Abstract
Human complement factor H (FH), an abundant 155-kDa plasma glycoprotein with 40 disulphide bonds, regulates the alternative-pathway complement cascade. Mutations and single nucleotide polymorphisms in the FH gene predispose to development of age-related macular degeneration, atypical haemolytic uraemic syndrome and dense deposit disease. Supplementation with FH variants protective against disease is an enticing therapeutic prospect. Current sources of therapeutic FH are restricted to human blood plasma highlighting a need for recombinant material. Previously FH expression in cultured plant, mammalian or insect cells yielded protein amounts inadequate for full characterisation, and orders of magnitude below therapeutic usefulness. Here, the V62,Y402 variant of FH has been produced recombinantly (rFH) in Pichia pastoris cells. Codon-optimisation proved essential whilst exploitation of the yeast mating α-factor peptide ensured secretion. We thereby produced multiple 10s-of-milligram of rFH. Following endoglycosidase H digestion of N-linked glycans, rFH (with eight residual N-acetylglucosamine moieties) was purified on heparin-affinity resin and anion-exchange chromatography. Full-length rFH was verified by mass spectrometry and Western blot using monoclonal antibodies to the C-terminus. Recombinant FH is a single non-aggregated species (by dynamic light scattering) and fully functional in biochemical and biological assays. An additional version of rFH was produced in which eight N-glycosylation sequons were ablated by Asn–Gln substitutions resulting in a glycan-devoid product. Successful production of rFH in this potentially very highly expressing system makes production of therapeutically useful quantities economically viable. Furthermore, ease of genetic manipulation in P. pastoris would allow production of engineered FH versions with enhanced pharmacokinetic and pharmacodynamic properties.
Keywords: Protein therapeutic, Age-related macular degeneration, Atypical haemolytic uraemic syndrome, Dense deposit disease, Synthetic gene
Introduction
The human glycoprotein known as complement factor H (FH1; 155 kDa) [1] and [2] is a complement regulator that circulates in plasma at 350-600 mg/l [3]. It contains 1213 amino acid residues, 40 disulphide bonds and eight N-glycans [4], and its protein component consists entirely of 20 CCP modules [5] joined by short linkers[6] ( Fig. 1). Factor H acts both in the fluid phase and on surfaces to limit the amplification of the activation-specific complement protein fragment, C3b [7]. It has a strong preference for self, as opposed to foreign, surfaces [8] and [9], i.e. it does not effectively suppress C3b accumulation and propagation on the surfaces of bacteria and other foreign particles. Surface-attached C3b binds to receptors on macrophages and thereby facilitates phagocytosis. It also initiates the complement cascade that has both inflammatory and cytolytic consequences [10] and [11]. Association of C3b with factor B forms C3bBb, which is a C3 convertase, able to cleave C3 to generate further copies of C3b [12]. A reactive thioester exposed upon C3 cleavage then mediates attachment of C3b to nearby nucleophiles such as may be found on a proximal surface. Consequently, C3b molecules are surface deposited in growing clusters around each progenitor molecule. Thus, in a “fire fighting” role, FH molecules diffuse over the self surface dealing with “outbreaks” or clusters of C3b molecules. It is, however, unclear how FH engages with each C3b molecule it encounters [13].
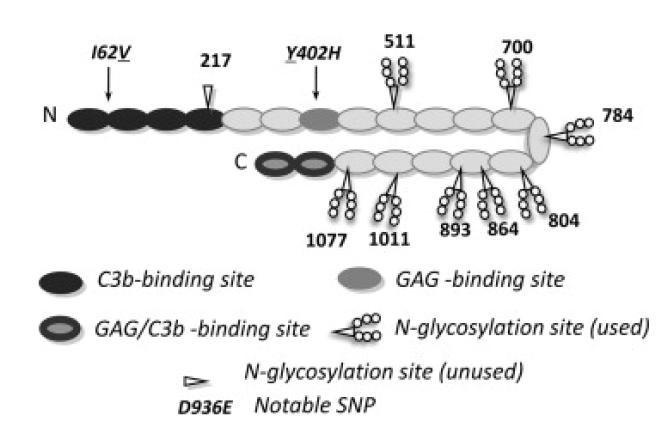
Figure One.
Summary of complement factor H structure and binding sites. Cartoon of human FH (SwissProt ID P08603) molecule that may have a bent-back overall architecture [72]. Each of 20 CCP modules (ovals) contains ~60 amino acid residues, is β-strand rich, and has two disulphide bonds. Positions of notable SNPs (see text) are indicated – underlines denote the variant produced in this study. Shading signifies binding sites for C3b and glycosaminoglycans (GAGs) (or both in the case of CCP modules 19 and 20 that also bind to sialic acid). Eight of nine potential N-glycosylation sites are occupied (as indicated) in the native protein – P. pastoris-derived glycans were enzymatically cleaved during preparation of recombinant (r)FH. The eight asparagine residues were mutated to glutamine residues in the “All-Q” mutant.
The two primary C3b-binding sites lie at either end of the FH molecule, and bind simultaneously to one, or possibly two, C3b molecules [13] and [14]. The N-terminal binding site alone is sufficient both to act as a cofactor for factor I-mediated proteolysis of C3b and to accelerate dissociation of any C3bBb complexes that do form [15]. Bivalent binding probably helps to retain FH on the host-cell surface over the course of multiple C3b-cleavage events. Polyanion-binding sites on FH specific for glycosaminoglycans and sialic acids selectively enforces residency at self-surfaces [16], [17] and [18]. Thus not only must FH prevail in a running battle against outbreaks of C3b deposition but it must also discriminate between self and foreign tissues. Deficiencies in either of these respects could be dangerous for the host.
Individuals with mutations [19], [20], [21], [22] and [23] in FH or autoantibodies to the C terminus [24], [25],[26] and [27] of FH are predisposed to developing the renal thrombotic microangiopathy, atypical haemolytic uraemic syndrome. People with FH deficiency resulting from truncating mutations that lead to low serum FH levels are prone to develop dense deposit disease (DDD) [20], [28], [29] and [30] like the FH−/− mouse [31]or FH-deficient Norwegian Yorkshire pig [32]. Two common polymorphisms in the FH gene, I62V in CCP 2 and Y402H in CCP 7 [33], [34], [35] and [36] are associated with a significantly increased life-time risk of developing age-related macular degeneration (AMD). All these diseases are characterised by complement-mediated damage to self tissue due to FH functional deficiency [37]. The observation that supplementation of FH by plasma infusion alone or as part of plasma exchange (plasmapheresis) (Reviewed by Waters et al.[38]) can control AP complement turnover in aHUS and limit disease progression has led to hopes that supplementation with FH in such cases could ameliorate symptoms. For therapy, large doses (100s of milligrams) of functional protein are required owing to high levels of circulating protein in healthy individuals. Plasma-derived therapies have, however, been associated with infective complications [39] and [40]. Hence there is an unmet need for a recombinant source of FH.
Efforts that have previously been expended on heterologous expression of the FH gene have met with only limited success. For example, cDNA encoding full-length human FH was expressed in insect cells [41]. Quantities were adequate for functional studies – the resultant FH matched or exceeded plasma-purified FH in cofactor activity and in its affinity for C3b on sheep erythrocytes. But full characterisation was not performed, e.g. glycosylation was not investigated, nor was SDS–polyacrylamide gel electrophoresis under reducing conditions (that would reveal proteolytic clipping of the chain) reported. Total protein production levels were probably too low for thorough biophysical characterisation; they certainly fell well short of the requirements of therapeutic utility. Insect cell-expressed material – including module-deletion mutants of FH – was employed in a further study [42] but use of these proteins appear not to have been reported subsequently. Complement factor H has also been expressed in mammalian cells [43] and more recently in plant cells [44] but yields were even lower than in insect cells; again the material could not be fully authenticated or biophysically characterised.
Protein production based on expression in engineered Escherichia coli, and refolding of insoluble material, has been used for manufacture of several FH fragments [45] and [46] but not for production of full-length FH. Yeast cells represent a viable alternative that avoids the unpredictability of refolding and produces proteins free of endotoxins and oncogenic entities as well as viral DNA [47] and [48]. The methylotropic yeast, Pichia pastoris has favourable properties for the secretion of high-molecular weight proteins that are inefficiently secreted in most other yeast species due to retention in the periplasmatic space [48]. Taking advantage of the AOX1 promoter – one of the strongest known in nature – good yields are possible from P. pastoris that can grow to very high cell densities in a fermentor [49]. Furthermore, P. pastoris grows on simple and inexpensive media, and allows genetic manipulation with relative ease [50]. This species recognises and processes many secretion signal leader sequences, which facilitates secretion of the recombinant protein into the growth medium. Secretion is advantageous for downstream protein collection and purification. P. pastoris, as a eukaryotic production system, is able to perform the appropriate post-translational modifications e.g. proteolytic maturation, glycosylation and disulphide bridge formation.
