Abstract
Background
SLC25A12, a susceptibility gene for autism spectrum disorders (ASDs) that is mutated in a neurodevelopmental syndrome, encodes a mitochondrial aspartate/glutamate carrier (AGC1). AGC1 is an important component of the malate/aspartate shuttle, a crucial system supporting oxidative phosphorylation and ATP production.
Methods
We characterized mice with a disruption of the Slc25a12 gene, followed by confirmatory in vitro studies.
Results
Slc25a12-knockout mice, which showed no AGC1 by immunoblotting, were born normally but displayed delayed development and died around 3 weeks after birth. In P13-14 knockout brains, the brains were smaller with no obvious alteration in gross structure. However, we found a reduction in myelin basic protein (MBP)-positive fibers, consistent with a previous report. Furthermore, the neocortex of knockout mice contained abnormal neurofilamentous accumulations in neurons, suggesting defective axonal transport and/or neurodegeneration. Slice cultures prepared from knockout mice also showed a myelination defect, and reduction of Slc25a12 in rat primary oligodendrocytes led to a cellautonomous reduction in MBP expression. Myelin deficits in slice cultures from knockout mice could be reversed by administration of pyruvate, indicating that reduction in AGC1 activity leads to reduced production of aspartate/N-acetyl aspartate (NAA) and/or alterations in the NADH/NAD+ ratio, resulting in myelin defects.
Conclusions
Our data implicate AGC1 activity in myelination and in neuronal structure, and indicate that while loss of AGC1 leads to hypomyelination and neuronal changes, subtle alterations in AGC1 expression could affect brain development contributing to increased autism susceptibility.
Keywords: Malate/aspartate shuttle, mitochondria, N-acetyl aspartate (NAA), neuron-oligodendrocyte interactions, pyruvate
Introduction
ASDs are neurodevelopmental disorders with strong genetic components (1, 2). SLC25A12 (solute carrier family 25 member 12) is a gene on chromosome 2q31 that was identified as an autism susceptibility gene through both linkage and association studies (3). Recently, homozygous mutations in SLC25A12 have been reported in a patient with seizures, severe hypotonia and arrested psychomotor development, with global hypomyelination (4). SLC25A12 encodes the Ca2+-dependent mitochondrial AGC1, which is expressed in brain and skeletal muscle. A peripheral AGC isoform, called AGC2 (encoded by SLC25A13 on chromosome 7q21) is mainly expressed in liver, kidney, and heart. AGC1 and AGC2 function in the transport of aspartate from the mitochondrial matrix to the intermembrane space in exchange for glutamate, and represent a component of the malate/aspartate shuttle (MAS), a crucial pathway that supports oxidative phosphorylation to produce ATP by the transport of NADH-reducing equivalents into the mitochondrial matrix (5).
We and others have reported linkage of the 2q31 region to ASDs, and two single nucleotide polymorphisms (SNPs) of the SLC25A12 gene – one immediately upstream of alternately spliced exon 4 (rs2056202) and one in the small intron between exons 16 and 17 (rs2292813) – have been shown to be associated with ASDs in 2 and 3 of 6 studies, respectively (3, 6–10). In each of the positive associations, the effect was in the same direction, providing very strong evidence for replication. A follow-up study to the first association study indicated that one of the SNPs (rs2056206) was associated with the levels of routines and rituals in ASDs (11), supporting a functional role for these SNPs in AGC1 activity. The SLC25A12 gene is expressed in developing human brain (12) and is expressed about 1.5 fold higher in individuals with ASDs in the dorsolateral frontal cortex in postmortem samples (12). An increase in AGC1 activity was also reported in postmortem samples, a finding that was attributed to altered calcium levels (10).
Based on these findings, we hypothesized that genetic alterations in SLC25A12 and/or other mechanisms that alter AGC1 activity will affect neurodevelopment, resulting in phenotypes that can contribute to disorders such as ASDs. To gain insight into the possible mechanisms by which SLC25A12 might affect neurodevelopment, we generated knockout mice with a disruption of Slc25a12. We analyzed Slc25a12-knockout mice from histological, molecular, and cell biological perspectives.
Materials and Methods
Development of Slc25a12-knockout mice, Genotyping, and Brain MRI analysis, as well as more detailed protocols for the methods summarize below, are found in supplemental materials.
Biochemical analyses
Immunoblotting on brain extract was performed using standard methods using alkaline phosphatase (Sigma) or HRP (Jackson Immunologicals) conjugated secondary antibodies.
The mRNA levels of the myelin genes, Cldn11, Cnp1, Mag, Mobp, Olig2, Plp1, Qk5/6, Sox10, and Erbb4 were measured by qPCR using TaqMan MGB probes and primer sets (Applied Biosystems) on RNA prepared from brains as described in the supplement.
Slice cultures
Littermates (P10) from heterozygote matings were used to prepare cerebellar slice cultures described in the supplement. Following treatment, cultures were fixed with 4% paraformaldehyde at day 7 in vitro and processed for immunohistochemistry using rabbit anti-MBP (Chemicon) and mouse anticalbindin (Sigma), and analyzed by the fluorescence microscopy.
OPC cultures
OPCs prepared from rat brain were nucleofected using the rat oligodendrocyte kit (VPG-1009, Amaxa) following the manufacturer’s protocol. After nucleofection, OPCs were plated in proliferation medium for 2 days, and then switched to differentiation medium (day 2). Cultures were fixed with 4% paraformaldehyde and immunostained with anti-MBP antibody (Calbiochem) followed by secondary antibody conjugated with Cy3 (Jackson). Cultures were analyzed by confocal microscopy in a blinded manner.
Histology
Sagittal and coronal sections (40μm-thick) were prepared on a Vibratome and immunostained for antibodies indicated. Secondary antibodies used were either fluorescently labeled or HRP-conjugated and visualized either by fluorescence microscopy or by bright field microscopy following DAB staining.
Statistical analysis
All data represent mean and standard error of the mean (SEM) for 3 or more experiments. When applicable, Student’s t tests were used for statistical analysis comparing two groups.
