Abstract
Enterohepatic Helicobacter species (EHS) often are associated with typhlocolitis and rectal prolapse in mice. We sought to describe rectal prolapses histologically, relate lesions to mouse genotype and EHS infection status, and characterize EHS pathogens on our campus. Our mouse population was housed among 6 facilities on our main campus and a seventh, nearby facility. We investigated cases of rectal prolapse over 1 y and included 76 mice, which were broadly categorized according to genotype. Microscopically, lesions ranged from mild to severe typhlocolitis, often with hyperplastic and dysplastic foci. Neoplastic foci tended to occur at the ileocecal–colic junction. Lesions were most severe in strains that had lower-bowel inflammatory disease, notably IL10, Rag1, and Rag2 knockout strains; prolapses occurred in these strains when housed both in areas with endemic EHS and in our Helicobacter-free barrier facility. Most mice with rectal prolapses were immunocompromised genetically modified mice; however, the most frequently sampled strain, the lamellipodin knockout, was noteworthy for its high incidence of rectal prolapse, localized distal colonic and rectal lesions, and lack of known immunodeficiency. This strain is being explored as a model of rectal carcinoma. Most of the colons examined tested PCR-positive for EHS, often with coinfections. Although H. bilis is prevalent on our campus, we did not find this organism in any mice exhibiting clinical signs of rectal prolapse. Identification of H. apodemus in 22% of cases has fueled increased surveillance on our campus to characterize this organism and differentiate it from the closely related H. rodentium.
Abbreviations: EHS, enterohepatic Helicobacter species; IBD, inflammatory bowel disease; RFLP, restriction-fragment–length polymorphism; RP, rectal prolapse
Rectal prolapse (RP) occurs commonly in laboratory mice and is often associated with lower-bowel inflammation. Mice have a relatively short and poorly supported distal colon, which lacks a serosal covering.30 This anatomic weakness, coupled with a microbial insult, toxic injury, or space-occupying neoplastic masses within the gastrointestinal tract, are the predisposing factors for tenesmus and RP (Figure 1). In the context of microbial insults, the pathogenesis involves diffuse or multifocal inflammation in the more proximal segments of colon or distal colon, which can result in thickened edematous tissue and tenesmus, triggering a prolapse.6,30,40 Bacteria most often associated with this condition are the enterohepatic Helicobacter species (EHS) and Citrobacter rodentium; although in theory any pathogenic bacteria causing colitis may predispose mice to RP.1,11,13,38
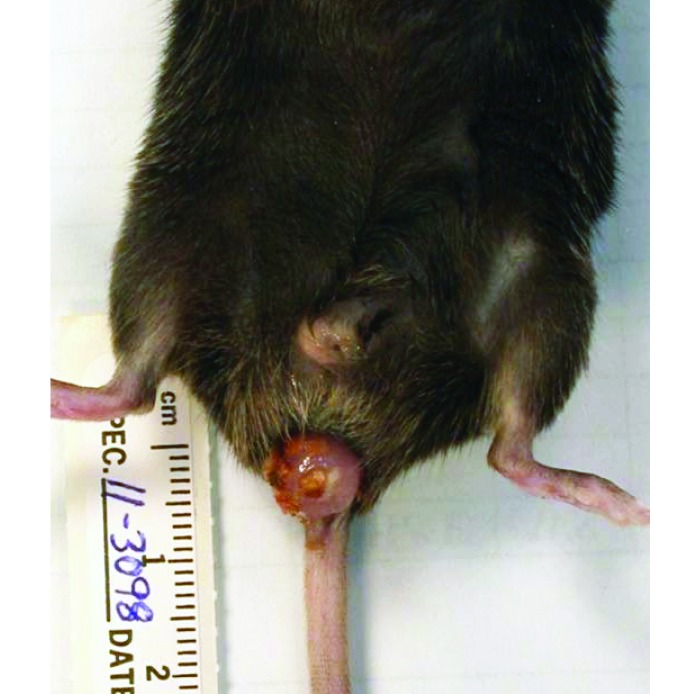
Figure 1.
