferase. The results were expressed as mean ‘fold induction'. Mean values of at least three independent experiments are displayed±S.D.
RNA extraction and qRT-PCR
RNA extraction using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), generation of cDNA with Superscript III reverse transcription Kit (Invitrogen) and PCR using the comparative CT method with the SYBR Green PCR Master Mix (Applied Biosystems, Grand Island, NY, USA) and the ABI Prism 7900TM Sequence Detection System have previously been described.53 Experiments were performed in triplicate using three independent cDNAs. Primer sequences are in Supplementary Table S2.
Chromatin immunoprecipitation assays
Panc04.03 cells were treated with the GSK-3i LY2064827 (0.5 μM) or diluent (DMSO) for 16 h. Treated cells were cross-linked and subjected to chromatin immunoprecipitation as described.55 The following antibodies were used for ChIP: rabbit polyclonal antibodies to p65 and p50 (Santa Cruz Biotechnologies), monoclonal H3K14ac, RNA polymerase II and rabbit polyclonal HDAC3 and SIRT1 (Millipore, Billerica, MA, USA), rabbit polyclonal anti-p65 (acetylated K310), and anti-acetylated lysine (Abcam, Cambridge, MA, USA). Three sets of qPCR primers were designed for IκBα, Bcl-xL and cIAP2 within promoter/enhancer or intergenic regions based on the location of NF-κB binding sites. The result was first normalized to input or control IgG, then to diluent control. Experiments were performed in triplicate. Primer sequences are presented in Supplementary Table S3.
Acknowledgments
This work was supported, in part by the Mayo Clinic Pancreatic Cancer SPORE P50CA102701 (DDB), Mayo Clinic Prostate Cancer SPORE Developmental Project (J-SZ), American Cancer Society Institutional New Investigator Award (J-SZ), R01 CA69008 (SHK), funds from Universidad del Pais Vasco and CIBERehd (MHV) a Mildred-Scheel fellowship of German Cancer Society (VE and AK), and Reserve Talent of Universities Overseas Research Program of Heilongjiang (ZD).
Glossary
- ChIP
chromatin immunoprecipitation
- cFLIP
cellular Flice-like inhibitory protein
- cIAP2
cellular inhibitor of apoptosis protein 2
- DISC
death-inducing signaling complex
- DR
death receptor
- EGFP
enhanced green fluorescent protein
- FADD
Fas-associated protein with death domain
- FITC
fluorescein isothiocyanate
- GSK-3
glycogen synthase kinase-3
- GSK-3i
glycogen synthase kinase-3 inhibitor
- MEF
mouse embryonic fibroblast
- NES
nuclear export signal
- NLS
nuclear localization signal
- NF-κB
nuclear factor kappa-light-chain-enhancer of activated B cells
- PDA
pancreatic ductal adenocarcinoma
- PLA
proximity ligation assay
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- shRNA
short hairpin RNA
- TNFα
tumor necrosis factor alpha
- TRAIL
tumor necrosis factorα-related apoptosis inducing ligand
- XIAP
X-linked inhibitor of apoptosis protein.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on Cell Death and Disease website (http://www.nature.com/cddis)
Edited by G Raschellá
Supplementary Material
References
- Fulda S. Apoptosis pathways and their therapeutic exploitation in pancreatic cancer. J Cell Mol Med. 2009;13:1221–1227. doi: 10.1111/j.1582-4934.2009.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Exploiting death receptor signaling pathways for tumor therapy. Biochim Biophys Acta. 2004;1705:27–41. doi: 10.1016/j.bbcan.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Micheau O, Shirley S, Dufour F. Death receptors as targets in cancer. Br J Pharmacol. 2013;169:1723–1744. doi: 10.1111/bph.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenazi A. Targeting the extrinsic apoptosis pathway in cancer. Cytokine Growth Factor Rev. 2008;19:325–331. doi: 10.1016/j.cytogfr.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- den Hollander MW, Gietema JA, de Jong S, Walenkamp AM, Reyners AK, Oldenhuis CN, et al. Translating TRAIL-receptor targeting agents to the clinic. Cancer Lett. 2013;332:194–201. doi: 10.1016/j.canlet.