Results
Developmental abnormalities in Slc25a12-knockout mice
To clarify the function of AGC1 in brain, we generated mice with a disruption of the Slc25a12 gene. The Slc25a12 gene in mice consists of 18 exons, spread over ~100 kb. We modified a mouse genomic DNA fragment by replacing exon 1 with a neolGFP cassette (Suppl. Fig. 1A). The targeting construct was introduced into C57BL/6 embryonic stem (ES) cells and targeted clones were used to establish a mouse line carrying the disrupted allele on the C57BL/6Tac genetic background. Matings between heterozygous animals produced wild type, heterozygous, and knockout progeny with apparently Mendelian frequencies (27, 52, and 21 percent, respectively, in 66 pups from 10 litters). AGC1 levels were below the level of detection in homozygous knockout mice, while in heterozygous mice AGC1 levels were about half of that found in wild-type animals (Suppl. Fig. 1B, C). Measurement of MAS activity using mitochondrial fractions prepared from P10 knockout brains showed very low MAS activity (wild type, 14±0.1 absorbance change/min/g; knockout, 1.4±0.1 absorbance change/min/g; n=3 per group), indicating that this pathway is functionally disrupted in the knockouts.
The knockouts were indistinguishable through the first few days of life from wild type or heterozygous animals, but around P7, knockouts could be identified by their smaller size with continuing growth retardation. At P13–14, the average body weight of wild type animals was 7.60 ± 0.73 grams (mean ± SEM; n = 8) and 8.24 ± 1.06 grams for heterozygotes (n = 17) (P=0.068 compared to wild type), whereas that of the knockouts was 3.79 ± 0.74 grams (n = 12) (P=1.35 × 10−9 compared to wild type, and P=9.9 × 10−15 compared to heterozygotes). Knockouts showed a wide-based, ataxic gait, as well as tremor, and died around 3 weeks postnatally. No knockouts survived by 4 weeks after birth.
Myelin and neuronal abnormalities in Slc25a12-knockout mice
We analyzed P13–P14 knockout and control brains by histology and immunohistochemistry and found 4 major alterations in Slc25a12-knockout mice. Cresyl violet and hematoxylin-eosin staining revealed reduction of brain size, consistent with a previous report (13) (Fig. 1). This reduction in size was observed in almost all brain structures, including hippocampus, striatum, and thalamus (Fig. 1C, D), suggesting global neurodevelopmental delay. However, other than brain size, we did not find any obvious gross histological differences between knockout and control mice in cortical and subcortical structures, including brainstem (Fig. 1A, B).
Figure 1. Slc25a12-knockout mice have smaller brains.
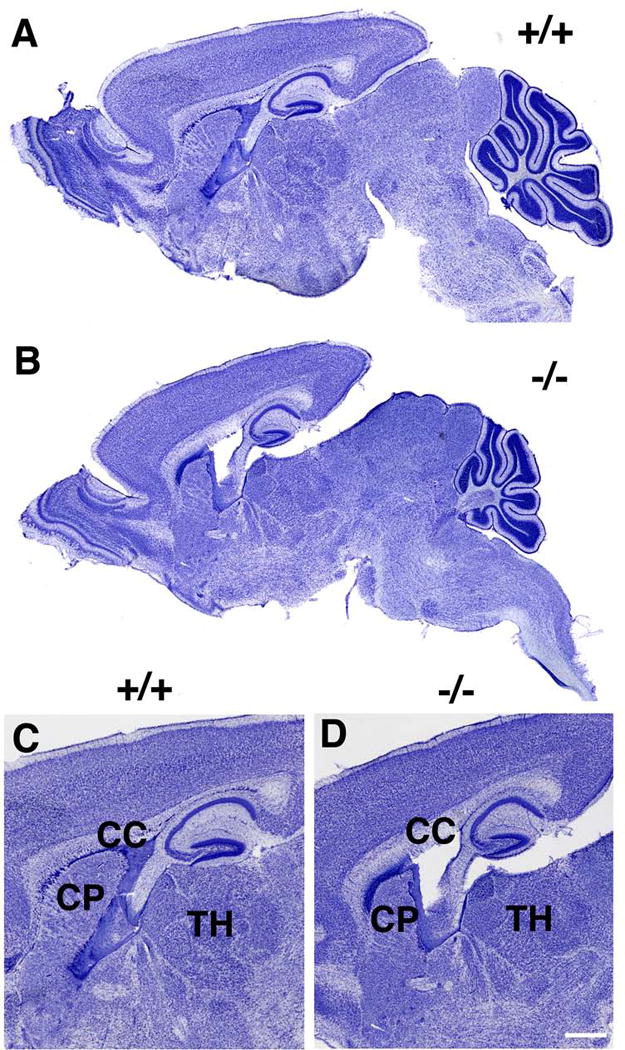
Sagittal sections of P13 brain from wild type (+/+, A, C) or knockout (−/−, B, D) mice are shown stained with Cresyl violet. Note the generally smaller size of all brain regions in the knockout animal. Panels C and D show higher power views of the images in A and B, with the corpus callosum (CC), caudate putamen (CP) and thalamus (TH) indicated. Scale bar: A and B, 750 μm; C and D, 500 μm.
In P13–14 knockout brains, we identified a reduction in myelin basic protein (MBP)-positive fibers (Fig. 2A). Areas affected included the corpus callosum, anterior commissure, internal capsule, and the habenulointerpedunclar tract. The alterations in myelination were also observed by proteolipid protein (PLP) staining (Fig. 2B). This myelin alteration was confirmed by quantitative immunoblotting of whole brain extracts prepared from P10 littermates. MBP levels were significantly reduced (to 75% of wild type) in knockouts at P10, whereas glial fibrillary acidic protein (GFAP) (an astrocyte marker), synaptophysin, and neurofilament (NF) (synaptic and neuronal markers) showed no differences in expression levels (Suppl. Fig. 1C). We obtained similar results using whole cortex instead of whole brains (data not shown).
Figure 2. Slc25a12-knockout mice show myelination deficits.