Herein we report successful production in P. pastoris, under non-optimised fermentation conditions, of multiple 10s-of-mg of pure fully authenticated, full-length human FH. This approach has also been applied to produce a glycan-free version of the protein. Our results demonstrate that P. pastoris is the method of choice for producing, in a relatively fast and economical procedure, the quantities of recombinant FH needed for investigations of disease mechanisms and therapeutic potential.
Materials and methods
Preparation of plasmids and transformation
Native DNA coding for human FH was amplified from the human universal Quick-clone cDNA library (Clontech) and cloned into pCR4Blunt-TOPO vector (Invitrogen). Codon-optimised DNA sequences (see Supplementary Fig. 1), encoding human FH or a mutant versions of FH (see Fig. 1), were purchased from GeneArt. These native and codon-optimised DNA sequences were each inserted into the yeast expression vector pPICZαB (Invitrogen), using PstI and XbaI restriction enzyme (New England Biolabs) sites, downstream of the alcohol oxidase (AOX1) promoter and behind the DNA coding for the prepro-α-factor secretion signal. Subsequently KM71H P. pastoris cells (Invitrogen) were transformed with SacI-linearised plasmid (using electroporation and standard settings on a Biorad GenePulser). Selection of P. pastorisclones containing the expression plasmid was achieved by streaking transformed yeast onto YPDS (i.e. 1% (w/v) yeast extract, 2% (w/v) peptone, 2% (w/v) dextrose, 1 M d-sorbitol) plates containing 300 mg/ml Zeocin. Cell colonies that grew (30 °C for three-four days) on these antibiotic-containing plates were thought likely to carry multiple copies of the target gene and were screened for protein expression.
Protein production
For small-scale production trials ten colonies were grown in 10 ml buffered minimal glycerol medium [BMGi.e. 100 mM potassium phosphate (pH 6.0), 1.34% (w/v) yeast nitrogen base, 4 × 10−5% (w/v) biotin, 1% (v/v) glycerol] for 2 days prior to transfer of the cell pellet (after spinning at 1500g for 5 min) into buffered minimal methanol medium [BMM i.e. 100 mM potassium phosphate (pH 6.0) 1.34% (w/v) yeast nitrogen base, 4 × 10−5% (w/v) biotin, 0.5% (v/v) methanol]. For three consecutive days the BMM was substituted with 1.0%, 1.1% and 1.1% (v/v) of methanol. Then the supernatant was harvested and a 15-μl aliquot of supernatant from each of the 10 colonies was screened for rFH expression by Western blotting. Large-scale protein production was attempted in a 5-l cylindrical Bioflow 3000 (New Brunswick Scientific) fermentor vessel based on Invitrogen guidelines. Briefly, in a typical fermentation the initial volume of growth media was 3–4 l. For the starter culture, a 500-ml culture in yeast-extract peptone dextrose medium was grown in a 2-l shaker flask and inoculated into the initial fermentation medium [2.5% (v/v) glycerol, 0.425% (w/v) potassium hydroxide, 1.5% (w/v) magnesium sulphate heptahydrate, 1.82% (w/v) potassium sulphate, 0.095% calcium sulphate, 2.3% (v/v) ortho-phosphoric acid, 0.025% (v/v) antifoam 206 (Sigma–Aldrich) and 0.4% (v/v) high-purity grade fermentation trace mineral salts (PTM1 salt, Amresco)] which had been adjusted to pH 5.0 using 34% (v/v) ammonia solution. Cell mass was grown in a glycerol-fed batch phase at 30 °C (feeds included 1.2 ml of PTM1 salts for every 100 ml glycerol added) to reach a wet-cell mass of about 200 g/l prior to induction with 0.5% (v/v) methanol at 15 °C. Following induction, methanol was fed in batches so as to maintain a level of up to 1.5% (v/v) (with 1.2 ml of PTM1 salts added for every 100 ml methanol) after consumption of the previous methanol feed.
Protein purification
After filtration to remove cells and addition of EDTA and PMSF to final concentrations of 5 and 0.5 mM, respectively, the supernatant from the fermentor was diluted one-in-five with distilled water and applied to a self-poured column containing Heparin FastFlow resin (see Table 1 for details of the purification). Fractions containing protein were pooled and the glycans were removed by incubating the sample with, typically, 30,000 units of the endoglycosidase-maltose binding protein fusion enzyme, Endo Hf (New England Biolabs) for 2-3 h at 37 °C. Protein was next applied to a Concanavalin A column to remove P. pastoris-derived glycans and then to amylose resin to remove Endo Hf. The sample was further purified on a self-poured Poros-Heparin chromatography column (Table 1; this step was used only in the initial preparation – it was omitted in subsequent preparations). The final purification step involved a strong anion-exchange step on a MonoQ or ResourceQ column (GE Healthcare). The protein was eluted by a gradient, over 20 column volumes (MonoQ) or 12.5 column volumes (ResourceQ), from 20 mM glycine buffer (pH 9.5) to the same buffer supplemented with 1 M NaCl. Fractions were run out on SDS–PAGE (4–12% acrylamide gradient gels) under both reducing (143 mM DTT) and non-reducing sample-loading conditions.
Table 1.
Purification of recombinant factor H.
| Step | Description | Equilibration buffer | Elution buffer | Gradient over ncolumn volumes | Conductivity measurement as FH elutes |
|---|---|---|---|---|---|
| 1 | Load on Heparin–sepharose fastFlowa; self-poured column | 20 mM potassium phosphate buffer, pH 6.0 | 20 mM potassium phosphate buffer, pH 6.0, with 1 M NaCl | n = 6 | 21 mS/cm |
| 2 | Deglycosylation with Endo Hf | For steps 2 and 3, fractions eluted from step 1 used directly, without buffer exchange | Flow-through collected in step 3 | n.a. | |
| 3 | Load on Concanavalin A Sepharosea; bench-top column | ||||
| 4 | Load on amylose resinb; bench-top column | Buffer as eluted from step 1 | Equilibration buffer (i.e.no gradient) | Flow-through collected | n.a. |
| 5c | Load on Poros heparin resind; self-poured column | Phosphate-buffered saline (PBS) | PBS substituted with 1 M NaCl | n = 20 | 30 mS/cm |
| 6 | Load on MonoQ or ResourceQ columna | 20 mM Glycine buffer, pH 9.5, 125 mM NaCl | 20 mM Glycine buffer, pH 9.5, 1 M NaCl | n = 20 (MonoQ) or 12 (ResourceQ) | 24 mS/cm |
From GE-Healthcare.
From New England Biolabs.
This step used only in initial preparation, not in subsequent batches.
From Invitrogen.
The “all-Q” FH was purified as above, except the protein was not treated with Endo Hf and, following elution from the self-poured XK-Heparin column, fractions were pooled and buffer-exchanged into 20 mM glycine, 150 mM NaCl (pH 9.5) and loaded onto a Resource-Q anion-exchange column. The protein was eluted over six column volumes in a linear gradient from 20 mM glycine, 120 mM NaCl (pH 9.5) to 20 mM glycine, 1 M NaCl (pH 9.5).
Western blots
Samples were electrophoresed on SDS–PAGE for Western blots. Primary antibodies (polyclonal anti-FH raised in goat (Calbiochem) or the mouse-derived monoclonal antibodies (MAb-SC47686_L20/3 and MAb-Abnova-0167 that recognise an epitope within the two C-terminal modules of FH) were used. Detection was achieved using horse-radish peroxidase-coupled secondary anti-goat IgG (Sigma) or anti-mouse IgG (Cell signalling) antibodies.
MALDI-ToF characterisation of rFH
For MALDI-time-of-flight mass spectrometry, a Voyager-DE STR Biospectrometry Workstation instrument (Applied Biosystems) was used. An internal standard (IgG1) and the candidate rFH were crystallised with 2,4,6-trihydroxyacetophenone matrix prior to ionisation and analysis as described previously [4].
