Abstract
Excess pressure integral (XSPI), a new index of surplus work performed by the left ventricle, can be calculated from blood pressure (BP) waveforms and may indicate circulatory dysfunction. We investigated whether XSPI predicted future cardiovascular (CV) events and target organ damage in treated hypertensive individuals.
Radial BP waveforms were acquired by tonometry in 2069 individuals (63±8y) in the Conduit Artery Functional Evaluation sub-study of the Anglo-Scandinavian Cardiac Outcomes trial. Measurements of left ventricular mass index (LVMI; n = 862) and common carotid artery intima media thickness (cIMT; n = 923) were also performed. XSPI and the integral of reservoir pressure (PRI) were lower in people treated with amlodipine ± perindopril than atenolol ± bendroflumethiazide, although brachial systolic BP was similar. A total of 134 CV events accrued over a median 3.4 years of follow-up; XSPI was a significant predictor of CV events after adjustment for age and sex and this relationship was unaffected by adjustment for conventional CV risk factors or Framingham risk score. XSPI, central systolic BP, central augmentation pressure (AP), central pulse pressure (cPP) and PRI were correlated with LVMI, but only XSPI, AP and cPP were positively associated with cIMT. Associations between LVMI and XSPI and PRI, and cIMT and XSPI were unaffected by multivariable adjustment for other covariates. XSPI is a novel indicator of CV dysfunction and independently predicts CV events and target organ damage in a prospective clinical trial.
Keywords: Blood Pressure, Cardiovascular events, Antihypertensive therapy
INTRODUCTION
Systolic and diastolic blood pressure (BP) are important modifiable risk factors for cardiovascular (CV) disease (1). Recently there has been increased interest in the potential predictive value of other parameters derived from the BP waveform. The complex interactions between the heart and the arterial system cause distinctive changes in the BP waveform with aging and disease. Two basic concepts have been advanced to explain these changes: Windkessel models and wave transmission models. Windkessel models describe the pressure waveform in terms of a compliant elastic arterial component coupled to an outflow resistance corresponding to the microcirculation. This is a simple and intuitive model of the circulation, but its limitations in systole are well recognised. This approach has been largely supplanted by models based on arterial wave travel (2;3). Westerhof et al. (4) devised a method for the separation of arterial waves into their forward and backward travelling components, but the original approach requires the simultaneous measurement of arterial pressure and flow at the same location, which is difficult to perform non-invasively. Studies using proxies for wave reflection such as central augmentation index (AIx) have not consistently shown associations with major adverse CV events (5-8), and a recent meta-analysis (9) found that although increased AIx was associated with increased risk of CV events overall, there was significant heterogeneity between studies. Recently we suggested that this may be because AIx is a composite index of arterial compliance and wave reflection (10). An alternative approach has been proposed by Wang et al. (11) where the arterial waveform may be simply described by a heuristic model incorporating elements from both Windkessel and wave analysis, termed the reservoir-wave model (12). Wang et al. used simultaneous measurement of arterial pressure and flow to permit the separation of the measured pressure into reservoir and excess pressure components. More recently, Parker et al. (13) have shown that reservoir pressure can be estimated from the pressure waveform alone and that the integral of reservoir pressure (PRI) corresponds to the theoretical minimum hydraulic work required to generate the required stroke volume. Reservoir pressure calculated from central BP waveforms has recently been shown to correlate with reflected pressure and to predict cardiovascular events in high risk patients (14). Importantly the integral of the excess pressure (XSPI), the difference between the measured BP waveform and PRI, is an index of unnecessary work done by the ventricle in each cardiac cycle and elevated XSPI is likely to be indicative of circulatory dysfunction. We therefore hypothesized that higher XSPI would be associated with increased target organ damage and future CV events and tested this hypothesis in a retrospective analysis of data collected in the Conduit Artery Functional Evaluation (CAFE) and Hypertension Associated Cardiovascular Disease (HACVD) sub-studies of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT).
METHODS
Details of ASCOT (15), CAFE (16) and HACVD (17) have been published previously. In brief, ASCOT recruited 19,257 hypertensive patients, on or off antihypertensive treatment, with three or more other risk factors for CV disease (male sex, age >55 years, a history of smoking, left ventricular hypertrophy, family history of early coronary heart disease (CHD), microalbuminuria or proteinuria, non-insulin dependent diabetes, peripheral vascular disease, previous stroke or transient ischaemic attack, or a ratio of plasma total cholesterol to HDL-cholesterol of six or higher). Participants were randomized to one of the two blood pressure-lowering strategies: either amlodipine±perindopril or atenolol±bendroflumethiazide as required. People with a fasting total cholesterol of <6.5mmol/L, who were untreated with a lipid lowering agent at randomization were additionally randomized to a nested factorial study of 10 mg atorvastatin vs. placebo. Patients randomized to the BP lowering study were followed for a period of 5.5 years. 174 individuals had a CV event prior to baseline assessment; these were excluded from the analysis. All studies were approved by the relevant institutional review committees; were adherent to the principles of the Declaration of Helsinki , and all participants gave informed consent.