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Arlt A, Muerkoster SS, Schafer H. Targeting apoptosis pathways in pancreatic cancer. Cancer Lett. 2013;332:346–358. doi: 10.1016/j.canlet.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29:4752–4765. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- Pennarun B, Meijer A, de Vries EG, Kleibeuker JH, Kruyt F, de Jong S. Playing the DISC: turning on TRAIL death receptor-mediated apoptosis in cancer. Biochim Biophys Acta. 2010;1805:123–140. doi: 10.1016/j.bbcan.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Roder C, Trauzold A, Kalthoff H. Impact of death receptor signaling on the malignancy of pancreatic ductal adenocarcinoma. Eur J Cell Biol. 2011;90:450–455. doi: 10.1016/j.ejcb.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19:5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Wermann H, Arlt A, Schutze S, Schafer H, Oestern S, et al. CD95 and TRAIL receptor-mediated activation of protein kinase C and NF-kappaB contributes to apoptosis resistance in ductal pancreatic adenocarcinoma cells. Oncogene. 2001;20:4258–4269. doi: 10.1038/sj.onc.1204559. [DOI] [PubMed] [Google Scholar]
- Azijli K, Weyhenmeyer B, Peters GJ, de Jong S, Kruyt FA. Non-canonical kinase signaling by the death ligand TRAIL in cancer cells: discord in the death receptor family. Cell Death Differ. 2013;20:858–868. doi: 10.1038/cdd.2013.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund D, Klose S, Zhou D, Baumann B, Roder C, Kalthoff H, et al. Role of caspases in CD95L- and TRAIL-induced non-apoptotic signalling in pancreatic tumour cells. Cell Signal. 2007;19:1172–1184. doi: 10.1016/j.cellsig.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Trauzold A, Siegmund D, Schniewind B, Sipos B, Egberts J, Zorenkov D, et al. TRAIL promotes metastasis of human pancreatic ductal adenocarcinoma. Oncogene. 2006;25:7434–7439. doi: 10.1038/sj.onc.1209719. [DOI] [PubMed] [Google Scholar]
- Braeuer SJ, Buneker C, Mohr A, Zwacka RM. Constitutively activated nuclear factor-kappaB, but not induced NF-kappaB, leads to TRAIL resistance by upregulation of X-linked inhibitor of apoptosis protein in human cancer cells. Mol Cancer Res. 2006;4:715–728. doi: 10.1158/1541-7786.MCR-05-0231. [DOI] [PubMed] [Google Scholar]
- Khanbolooki S, Nawrocki ST, Arumugam T, Andtbacka R, Pino MS, Kurzrock R, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- Doble BW, Woodgett JR. GSK-3: tricks of the trade for a multi-tasking kinase. J Cell Sci. 2003;116 (Pt 7:1175–1186. doi: 10.1242/jcs.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–189. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Kim NG, Gumbiner BM. Regulation of protein stability by GSK3 mediated phosphorylation. Cell Cycle. 2009;8:4032–4039. doi: 10.4161/cc.8.24.10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Carbone C, Melisi D. NF-kappaB as a target for pancreatic cancer therapy. Expert Opin Ther Targets. 2012;16 (Suppl 2:S1–S10. doi: 10.1517/14728222.2011.645806. [DOI] [PubMed] [Google Scholar]
- Kitano A, Shimasaki T, Chikano Y, Nakada M, Hirose M, Higashi T, et al. Aberrant glycogen synthase kinase 3 beta is involved in pancreatic cancer cell invasion and resistance to therapy. PloS One. 2013;8:e55289. doi: 10.1371/journal.pone.0055289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougolkov AV, Fernandez-Zapico ME, Bilim VN, Smyrk TC, Chari ST, Billadeau DD. Aberrant nuclear accumulation of glycogen synthase kinase-3beta in human pancreatic cancer: association with kinase activity and tumor dedifferentiation. Clin Cancer Res. 2006;12:5074–5081. doi: 10.1158/1078-0432.CCR-06-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ougolkov AV, Fernandez-Zapico ME, Savoy DN, Urrutia RA, Billadeau DD. Glycogen synthase kinase-3beta participates in nuclear factor kappaB-mediated gene transcription and cell survival in pancreatic cancer cells. Cancer Res. 2005;65:2076–2081. doi: 10.1158/0008-5472.CAN-04-3642. [DOI] [PubMed] [Google Scholar]