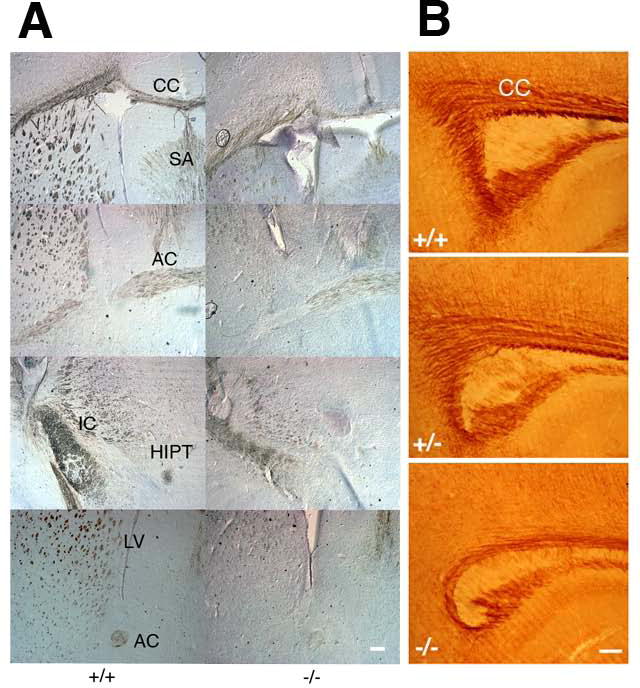
A. Coronal sections through frontal cortex from P14 wild type (+/+) and knockout (−/−) brains were stained with anti-MBP antibody followed by HRP-conjugated secondary antibody visualized by the DAB reaction. B. Sagittal sections from P13 wild type (+/+), heterozygous (+/−) and knockout (−/−) brains were stained with an anti-PLP antibody. CC, corpus callosum; SA, septal area; IC, internal capsule; AC, anterior commissure; HIPT, habenulo-interpedunclar tract; LV, lateral ventricles. Scale bars, 200 μm.
As a general assessment of neuronal integrity we also performed a variety of immunostains on P13-P14 brains, including staining for the NF triplet proteins (NF-L, NF-M and NF-H). NF staining was found widely in all brain regions in both knockout and control brains and in large fiber tracts such as the corpus callosum or fimbria; the staining was comparable in knockouts and controls. However, throughout the neocortex there were fewer fine processes that exhibited NF staining. These changes were best seen with the anti-NF antibody SMI-31 which recognizes phosphorylated forms of the mouse NF-M and NF-H proteins that are found mainly in axons (Fig. 3). Many more fine neuronal processes were present in layers IV-VI in the control animals (arrows in A and C) than in the homozygous knockouts (B, D), whereas in the knockouts there were additional punctate accumulations of NF proteins in the deeper cortical layers that were not seen in the wild type animals (arrowheads in D). In contrast, there was increased NF perikaryal staining in many neurons of the knockouts, especially in layers II-III (B). By confocal imaging, there was decreased staining of the fine axons normally found in layer I (arrow in E) in the knockouts (F) with increased cell body staining in layers II/III (F). A very similar staining pattern was seen with antibody RMO108 that recognizes phosphorylated forms of the mouse NF-M (data not shown). While the neurofilament accumulations and loss of fine processes were most prominent in layers II/III, they were also seen in deeper cortical layers as well, and thus do not appear to be specific to a neuronal subset or lamina but rather an indication of a generalized alteration in neurofilament processing or transport. These findings could reflect a defect in the development of fine axonal processes or defective NF transport. Alternatively they could also be a sign of neurodegeneration in Slc25a12-knockouts.
Figure 3. Altered neurofilament distribution in Slc25a12-knockout mice.
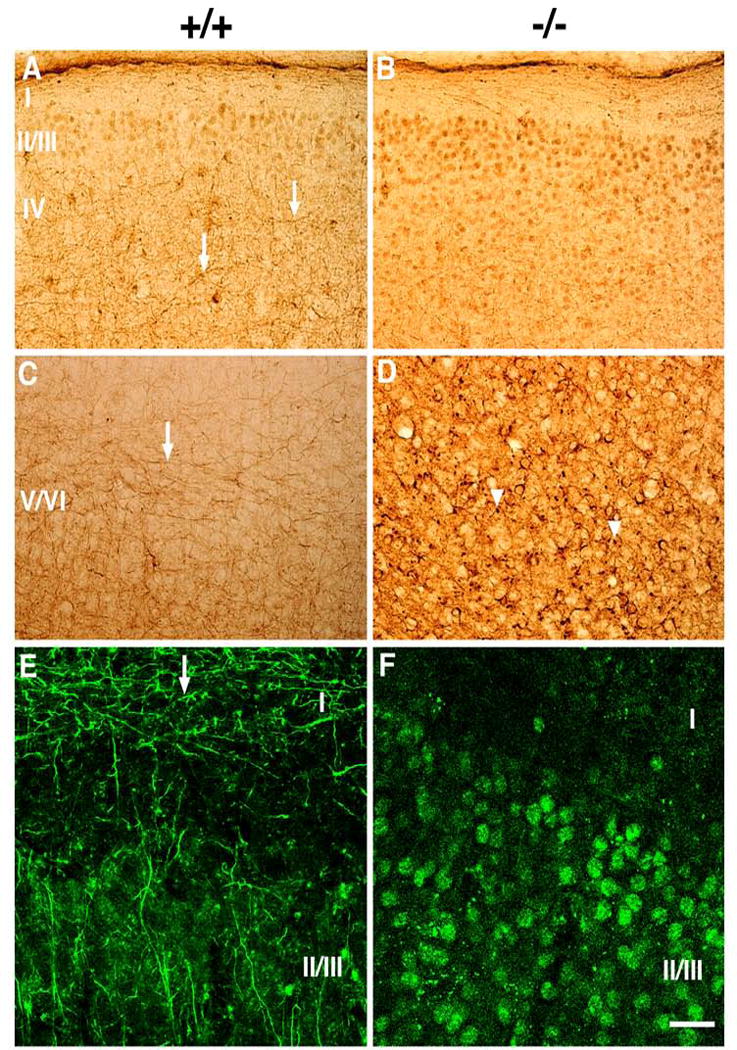
Sagittal sections from P13 cortex of wild type (+/+, A, C, E) and knockout (−/−, B, D, F) mice were stained with antibody SMI-31, which recognizes phosphorylated forms of the mouse mid-sized and heavy NF proteins that are normally found primarily in axons. Sections are shown from the somatosensory cortex (A–D) and auditory cortex (E, F), the latter with confocal imaging in superficial cortical layers. Cortical layers I–VI are indicated. Arrows in A, C and E point to many fine axonal processes that are labeled in wild type animals, but absent in knockout animals. Arrowheads in D indicate neurofilamentous accumulations that are present in the knockout mice. Scale bar: A and B, 100 μm; C and D, 50 μm; E and F, 25 μm.