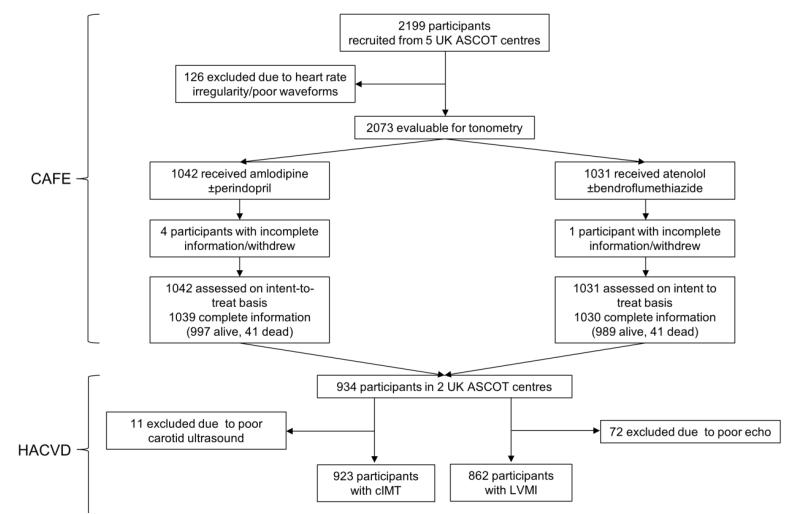
The CAFE sub-study included 2199 patients enrolled in five ASCOT centres in UK and Ireland (figure 1). Initial measurements were made in CAFE approximately 1 year (median (range) 9 (6 - 18) months) after randomization. Radial tonometry was performed using a Sphygmocor device (Atcor Medical, Australia) and central systolic blood pressure (cSBP), augmentation index (AIx) and heart rate corrected augmentation index (AIx75) calculated as previously described (16). A composite of total CV events and procedures as defined in ASCOT was used as the primary endpoint. Total CV events were defined as cardiovascular mortality, non-fatal myocardial infarction (symptomatic and silent), unstable angina, chronic stable angina, fife threatening arrhythmias, non-fatal heart failure, non-fatal stroke, peripheral arterial disease, revascularization procedures, and cerebrovascular events including transient ischaemic attack, retinal vascular thrombosis and reversible ischaemic neurological deficit. This endpoint differs from that used in the original analysis reported in the CAFE study (16), which also included development of renal impairment as an additional post hoc endpoint. To allow comparison with the earlier work the CAFE endpoint was also examined as a secondary objective. Waveforms were calibrated to brachial cuff blood pressure measurements according to manufacturer’s instructions.
Figure 1. Flow diagram of participants.
Abbreviations ASCOT – Anglo Scandinavian Cardiac Outcomes Trial, CAFE - the Conduit Artery Functional Endpoint Study, cIMT – common carotid artery intima-media thickness, HACVD - the Hypertension Associated Cardiovascular Disease study, LVMI – left ventricular mass index.
HACVD recruited a total of 1006 individuals from two of the five ASCOT centres that also participated in CAFE. These individuals underwent extensive additional cardiovascular phenotyping; 933 of these patients also had baseline measurements of central BP as part of the CAFE sub-study (Figure 1) of these 862 had valid measurements of left ventricular (LV) mass and 924 had valid measurements of common carotid intima media thickness (cIMT). In addition to brachial and central BP, these participants underwent measurement of LV mass indexed to height2.7 (LVMI) and cIMT in both common carotid arteries using a HDI 5000 ultrasound device (Philips Healthcare, UK) by 3 experienced echocardiographers and performed according to American Society of Echocardiography guidelines(18;19). LV mass was calculated as:
where LVIDd is LV internal dimension at end diastole, PWTd is posterior wall thickness at end diastole and SWTd is septal wall thickness at end diastole. Mean far wall cIMT was quantified using a validated automated program (AMS) (20). Intraclass correlation coefficients for LV mass and cIMT between observers were 0.88 and 0.94 respectively (n=12).
Calculation of reservoir and excess pressures and their relationship to left ventricular work
Reservoir pressure (figure 2) was calculated from the ensemble averaged radial tonometric waveforms recorded by the Sphygmocor device without the application of a generalized transfer function. In brief, sphygmocor *.txt files were saved and data subsequently imported into Matlab (Mathworks, Inc, Natick, Massachusetts, USA) for analysis using customised programs based on (10;21). Further details are provided in Supplementary data. Reservoir pressure is assumed to vary temporally in the same way throughout aorta and large elastic arteries, but with a time lag that depends on the location and wave propagation characteristics of the arteries (13). Mass conservation in an arterial system containing N vessels requires
| (1) |
where Q0(t) is the volume flow rate at the aortic root, Cn is the compliance of the vessel segment n, is the reservoir pressure at the aortic root, is the reservoir pressure in vessel n, Rn is the resistance of vessel n, τn is the time it takes for a wave to travel from the aortic root to vessel n and P∞ is the pressure at zero flow.
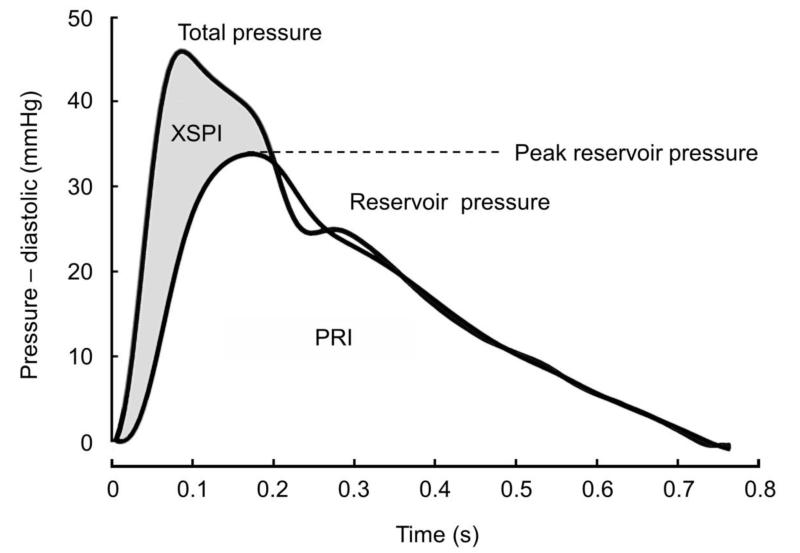
Figure 2. A representative example of separation of the pressure waveform into excess and reservoir pressure.
The trace shows a pressure waveform recorded using an arterial tonometer and calibrated to brachial systolic and diastolic pressure. The reservoir pressure waveform was then calculated the algorithm described in methods. Excess pressure (shaded grey) was calculated by subtracting the reservoir pressure from the measured pressure waveform. Peak reservoir pressure is indicated.
Excess pressure in vessel n (XSPn) is defined as the difference between the measured pressure Pn(t) and the reservoir pressure
| (2) |
Hydraulic work done by the ventricle (W) depends on the volume flow rate Qot and the pressure in the aortic root
| (3) |
where t corresponds to the cardiac period. This work can be separated into reservoir and excess work
| (4) |
where reservoir work is the hydraulic work done by the ventricle against the reservoir pressure and the excess work (XSW) is the work done against the excess pressure at the aortic root. For a given flow, Qo(t) the integral of the excess pressure (XSPI) is therefore an index of the excess work done by the ventricle.