Affinity for C3b
Surface-plasmon resonance (SPR) (on a Biacore T 100, GE Healthcare) was employed to measure affinities for amine-coupled C3b (on a CM5 sensor chip). Duplicate sensorgrams were accumulated for a series of concentrations of recombinant and reference FH (plasma-purified material from Complement Technology, Texas). Both proteins were analysed at the following concentrations (as estimated by A280, using a calculated molar extinction coefficient of 246,800): 5.4, 1.0, 0.5 and 0.1 μM. For fitting of the dissociation constant, the SPR responses obtained for two independent sensor-chip surfaces that had been coupled with different amounts of C3b (1540 and 3030 response units of C3b immobilised), were plotted against the above range of analyte concentrations flowed over the chip surfaces (in duplicate).
Cofactor activity
A fluid-phase assay [51] was used to measure cofactor activity for factor I-mediated proteolytic cleavage of C3b. Factor I, FH and C3b were all purchased from Complement Technology. The reaction mix in PBS buffer contained Factor I at 0.11 μM, C3b at 0.86 μM and either reference FH or rFH or soluble complement receptor type 1 (sCR1, Celldex Therapeutics, MA) at 0.3 μM. The reaction mixtures were vortexed and incubated in a water bath at 37 °C for a time course. Aliquots of 20 μL were taken at 0, 2, 5, 15 and 30 min. An aliquot of 8 μL reducing (143 mM dithiothreitol) lithium dodecyl sulfate sample buffer was added to stop the reaction and the samples were heated immediately. The Factor I/FH or Factor I/sCR1 catalysed cleavage of the α′-chain can be followed by visualising the products and unused substrates following SDS–PAGE and Coomassie blue staining.
Decay-accelerating activity
An assay of convertase decay-accelerating activity was performed using a Biacore T 100 instrument, as described previously [52], [53] and [54]. Briefly, about 134 resonance units (RU) of C3b (Complement Technology) were coupled to the CM5-sensorchip via standard amine coupling. Subsequently, a mixture of Factor B (500 nM) and fD (60 nM) (both from Complement Technology) were flowed over the surface for 120 s, at a rate of 10 μl/min, to form the C3 convertase. Proteins were dialysed into the running buffer (HBS-P+ i.e. 10 mM HEPES-buffered 150 mM saline (pH 7.4), 0.05% (v/v) surfactant p20, 1 mM MgCl2) prior to analysis. The concentration of proteins solutions flowed over the chip surface was estimated using their absorbance at 280 nm and their calculated extinction coefficients. Convertase decay was visualised in real time; after observing regular decay for 210 s, either bovine serum albumin (1 μM), FH19-20 (1 μM), plasma-purified FH (0.5 μM) or rFH (0.5 μM) was injected (in duplicates) for 60 s. Between injections, the surface was regenerated using a 1 μM FH1–4 in HBS-P+ (contact time 45 s) followed by injection of 1 M NaCl (contact time 45 s). Data were evaluated using the Biaevaluation software (GE healthcare). From each response curve (sensorgram) showing analyte protein binding to the convertase (followed by convertase decay), was subtracted the sensorgram that had been recorded when the same analyte was flowed over the C3b-coated surface but with no convertase present.
Haemolytic protection assay
This assay was adapted from that originally described by Sanchez-Corral et al. [55], and from an investigation of the recombinantly expressed C-terminus of FH reported by Ferrreira et al. [18].
An aliquot of 1 ml of defibrinated sheep blood (TCS Biosciences) was transferred to a 15-ml falcon tube and diluted with 10 ml of washing buffer containing 20 mM HEPES, 145 mM NaCl, 0.1% (w/v) gelatin (from pig skin, Fluka), 10 mM EDTA (pH 7.3) at 25 °C. The cell suspension was mixed gently and spun for 10 min at 500g (4 °C). The supernatant along with a thin white layer of leucocytes were discarded and the procedure was repeated twice more. Subsequently, the washing buffer was replaced by an EDTA-free equivalent. Three more washing steps were performed with the EDTA-free buffer and centrifugation at 1000g.
In preliminary experiments, 2.5-μl aliquots of various dilutions of the original cell suspension were lysed by addition of 197.5 μl distilled water. Then 100-μl aliquots of the lysed solutions were transferred to separate wells of a 96-well plate and the A412 was measured for each well. A sheep erythrocyte stock suspension yielding A412 = 0.5-0.6 units (1-cm path length) in this procedure was then selected for use in the assay.
A mixture of the following in a 16:1:2:1 ratio was prepared: FH-depleted human serum (ΔFH-HS, purchased from Complement Technology), 0.1 M Mg-EGTA (i.e. 0.1 M MgCl2, 0.1 M ethylene glycol-bis(2-aminoethyl)-N,N,N′,N′-tetraacetic acid), sheep erythrocyte stock suspension (see above) and haemolytic assay buffer (20 mM HEPES, 141 mM NaCl, 5 mM MgEGTA, 0.1% gelatin (w/v) (pH 7.3)). To 25-μl batches of this mixture were added 25 μl aliquots of phosphate buffered saline (PBS; i.e. no-protein control) or various concentrations of FH, rFH or FH6–8 in PBS. The resultant 50-μl reaction mixtures were incubated at 37 °C for 20 min and the reaction was stopped by addition of 150 μl cold quenching buffer (20 mM HEPES, 145 mM NaCl, 5 mM EDTA (pH 7.3)). Remaining cells were spun down for 5 min (1500g, 4 °C) in a 96-well round bottom plate and 100 μl of supernatant was transferred from each well to a fresh 96-well plate, which was then used for A412 measurement. Each experiment was repeated four times in total and a standard error was calculated for each sample. As a further negative control a well was filled with the same reaction mixture but containing ΔFH-HS which had been heat-inactivated at 56 °C for 30 min [56].
Results
Attempted production using FH cDNA did not yield detectable amounts of protein
Following transformation with pPICZαB containing native-sequence DNA encoding FH under the control of the AOX1 promoter, several P. pastoris clones grew on plates containing 300 μg/ml Zeocin, consistent with the presence of multiple copies of the insert in the transformed cells. These were screened for protein production in BMG, first in 10-ml and then in 100-ml shaking cultures. We failed, however, to detect (on SDS–PAGE, stained with Coomassie blue) any evidence of FH production (not shown). We next checked to see if protein production by transformed cells could be detected under more favourable expression conditions in a fermentor where levels of nutrients and dissolved oxygen are controlled. A Western dot blot (using a commercial goat polyclonal anti-human FH antibody and secondary antibody coupled to horse radish peroxidase) performed on a 20-fold concentrate of the growth medium (not shown) failed to detect any product. Likewise a step-wise elution (1 M NaCl) from a 1-ml heparin-affinity column that had been loaded with 10 ml (diluted to 60 ml to reduce ionic strength) of growth medium from a fermentor did not yield enough recombinant FH to be detected by Western blot.
Successful production and purification of factor H encoded by a synthetic gene
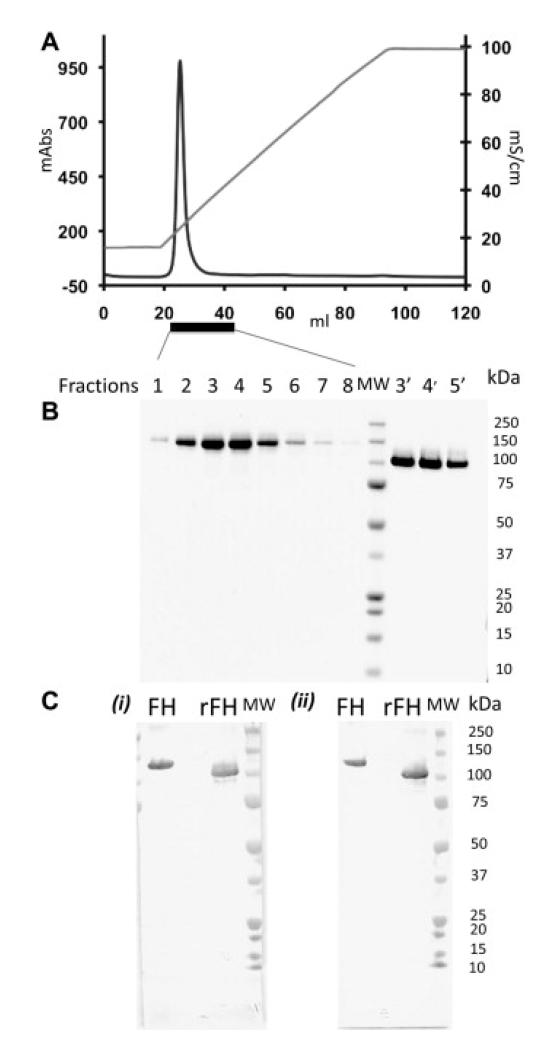
A codon-optimised DNA sequence (Geneart) was therefore cloned into the pPICZαB plasmid and the resultant vector was amplified then transformed into P. pastoris strain, KM71H. As before, P. pastoris cell colonies that grew on high Zeocin-containing plates were screened for protein expression. In contrast to the results with native-sequence FH, these cells produced a protein of the expected size that tested positive by Western blot (not shown). Production of cells was subsequently undertaken in a 4-l fermentor. Supernatant from the fermentor was diluted and applied to a heparin-affinity chromatography column, then fractionated on a linear NaCl gradient (see Table 1 for details of the purification steps). It is well established that P. pastorissynthesises glycans on N-glycosylation sites, resulting in heterogeneous preparations of glycoproteins with non-mammalian glycosylation patterns. It was decided to remove these enzymatically using a fusion protein of endoglycosidase H and mannose binding protein (Endo Hf), which trims N-glycans back to N-acetyl-d-glucosamine moieties. Endo Hf-treated FH-containing fragments were pooled and processed by sequential applications to a Concanavalin A-coupled resin and an amylose resin. Further purification was effected by heparin-affinity chromatography, eluting with a linear salt gradient in PBS; the main peak co-elutes, at 270 mM NaCl, with plasma-purified FH (not shown) indicating that recombinant FH has a similar affinity for heparin. Finally, strong anion-exchange chromatography was performed on the sample, resulting in a single sharp peak (Fig. 2A). Table 1 summarises details of the purification steps.