Mouse rectal prolapse. An example of the clinical presentation of rectal prolapse in laboratory mice. Note the attachment of bedding and nesting material in the film of mucous that frequently is seen covering the exposed rectal tissue. Generally the tissue becomes severely erythematous, as can be appreciated in this photograph.
Although the clinical presentation of RP may occur in immunocompetent mice, it is most often associated with mice that have a spontaneous or transgenic mutation causing immunodeficiency.11,13,38 Indeed, these naturally occurring murine pathogens are used to model inflammatory bowel disease in strains that are highly susceptible to typhlocolitis with EHS infection; examples include Il10−/− and Rag-deficient mice.3,5,8,9,13,16,19,20,22,40 In addition, H. hepaticus and other EHS including H. typhlonius, H. rodentium, and H. bilis, which are known to persistently colonize the intestinal crypt of the lower bowel, have been shown to induce colitis-associated cancer in susceptible immunodeficient strains of mice.4,7,9,23,24,27,29,31
In 1999, our institution introduced a rodent importation policy to reduce the introduction of murine pathogens. As part of this program, all approved commercial vendors were screened to ensure animals were SPF for EHS. Any random-source mice (typically imported from other academic institutions for collaborative projects) were required to be rederived by embryo transfer. In comparing PCR data between 1999 (prior to implementing the ET policy) and 2009, we found that after more than a decade of strict rederivation and husbandry practices that reduce fecal–oral transmission, EHS prevalence was markedly reduced.21 Despite this success, these practices did not completely eradicate rodent EHS. Of particular note, 2 facilities on campus house well-established long-term breeding colonies, many of which are unique transgenic lines with various immunodeficiencies, that are used primarily for immunology and cancer research. Rederivation of each of these strains was considered to be cost-prohibitive; thus EHS has remained endemic in these breeding colonies for more than a decade, as evident by our recent surveillance for EHS prevalence.21 The species known to be prevalent on our campus prior to the current study included H. hepaticus, H. rodentium, H. typhlonius, and H. bilis; in a few isolated areas, H. mastomyrinus was identified also.21
Although EHS infections often are subclinical, we sought to correlate the presence of EHS-endemic areas with clinical lower-bowel inflammation (evident by rectal prolapse). In this survey of laboratory mice at our institution, we identified patterns in mouse strain susceptibility to RP, RP association with EHS, and histopathologic findings and correlated specific EHS species with clinical disease. Because we sought to study spontaneous infections, we excluded any mice on study with experimentally induced inflammatory bowel disease (IBD), including Helicobacter-induced IBD and chemically induced colitis models.
From July 2011 to July 2012, a total of 63 mice with RP from these 6 facilities at our institution were necropsied as part of this investigation. In addition, 13 mice with RP were identified at a nearby research institute housing mice known to have endemic EHS.
Materials and Methods
Animals.
Over a 1-y period, mice with rectal prolapse at our institution (Massachusetts Institute of Technology, Cambridge, MA) initially were identified through our laboratory animal health monitoring system. The standard endpoint criteria for clinical rectal prolapse cases were exposed tissue of 0.5 cm or more, declining body condition (according to a 5-point scoring system), hemorrhage, concurrent diarrhea, or other concurrent nonspecific signs such as rough haircoat, dehydration, hunched posture, and inactivity. Mice sometimes were donated by investigators prior to reaching these endpoints; otherwise, animals were donated when endpoint criteria were reached. Excluded pathogens in MIT facilities include most known parasitic, viral, and bacterial mouse pathogens (as determined by our dirty-bedding sentinel program), although mouse norovirus is not included routinely in our surveillance program. Excluded agents include Sendai virus, pneumonia virus of mice, mouse hepatitis virus, mouse parvoviruses, reovirus, epizootic diarrhea of infant mice, mouse encephalomyelitis virus, ectromelia virus, lymphocytic choriomeningitis virus, murine adenovirus, murine cytomegalovirus, K virus, polyoma virus, cilia-associated respiratory bacillus, Mycoplasma spp. and endo- and ectoparasites. In addition, the respiratory and gastrointestinal tracts are cultured semiannually to detect any bacterial pathogens, including C. rodentium. With the exception of one barrier facility, mice are not maintained as Helicobacter-free.