Statistical analysis
Continuous data are presented as means (SD), medians (interquartile range) or means (95% confidence interval) as indicated. Skewed data were log transformed for analysis. Statistical analysis was performed in STATA 12.1 (StataCorp, College Station, Texas, USA). Treatment groups were compared using a Student’s t-test for continuous data and a Chi2 test for categorical data. Correlations were assessed using Pearson’s correlation coefficients (r) and multivariable linear regression was also performed. Age, sex, mean arterial pressure, central augmentation pressure (AP), diabetes, total and high density lipoprotein smoking and BP treatment allocation were included a priori as covariates for cIMT while age, sex, mean arterial pressure, AP and BP treatment allocation were chosen a priori for inclusion in models of LVMI based on associations reported in previous studies. Regression diagnostics including assessment of collinearity was performed. Survival analysis of the relationship between cardiovascular events and BP parameters was assessed using Nelson Aalen analysis and univariate and multivariable Cox regression. The proportional hazards assumption was examined using scaled Schoenfeld residuals. For multivariable Cox regression, classical cardiovascular risk factors: age, sex, systolic blood pressure, diabetes, total and high density lipoprotein and smoking were included a priori as covariates. In an alternative model a 10 year cardiovascular risk estimate based on the Framingham risk score (22) was included to examine further the possible independent predictive utility of XSPI and other hemodynamic parameters. To compare the predictive ability of survival models with and without XSPI we calculated Harrell’s C statistic using the somersd command in Stata(23). We also computed net reclassification improvement (NRI) and integrated discrimination improvement (IDI) as measures of the incremental value of XSPI.(24)
RESULTS
The characteristics of the participants at randomization and at the time of the first visit are shown in table 1. The majority of participants were men and approximately a quarter were current smokers or were diabetic. Participants in the HACVD subgroup were very similar to the whole group (table 1). A total of 134 total CV first events accrued over a median follow-up period of 3.45 y.
Table 1. Characteristics and risk factors at time of randomisation and at visit 1.
| All participants | Atenolol ±bendroflumethiazide | Amlodipine ±perindopril | HACVD | |
|---|---|---|---|---|
|
| ||||
| BASELINE | ||||
| N | 2,069 | 1030 | 1039 | 934 |
| Female, n (%) | 390 (18.9) | 187 (18.2) | 203 (19.5) | 196 (21.0) |
| Age, y | 62.8 (8.2) | 62.6 (8.3) | 62.9 (8.2) | 62.2 (8.0) |
| BMI, kg/m2 | 29.0 (4.6) | 29.0 (4.5) | 29.1 (4.7) | 29.1 (4.7) |
| bSBP, mmHg | 160.4 (17.6) | 159.9 (16.6) | 160.9 (18.5) | 159.7 (18.0) |
| DBP, mmHg | 92.5 (9.7) | 92.4 (9.6) | 92.6 (9.9) | 92.5 (9.9) |
| HR, bpm | 71.5 (12.3) | 71.8 (12.3) | 71.3 (12.4) | 71.1 (12.2) |
| Total cholesterol, mmol/l | 5.8 (1.0) | 5.8 (1.0) | 5.8 (1.1) | 5.8 (1.0) |
| HDL cholesterol, mmol/l | 1.2 (1.1, 1.5) | 1.3 (1.1, 1.5) | 1.2 (1.0, 1.5) | 1.2 (1.0,1.5) |
| Triglycerides, mmol/l | 1.6 (1.1, 2.2) | 1.6 (1.1, 2.2) | 1.6 (1.1, 2.2) | 1.6 (1.1, 2.1) |
| Glucose, mmol/l | 5.5 (5.1, 6.3) | 5.5 (5.0, 6.3) | 5.5 (5.1, 6.2) | 5.3 (4.9, 6.1) |
| Creatinine, mmol/l | 99 (17) | 98 (17) | 99 (17) | 98 (17) |
| Current smoker, n (%) | 518 (25) | 251 (24) | 267 (26) | 204 (22) |
| Diabetes, n (%) | 462 (22) | 237 (23) | 225 (22) | 205 (22) |
| ASCOT-LLA, n (%) | 1089 (53) | 542 (53) | 547 (53) | 479 (51) |
| Aspirin use, n (%) | 517 (25) | 243 (24) | 274 (26) | 437 (47) |
|
| ||||
| VISIT 1 | ||||
|
| ||||
| bSBP, mmHg | 134.4 (15.1) | 134.5 (16.3) | 134.3 (13.7) | 134.9 (16.2) |
| DBP, mmHg | 78.5 (9.4) | 79.1 (9.5) | 78.0 (9.2)** | 79.6 (9.6) |
| HR, bpm | 63.6 (12.3) | 57.7 (10.1) | 69.4 (11.4)*** | 65.8 (13.3) |
| cSBP, mmHg | 124.4 (14.8) | 126.5 (15.8) | 122.4 (13.4)*** | 125.7 (16.1) |
| cPP, mmHg | 45.2 (12.3) | 46.8 (13.2) | 43.5 (11.2)*** | 45.4 (13.0) |
| AP, mmHg | 13.7 (7.3) | 15.7 (7.5) | 11.7 (6.4) | 14.4 (7.5) |
| AIx, % | 28.7 (10.0) | 32.0 (9.0) | 25.4 (9.7)*** | 29.9 (9.7) |
| AIx75, % | 23.3 (7.9) | 23.8 (7.6) | 22.8 (8.1)*** | 24.0 (8.1) |
| XSPI, mmHg.s | 6.0 (1.8, 17.0) | 6.1 (1.8, 17.0) | 5.8 (2.0, 14.2)*** | 6.1 (1.8, 15.5) |
| PRI, mmHg.s | 90.0 (40.7, 185.5) | 99.2 (45.3, 185.5) | 80.9 (40.7, 155.2)** | 93.5 (41.4, 185.5) |
| Peak Pres, mmHg | 110.5 (12.9) | 112.5 (13.5) | 108.6 (12.0)*** | 111.5 (13.7) |
Results are mean (SD), median (interquartile range) for skewed data or n (%) for categorical data. AIx – augmentation index, AIx75 – heart rate corrected augmentation index, AP – augmentation pressure, ASCOT-LLA – lipid lowering arm in ASCOT, BMI – body mass index, bSBP – brachial systolic blood pressure, cPP – central pulse pressure, cSBP – systolic blood pressure, DBP – diastolic blood pressure, HR – heart rate, HDL – high density lipoprotein, Pres – reservoir pressure, PRI –reservoir pressure intergral, XSPI – excess pressure integral.