Figure Two.
Production of recombinant complement factor H. (A) In the final purification step (see Table 1) FH emerges from an anion-exchange column (MonoQ) as a single sharp peak (A280 in milli-absorbance units on left-hand y-axis) upon elution with a salt gradient (20 mM glycine buffer, pH 9.5, 0.12–1 M NaCl; conductivity on right-hand y-axis). (B) Fractions eluted from the MonoQ column (see Fig. 2A) were subjected to SDS–PAGE and protein bands were visualised with Coomassie blue. Lanes 1-8 (reducing conditions) correspond to elution volumes 23-30 mL. Lane 9 contains molecular weight markers (MW) as indicated on the right-hand side. Lanes 3′, 4′ and 5′ correspond to lanes 3, 4 and 5 but were run under non-reducing conditions. (C) Two antibodies that recognise epitopes within the C-terminal CCP modules of FH, were used in Western blots. Plasma FH (left lane) and recombinant rFH (middle lane) were detected with (i) MAb-SC47686_L20/3 or (ii) Mab-Abnova-0167. MW = molecular weight markers – see right-hand side of gel (ii).
Fractions were electrophoresed on SDS–polyacrylamide gels stained with Coomassie blue (Fig. 2B). Single bands were obtained, under both reducing and non-reducing conditions, with an apparent molecular weight consistent with deglycosylated FH. The non-reduced form runs ahead of the reduced form as expected for a protein with multiple disulphide bonds. These results confirm a good level of purity and indicate that the polypeptide chain had not been cleaved to a significant extent. To confirm identity, Western blots were performed using a polyclonal antibody (raised in goat against FH purified from human plasma) (not shown), and two monoclonal antibodies that recognise epitopes within C-terminal CCPs 19-20.
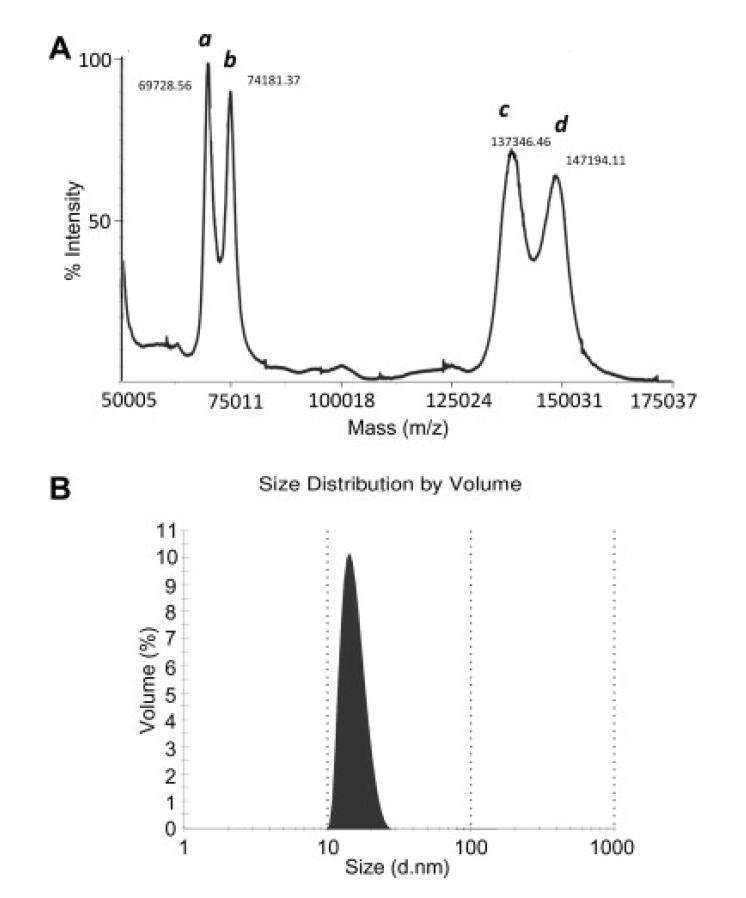
MALDI-TOF mass spectrometry was used to confirm the mass of the P. pastoris-produced FH ( Fig. 3A). The mass of ~138,000 Da is in excellent agreement with the theoretical mass of FH (137,052-138,596 Da including the polypeptide chain and up to eight N-acetyl-d-glucosamine moieties that remain attached to the polypeptide following the catalytic action of the endoglycosidase). Dynamic light scattering (Fig. 3B) demonstrated that rFH behaves as mondispersed particles with hydrodynamic radii of 14 nm (in PBS at 20 °C). We next set out to establish whether the rFH is able to bind its primary ligand, C3b.
Figure 3.
Validation of rFH by mass spectrometry and dynamic light scattering. (A) The candidate recombinant FH (peaks a and c correspond to double-charged and single-charged species, respectively) and an internal standard (IgG1; peaks b and d correspond to double-charged and single-charged species, respectively) were analysed on a MALDI-ToF mass spectrometer. The experimentally derived mass of ~138,000 Da is in good agreement with the theoretical mass of rFH. (B) Dynamic light scattering was performed on rFH in PBS at a concentration of 1 mg/ml. No indication of aggregation was observed.
Recombinant FH binds to C3b
Recombinant FH and plasma-purified FH were tested for C3b-binding affinity using a surface-plasmon resonance (SPR) instrument (Fig. 4). The two versions of FH, at a range of apparent concentrations (estimated on the basis of A280), were each flowed over two independent sensor-chip (CM5) surfaces to which different amounts of C3b had been attached (loaded) via amine coupling. In this comparison, the recombinant version (KD = 1.5 μM) apparently binds more tightly than plasma-purified FH (KD = 2.9 μM). In previous work, SPR (using a CM5 chip) yielded KD values of between 1.5 and 2.2 μM for the C3b:FH interaction [14]. Note also that a significantly larger number of response units were obtained for the binding of rFH (at half-saturation) compared to plasma-purified FH. These values demonstrate that more molecules of rFH are binding to C3b on the sensor chip and hence it implies a higher proportion of active molecules in our current preparation of recombinant material relative to that particular batch of plasma-purified FH. We subsequently performed assays to test whether rFH is functionally active.
Figure Four.
Measurement of affinity for C3b. (A and B) Use of surface-plasmon resonance (SPR) to measure affinity of plasma-purified rFH (Fig. 4A) and plasma-purified FH (Fig. 4D) for amine-coupled C3b on a CM5 sensor chip (Biacore). Duplicate sensorgrams are shown for a concentration series (5.4, 1.0, 0.5, 0.1 μM) flowed over 1540 response units of immobilised C3b. (C and D) Plots of response units versus (C) rFH or (D) FH concentrations are shown for two different flow cells with either 1540 RUs (lower curve in each plot) or 3030 RUs (upper curve in each plot) of C3b. The dashed vertical line indicates the KD fitted in each case to both plots simultaneously, and yielding 1.4 μM for rFH and 2.9 μM for plasma-purified FH.