As an additional indicator of neuronal integrity we also performed calbindin immunostaining. Calbindin is a calcium binding protein that labels a subset of GABAergic neurons as well as other neuronal types including cerebellar Purkinje cells. In the cerebellum, where a disrupted alignment of the Purkinje cell layer was observed in Slc25a12-knockouts (Fig. 4); in contrast to wild-type mice, calbindin-immunoreactive Purkinje cells in the knockout mice frequently formed a layer that was several cells thick (A–D). The Purkinje cell dendrites were also less elaborate in the knockouts and the molecular layer appeared generally thinner (compare E and F). No differences in calbindin immunostaining were observed in the hippocampus and neocortex (data not shown).
Figure 4. Altered Purkinje cell alignment in the cerebellum of Slc25a12-knockout mice.
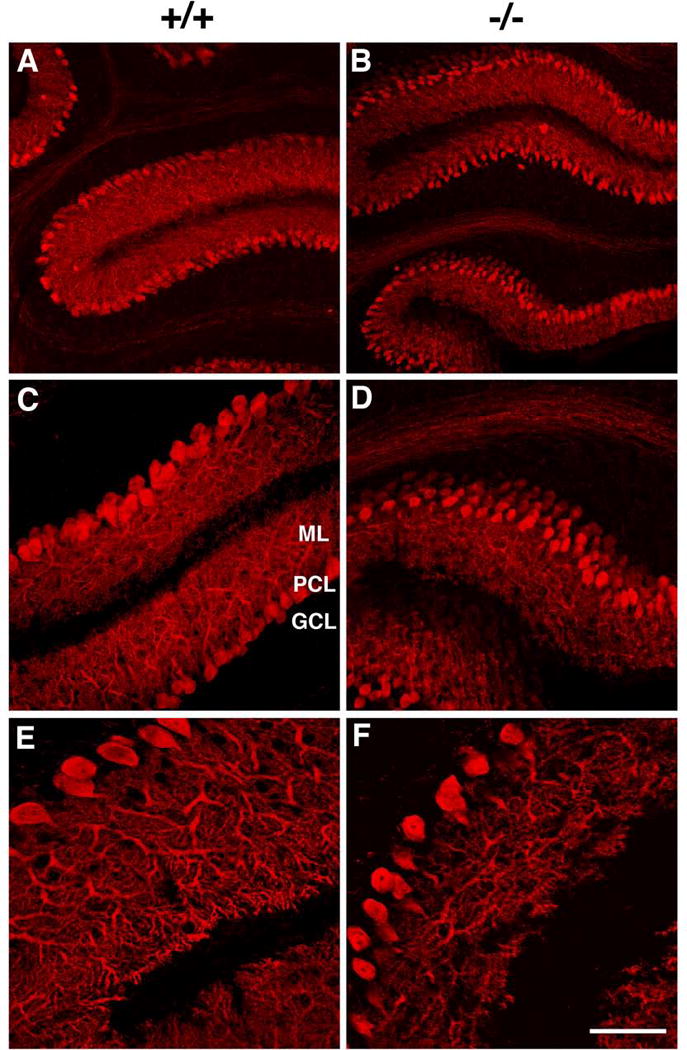
Confocal imaging of sagittal sections from P13 wild type (+/+, A, C, E) and knockout (−/−, B, D, F) cerebella stained with an anti-calbindin antibody are shown. The location of the granule cell layer (GCL), Purkinje cell layer (PCL) and molecular layer (ML) are indicated. Note that the Purkinje cells in the mutant frequently form a layer several cells thick (D) and that Purkinje cell dendrites appear attenuated in the knockout (F). Scale bar: A and B, 100 μm; C and D, 50 μm; E and F, 25 μm.
Altered Mag expression in male heterozygous mice
We also looked at adult heterozygous mice. In these mice, we did not detect any structural changes by immunohistochemistry (data not shown). Furthermore, we did not identify any structural changes when we used MRI to measure ventricular size (male wild type, 2442±101 Arbitrary Unit/AU; male heterozygotes, 2458±198 AU; P = 0.47); female wild type, 2447±135 AU; female heterozygotes, 2300±169 AU; P = 0.26). However, when we analyzed gene expression in adult brains focusing on several oligodendrocyte/myelin related genes (i.e., Cldn11, Cnp1, Qk5/6, Mobp, Mbp, Olig2, Plp1, Sox10, Erbb4, and Mag), we did observe a reduction in Mag expression (coding for myelin associated glycoprotein) in male heterozygotes compared to wild type males (61% of control, P = 0.017 for the first set, 9 wild type and 8 heterozygous males, and 62% of control, P = 0.032 for the second set, 10 wild type and 13 heterozygous males), indicating the presence of subtle alterations in myelination-associated gene expression in the heterozygous animals. Interestingly, we did not observe change in females (data not shown). Other oligodendrocyte/myelin genes did not show significant changes.
Myelination deficits in cerebellar slices from Slc25a12-knockout mice
To further study the role of AGC1 in neuronal development and myelination further, we used in vitro systems. We prepared cerebellar slice cultures from P10 wild type, heterozygous, and knockout mice and cultured them in vitro for one week. In wild type and heterozygous cultures, normal development of Purkinje cell dendrites and axons was confirmed by calbindin immunostaining. Furthermore, normal myelination in vitro was confirmed by MBP staining (Fig. 5A, left panel, and not shown). In contrast, in cultures from knockout animals, the axons of Purkinje cells were less myelinated than controls (Fig. 5A, left panel).
Figure 5. Pyruvate administration rescues myelination deficits of Slc25a12-knockout cultures in vitro.