P<0.01;
P<0.001 comparing amlodipine ±perindopril with atenolol ±bendroflumethiazide by Student’s t-test or Mann Whitney U test for skewed data.
Differential effects of antihypertensive treatment regimen on blood pressure and derived variables
As previously reported (16), treatment with atenolol ± bendroflumenthiazide and amlodipine ± perindopril achieved similar levels of control of brachial systolic BP, although diastolic BP was lower in the amlodipine-based regimen. XSPI, PRI and peak reservoir pressure were significantly lower in individuals randomised to amlodipine ± perindopril (table 1). Amlodipine ± perindopril treatment was also associated with lower cSBP, lower AIx and AIx75, and higher heart rates than atenolol±bendroflumenthiazide.
Inter-relationships between BP measures
Interrelationships between hemodynamic measures are shown in table 2. XSPI and cPP showed a close correlation (r = 0.84; p<0.001). XSPI also correlated with augmentation pressure (AP; r = 0.69; p <0.001) and showed a weak inverse correlation with heart rate (r = −0.23; p <0.001). There was a moderate correlation between PRI and AP (r = 0.57; p<0.001) or AIx (r = 0.60; p<0.001) and a strong inverse correlation with heart rate (r = −0.87; p<0.001). The correlation between XSPI and PRI was modest (r = 0.21; p <0.001).
Table 2. Correlations between hemodynamic measures.
| Variable | XSPI, mmHg.s | PRI, mmHg.s | Peak Pres mmHg | bSBP, mmHg | DBP, mmHg | cSBP, mmHg | cPP, mmHg | Heart rate, bpm | AP, mmHg | AIx, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PRI, mmHg.s | r | 0.21 | |||||||||
| p | <0.001 | ||||||||||
| Peak Pres, mmHg | r | 0.23 | 0.64 | ||||||||
| p | <0.001 | <0.001 | |||||||||
| bSBP, mmHg | r | 0.59 | 0.37 | 0.83 | |||||||
| p | <0.001 | <0.001 | <0.001 | ||||||||
| DBP, mmHg | r | −0.13 | 0.48 | 0.71 | 0.49 | ||||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
| cSBP, mmHg | r | 0.62 | 0.58 | 0.89 | 0.94 | 0.56 | |||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||||||
| cPP, mmHg | r | 0.84 | 0.35 | 0.52 | 0.75 | −0.10 | 0.77 | ||||
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | |||||
| Heart rate, bpm | r | −0.23 | −0.87 | −0.25 | −0.05 | −0.03 | −0.27 | −0.33 | |||
| p | <0.001 | <0.001 | <0.001 | 0.03 | 0.3 | <0.001 | <0.001 | ||||
| AP, mmHg | r | 0.69 | 0.57 | 0.51 | 0.51 | 0.02 | 0.70 | 0.83 | −0.55 | ||
| p | <0.001 | <0.001 | <0.001 | <0.001 | 0.3 | <0.001 | <0.001 | <0.001 | |||
| AIx, % | r | 0.40 | 0.60 | 0.33 | 0.13 | 0.08 | 0.41 | 0.44 | −0.61 | 0.84 | |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | ||
| AIx75, % | r | 0.34 | 0.12 | 0.23 | 0.14 | 0.09 | 0.33 | 0.33 | −0.06 | 0.68 | 0.8142 |
| p | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | |
Data are Pearson’s correlation coefficients and respective p values (italics). AIx – augmentation index, AIx75 heart rate corrected augmentation index, AP – augmentation pressure, bSBP – brachial systolic blood pressure, cPP – central pulse pressure, cSBP – systolic blood pressure, DBP – diastolic blood pressure, Pres – reservoir pressure, PRI –reservoir pressure intergral, XSPI – excess pressure integral.
Excess pressure integral and reservoir pressure integral as predictors of cardiovascular events
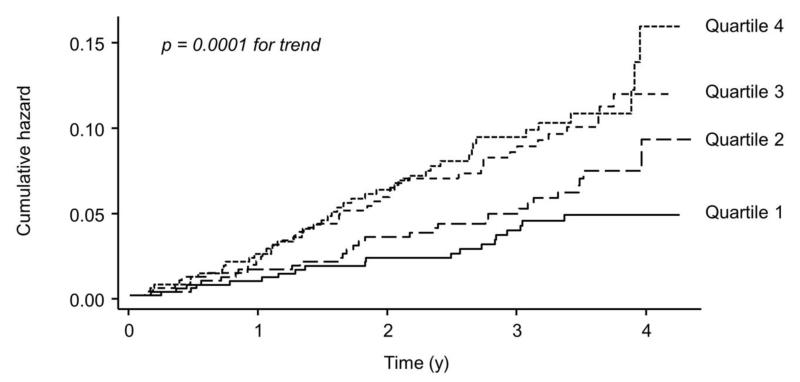
Of the measured BP parameters, XSPI, AP, AIx and AIx 75 were significant predictors of CV events in a Cox regression analysis after adjustment for age and sex (table 3), with XSPI being the strongest predictor. The association between XSPI and CV events was also evident when XSPI was subdivided into quartiles (figure 3). The association between XSPI and CV events was little altered by inclusion of conventional CV risk factors, either individually (not shown), collectively (table 4) or when summarized by Framingham risk score (table 4). Inclusion of heart rate or body mass index (BMI) in models had no effect on the association between XSPI and CV events (data not shown). cPP (table 4) or AP (data not shown) were not significant predictors of CV events when forced into a model containing XSPI and Framingham risk score although the latter two variables remained independent predictors of CV events. Inclusion of other BP measures (e.g. diastolic pressure, brachial pulse pressure) or antihypertensive treatment regimen had no substantial effect on models. Harrell’s C statistic increased from 0.576 to 0.615 (p = 0.04) following inclusion of quartiles of XSPI into the Cox model containing Framingham risk score and both NRI = 0.262 (95% confidence limits 0.129, 0.401) and IDI = 0.005 (95% confidence limits 0.000, 0.013) increased significantly.