Recombinant factor H has complement regulatory activity
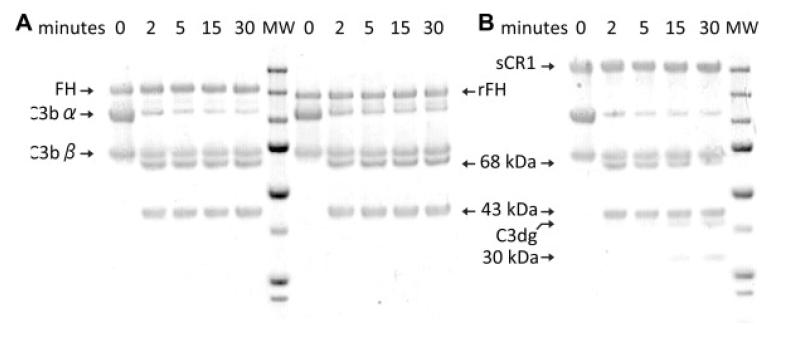
Factor H acts as an essential co-factor for the serine protease factor I-mediated proteolysis of C3b. This yields iC3b that consists of the unaltered β-chain and two cleavage products – 68 and 43 kDa – of the α′-chain. A sample of rFH (0.3 μM final concentration), along with a positive control containing the same amount of plasma-purified FH, was incubated over a time course with C3b and factor I in PBS. Samples were taken at intervals and analysed by SDS–PAGE (Fig. 5). Over time both rFH and plasma-purified FH cause appearance of the α′-chain fragments consistent with iC3b production. This comparison demonstrates that rFH not only interacts with C3b but also mediates productive interactions between C3b and factor I; thus it provides further evidence of the integrity of the recombinant material.
Figure Five.
Cofactor assay. (A) The abilities of FH (lanes 1-5) and rFH (lanes 7-11) to act as cofactors for factor I-catalysed cleavage of C3b to iC3b were assessed by visualising the 43-kDa and 68-kDa proteolytic fragments of the α′-chain using SDS–PAGE followed by Coomassie blue staining. Incubation times were 0-30 min, as indicated. Both versions of FH have similar activities such that the α′-chain of C3b is completely processed within 5 min. MW = molecular weight markers of (from top) 250, 150, 100, 75, 50, 37, 25 and 20 kDa. (B) For comparison, the cofactor activity of soluble complement receptor type 1 (sCR1), at the same concentration was followed over the same time intervals. Note that (in agreement with literature [73]) sCR1, but neither rFH nor plasma-purified FH, promoted the further degradation of the α′-chain to C3dg and a 30-kDa fragment. MW, as in Fig. 5A.
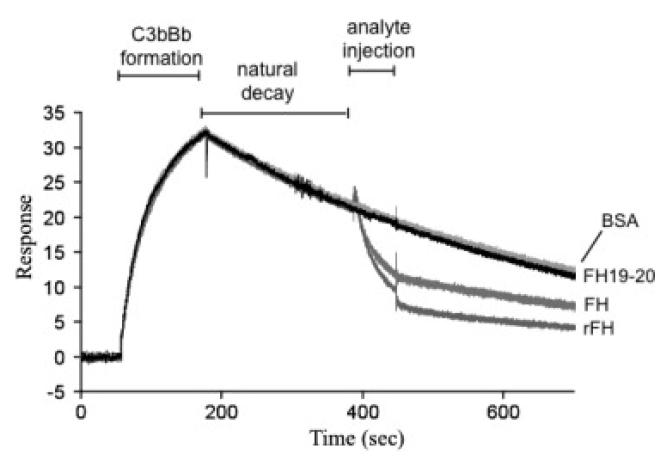
In addition to being the cofactor for factor I, FH accelerates dissociation (or decay) of the bimolecular C3 convertase of the alternative pathway (i.e. C3bBb). The ability of rFH to expedite dissociation of C3bBb was monitored in a SPR-based assay. In this experiment, C3b was first immobilised on a CM5 chip via amine coupling. Then a mixture of factor B and factor D was flowed over the C3b-loaded chip surface (Fig. 6). This produced a rise in response units consistent with formation of a complex of surface-bound factor B with C3b and its cleavage in this context by factor D to produce C3bBb. Subsequently, the loss in response units over time corresponds to natural decay of the complex, which is intrinsically unstable, to C3b and Bb (these components cannot then re-associate). Both plasma-purified FH and rFH, at identical apparent protein concentrations (500 nM), caused a significant acceleration of the decay process (Fig. 6). Decay acceleration probably entails interaction of FH with both C3b and Bb within the convertase and these results provide additional support for the integrity of the rFH. Indeed, the rFH appears to be more potent in this respect than plasma-purified FH; this is consistent with their measured affinities for C3b (Fig. 4) and, again, could be ascribed to a higher proportion of fully functional molecules within the sample of rFH.
Figure Six.
Decay-accelerating activity. Surface-plasmon resonance was used to monitor formation of the C3bBb (convertase) complex as factor D and factor B were flowed together over C3b that was amine-coupled to a CM5 (Biacore) sensor chip. The subsequent decline in response reflects decay of the complex as Bb is released from the chip surface. The rate of decay is accelerated by initiating (in this case 210 s into the natural decay process) a flow of reference FH or rFH. At similar concentrations (0.5 μM), rFH is a more effective decay accelerator in this assay than plasma-purified FH. The control proteins, BSA and FH modules 19-20, have no effect on decay. These are subtracted sensorgrams – details in Methods.
Recombinant factor H is functional in a biological context
A hallmark of FH is its ability to protect from human complement-mediated haemolysis sheep erythrocytes that, like human erythrocytes, have cell surfaces rich in polyanions such as sialic acid. When sheep erythrocytes were incubated with ΔFH-HS, haemolysis, as monitored by release of haemoglobin, occurs in 20 min (Fig. 7). When either plasma-purified FH or rFH is added to a final concentration of 0.4 μM, haemolysis in the reaction mixture is significantly suppressed. Thus, consistent with the integrity of this recombinant material, rFH can recognise C3b (or C3bBb) deposited on a polyanion-rich surface and prevent its amplification. It appeared somewhat more effective in this role than plasma-purified FH.
Figure Seven.
Ability of rFH to protect sheep erythrocytes from haemolysis. Sheep erythrocytes were incubated in physiological buffer, with 1.5 μM FH modules 6-8 (negative control), 0.4 μM plasma-purified FH or 0.4 μM rFH, prior to exposure (for 20 min at 37 °C) to human serum that had been depleted of FH. The reaction was quenched and A412 was measured. The results shown were the average (plus or minus standard deviation) of four experiments.
Production of other versions of rFH
Fig. 8 illustrates that the method described herein for production of multiple-10s of mg (and potentially gram-quantities) of rFH can also be applied to a mutant version of the protein. In “all-Q” rFH, eight asparagines within N-glycosylation sites (which are used [4]) have been replaced with glutamine residues to create a null-glycosylation mutant. This will enable production of homogeneous FH in P. pastoris without the need for incubation with endoglycosidase and the attendant risk of damage due to residual proteases.
Figure Eight.
Production of “all-Q” mutant. The sample of “all-Q” mutant of rFH (left-hand gel) migrates as a single band during SDS–PAGE under reducing (R) and non-reducing (NR) conditions (stained by Coomassie blue). Endo Hf (77 kDa) treatment causes no change in migration rate. This is consistent with the “all-Q” mutant having no N-glycosylation sites and being glycan-free. For comparison, rFH (prior to purification) migrates as a fuzzy band until it is Endo Hf treated (right-hand gel). MW = molecular weight markers as indicated to left and right of the two gels.
Discussion
The production of rFH from native-sequence FH-encoding DNA proved problematic despite use of a heterologous expression system that is particularly suitable for production of large extracellular proteins containing disulphides [57] and that was used successfully, in previous studies, for expression of numerous shorter segments of FH [58], [59] and [60]. One possibility, given the relatively large size of the gene for full-length FH, is that a cumulative effect of the presence of rare codons, repeated codon and/or nucleotides (potentially causing frame-shift errors), cryptic translation initiation sites, endoribonuclease sites and mRNA stem-loop structures (promoting early termination of translation) results in a near-zero yield [61]. Hence the DNA sequence encoding FH was redesigned in an attempt to minimise these putative obstacles to recombinant protein production.
Using a codon-optimised gene we were able to largely overcome the aforementioned problems and produce authentic FH polypeptide using the potentially very highly expressing P. pastoris system. The yields of protein from this procedure, which had not been optimised, are in the region of 3-5 mg/l of fermentation supernatant. This can almost certainly be significantly enhanced [62], however, by one or more of the following: amplifying the genomic copy number of the codon-optimised, FH-encoding DNA; further codon optimisation; exploring other P. pastoris strains; surveying a range of growth conditions such as pH and temperature; sorbitol–methanol co-feeding that has delivered an eight-ninefold yield enhancement [63]; and co-expression with genes for chaperones such as disulphide isomerase that produced a sixfold improvement[64].