Mice housed at the nearby research institute (facility G) have an identical surveillance program, and pinworms were noted during a testing period concurrent with this survey. Mice were treated with antihelminthics at that time and have been free of pinworms since completion of the treatment.
All donated animals included in the current study had been used humanely on protocols approved by the Massachusetts Institute of Technology Committee on Animal Care or the IACUC of the nearby research institute. The use of the mice pertaining to the current project occurred postmortem.
Mice at our institution are housed in both static microisolation and IVC systems containing heat-treated hardwood bedding (Beta Chip, Nepco, Warrensburg, NY). The standard pelleted diet for all facilities is RMH 3000 (Purina Mills, Richmond, IN), and water is either filtered or prepared by reverse osmosis. When cages are changed, mice are handled with sanitized forceps (Quatracide PV, Pharmacal Research Laboratories, Naugatuck, CT), and animal caretakers provide husbandry from rooms of known EHS-free status to those with mice of unknown status or housing known-contaminated mice. This clean-to-dirty traffic flow applies to both personnel and equipment.
Necropsy.
Mice were euthanized and submitted for a complete necropsy and specimen processing through the animal histopathology laboratory of our institution. Liver and gastrointestinal tract samples were collected and stored at −20 °C (for PCR assays), at −80 °C (culture), or in formalin (histology) at room temperature. Samples of small intestine were prepared as strips representing each region (duodenum, jejunum, and ileum), and in a separate paraffin block, the entire colon was prepared with the cecum. Samples of these regions for PCR and culture assays consisted of 1.0- to 1.5-mm segments of tissue from the distal tip of the cecum and from the colon approximately 1.5 cm distal to the ileocecocolic junction. Sections of the rectum used for these assays included a 1-mm full-thickness wedge section of the prolapsed portion. Parasitologic testing included purified sodium nitrate floatation (Fecasol, Vetoquinol, Fort Worth, TX), anal tape tests, and direct smears of cecal contents. For all mice, histologic submissions included liver, the entire gastrointestinal tract from stomach to rectum, and mesenteric lymph node.
Histopathology.
Gastrointestinal and liver tissues were formalin-fixed, processed, and embedded in paraffin; 5-µm sections were stained with hematoxylin and eosin. Slides were evaluated in a blinded fashion by a board-certified veterinary pathologist. Lesions in different regions of the gastrointestinal tract and liver were assessed qualitatively for characteristics consistent with murine colitis including inflammation, epithelial defects, edema, crypt atrophy, hyperplasia, and dysplasia/neoplasia.32 To specifically delineate the extent of inflammation in different segments of the intestine, pathologic assessments were done at 4 separate locations (cecum with ileocecocolic junction, proximal and transverse colon, distal colon, and rectum). Criteria for malignancy, when relevant, were determined by using guidelines recommended by the Mouse Models of Human Cancers Consortium's consensus report on colorectal neoplasia.2
Helicobacter PCR and RFLP analysis.
DNA was extracted (High Pure PCR Template Preparation Kit, Roche Applied Science Indianapolis, IN) from tissue samples (colonic, cecal, and rectal) according to manufacturer instructions. Genus-level Helicobacter spp. primers (forward, C97; reverse, C05)12 were used to amplify a 1.2-kb PCR product, which was detected by electrophoresis (on 1% agarose gel) and ethidium bromide staining followed by visualization with UV light. Positive samples were analyzed by using HhaI and AluI restriction-fragment–length polymorphism (RFLP) as previously described.35 RFLP patterns were confirmed using PCR assays with species-specific primers for H. hepaticus,34 H. typhlonius,10 H. rodentium,36 H. bilis,14 and H. mastomyrinus,37 with positive controls consisting of DNA extracted from pure cultures of each Helicobacter species. Additional primers to distinguish H. rodentium and H. apodemus were designed for the current study. The sequences of all primers used are listed in Table 1.
Table 1.