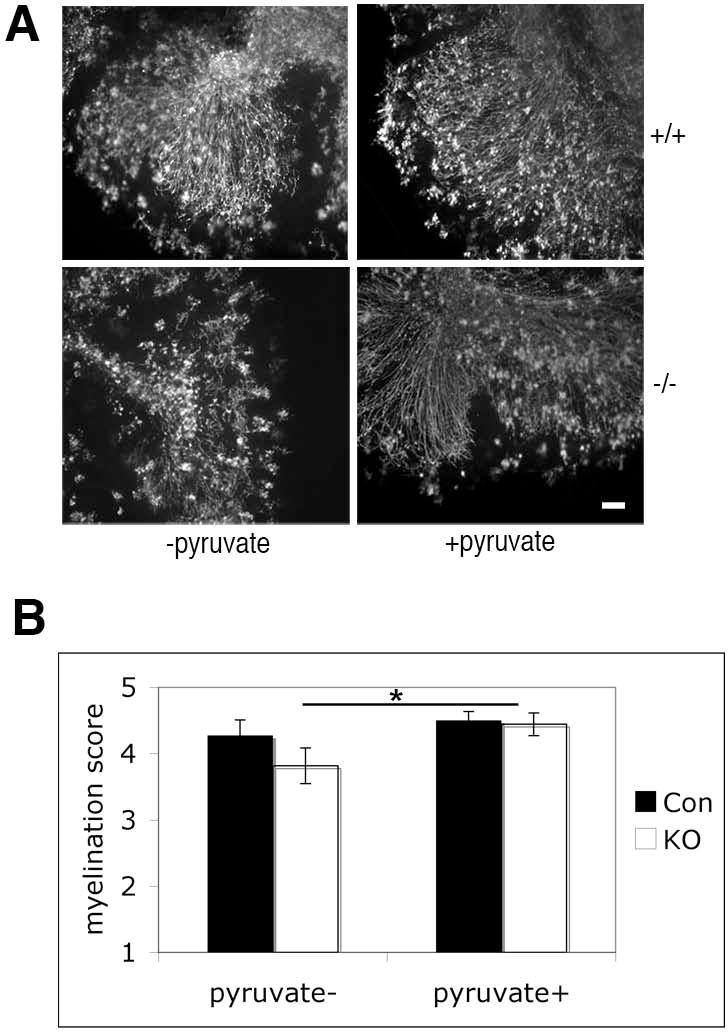
A. Cerebellar slice cultures, prepared from P10 wild type (+/+) and knockout (−/−) mice, were treated with or without pyruvate for 7 days and stained for MBP. Scale bar: 100 μm. B. Slice cultures were quantified by an observer blind to genotype and treatment condition, using a scale from 1 to 5 (see Materials and Methods). Con, control (wild type and heterozygous) animals; KO, knockouts. *, P = 0.038.
Rescue of myelination deficits in vitro by pyruvate administration
Mutations in SLC25A13, which codes for AGC2, cause adult-onset type II citrullinemia in humans. It has been shown that pyruvate administration in Slc25a13-knockout mouse liver explants ameliorates the deficient phenotype ex vivo through modulation of cellular metabolism involving mitochondria (14). We hypothesized that pyruvate may be able to change cellular metabolism in the absence of AGC1, ultimately leading to recovery of myelination. To test if pyruvate administration would ameliorate the myelination deficits in Slc25a12-knockouts, we added pyruvate to cerebellar slice cultures. After 7 days of culture in the presence of pyruvate, more MBP-positive axons were observed in knockout cultures (Fig. 5), indicating that myelination was significantly enhanced by myelination.
Reduced MBP expression in Slc25a12-deficient oligodendrocyte progenitor cells
While AGC1 is highly expressed in neurons (15), we also observed expression of AGC1 in oligodendrocyte progenitor cells (OPCs) at their earliest stage of differentiation in vitro (data not shown), and hence it was of interest to determine whether perturbation of AGC1 in oligodendrocytes can alter myelination. When we nucleofected short hairpin RNA (shRNA) against AGC1 into purified OPCs, we observed that fewer cells expressed MBP at day 5 compared to controls (Fig. 6). In contrast, with the introduction of control (scrambled) shRNAs, most cells expressed high levels of MBP, comparable to the control cultures. Quantitative PCR showed that shRNA treatment resulted in an ~60% reduction of Agc1 gene expression and ~60% reduction in Mbp gene expression at day 5 (note that transfection efficiency was ~60%). To exclude the possibility of off-target effects of shRNA, we also nucleofected shRNA together with AGC1 cDNA in a rescue experiment and found that the majority of cells were high MBP expressers, similar to controls (Fig. 6). These results indicate that perturbation of AGC1 expression in oligodendrocytes is sufficient to alter their development, i.e. that there is an oligodendrocyte-autonomous effect of AGC1 expression on myelination. This, however, does not exclude the possibility that reduced expression of AGC1 in neurons also contributes to the observed myelin deficits.
Figure 6. Inhibition of Slc25a12 in OPCs inhibits MBP expression in vitro.