Table 3. Cox regression analysis of associations with cardiovascular events.
| Variable | standardized hazard ratio | (95% CI) | p | |
|---|---|---|---|---|
| XSPI | 1.30 | 1.07 | 1.57 | 0.007 |
| PRI | 1.00 | 0.84 | 1.19 | >0.9 |
| Peak Pres | 1.05 | 0.88 | 1.26 | 0.6 |
| bSBP | 1.11 | 0.93 | 1.32 | 0.2 |
| DBP | 0.99 | 0.82 | 1.19 | 0.9 |
| cSBP | 1.13 | 0.95 | 1.34 | 0.2 |
| cPP | 1.18 | 0.99 | 1.40 | 0.07 |
| Heart rate | 1.01 | 0.86 | 1.20 | 0.9 |
| AP | 1.22 | 1.02 | 1.45 | 0.03 |
| AIx | 1.22 | 1.01 | 1.48 | 0.04 |
| AIx75 | 1.27 | 1.04 | 1.56 | 0.02 |
Data are presented as standardized hazard ratio (i.e. per unit SD) to permit direct comparisons. All data are adjusted for age and sex. Abbreviations: AIx – augmentation index, AIx75 heart rate corrected augmentation index, AP – augmentation pressure, bSBP – brachial systolic blood pressure, cPP – central pulse pressure, cSBP – systolic blood pressure, DBP – diastolic blood pressure, Pres – reservoir pressure, PRI – reservoir pressure intergral, XSPI – excess pressure integral.
Figure 3. Nelson-Aalen cumulative hazard plot.
The plot shows the relationship between quartiles (quartile 1 = 232.2 to 581.9 mmHg.s ; quartile 2 = 582.3 to 720.5 mmHg.s ; quartile 3 = 720.9 to 897.7 mmHg.s; quartile 4 = 899.2 to 2174.9 mmHg.s) of excess pressure integral and total cardiovascular events and procedures (fatal and non-fatal myocardial infarction, coronary revascularization procedures, new-onset angina (stable or unstable), fatal and non-fatal heart failure, and life-threatening arrhythmias and stroke). A logrank test was used to look for trend in survivor function across the four ordered groups.
Table 4. Multivariable Cox regression analysis of excess pressure time integral and cardiovascular events after adjusting for other risk factors.
| Variable | Hazard Ratio | (95% CI) | P |
|---|---|---|---|
| Model 1 | |||
| XSPI | 2.13 | (1.23, 3.70) | 0.007 |
| Age | 1.34 | (1.06, 1.68) | 0.013 |
| Sex | 1.79 | (1.08, 2.95) | 0.023 |
| Model 2 | |||
| XSPI | 2.28 | (1.09, 4.78) | 0.029 |
| Age | 1.43 | (1.12, 1.83) | 0.004 |
| Sex | 1.87 | (1.11, 3.14) | 0.02 |
| bSBP | 1.00 | (0.98, 1.01) | 0.6 |
| Smoking | 1.52 | (1.03, 2.24) | 0.04 |
| Diabetes | 1.26 | (0.83, 1.91) | 0.3 |
| Total cholesterol | 1.16 | (0.98, 1.38) | 0.08 |
| HDL cholesterol | 0.58 | (0.33, 1.00) | 0.05 |
| Treatment arm | 0.94 | (0.67, 1.32) | 0.7 |
| Model 3 | |||
| XSPI | 1.91 | (1.13, 3.23) | 0.015 |
| Framingham risk | 7.01 | (1.87, 26.21) | 0.004 |
| Model 4 | |||
| XSPI | 1.53 | (1.07 2.20) | 0.02 |
| cPP | 0.79 | (0.56 1.13) | 0.2 |
| Framingham risk | 7.65 | (2.03 28.85) | 0.003 |
Abbreviations: bSBP – brachial systolic blood pressure, cPP – central pulse pressure, DBP – diastolic blood pressure, HDL – high density lipoprotein, PRI – reservoir pressure intergral, XSPI – excess pressure integral.
XSPI also predicted the post-hoc defined composite outcome of total cardiovascular events/procedures and development of renal impairment used in the original CAFE study (standardized HR (95% confidence interval) = 1.42 (1.21, 1.67); p < 0.001); cPP was also a significant predictor of events in this post-hoc model as previously reported (16).
Associations with target organ damage and left ventricular function
The majority of hemodynamic measures with the exception of diastolic BP and AIx or AIx75 were significantly correlated with LVMI (table 5A). In contrast only XSPI (positively), diastolic BP (negatively) and central pulse pressure and AP (positively) were associated with cIMT (table 3). XSPI was also correlated with measures of LV systolic and diastolic function (Table 5B). XSPI In multivariable models XSPI and PRI remained significantly associated with LVMI when other hemodynamic measures were included in models (table 6). The association between XSPI and cIMT was also unaffected after adjustment for other covariates (table 6); additional adjustment for BMI also failed to attenuated the association between XSPI and cIMT (not shown).
Table 5. A) Correlations between hemodynamic measures and left ventricular mass and common carotid artery intima media thickness.
| LVMI (n = 862) | cIMT (n = 924) | |||
|---|---|---|---|---|
| Variable | r | p | r | p |
| XSPI, mmHg.s | 0.15 | <0.001 | 0.15 | <0.001 |
| PRI, mmHg.s | 0.17 | <0.001 | −0.03 | 0.3 |
| Peak Pres, mmHg | 0.18 | <0.001 | −0.06 | 0.1 |
| SBP, mmHg | 0.21 | <0.001 | 0.02 | 0.5 |
| DBP, mmHg | 0.06 | 0.08 | −0.15 | <0.001 |
| cSBP, mmHg | 0.20 | <0.001 | 0.01 | 0.7 |
| cPP, mmHg | 0.21 | <0.001 | 0.13 | <0.001 |
| AP, mmHg | 0.13 | 0.001 | 0.10 | 0.004 |
| AIx, % | 0.03 | 0.3 | 0.07 | 0.1 |
| AIx75, % | −0.05 | 0.1 | 0.04 | 0.2 |
B) Correlations between XSPI and measures of left ventricular systolic and diastolic function.
| Variable | r | p |
|---|---|---|
| Ejection fraction, % | 0.10 | 0.003 |
| Midwall fractional shortening | 0.10 | 0.005 |
| s′, cm/s | −0.29 | <0.001 |
| E/e′ | 0.12 | <0.001 |
| B-type natriuretic peptide), pg/ml¶ | 0.18 | <0.001 |
Data are Pearson’s correlation coefficients and respective p values (italics). Abbreviations: AIx – augmentation index, AIx75 heart rate corrected augmentation index, AP – augmentation pressure, bSBP – brachial systolic blood pressure, cPP – central pulse pressure, cIMT – common carotid artery intima media thickness, cSBP – systolic blood pressure, DBP – diastolic blood pressure, E/e′ - ratio of peak early transmitral flow velocity to peak early diastolic mitral annulus velocity, LVMI- left ventricular mass index, Pres – reservoir pressure, PRI –reservoir pressure intergral, s′ – peak systolic mitral annulus velocity, XSPI – excess pressure integral.