Primers used for species-specific EHS PCR assays
| Species | Forward primer | Reverse primer | Reference |
| H. hepaticus | 5′ GCAUUUGAAACUGUUACUCUG 3′ | 5′ GGGGAGCUUGAAAACAG 3′ | 34 |
| H. bilis | 5′ AGAACTGCATTTGAAACTACTTT 3′ | 5′ GGTATTGCATCTCTTTGTATGT 3′ | 14 |
| H. typhlonius | 5′ AGGGACTCTTAAATATGCTCCTAGAGT 3′ | 5′ ATTCATCGTGTTTGAATGCGTCAA 3′ | 10 |
| H. rodentium | 5‘ GTCTT AGT TGC TAA CTA TT 3′ | 5′ AGA T'IT GCT CCA TTT CAC AA 3′ | 36 |
| H. rodentium* | 5′ GTGGAGTGCTAGCTTGCTAGAA 3′ | 5′ ACCGTAGCATAGCTGATCTA 3′ | current study |
| H. apodemus | 5′ TGGGAGTGCCCTTTTAGGGAG 3′ | 5′ TGAGATTTGCTCCATTTCAC 3′ | current study |
| H. mastomyrinus | 5′ AGAACTGCATTTGAAACTATGAG 3′ | 5′ CAGTATTGCGTCTCTTTGTA 3′ | 35 |
The H. apodemus-specific primers and H. rodentium* primers were designed for the current study and used to distinguish H. rodentium from H. apodemus.
Culture.
Fresh tissue and fecal samples were collected aseptically at necropsy and stored at −80 °C in Brucella broth with 15% glycerol. After thawing and homogenization, tissue slurries were gently passed through 0.45-µm syringe filters for culture on 5% sheep blood agar plates (Remel Laboratories, Lenexa, KS). In addition, unfiltered samples were cultured onto cefoperazone-, vancomycin-, and amphotericin B-impregnated selective media (CVA plates, BD, Franklin Lakes, NJ). All samples were prepared in duplicate and cultured at 37 °C in microaerobic conditions of N2, H2, and CO2 (80:10:10). Plates were monitored frequently for growth for as long as 4 wk. Any growth with visible characteristics suggestive of EHS was harvested for additional PCR assays or 16S rRNA sequencing.
16S rRNA sequencing.
Because the organisms in some samples could not be identified solely by species-specific PCR (mainly due to coinfections that created complex RFLP patterns), additional analysis was done by 16S rRNA gene sequencing. Either pure isolates (cultured form cecal contents) or PCR products from the C05 and C97 genus-level primers (purified by using the QIAquick PCR purification kit; Qiagen, Valencia, CA) were sequenced by using the BigDye Terminator Cycle Sequencing Kit (version 3.1, Applied Biosystems, Foster City, CA) on a genetic analyzer (model 3500, Applied Biosystems). Results then were compared with data in the NCBI GenBank nucleotide database by using a BLAST search (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
Necropsy and RP prevalence.
Six rodent facilities on our campus and a seventh facility at a research institution adjacent to our campus (facility G) were included in this study. Mice were screened for pinworms at the time of necropsy, because these common helminth infections have been implicated in RP.39 All mice from our institution were negative by both anal tape tests and direct examination of cecal contents. However, a subset of mice from facility G was infected with pinworms, according to both direct cecal exam and histopathology (4 of 13 mice; all with Aspiculuris teraptera).