Despite the near-complete removal of N-glycans, the recombinant material showed little propensity to self-associate under physiologically relevant conditions. Moreover the recombinant material has native-like affinity for heparin, which is considered a good model compound for glycosaminoglycans that are, apparently, used by FH to assist in recognition of self-cell surfaces. Factor H probably engages with C3b via simultaneous deployment of two principal binding sites within the FH molecule, one within N-terminal CCP modules 1-4 and the other within C-terminal CCP modules 19-20. The avidity arising from this putatively bivalent interaction is needed for efficient complement regulation since it presumably assists retention of FH on a C3b-coated surface. Previous studies established KD values for binding to C3b of 10 and 4 μM for the recombinant N-terminal four-module FH fragment (rFH1-4), and the C-terminal two-module FH fragment (rFH19-20), respectively [14] and [65] while the KD for C3b of the full-length protein is between 1.5 and 2.2 μM. This range of values for plasma-purified protein probably reflects batch-to-batch variation, the source of the material (from an individual or from pooled plasma), and might also depend upon the age of the protein sample and how it has been stored Thus, the affinity for C3b of rFH lies at the tight end of the range of values for plasma-purified batches of FH, while full-length rFH binds to C3b more tightly than either rFH1-4 or rFH19-20. These results are consistent with the expected cooperation between the N- and C-terminal C3b-binding sites within rFH that may be mediated by architectural properties of the more central modules [66]. Thus these data provide strong support for the structural and functional integrity of the entire recombinant protein.
Taken together, the functional analyses of rFH have three implications. First, P. pastoris is able to synthesise a version of FH that emulates native material with respect to both its structure and function. Second, the eight native biantennary N-linked glycans of the native FH are not required for folding and are entirely dispensable in respect of the little-understood mechanisms employed by FH for self-surface recognition and complement regulation. This is in agreement with previous work in which glycans were enzymatically removed [4] and [67]. It is noteworthy ( Fig. 1) that all of the utilised N-glycosylation sites are located on central modules and away from the principal ligand-binding sites of FH. Third, the comparison with plasma-purified FH suggests that heterologous expression affords an advantage in terms of the proportion of active molecules present in the preparation. This is not really surprising considering that native material used in most experiments on FH is harvested from pooled plasma; it will inevitably contain a range of polymorphic variants and, potentially, protein molecules damaged by glycation, oxidation, nitration, acetylation or partial proteolytic cleavage.
Unlike in previous work, sufficient site-directed mutants can be produced in P. pastoris for quantitative functional, biochemical and biophysical studies aimed at an understanding of mechanism. These results exemplify the scope for production of specific sequence variants of human FH that are difficult to access from plasma without recourse to expensive immunoaffinity-based chromatography. This could be especially useful in therapeutic applications since protective haplotypes of FH, in diseases such as AMD, aHUS and DDD, have been identified that would be good candidates for use in therapies based on supplementation of FH in patients who carry at-risk alleles [33], [68] and [69]. As understanding of mechanism improves, rationally engineered versions of FH that are more compact or more potent could be produced easily in P. pastoris, tested in vitro, and scaled up for in vivo studies. The incorporation of human-like glycosylation is feasible in P. pastoris [70]. An additional potential benefit of P. pastoris as an expression system is the potential for incorporation of unnatural amino acids [71] in order to multiply the options for protein engineering or to serve as anchors for site-specific bioconjugations.
Supplementary Material
Acknowledgments
We thank Dr John White, Dr Bruce Ward and Ms Pamela Beattie and the staff of the Edinburgh Protein Production Facility for assistance with protein production and purification. We thank Dr Henry March of Celldex Therapeutics for the gift of sCR1. PNB, CQS and FCS are supported by grants from the Wellcome Trust (081179), and the Chief Scientist’s Office; AR is a Wellcome Trust Intermediate Clinical Fellow.
Abbreviations used
- aHUS
atypical haemolytic uraemic syndrome
- AMD
age related-macular degeneration
- CCP
complement control protein module
- DDD
dense deposit disease
- FH
factor H
- RU
response units
- sCR1
soluble complement receptor type 1
- SPR
surface-plasmon resonance
- ΔFH-HS
FH-depleted normal human serum
References
- [1].Sim RB, DiScipio RG. Purification and structural studies on the complement-system control protein beta 1H (Factor H) Biochem. J. 1982;205:285–293. doi: 10.1042/bj2050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ripoche J, Day AJ, Willis AC, Belt KT, Campbell RD, Sim RB. Partial characterization of human complement factor H by protein and cDNA sequencing: homology with other complement and non-complement proteins. Biosci. Rep. 1986;6:65–72. doi: 10.1007/BF01145180. [DOI] [PubMed] [Google Scholar]
- [3].Weiler JM, Daha MR, Austen KF, Fearon DT. Control of the amplification convertase of complement by the plasma protein beta1H. Proc. Natl. Acad. Sci. USA. 1976;73:3268–3272. doi: 10.1073/pnas.73.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fenaille F, Le Mignon M, Groseil C, Ramon C, Riande S, Siret L, Bihoreau N. Site-specific N-glycan characterization of human complement factor H. Glycobiology. 2007;17:932–944. doi: 10.1093/glycob/cwm060. [DOI] [PubMed] [Google Scholar]
- [5].Soares DC, Barlow PN. Complement control protein modules in the regulators of complement activation. In: Morikis D, Lambris JD, editors. Structural Biology of the Complement System. CRC Press, Taylor & Francis Group; Boca Raton: 2005. pp. 19–62. [Google Scholar]
- [6].Kristensen T, Tack BF. Murine protein H is comprised of 20 repeating units, 61 amino acids in length. Proc. Natl. Acad. Sci. USA. 1986;83:3963–3967. doi: 10.1073/pnas.83.11.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pangburn MK, Schreiber RD, Muller-Eberhard HJ. Human complement C3b inactivator: isolation, characterization, and demonstration of an absolute requirement for the serum protein beta1H for cleavage of C3b and C4b in solution. J. Exp. Med. 1977;146:257–270. doi: 10.1084/jem.146.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Meri S, Pangburn MK. Discrimination between activators and nonactivators of the alternative pathway of complement: regulation via a sialic acid/ polyanion binding site on factor H. Proc. Natl. Acad. Sci. USA. 1990;87:3982–3986. doi: 10.1073/pnas.87.10.3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pangburn MK. Host recognition and target differentiation by factor H. A regulator of the alternative pathway of complement. Immunopharmacology. 2000;49:149–157. doi: 10.1016/s0162-3109(00)80300-8. [DOI] [PubMed] [Google Scholar]
- [10].Walport MJ. Complement. Second of two parts. N. Engl. J. Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- [11].Walport MJ. Complement. First of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- [12].Pangburn MK, Muller-Eberhard HJ. The alternative pathway of complement. Springer Semin Immunopathol. 1984;7:163–192. doi: 10.1007/BF01893019. [DOI] [PubMed] [Google Scholar]
- [13].Schmidt CQ, Herbert AP, Hocking HG, Uhrin D, Barlow PN. Translational Mini-Review Series on Complement Factor H: structural and functional correlations for factor H. Clin. Exp. Immunol. 2008;151:14–24. doi: 10.1111/j.1365-2249.2007.03553.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Schmidt CQ, Herbert AP, Kavanagh D, Gandy C, Fenton CJ, Blaum BS, Lyon M, Uhrin D, Barlow PN. A new map of glycosaminoglycan and C3b binding sites on factor H. J. Immunol. 2008;181:2610–2619. doi: 10.4049/jimmunol.181.4.2610. [DOI] [PubMed] [Google Scholar]