B-type natriuretic peptide was measured in 898 individuals and log transformed prior to performing correlation.
Table 6. Multivariable models of predictors of A) left ventricular mass index (LVMI) B) carotid intima-media thickness (cIMT).
| A) LVMI | Adjusted r2 = 0.05 | ||
|---|---|---|---|
| Variables | Coefficient | (se) | p |
| XSPI¥ | 7.04 | 2.26 | 0.002 |
| PRI¥ | 9.67 | 2.43 | <0.001 |
| Age | −0.14 | 0.08 | 0.06 |
| Male sex | −1.65 | 1.50 | 0.3 |
| MAP | 0.08 | 0.06 | 0.2 |
| AP | −0.36 | 0.16 | 0.02 |
| Amlodipine±perindopril | −0.35 | 1.18 | 0.8 |
| Constant | −29.91 | 9.56 | 0.002 |
| B) cIMT | r2 = 0.12 | ||
|---|---|---|---|
| Coefficient | (se) | p | |
| XSPI¥ | 0.067 | 0.025 | 0.008 |
| Age, y | 0.007 | 0.001 | <0.001 |
| Male sex | 0.019 | 0.017 | 0.3 |
| Total cholesterol | 0.009 | 0.005 | 0.1 |
| MAP | −0.001 | 0.001 | 0.2 |
| AP | −0.002 | 0.001 | 0.2 |
| Smoking | 0.027 | 0.015 | 0.08 |
| HDL cholesterol, mmol/l | −0.028 | 0.018 | 0.1 |
| Diabetes | 0.004 | 0.015 | 0.8 |
| Amlodipine±perindopril | −0.046 | 0.013 | <0.001 |
| Constant | −0.318 | 0.103 | 0.002 |
Abbreviations: AP – augmentation pressure, HDL – high density lipoprotein, MAP – mean arterial pressure, PRI –reservoir pressure intergral, XSPI – excess pressure integral. Central pulse pressure was omited from the models due to strong collinearity with XSPI and AP.
DISCUSSION
Elevated XSPI, a novel measure of circulatory dysfunction, was associated with cardiovascular target organ damage and predicts incident CV events in people with well controlled hypertension. Higher reservoir pressure was associated with increased left ventricular mass, but was not associated with increased cIMT, and was not associated with an increased risk of cardiovascular events.
High BP is a well-established risk factor for CV events but previous studies have reported conflicting results regarding the prognostic value of parameters derived from the BP waveform (5-8). There is some evidence that central pressure may be a better predictor of CV events than conventional BP measures (9), although this remains to be convincingly established (25). In contrast, assessment of wave reflection has been more consistently associated with increased risk of CV events (26), but the requirement for simultaneous measurements of pressure and flow limits the general applicability of this approach outside a research setting. Use of pressure-based approximative methods to estimate the magnitude of reflection give values that differ substantially from those obtained using measured pressure and flow (27), although the amplitude of the reflected wave calculated by this approach does independently predict target organ damage and CV events (28-30).
XSPI is a novel measure derived from the pulse waveform that does not require use of a transfer function and is easily implemented with potential to be automated and used in a clinical setting. Theoretical analysis (13) suggests that it provides information about surplus work performed by the ventricle and when radial measurements are employed it may also incorporate information regarding the state of the peripheral arterial system. Previous studies have also reported that central pressure is more strongly associated with cIMT (31;32) and left ventricular hypertrophy (32;33) and a recent study of high risk patients with suspected coronary artery disease both XSPI and PRI calculated from estimated central waveforms were predictive of subsequent cardiovascular events (14). Our data based on radial waveforms confirm and extend these observations. We also show that while both excess pressure and reservoir pressure are related to LV mass and function (consistent with both of these parameters reflecting hemodynamic load), only excess pressure correlates with cIMT, suggesting that this measure is more closely related to atherosclerotic disease, possibly through endothelial dysfunction accounting for poor circulatory function and enhanced propensity to atherosclerosis.
Interestingly, in this study AIx was more closely related to reservoir pressure than excess pressure. This is consistent with previous reports in people undergoing coronary angiography (34;35) and suggests that central AIx may be viewed more as an indicator of central aortic compliance than reflection of large discrete waves. This observation is also consistent with a recent meta-analysis which suggested only a minor role for wave reflection in the pressure augmentation that occurs with aging (36).
This study has several limitations: CV outcomes were pooled and there were insufficient events to allow an analysis by individual subtypes of CV event; all participants were hypertensive individuals participating in a randomized clinical trial; and the sample did not include a large proportion of women. The findings may therefore not be applicable to other populations. The study used a retrospective analysis of prospective data although it seems unlikely that this will have introduced bias into the findings.
Perspectives
Calculation of the reservoir and excess pressure from the measured pressure waveform is straightforward and can be applied to any prospective or retrospective data sets where pressure waveforms are recorded and use of a generalized transfer function is not required. The recent development of oscillometric cuff-based approaches (reviewed in (37)) that record the pressure waveform in addition to measuring BP may make this approach more widely applicable. The method has a sound mathematical and theoretical basis linking it to excess ventricular work and to future cardiovascular events, and it provides unique insight into cardiovascular physiology. Excess pressure time integral predicted cardiovascular events in a moderate sized sub-set (10%) of the ASCOT participants and is additive to the predictive value of conventional risk factors. Finally, by differentially discriminating between different drug classes this measurement offers a potentially new tool for selection of pharmacological therapies on a patient specific basis.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
1) What Is New
A novel measure of surplus cardiac workload, excess pressure integral, can be simply derived from blood pressure waveforms without the need for transfer functions or other mathematical transformations. Excess pressure integral predicts cardiovascular events more powerfully than central blood pressure or other parameters derived from the blood pressure waveform and is also associated with target organ damage in people with treated hypertension
2) What is relevant
Measurement of blood pressure waveforms by means of tonometry or cuff techniques is becoming more widespread. Calculation of excess pressure integral may prove useful in cardiovascular risk evaluation in hypertension and to assess the differential effects of antihypertensive therapies.