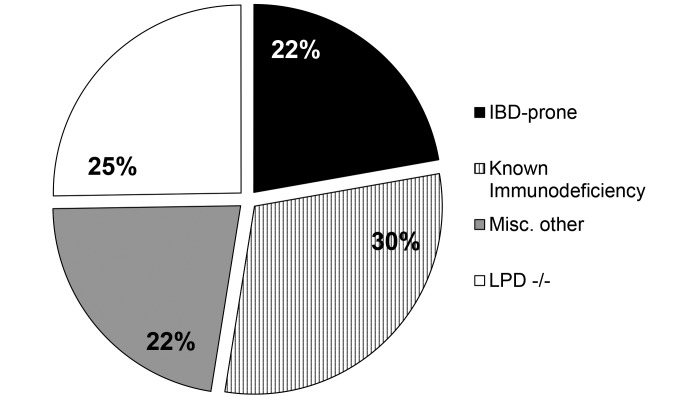
RP occurred in diverse mouse genotypes, predominantly on either C57BL/6 or 129 backgrounds. Because repeat occurrences of RP within a mouse strain were rare, it is difficult to assess data in terms of strain susceptibility. An exception to this difficulty was the high prevalence of RP in lamellipodin (Lpd)-deficient mice (C57BL/6-Raph1tm1Fbg), which were housed in facility D and comprised 25% of all RP cases. For the remaining mice with RP, strains were broadly categorized according to their immune profile (Figure 2). The largest group of mice had some form of a known immunodeficiency; the variety of deficiencies is too extensive to list but included mutations in components such as T-cell receptors, p53, Kras, and MHC proteins. A second group of immunodeficient mice was differentiated because of their known susceptibilities to EHS-induced disease and their use as models of IBD and lower-bowel cancer; the majority of these mice (14 of 18) were homozygous null for IL10 (although the genetic background varied and was either 129 or B6). In some cases, mice lacking Il10 had additional mutations, such as Rag2−/−,OPN−/−,Tlr2−/−, and ApcMin. The final group was categorized as ‘miscellaneous;’ these mice had no known immunodeficiency or other propensity to develop lower-bowel inflammatory disease and represented a wide range of genotypes. Although the age of affected mice varied widely across all groups, mice tended to be older; the mean age of mice with RP was 7 mo, with a range of 2 to 24 mo. This propensity occurred even in the genotypically uniform groups of Lpd−/− and IBD-prone mice, for which age at RP presentation was quite variable.
Figure 2.
Classification of mouse strains with RP. In general, there were very few repeat occurrences of RP in a single strain of mice. The one exception was Lpd −/− mice, which accounted for approximately 25% of all cases. The remaining mice were broadly categorized into 3 primary groups: strains prone to inflammatory bowel disease (IBD-prone), those with known immunodeficiencies, and a miscellaneous group.
Histopathology.
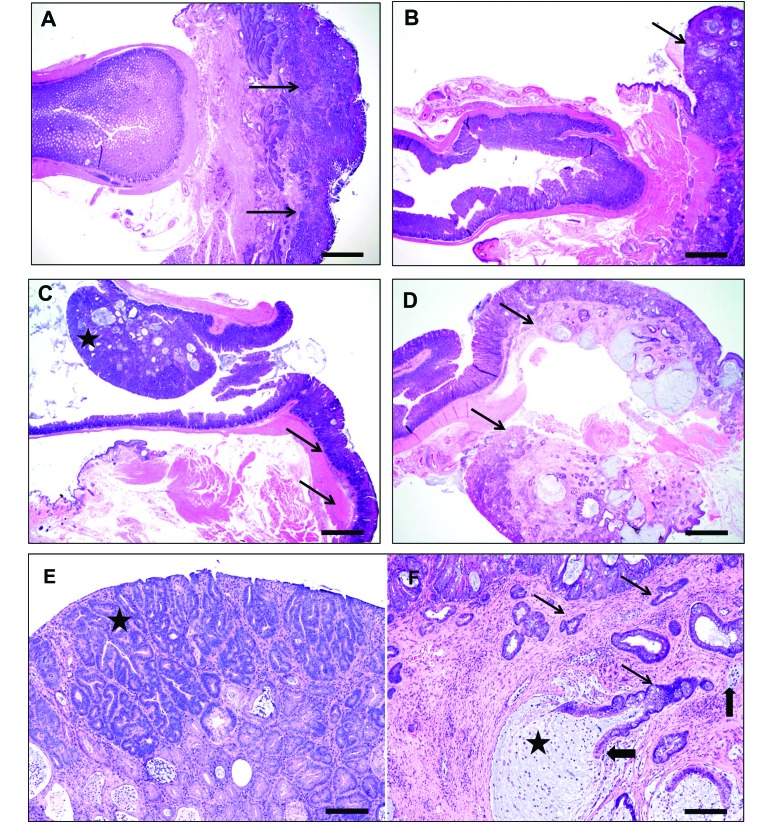
The findings from qualitative histologic assessment of the lower bowel were generalized for each of the 4 groups of mice described earlier, and representative images from each group are shown in Figure 3.