- [15].Kuhn S, Skerka C, Zipfel PF. Mapping of the complement regulatory domains in the human factor H-like protein 1 and in factor H1. J. Immunol. 1995;155:5663–5670. [PubMed] [Google Scholar]
- [16].Pangburn MK, Atkinson MA, Meri S. Localization of the heparin-binding site on complement factor H. J. Biol. Chem. 1991;266:16847–16853. [PubMed] [Google Scholar]
- [17].Meri S, Pangburn MK. Regulation of alternative pathway complement activation by glycosaminoglycans: specificity of the polyanion binding site on factor H. Biochem. Biophys. Res. Commun. 1994;198:52–59. doi: 10.1006/bbrc.1994.1008. [DOI] [PubMed] [Google Scholar]
- [18].Ferreira VP, Herbert AP, Hocking HG, Barlow PN, Pangburn MK. Critical role of the C-terminal domains of factor H in regulating complement activation at cell surfaces. J. Immunol. 2006;177:6308–6316. doi: 10.4049/jimmunol.177.9.6308. [DOI] [PubMed] [Google Scholar]
- [19].Warwicker P, Goodship TH, Donne RL, Pirson Y, Nicholls A, Ward RM, Turnpenny P, Goodship JA. Genetic studies into inherited and sporadic hemolytic uremic syndrome. Kidney Int. 1998;53:836–844. doi: 10.1111/j.1523-1755.1998.00824.x. [DOI] [PubMed] [Google Scholar]
- [20].Dragon-Durey MA, Fremeaux-Bacchi V. Atypical haemolytic uraemic syndrome and mutations in complement regulator genes. Springer Semin Immunopathol. 2005;27:359–374. doi: 10.1007/s00281-005-0003-2. [DOI] [PubMed] [Google Scholar]
- [21].Richards A, Buddles MR, Donne RL, Kaplan BS, Kirk E, Venning MC, Tielemans CL, Goodship JA, Goodship TH. Factor H mutations in hemolytic uremic syndrome cluster in exons 18-20. A domain important for host cell recognition. Am. J. Hum. Genet. 2001;68:485–490. doi: 10.1086/318203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Perez-Caballero D, Gonzalez-Rubio C, Gallardo ME, Vera M, Lopez-Trascasa M, Rodriguez de Cordoba S, Sanchez-Corral P. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 2001;68:478–484. doi: 10.1086/318201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Caprioli J, Bettinaglio P, Zipfel PF, Amadei B, Daina E, Gamba S, Skerka C, Marziliano N, Remuzzi G, Noris M. The molecular basis of familial hemolytic uremic syndrome: mutation analysis of factor H gene reveals a hot spot in short consensus repeat 20. J. Am. Soc. Nephrol. 2001;12:297–307. doi: 10.1681/ASN.V122297. [DOI] [PubMed] [Google Scholar]
- [24].Dragon-Durey MA, Loirat C, Cloarec S, Macher MA, Blouin J, Nivet H, Weiss L, Fridman WH, Fremeaux-Bacchi V. Anti-Factor H autoantibodies associated with atypical hemolytic uremic syndrome. J. Am. Soc. Nephrol. 2005;16:555–563. doi: 10.1681/ASN.2004050380. [DOI] [PubMed] [Google Scholar]
- [25].Jozsi M, Strobel S, Dahse HM, Liu WS, Hoyer PF, Oppermann M, Skerka C, Zipfel PF. Anti factor H autoantibodies block C-terminal recognition function of factor H in hemolytic uremic syndrome. Blood. 2007;110:1516–1518. doi: 10.1182/blood-2007-02-071472. [DOI] [PubMed] [Google Scholar]
- [26].Jozsi M, Licht C, Strobel S, Zipfel SL, Richter H, Heinen S, Zipfel PF, Skerka C. Factor H autoantibodies in atypical hemolytic uremic syndrome correlate with FHR1/FHR3 deficiency. Blood. 2008;111:1512–1514. doi: 10.1182/blood-2007-09-109876. [DOI] [PubMed] [Google Scholar]
- [27].Moore I, Strain L, Pappworth I, Kavanagh D, Barlow PN, Herbert AP, Schmidt CQ, Staniforth SJ, Holmes LV, Ward R, Morgan L, Goodship TH, Marchbank KJ. Association of factor H autoantibodies with deletions of FHR1, FHR3, FHR4, and with mutations in FH, CFI, CD46, and C3 in patients with atypical hemolytic uremic syndrome. Blood. 2010;115:379–387. doi: 10.1182/blood-2009-05-221549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Dragon-Durey MA, Fremeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Fridman W. Herman, Weiss L. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J. Am. Soc. Nephrol. 2004;15:787–795. doi: 10.1097/01.asn.0000115702.28859.a7. [DOI] [PubMed] [Google Scholar]
- [29].Licht C, Heinen S, Jozsi M, Loschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B, Zipfel PF. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II) Kidney Int. 2006;70:42–50. doi: 10.1038/sj.ki.5000269. [DOI] [PubMed] [Google Scholar]
- [30].Montes T, de Jorge E. Goicoechea, Ramos R, Goma M, Pujol O, Sanchez-Corral P, Rodriguez de Cordoba S. Genetic deficiency of complement factor H in a patient with age-related macular degeneration and membranoproliferative glomerulonephritis. Mol. Immunol. 2008;45:2897–2904. doi: 10.1016/j.molimm.2008.01.027. [DOI] [PubMed] [Google Scholar]
- [31].Pickering MC, Cook HT, Warren J, Bygrave AE, Moss J, Walport MJ, Botto M. Uncontrolled C3 activation causes membranoproliferative glomerulonephritis in mice deficient in complement factor H. Nat. Genet. 2002;31:424–428. doi: 10.1038/ng912. [DOI] [PubMed] [Google Scholar]
- [32].Jansen JH, Hogasen K, Grondahl AM. Porcine membranoproliferative glomerulonephritis type II: an autosomal recessive deficiency of factor H. Vet. Rec. 1995;137:240–244. doi: 10.1136/vr.137.10.240. [DOI] [PubMed] [Google Scholar]
- [33].Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R. A common haplotype in the complement regulatory gene factor H (HF1/FH) predisposes individuals to age-related macular degeneration. Proc. Natl. Acad. Sci. USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Edwards AO, Ritter R, 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA. Complement factor H polymorphism and age-related macular degeneration. Science. 2005;308:421–424. doi: 10.1126/science.1110189. [DOI] [PubMed] [Google Scholar]
- [36].Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA. Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005;308:419–421. doi: 10.1126/science.1110359. [DOI] [PubMed] [Google Scholar]
- [37].Richards A, Kavanagh D, Atkinson JP. Inherited complement regulatory protein deficiency predisposes to human disease in acute injury and chronic inflammatory states the examples of vascular damage in atypical hemolytic uremic syndrome and debris accumulation in age-related macular degeneration. Adv. Immunol. 2007;96:141–177. doi: 10.1016/S0065-2776(07)96004-6. [DOI] [PubMed] [Google Scholar]
- [38].Waters AM, Licht C. aHUS caused by complement dysregulation: new therapies on the horizon. Pediatr. Nephrol. 2011;26:41–57. doi: 10.1007/s00467-010-1556-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hoots WK. History of plasma-product safety. Transfus. Med. Rev. 2001;15:3–10. doi: 10.1053/tm.2001.25377. [DOI] [PubMed] [Google Scholar]
- [40].Carbone J. Adverse reactions and pathogen safety of intravenous immunoglobulin. Curr. Drug Saf. 2007;2:9–18. doi: 10.2174/157488607779315480. [DOI] [PubMed] [Google Scholar]
- [41].Sharma AK, Pangburn MK. Biologically active recombinant human complement factor H: synthesis and secretion by the baculovirus system. Gene. 1994;143:301–302. doi: 10.1016/0378-1119(94)90116-3. [DOI] [PubMed] [Google Scholar]
- [42].Sharma AK, Pangburn MK. Identification of three physically and functionally distinct binding sites for C3b in human complement factor H by deletion mutagenesis. Proc. Natl. Acad. Sci. USA. 1996;93:10996–11001. doi: 10.1073/pnas.93.20.10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sanchez-Corral P, Perez-Caballero D, Huarte O, Simckes AM, Goicoechea E, Lopez-Trascasa M, De Cordoba SR. Structural and functional characterization of factor H mutations associated with atypical hemolytic uremic syndrome. Am. J. Hum. Genet. 2002;71:1285–1295. doi: 10.1086/344515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Buttner-Mainik A, Parsons J, Jerome H, Hartmann A, Lamer S, Schaaf A, Schlosser A, Zipfel PF, Reski R, Decker EL. Production of biologically active recombinant human factor H in Physcomitrella. Plant Biotechnol J. 2010 doi: 10.1111/j.1467-7652.2010.00552.x. in press. [DOI] [PubMed] [Google Scholar]