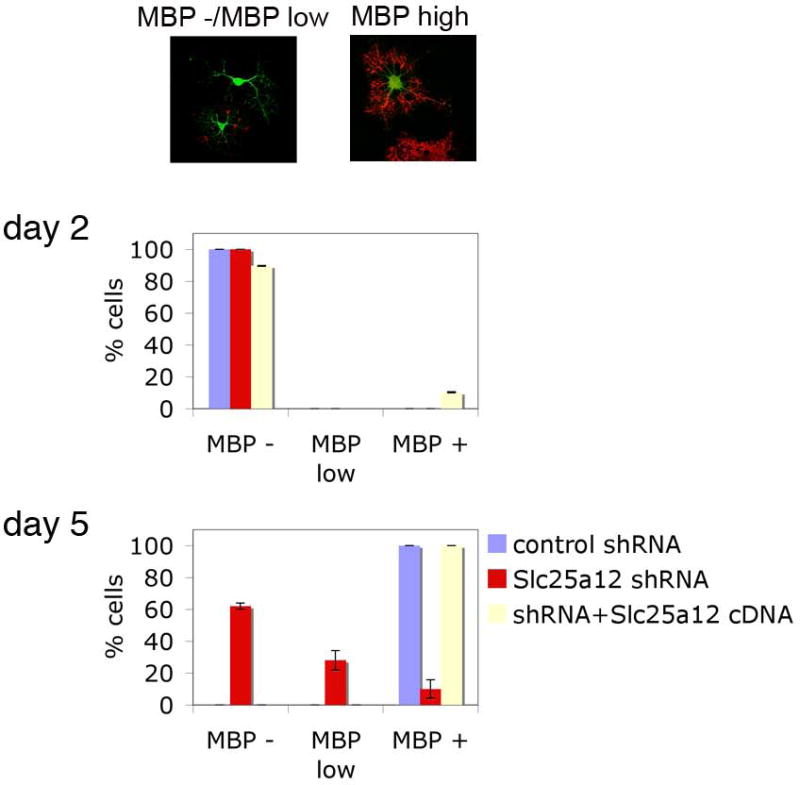
OPC cultures prepared from P0 rat brains were nucleofected with a GFP construct, together with either control (scrambled sequence) shRNA (blue), shRNA against Slc25a12 (red) or shRNA against Slc25a12 with SLC25A12 cDNA (white). Transfected (GFP-positive) cells were classified as having no MBP expression (MBP -, see example in top left panel), low MBP expression (MBP low, see example in top left panel) or high MBP expression (MBP +, see example in top right panel). Cultures were assessed in a blinded fashion at day 2 (middle) and day 5 (bottom), and the proportion of cells in each stage shown. P = 0.036
Discussion
SLC25A12 is a putative ASD susceptibility gene and a gene involved in a severe neurodevelopmental disorder. The gene encodes AGC1, a mitochondrial protein. In order to clarify its involvement in disease, understanding AGC1 function in brain development and function is important. In this report, we characterized AGC1 knockout mice morphologically. Four major alterations in AGC1 knockout mice were identified: 1) smaller brain size, 2) myelination defects in the brain, 3) altered neurofilament distribution in the cortex, and 4) Purkinje cell abnormalities in the cerebellum. Some of these deficits are similar to what was recently described in the newly identified syndrome, including brain size and myelin deficits, as well as some behavioral changes (4). Below we discuss these abnormalities and their relevance to neurodevelopment.
AGC1 affects brain development
AGC1 is a Ca2+-dependent mitochondrial aspartate/glutamate carrier involved in the MAS system, crucial for effective oxidative phosphorylation to produce ATP (5). AGC1-deficient cells may not be able to produce ATP in an efficient manner. Since AGC1 is mainly expressed in brain and skeletal muscle, when energy demand in those tissues is high, particularly during development, AGC1 deficiency may affect development of those tissues. Consistent with this idea, AGC1 knockout mice appear normal at birth but as development proceeds, they became lethargic, showing severe delays in growth and development. We found that increase of body weight stops and that brain size is smaller compared to the wild type or heterozygous littermates at P13–14 (Fig. 1). This is consistent with the previous report by Jalil et al. (13) who also developed Slc25a12-knockout mice, but in a different genetic background.
We also found Purkinje cell alterations in the cerebellum. Purkinje cells were frequently misaligned and had a less developed dendritic structure, producing a thinner molecular layer (Fig. 4). This may be a consequence of developmental delay as Purkinje cell development can be readily affected by developmental insults. Interestingly, AGC1 knockouts exhibited an ataxic gait and tremor, the underlying basis of which may be these cerebellar alterations, although we can not exclude the possibility that general developmental delay of animals as well as specific effects on skeletal muscle development might contribute to the phenotype (AGC1 is also highly expressed in skeletal muscle). Given the role of AGC1 in mitochondrial function, these alterations may also be related to insufficient ATP generation during the high-energy demand periods of development.
AGC1 is important for myelin formation
AGC1 is involved in the MAS system that is important for ATP production and therefore, it was at first surprising that the knockout mice showed major defects in myelination (13, and see Fig. 2). It appears, however, that AGC1 is also crucial for providing proper levels of aspartate to the cytoplasm, where it can be metabolized to NAA, as suggested by Jail et al. (13). Jail et al. demonstrated that in Slc25a12-knockout mice, NAA levels in the brain are reduced. Myelination requires the coordinated interactions between neurons and oligodendrocytes, and considering the presence of neuronal alterations, the deficits in myelin in the knockout mice might thus be attributed to alterations in neuron-oligodendrocyte interactions. As AGC1 is highly expressed in neurons, it was proposed that NAA produced in neurons is transported to oligodendrocytes to provide a source for acetyl residues, necessary for myelin lipid formation (13, 15). However, when we looked at purified OPCs, we observed that when AGC1 expression was perturbed, OPC differentiation and MBP expression were altered (Fig. 6), demonstrating that OPCs themselves are directly affected by the absence of AGC1 and this in turn affects myelination.
Administration of pyruvate restored myelination in our studies, as shown by MBP expression in slice cultures (Fig. 5). Based on the possibility that myelin deficits are caused by the reduction of NAA levels in Slc25a12-knockout mice, we can hypothesize that pyruvate administration provides an alternate source for generating aspartate, that can be converted to NAA, and in turn provide lipids for myelin formation (Suppl. Fig. 2). Furthermore, pyruvate administration may also restore the NADH/NAD+ ratio, so that mitochondria can produce ATP more efficiently (Suppl. Fig. 2). Pyruvate has been shown to be effective in treating some of the clinical features related to mitochondrial dysfunction (16; M. Tanaka, personal communication). It would be interesting to determine whether pyruvate administration to neonatal pups would rescue some of the Slc25a12-knockout phenotype and restore myelin formation. In any event, our pyruvate administration results further support the involvement of AGC1 in myelinating processes in the brain. Recently, a case of an AGC1 deficiency has been reported (4). The patient is homozygous for a missense mutation in the SLC25A12 gene that abolishes AGC1 activity, and the child shows arrested psychomotor development, hypotonia, and seizures. MRI scans indicate that there is global hypomyelination in the cereberal hemispheres. Experimental interventions in the knockout animals (including those providing alternate sources of pyruvate and aspartate) might someday lead to potential interventions in such conditions.
In AGC1 heterozygotes, we have not seen any obvious defects in myelination, other than reduction of Mag expression (to the ~60% of control). Interestingly, although reproducible, we only detect this change in males. The basis for this difference is still not clear. MAG is the first myelin membrane protein that appears at the contact points of axons with the processes of oligodendrocytes, supporting axon-oligodendrocyte interactions that are important for myelination. Since myelin is dynamic, even in adult brains where there is constitutive turnover related to remodeling and changes associated with neuronal plasticity, a slight reduction of MAG might mean that myelin cannot be as dynamically regulated in AGC1 heterozygous mice.