3) Summary
Excess pressure integral, a novel parameter calculated from the blood pressure waveform is an indicator of CV dysfunction and independently predicts CV events and target organ damage in a prospective clinical trial.
Acknowledgments
SOURCES OF FUNDING: The CAFE and HACVD studies were independent, investigator-initiated, investigator-designed, and investigator-led study funded by a grant program from Pfizer UK. D Francis, A Hughes, J Mayet and S Thom received support from the Biomedical Research Centre Award to Imperial College Academic Health Sciences Centre and the BHF Research Centre Excellence Award to Imperial College London. D Francis was supported by a British Heart Foundation Senior Clinical Fellowship. J Davies was supported by a National Institute Heath Research (Walport) Clinical Lectureship. B Williams is an NIHR Senior Investigator and receives support from the NIHR UCL Hospitals Biomedical Research Centre. We acknowledge the contribution of Tom Williams, University of Leicester, UK, for extraction and collation of the raw waveform data used in this analysis. The investigators also acknowledge the invaluable support of the clinical trial doctors, nurses, and support staff for their important contributions. In addition, we thank all the patients who participated in the study.
The CAFE and HACVD sub-studies were supported by investigational grants from Pfizer International, New York, NY, USA. The principal funding source for ASCOT was Pfizer, New York, NY, USA, additional funding was also provided by Servier Research Group, Paris, France, and Leo Laboratories, Copenhagen, Denmark.
JK Cruickshank has received honoraria and served as a consultant/advisory board member for Pfizer. A Stanton has received honoraria from Pfizer. S Thom reports receiving research funding and acting in a consultant/advisory board capacity for Pfizer. B Williams has received travel grant support and honoraria from Pfizer for lectures at international conferences.
Footnotes
DISCLOSURES: D Collier, J Davies, D Francis, A Hughes, P Lacy, A Malaweera, J Mayet, K Parker and T Tillin report no conflicts within the last 2 years.
REFERENCES
- (1).Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- (2).Westerhof N, Lankhaar JW, Westerhof BE. The arterial Windkessel. Med Biol Eng Comput. 2009;47:131–141. doi: 10.1007/s11517-008-0359-2. [DOI] [PubMed] [Google Scholar]
- (3).Parker KH. An introduction to wave intensity analysis. Med Biol Eng Comput. 2009;47:175–188. doi: 10.1007/s11517-009-0439-y. [DOI] [PubMed] [Google Scholar]
- (4).Westerhof N, Sipkema P, Van den Bos GC, Elzinga G. Forward and backward waves in the arterial system. Cardiovasc Res. 1972;6:648–656. doi: 10.1093/cvr/6.6.648. [DOI] [PubMed] [Google Scholar]
- (5).Chirinos JA, Zambrano JP, Chakko S, Veerani A, Schob A, Willens HJ, Perez G, Mendez AJ. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45:980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- (6).Weber T, Auer J, O’Rourke MF, Kvas E, Lassnig E, Lamm G, Stark N, Rammer M, Eber B. Increased arterial wave reflections predict severe cardiovascular events in patients undergoing percutaneous coronary interventions. Eur Heart J. 2005;26:2657–2663. doi: 10.1093/eurheartj/ehi504. [DOI] [PubMed] [Google Scholar]
- (7).Dart AM, Gatzka CD, Kingwell BA, Willson K, Cameron JD, Liang YL, Berry KL, Wing LM, Reid CM, Ryan P, Beilin LJ, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Morgan TO, West MJ. Brachial blood pressure but not carotid arterial waveforms predict cardiovascular events in elderly female hypertensives. Hypertension. 2006;47:785–790. doi: 10.1161/01.HYP.0000209340.33592.50. [DOI] [PubMed] [Google Scholar]
- (8).Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, Vita JA, Levy D, Benjamin EJ. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation. 2010;121:505–511. doi: 10.1161/CIRCULATIONAHA.109.886655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Vlachopoulos C, Aznaouridis K, O’Rourke MF, Safar ME, Baou K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur Heart J. 2010;31:1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- (10).Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586. doi: 10.1152/ajpheart.00875.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Wang JJ, O’Brien AB, Shrive NG, Parker KH, Tyberg JV. Time-domain representation of ventricular-arterial coupling as a windkessel and wave system. Am J Physiol Heart Circ Physiol. 2003;284:H1358–H1368. doi: 10.1152/ajpheart.00175.2002. [DOI] [PubMed] [Google Scholar]
- (12).Tyberg JV, Davies JE, Wang Z, Whitelaw WA, Flewitt JA, Shrive NG, Francis DP, Hughes AD, Parker KH, Wang JJ. Wave intensity analysis and the development of the reservoir-wave approach. Med Biol Eng Comput. 2009;47:221–232. doi: 10.1007/s11517-008-0430-z. [DOI] [PubMed] [Google Scholar]
- (13).Parker KH, Alastruey J, Stan GB. Arterial reservoir-excess pressure and ventricular work. Med Biol Eng Comput. 2012;50:419–424. doi: 10.1007/s11517-012-0872-1. [DOI] [PubMed] [Google Scholar]
- (14).Hametner B, Wassertheurer S, Hughes AD, Parker KH, Weber T, Eber B. Reservoir and excess pressures predict cardiovascular events in high-risk patients. Int J Cardiol. 2014;171:31–36. doi: 10.1016/j.ijcard.2013.11.039. [DOI] [PubMed] [Google Scholar]
- (15).Dahlof B, Sever PS, Poulter NR, Wedel H, Beevers DG, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet. 2005;366:895–906. doi: 10.1016/S0140-6736(05)67185-1. [DOI] [PubMed] [Google Scholar]