- Shimasaki T, Kitano A, Motoo Y, Minamoto T. Aberrant glycogen synthase kinase 3beta in the development of pancreatic cancer. J Carcinog. 2012;11:15. doi: 10.4103/1477-3163.100866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bang D, Wilson W, Ryan M, Yeh JJ, Baldwin AS. GSK-3alpha promotes oncogenic KRAS function in pancreatic cancer via TAK1-TAB stabilization and regulation of noncanonical NF-kappaB. Cancer Discov. 2013;3:690–703. doi: 10.1158/2159-8290.CD-12-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W, 3rd, Baldwin AS. Maintenance of constitutive IkappaB kinase activity by glycogen synthase kinase-3alpha/beta in pancreatic cancer. Cancer Res. 2008;68:8156–8163. doi: 10.1158/0008-5472.CAN-08-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X, Zhang L, Thrasher JB, Du J, Li B. Glycogen synthase kinase-3beta suppression eliminates tumor necrosis factor-related apoptosis-inducing ligand resistance in prostate cancer. Mol Cancer Ther. 2003;2:1215–1222. [PubMed] [Google Scholar]
- Mamaghani S, Simpson CD, Cao PM, Cheung M, Chow S, Bandarchi B, et al. Glycogen synthase kinase-3 inhibition sensitizes pancreatic cancer cells to TRAIL-induced apoptosis. PloS One. 2012;7:e41102. doi: 10.1371/journal.pone.0041102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrell WB, Szabo-Rogers HL, Liu KJ. Novel reporter alleles of GSK-3alpha and GSK-3beta. PloS One. 2012;7:e50422. doi: 10.1371/journal.pone.0050422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak C, Miyamoto S. A new alpha in line between KRAS and NF-kappaB activation. Cancer Discov. 2013;3:613–615. doi: 10.1158/2159-8290.CD-13-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doble BW, Patel S, Wood GA, Kockeritz LK, Woodgett JR. Functional redundancy of GSK-3alpha and GSK-3beta in Wnt/beta-catenin signaling shown by using an allelic series of embryonic stem cell lines. Dev Cell. 2007;12:957–971. doi: 10.1016/j.devcel.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KJ, Arron JR, Stankunas K, Crabtree GR, Longaker MT. Chemical rescue of cleft palate and midline defects in conditional GSK-3beta mice. Nature. 2007;446:79–82. doi: 10.1038/nature05557. [DOI] [PubMed] [Google Scholar]
- Zhou J, Freeman TA, Ahmad F, Shang X, Mangano E, Gao E, et al. GSK-3alpha is a central regulator of age-related pathologies in mice. J Clin Invest. 2013;123:1821–1832. doi: 10.1172/JCI64398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfeen M, Bharatam PV. Design of glycogen synthase kinase-3 inhibitors: an overview on recent advancements. Curr Pharm Des. 2013;19:4755–4775. doi: 10.2174/1381612811319260007. [DOI] [PubMed] [Google Scholar]
- Sun M, Zhou T, Jonasch E, Jope RS. DDX3 regulates DNA damage-induced apoptosis and p53 stabilization. Biochim Biophys Acta. 2013;1833:1489–1497. doi: 10.1016/j.bbamcr.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwabe RF, Brenner DA. Role of glycogen synthase kinase-3 in TNF-alpha-induced NF-kappaB activation and apoptosis in hepatocytes. Am J Physiol Gastrointest Liver Physiol. 2002;283:G204–G211. doi: 10.1152/ajpgi.00016.2002. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wilson W, 3rd, Cogswell PC, Baldwin AS. Glycogen synthase kinase 3beta functions to specify gene-specific, NF-kappaB-dependent transcription. Mol Cell Biol. 2005;25:8444–8455. doi: 10.1128/MCB.25.19.8444-8455.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Fang X, Jamaluddin MS, Boyd DD, Aggarwal BB. Genetic deletion of glycogen synthase kinase-3beta abrogates activation of IkappaBalpha kinase, JNK, Akt, and p44/p42 MAPK but potentiates apoptosis induced by tumor necrosis factor. J Biol Chem. 2004;279:39541–39554. doi: 10.1074/jbc.M403449200. [DOI] [PubMed] [Google Scholar]
- Buss H, Dorrie A, Schmitz ML, Frank R, Livingstone M, Resch K, et al. Phosphorylation of serine 468 by GSK-3beta negatively regulates basal p65 NF-kappaB activity. J Biol Chem. 279:49571–49574. doi: 10.1074/jbc.C400442200. [DOI] [PubMed] [Google Scholar]