Slc25a12-knockout mice show neuronal defects
In addition to myelination defects, we found that Slc25a12-knockout mice showed neuronal deficits, namely accumulation of NFs in axons of cortical neurons across multiple cortical layers (Fig. 3). We do not know whether this is a direct effect of absence of AGC1 in neurons or an indirect effect of the myelination deficits. The accumulation of phosphorylated NFs detected by antibody SMI-31 within cell bodies is suggestive of an intrinsic defect in axonal transport or could be reflective of a neurodegenerative effect. Defects in myelination could cause axonal damage, leading to impaired NF transport and their accumulation within neuronal perikarya. Alternatively, AGC1 in neurons may be important for axonal transport, perhaps by facilitating the production of ATP. In either case, neuronal deficits could contribute to phenotype with altered AGC1 expression. However, neuronal deficits in heterozygotes may be subtle, as we have not observed morphological changes in these mice.
Autism susceptibility and AGC1
SLC25A12/AGC1 has been shown to be associated with autism susceptibility (3, 6, 9, 10), although there are studies showing no association (7, 8). It is not surprising that genetic association studies of susceptibility genes do not replicate previous findings using different cohorts as the effect sizes are generally quite weak and the sample sizes likely too small to have adequate power in every case. Interestingly, a recent large-scale genome-wide association study has demonstrated that the SLC25A12 region is nominally associated with autism, although it did not reach genome-wide significance (Sutcliffe et al, 2009, International Meeting for Autism Research Abstract), further supporting possible involvement of AGC1 in autism susceptibility. However, the replicated SNPs are all intronic and their functional relevance to AGC1 expression is not yet clear. In postmortem brain samples, AGC1 is upregulated in dorsolateral frontal cortex of patients with autism (10, 12). This does not indicate that either overexpression or underexpression of AGC1 is associated with autism, as changes in expression may be a consequence of other aspects of the disorder and/or may reflect compensatory effects.
Since SLC25A12 is a putative autism susceptibility gene that at most increases genetic risk, AGC1 animal models that have either elevated or reduced AGC1 expression would not be expected to show clear ASD phenotypes. In this study, we rather intended to clarify the role of AGC1 in brain development and function, as well as to clarify the processes that may be affected when AGC1 expression is changed, which may be involved in increasing risk for ASDs.
We found that the absence of AGC1 affects several processes in brain development, namely general brain growth, myelination, and neuronal development, the latter associated with NFs. Thus, when AGC1 activity is modulated genetically, global effects mediated by altered mitochondrial function could affect a wide range of processes related to brain development. Such global effects on brain development may be the basis for involvement of AGC1 in ASD susceptibility.
Our results support the idea that alterations in AGC1 activity could affect NAA levels as well as ATP production in brain. There are several reports on NAA levels in brains of individuals with ASDs using magnetic resonance spectroscopy, with most studies pointing to decreased NAA levels, although some studies have found no differences compared to controls and one study found elevated levels (17–28). Additional studies suggest that there may be alterations in white matter and myelination in ASDs (reviewed in 29).
Synapse formation and plasticity, as well as myelination, demand a high-energy supply and therefore, these processes may also be altered when AGC1 function is modulated. In fact, AGC1 overexpression in primary neuronal cultures in vitro induces rapid movement of mitochondria into dendrites (12), which is thought to be associated with synaptic plasticity (30).
AGC1 is also a Ca2+ sensor modulating mitochondrial activity in cells (5). Recently, it has been shown that Ca2+ levels are elevated in postmortem brains from patients with autism compared to controls, and AGC1 activity itself is upregulated (10). Because AGC1 is involved in Ca2+ signals that affect global cell metabolism, sustained elevations of cellular Ca2+ may result in oxidative stress and cellular dysfunction through excessive activation of AGC1 (10).
From our studies, we cannot pinpoint specific pathways that are affected in Slc25a12-knockout mice, but rather found global effects in several aspects of brain development. Although we have not observed any obvious morphological changes in AGC1 heterozygous brains, either at the synaptic level or in neural circuitry, it is possible that subtle effects on several processes during brain development may lead to a behavioral phenotype. If so, then even subtle modulation of AGC1 expression may contribute to a behavioral phenotype, which may lead to an increased susceptibility to ASDs. In contrast, loss of functional AGC1 will lead to myelin abnormalities and severe neurodevelopmental deficits.
Supplementary Material
Acknowledgments
TS is a Seaver Fellow and supported in part by NYS SCI contract (C020935) and Stanley Medical Research Institute research grant (06R-1427). This research was supported by the Seaver Foundation (TS and JDB) and the NIMH (MH066673, JDB). Technical services at the MSKCC Small-Animal Imaging Core Facility were supported in part by NIH Small-Animal Imaging Research Program (SAIRP) Grant R24CA83084 and NIH Center Grant P30CA08748. We thank Dr. Jason Koutcher for helpful suggestions and Dov Winkleman for technical support for brain imaging. We thank Bill Janssen for assistance with confocal microscopy, Dr. Araceli del Arco (Universidad Autonoma) for anti-AGC1 antibodies, Dr. Robert Lazzarini (Mount Sinai School of Medicine) for anti-PLP antibodies, Dr. Virginia Lee (University of Pennsylvania) for anti-NF antibodies and Dr. Masashi Tanaka (Tokyo Metropolitan Institute for Gerontology) for helpful discussion.
Footnotes
Financial Disclosures
JDB and NR hold a patent on the SLC25A12 gene in autism and receive shared royalties on this gene. Other authors reported no financial interests or potential conflicts of interest.