- (16).Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O’Rourke M. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. doi: 10.1161/CIRCULATIONAHA.105.595496. [DOI] [PubMed] [Google Scholar]
- (17).Stanton A, FitzGerald D, Hughes A, Mayet J, O’Brien E, Poulter NR, Sever PS, Shields D, Thom S. An intensive phenotyping study to enable the future examination of genetic influences on hypertension-associated cardiovascular disease. J Hum Hypertens. 2001;15(Suppl 1):S13–S18. doi: 10.1038/sj.jhh.1001089. [DOI] [PubMed] [Google Scholar]
- (18).Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- (19).Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21:93–111. doi: 10.1016/j.echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- (20).Wendelhag I, Liang Q, Gustavsson T, Wikstrand J. A new automated computerized analyzing system simplifies readings and reduces the variability in ultrasound measurement of intima-media thickness. Stroke. 1997;28:2195–2200. doi: 10.1161/01.str.28.11.2195. [DOI] [PubMed] [Google Scholar]
- (21).Aguado-Sierra J, Davies JE, Hadjiloizou N, Francis D, Mayet J, Hughes AD, Parker KH. Reservoir-wave separation and wave intensity analysis applied to carotid arteries: a hybrid 1D model to understand haemodynamics. Conf Proc IEEE Eng Med Biol Soc. 2008;2008:1381–1384. doi: 10.1109/IEMBS.2008.4649422. [DOI] [PubMed] [Google Scholar]
- (22).Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- (23).Newson RB. Comparing the predictive powers of survival models using Harrell’s C or Somers’ D. Stata J. 2010;10:339–358. [Google Scholar]
- (24).Pencina MJ, Agostino RBD, Agostino RBD, Vasan RS. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- (25).Williams B, Lacy PS. Central aortic pressure and clinical outcomes. J Hypertens. 2009;27:1123–1125. doi: 10.1097/HJH.0b013e32832b6566. [DOI] [PubMed] [Google Scholar]
- (26).Manisty C, Mayet J, Tapp RJ, Parker KH, Sever P, Poulter NR, Thom SA, Hughes AD. Wave reflection predicts cardiovascular events in hypertensive individuals independent of blood pressure and other cardiovascular risk factors: an ASCOT (Anglo-Scandinavian Cardiac Outcome Trial) substudy. J Am Coll Cardiol. 2010;56:24–30. doi: 10.1016/j.jacc.2010.03.030. [DOI] [PubMed] [Google Scholar]
- (27).Kips JG, Rietzschel ER, De Buyzere ML, Westerhof BE, Gillebert TC, Van Bortel L, Segers P. Evaluation of noninvasive methods to assess wave reflection and pulse transit time from the pressure waveform alone. Hypertension. 2009;53:142–149. doi: 10.1161/HYPERTENSIONAHA.108.123109. [DOI] [PubMed] [Google Scholar]
- (28).Wang KL, Cheng HM, Sung SH, Chuang SY, Li CH, Spurgeon H, Ting CT, Najjar SS, Lakatta EG, Yin FCP, Chou P, Chen CH. Wave reflection and arterial stiffness in the prediction of 15-year all-cause and cardiovascular mortalities: a community-based study. Hypertension. 2010;55:799–805. doi: 10.1161/HYPERTENSIONAHA.109.139964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Weber T, Wassertheurer S, Rammer M, Haiden A, Hametner B, Eber B. Wave Reflections, Assessed With a Novel Method for Pulse Wave Separation, Are Associated With End-Organ Damage and Clinical Outcomes. Hypertension. 2012;60:534–41. doi: 10.1161/HYPERTENSIONAHA.112.194571. [DOI] [PubMed] [Google Scholar]
- (30).Chirinos J, Kips J, Jacobs D, Brumback L, Duprez D, Kronmal R, Bluemke D, Townsend R, Vermeersch S, Segers P. Arterial Wave Reflections and Incident Cardiovascular Events and Heart Failure. J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Roman MJ, Devereux RB, Kizer JR, Lee ET, Galloway JM, Ali T, Umans JG, Howard BV. Central pressure more strongly relates to vascular disease and outcome than does brachial pressure: the Strong Heart Study. Hypertension. 2007;50:197–203. doi: 10.1161/HYPERTENSIONAHA.107.089078. [DOI] [PubMed] [Google Scholar]
- (32).Wang KL, Cheng HM, Chuang SY, Spurgeon HA, Ting CT, Lakatta EG, Yin FCP, Chou P, Chen CH. Central or peripheral systolic or pulse pressure: which best relates to target organs and future mortality? J Hypertens. 2009;27:461–467. doi: 10.1097/hjh.0b013e3283220ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Roman MJ, Okin PM, Kizer JR, Lee ET, Howard BV, Devereux RB. Relations of central and brachial blood pressure to left ventricular hypertrophy and geometry: the Strong Heart Study. J Hypertens. 2010;28:384–388. doi: 10.1097/HJH.0b013e328333d228. [DOI] [PubMed] [Google Scholar]
- (34).Davies JE, Baksi J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Foale RA, Malik IS, Tyberg JV, Parker KH, Mayet J, Hughes AD. The arterial reservoir pressure increases with aging and is the major determinant of the aortic augmentation index. Am J Physiol Heart Circ Physiol. 2010;298:H580–H586. doi: 10.1152/ajpheart.00875.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Davies JE, Alastruey J, Francis DP, Hadjiloizou N, Whinnett ZI, Manisty CH, Aguado-Sierra J, Willson K, Foale RA, Malik IS, Hughes AD, Parker KH, Mayet J. Attenuation of wave reflection by wave entrapment creates a “horizon effect” in the human aorta. Hypertension. 2012;60:778–785. doi: 10.1161/HYPERTENSIONAHA.111.180604. [DOI] [PubMed] [Google Scholar]
- (36).Baksi AJ, Treibel TA, Davies JE, Hadjiloizou N, Foale RA, Parker KH, Francis DP, Mayet J, Hughes AD. A meta-analysis of the mechanism of blood pressure change with aging. J Am Coll Cardiol. 2009;54:2087–2092. doi: 10.1016/j.jacc.2009.06.049. [DOI] [PubMed] [Google Scholar]
- (37).Avolio AP, Butlin M, Walsh A. Arterial blood pressure measurement and pulse wave analysis--their role in enhancing cardiovascular assessment. Physiol Meas. 2010;31:R1–47. doi: 10.1088/0967-3334/31/1/R01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.