- Itoh S, Saito T, Hirata M, Ushita M, Ikeda T, Woodgett JR, et al. GSK-3alpha and GSK-3beta proteins are involved in early stages of chondrocyte differentiation with functional redundancy through RelA protein phosphorylation. J Biol Chem. 2012;287:29227–29236. doi: 10.1074/jbc.M112.372086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarchi F, Bertoli C, Sandy P, Schneider C. Glycogen synthase kinase-3 beta regulates NF-kappa B1/p105 stability. J Biol Chem. 2003;278:39583–39590. doi: 10.1074/jbc.M305676200. [DOI] [PubMed] [Google Scholar]
- O'Dea E, Hoffmann A. The regulatory logic of the NF-kappaB signaling system. Cold Spring Harb Perspect Biol. 2010;2:a000216. doi: 10.1101/cshperspect.a000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siggers T, Chang AB, Teixeira A, Wong D, Williams KJ, Ahmed B, et al. Principles of dimer-specific gene regulation revealed by a comprehensive characterization of NF-kappaB family DNA binding. Nat Immunol. 2012;13:95–102. doi: 10.1038/ni.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale ST. Dimer-specific regulatory mechanisms within the NF-kappaB family of transcription factors. Immunol Rev. 2012;246:193–204. doi: 10.1111/j.1600-065X.2011.01091.x. [DOI] [PubMed] [Google Scholar]
- Safa AR. c-FLIP, a master anti-apoptotic regulator. Exp Oncol. 2012;34:176–184. [PMC free article] [PubMed] [Google Scholar]
- Haag C, Stadel D, Zhou S, Bachem MG, Moller P, Debatin KM, et al. Identification of c-FLIP(L) and c-FLIP(S) as critical regulators of death receptor-induced apoptosis in pancreatic cancer cells. Gut. 2011;60:225–237. doi: 10.1136/gut.2009.202325. [DOI] [PubMed] [Google Scholar]
- Bai J, Sui J, Demirjian A, Vollmer CM, Jr, Marasco W, Callery MP. Predominant Bcl-XL knockdown disables antiapoptotic mechanisms: tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res. 2005;65:2344–2352. doi: 10.1158/0008-5472.CAN-04-3502. [DOI] [PubMed] [Google Scholar]
- Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69:2425–2434. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Targeting XIAP bypasses Bcl-2-mediated resistance to TRAIL and cooperates with TRAIL to suppress pancreatic cancer growth in vitro and in vivo. Cancer Res. 2008;68:7956–7965. doi: 10.1158/0008-5472.CAN-08-1296. [DOI] [PubMed] [Google Scholar]
- Stadel D, Mohr A, Ref C, MacFarlane M, Zhou S, Humphreys R, et al. TRAIL-induced apoptosis is preferentially mediated via TRAIL receptor 1 in pancreatic carcinoma cells and profoundly enhanced by XIAP inhibitors. Clin Cancer Res. 2010;16:5734–5749. doi: 10.1158/1078-0432.CCR-10-0985. [DOI] [PubMed] [Google Scholar]
- Zhang JS, Koenig A, Young C, Billadeau DD. GRB2 couples RhoU to epidermal growth factor receptor signaling and cell migration. Mol Biol Cell. 2011;22:2119–2130. doi: 10.1091/mbc.E10-12-0969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreros-Villanueva M, Zhang JS, Koenig A, Abel EV, Smyrk TC, Bamlet WR, et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis. 2013;2:e61. doi: 10.1038/oncsis.2013.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Koenig A, Harrison A, Ugolkov AV, Fernandez-Zapico ME, Couch FJ, et al. Mutant K-Ras increases GSK-3beta gene expression via an ETS-p300 transcriptional complex in pancreatic cancer. Oncogene. 2011;30:3705–3715. doi: 10.1038/onc.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XW, Peterson KL, Dai H, Schneider P, Lee SH, Zhang JS, et al. High cell surface death receptor expression determines type I versus type II signaling. J Biol Chem. 2011;286:35823–35833. doi: 10.1074/jbc.M111.240432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JS, Moncrieffe MC, Kaczynski J, Ellenrieder V, Prendergast FG, Urrutia R. A conserved alpha-helical motif mediates the interaction of Sp1-like transcriptional repressors with the corepressor mSin3A. Mol Cell Biol. 2001;21:5041–5049. doi: 10.1128/MCB.21.15.5041-5049.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.