References
- 1.Folstein SE, Rosen-Sheidley B. Genetics of autism: complex aetiology for a heterogeneous disorder. Nat Rev Genet. 2001;2(12):943–55. doi: 10.1038/35103559. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams BS, Geschwind DH. Advances in autism genetics: on the threshold of a new neurobiology. Nat Rev Genet. 2008;9(5):341–55. doi: 10.1038/nrg2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramoz N, et al. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161(4):662–9. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- 4.Wibom R, et al. AGC1 deficiency associated with global cerebral hypomyelination. N Engl J Med. 2009;361(5):489–95. doi: 10.1056/NEJMoa0900591. [DOI] [PubMed] [Google Scholar]
- 5.Satrustegui J, et al. Role of aralar, the mitochondrial transporter of aspartate-glutamate, in brain N-acetylaspartate formation and Ca(2+) signaling in neuronal mitochondria. J Neurosci Res. 2007;85(15):3359–66. doi: 10.1002/jnr.21299. [DOI] [PubMed] [Google Scholar]
- 6.Segurado R, et al. Confirmation of association between autism and the mitochondrial aspartate/glutamate carrier SLC25A12 gene on chromosome 2q31. Am J Psychiatry. 2005;162(11):2182–4. doi: 10.1176/appi.ajp.162.11.2182. [DOI] [PubMed] [Google Scholar]
- 7.Rabionet R, et al. Lack of association between autism and SLC25A12. Am J Psychiatry. 2006;163(5):929–31. doi: 10.1176/ajp.2006.163.5.929. [DOI] [PubMed] [Google Scholar]
- 8.Blasi F, et al. SLC25A12 and CMYA3 gene variants are not associated with autism in the IMGSAC multiplex family sample. Eur J Hum Genet. 2006;14(1):123–6. doi: 10.1038/sj.ejhg.5201444. [DOI] [PubMed] [Google Scholar]
- 9.Turunen JA, et al. Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Research. 2008;1(3) doi: 10.1002/aur.25. [DOI] [PubMed] [Google Scholar]
- 10.Palmieri L, et al. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- 11.Silverman JM, et al. Autism-related routines and rituals associated with a mitochondrial aspartate/glutamate carrier SLC25A12 polymorphism. Am J Med Genet B Neuropsychiatr Genet. 2008;147(3):408–10. doi: 10.1002/ajmg.b.30614. [DOI] [PubMed] [Google Scholar]
- 12.Lepagnol-Bestel AM, et al. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13(4):385–97. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- 13.Jalil MA, et al. Reduced N-acetylaspartate levels in mice lacking aralar, a brain- and muscle-type mitochondrial aspartate-glutamate carrier. J Biol Chem. 2005;280(35):31333–9. doi: 10.1074/jbc.M505286200. [DOI] [PubMed] [Google Scholar]
- 14.Moriyama M, et al. Pyruvate ameliorates the defect in ureogenesis from ammonia in citrin-deficient mice. J Hepatol. 2006;44(5):930–8. doi: 10.1016/j.jhep.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 15.Ramos M, et al. Developmental changes in the Ca2+-regulated mitochondrial aspartate-glutamate carrier aralar1 in brain and prominent expression in the spinal cord. Brain Res Dev Brain Res. 2003;143(1):33–46. doi: 10.1016/s0165-3806(03)00097-x. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka M, et al. Therapeutic potential of pyruvate therapy for mitochondrial diseases. Mitochondrion. 2007;7(6):399–401. doi: 10.1016/j.mito.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Hardan AY, et al. An MRI and proton spectroscopy study of the thalamus in children with autism. Psychiatry Res. 2008;163(2):97–105. doi: 10.1016/j.pscychresns.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gabis L, et al. 1H-Magnetic Resonance Spectroscopy Markers of Cognitive and Language Ability in Clinical Subtypes of Autism Spectrum Disorders. J Child Neurol. 2008 doi: 10.1177/0883073808315423. [DOI] [PubMed] [Google Scholar]
- 19.Endo T, et al. Altered chemical metabolites in the amygdala-hippocampus region contribute to autistic symptoms of autism spectrum disorders. Biol Psychiatry. 2007;62(9):1030–7. doi: 10.1016/j.biopsych.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Kleinhans NM, et al. N-acetyl aspartate in autism spectrum disorders: regional effects and relationship to fMRI activation. Brain Res. 2007;1162:85–97. doi: 10.1016/j.brainres.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeVito TJ, et al. Evidence for cortical dysfunction in autism: a proton magnetic resonance spectroscopic imaging study. Biol Psychiatry. 2007;61(4):465–73. doi: 10.1016/j.biopsych.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Friedman SD, et al. Gray and white matter brain chemistry in young children with autism. Arch Gen Psychiatry. 2006;63(7):786–94. doi: 10.1001/archpsyc.63.7.786. [DOI] [PubMed] [Google Scholar]
- 23.Murphy DG, et al. Asperger syndrome: a proton magnetic resonance spectroscopy study of brain. Arch Gen Psychiatry. 2002;59(10):885–91. doi: 10.1001/archpsyc.59.10.885. [DOI] [PubMed] [Google Scholar]
- 24.Hisaoka S, et al. Regional magnetic resonance spectroscopy of the brain in autistic individuals. Neuroradiology. 2001;43(6):496–8. doi: 10.1007/s002340000520. [DOI] [PubMed] [Google Scholar]
- 25.Otsuka H, et al. Brain metabolites in the hippocampus-amygdala region and cerebellum in autism: an 1H-MR spectroscopy study. Neuroradiology. 1999;41(7):517–9. doi: 10.1007/s002340050795. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto T, et al. Differences in brain metabolites between patients with autism and mental retardation as detected by in vivo localized proton magnetic resonance spectroscopy. J Child Neurol. 1997;12(2):91–6. doi: 10.1177/088307389701200204. [DOI] [PubMed] [Google Scholar]
- 27.Chugani DC, et al. Evidence of altered energy metabolism in autistic children. Prog Neuropsychopharmacol Biol Psychiatry. 1999;23(4):635–41. doi: 10.1016/s0278-5846(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 28.Fayed N, Modrego PJ. Comparative study of cerebral white matter in autism and attention-deficit/hyperactivity disorder by means of magnetic resonance spectroscopy. Acad Radiol. 2005;12(5):566–9. doi: 10.1016/j.acra.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Hughes JR. Autism: the first firm finding = underconnectivity? Epilepsy Behav. 2007;11(1):20–4. doi: 10.1016/j.yebeh.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Li Z, et al. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell. 2004;119(6):873–87. doi: 10.1016/j.cell.2004.11.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