- [45].Prosser BE, Johnson S, Roversi P, Herbert AP, Blaum BS, Tyrrell J, Jowitt TA, Clark SJ, Tarelli E, Uhrin D, Barlow PN, Sim RB, Day AJ, Lea SM. Structural basis for complement factor H linked age-related macular degeneration. J. Exp. Med. 2007;204:2277–2283. doi: 10.1084/jem.20071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jokiranta TS, Jaakola VP, Lehtinen MJ, Parepalo M, Meri S, Goldman A. Structure of complement factor H carboxyl-terminus reveals molecular basis of atypical haemolytic uremic syndrome. EMBO J. 2006;25:1784–1794. doi: 10.1038/sj.emboj.7601052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Eckart MR, Bussineau CM. Quality and authenticity of heterologous proteins synthesized in yeast. Curr. Opin. Biotechnol. 1996;7:525–530. doi: 10.1016/s0958-1669(96)80056-5. [DOI] [PubMed] [Google Scholar]
- [48].Hartner FS, Glieder A. Regulation of methanol utilisation pathway genes in yeasts. Microb. Cell Fact. 2006;5:39. doi: 10.1186/1475-2859-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Murasugi A. Secretory expression of human protein in the Yeast Pichia pastoris by controlled fermentor culture. Recent Pat. Biotechnol. 2010;4:153–166. doi: 10.2174/187220810791110679. [DOI] [PubMed] [Google Scholar]
- [50].Cereghino GP, Cregg JM. Applications of yeast in biotechnology: protein production and genetic analysis. Curr. Opin. Biotechnol. 1999;10:422–427. doi: 10.1016/s0958-1669(99)00004-x. [DOI] [PubMed] [Google Scholar]
- [51].Sahu A, Isaacs SN, Soulika AM, Lambris JD. Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J. Immunol. 1998;160:5596–5604. [PubMed] [Google Scholar]
- [52].Jokiranta TS, Westin J, Nilsson UR, Nilsson B, Hellwage J, Lofas S, Gordon DL, Ekdahl KN, Meri S. Complement C3b interactions studied with surface plasmon resonance technique. Int. Immunopharmacol. 2001;1:495–506. doi: 10.1016/s1567-5769(00)00042-4. [DOI] [PubMed] [Google Scholar]
- [53].Harris CL, Abbott RJ, Smith RA, Morgan BP, Lea SM. Molecular dissection of interactions between components of the alternative pathway ofcomplement and decay accelerating factor (CD55) J. Biol. Chem. 2005;280:2569–2578. doi: 10.1074/jbc.M410179200. [DOI] [PubMed] [Google Scholar]
- [54].Wu J, Wu YQ, Ricklin D, Janssen BJ, Lambris JD, Gros P. Structure of complement fragment C3b-factor H and implications for host protection by complement regulators. Nat. Immunol. 2009;10:728–733. doi: 10.1038/ni.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Sanchez-Corral P, Gonzalez-Rubio C, Rodriguez de Cordoba S, Lopez-Trascasa M. Functional analysis in serum from atypical hemolytic uremic syndrome patients reveals impaired protection of host cells associated with mutations in factor H. Mol. Immunol. 2004;41:81–84. doi: 10.1016/j.molimm.2004.01.003. [DOI] [PubMed] [Google Scholar]
- [56].Kavanagh D, Burgess R, Spitzer D, Richards A, Diaz-Torres ML, Goodship JA, Hourcade DE, Atkinson JP, Goodship TH. The decay accelerating factor mutation I197V found in hemolytic uraemic syndrome does not impair complement regulation. Mol. Immunol. 2007;44:3162–3167. doi: 10.1016/j.molimm.2007.01.036. [DOI] [PubMed] [Google Scholar]
- [57].Daly R, Hearn MT. Expression of heterologous proteins in Pichia pastoris: a useful experimental tool in protein engineering and production. J. Mol. Recognit. 2005;18:119–138. doi: 10.1002/jmr.687. [DOI] [PubMed] [Google Scholar]
- [58].Wiles AP, Shaw G, Bright J, Perczel A, Campbell ID, Barlow PN. NMR studies of a viral protein that mimics the regulators of complement activation. J. Mol. Biol. 1997;272:253–265. doi: 10.1006/jmbi.1997.1241. [DOI] [PubMed] [Google Scholar]
- [59].Smith BO, Mallin RL, Krych-Goldberg M, Wang X, Hauhart RE, Bromek K, Uhrin D, Atkinson JP, Barlow PN. Structure of the C3b binding site of CR1 (CD35). The immune adherence receptor. Cell. 2002;108:769–780. doi: 10.1016/s0092-8674(02)00672-4. [DOI] [PubMed] [Google Scholar]
- [60].Hocking HG, Herbert AP, Kavanagh D, Soares DC, Ferreira VP, Pangburn MK, Uhrin D, Barlow PN. Structure of the N-terminal region of complement factor h and conformational implications of disease-linked sequence variations. J. Biol. Chem. 2008;283:9475–9487. doi: 10.1074/jbc.M709587200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lorimer D, Raymond A, Walchli J, Mixon M, Barrow A, Wallace E, Grice R, Burgin A, Stewart L. Gene composer: database software for protein construct design. Codon engineering, and gene synthesis. BMC Biotechnol. 2009;9:36. doi: 10.1186/1472-6750-9-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].dAnjou MC, Daugulis AJ. A rational approach to improving productivity in recombinant Pichia pastoris fermentation. Biotechnol. Bioeng. 2001;72:1–11. doi: 10.1002/1097-0290(20010105)72:1<1::aid-bit1>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- [63].Ramon R, Ferrer P, Valero F. Sorbitol co-feeding reduces metabolic burden caused by the overexpression of a Rhizopus oryzae lipase in Pichia pastoris. J. Biotechnol. 2007;130:39–46. doi: 10.1016/j.jbiotec.2007.02.025. [DOI] [PubMed] [Google Scholar]
- [64].Huo X, Liu Y, Wang X, Ouyang P, Niu Z, Shi Y, Qiu B. Co-expression of human protein disulfide isomerase (hPDI) enhances secretion of bovine follicle-stimulating hormone (bFSH) in Pichia pastoris. Protein Expr. Purif. 2007;54:234–239. doi: 10.1016/j.pep.2007.03.016. [DOI] [PubMed] [Google Scholar]
- [65].Ferreira VP, Herbert AP, Cortes C, McKee KA, Blaum BS, Esswein ST, Uhrin D, Barlow PN, Pangburn MK, Kavanagh D. The binding of factor H to a complex of physiological polyanions and C3b on cells is impaired in atypical hemolytic uremic syndrome. J. Immunol. 2009;182:7009–7018. doi: 10.4049/jimmunol.0804031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Schmidt CQ, Herbert AP, Mertens HD, Guariento M, Soares DC, Uhrin D, Rowe AJ, Svergun DI, Barlow PN. The central portion of factor H (modules 10-15) is compact and contains a structurally deviant CCP module. J. Mol. Biol. 2010;395:105–122. doi: 10.1016/j.jmb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Jouvin MH, Kazatchkine MD, Cahour A, Bernard N. Lysine residues, but not carbohydrates, are required for the regulatory function of H on the amplification C3 convertase of complement. J. Immunol. 1984;133:3250–3254. [PubMed] [Google Scholar]
- [68].Abrera-Abeleda MA, Nishimura C, Smith JL, Sethi S, McRae JL, Murphy BF, Silvestri G, Skerka C, Jozsi M, Zipfel PF, Hageman GS, Smith RJ. Variations in the complement regulatory genes factor H (FH) and factor H related 5 (FHR5) are associated with membranoproliferative glomerulonephritis type II (dense deposit disease) J. Med. Genet. 2006;43:582–589. doi: 10.1136/jmg.2005.038315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fremeaux-Bacchi V, Kemp EJ, Goodship JA, Dragon-Durey MA, Strain L, Loirat C, Deng HW, Goodship TH. The development of atypical HUS is influenced by susceptibility factors in factor H and membrane cofactor protein-evidence from two independent cohorts. J. Med. Genet. 2005;42:852–856. doi: 10.1136/jmg.2005.030783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Vervecken W, Callewaert N, Kaigorodov V, Geysens S, Contreras R. Modification of the N-glycosylation pathway to produce homogeneous. Human-like glycans using GlycoSwitch plasmids. Methods Mol. Biol. 2007;389:119–138. doi: 10.1007/978-1-59745-456-8_9. [DOI] [PubMed] [Google Scholar]
- [71].Young TS, Ahmad I, Brock A, Schultz PG. Expanding the genetic repertoire of the methylotrophic yeast Pichia pastoris. Biochemistry. 2009;48:2643–2653. doi: 10.1021/bi802178k. [DOI] [PubMed] [Google Scholar]
- [72].Aslam M, Perkins SJ. Folded-back solution structure of monomeric factor H of human complement by synchrotron X-ray and neutron scattering. Analytical ultracentrifugation and constrained molecular modelling. J. Mol. Biol. 2001;309:1117–1138. doi: 10.1006/jmbi.2001.4720. [DOI] [PubMed] [Google Scholar]
- [73].Lachmann PJ. The amplification loop of the complement pathways. Adv. Immunol. 2010;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.