Figure 3.
Representative histology from categories of RP mice. (A) Distal gastrointestinal tract of an IL10−/− mouse, with prolapsed rectum (arrows) showing severe mucosal inflammation, ulcerations with marked glandular hyperproliferation, and dysplasia. Note the patchy multifocal inflammatory aggregates in the nonprolapsed distal colon. (B) Distal colon and prolapsed colorectum of a mouse from the immunocompromised group, showing prominent inflammation, glandular hyperplasia, distortion, and mucin-filled cystic glands in the prolapsed segment (arrow) and milder changes in the nonprolapsed distal colon. (C) Rectum of a mouse in the miscellaneous group, showing a distal colonic polypoid adenoma (star) and associated prolapsed segment (arrow) with inflammation, epithelial tethering, and hyperplasia. (D) Rectal prolapse (with junctional distal colon) from an Lpd−/− mouse, showing mucosal erosions, inflammation, and prominent hyperplastic, dysplastic and mucous-filled cystically distended invasive glands (arrow). Note that the lesions are mostly within the prolapsed segment and that the adjacent nonprolapsed colon is relatively unaffected. (E) Higher magnification of the mouse in panel C, showing darker staining adenomatous tubules (star) in superficial half arising from a background of hyperplastic, dysplastic, and cystic tubules with moderate stromal inflammation. (F) Higher magnification of the mouse in panel D, showing multiple irregular angular horizontally spreading proliferating dysplastic glands (thin arrows) deep in the submucos and musculature with nuclear atypia and piling and budding in a thickened stroma by fibrosis and inflammation. Frequently, the invasive cystic glands are filled with mucous (star) and have either partial or complete cell loss (thick arrows) from the lining epithelium and intaluminal cellular debris. Hematoxylin and eosin stain; bar, 400 µm (A through D); 80 µm (E and F).
The Lpd -deficient mice had unique lesions, which were unlike typical EHS-induced IBD. Although RP typically presented with inflammation spanning most of the colon and cecum, the lesions in Lpd −/− mice were localized predominantly to the rectum and rectocolic junction, with extension into the immediate adjacent distal colon, but lacked a florid typhlocolitis. The prolapsed rectum was characterized by moderate to severe mixed inflammation consisting of neutrophils, macrophages, lymphocytes, and eosinophils, as well as epithelial erosions and ulcerations, prominent glandular hyperproliferation, and severe dysplasia or neoplasia. More than half of Lpd −/− mice with RP developed carcinoma of the rectum.
The IBD-prone group of mice exhibited lesions representative of EHS models of IBD, with generally moderate to severe typhlocolitis with diffuse lymphoplasmacytic, histiocytic, and granulocytic inflammation; glandular abscessation; epithelial defects and hyperplasia; and erosive proctitis.
The largest group of mice with RP—those with known immunodeficiencies but not previously established IBD models—had lesions that were similar to but generally less severe than those of the IBD-prone group. Similar results were seen in the miscellaneous, immunocompetent group.
Beyond the Lpd -deficient group, neoplastic lesions were rare (4 mice in the immunocompromised group). However, when appropriate, this morphologic diagnosis was assigned according to previously established criteria outlined for mouse models of lower-bowel cancers.2 These 4 mice varied in their genetic mutations: a 1-y-old male mouse with a conditional Kras mutation (B6.129S4-Krastm4Tyj/J); a 6-mo-old male mouse that was a cross between Rag2−/− and CTLA4−/− and that was congenic for CD45.1 on a C57BL/6 background; another male mouse of unknown age with the Rag2 knockout crossed onto the congenic CD45.1 locus on the C57BL/6 background; and a male mouse of unknown age with a transgenic T-cell receptor mutation (C57BL/6-Tg(TcraTcrb)1100Mjb/J). Multisystemic lymphoma was a frequent finding, was judged to be unrelated to any typhlocolitis, and occurred in 8 mice (7 in the immunocompromised group, and a Il10−/−Rag2+/– mouse in the IBD-prone group).
All livers were examined in light of the ability of H. hepaticus and H. bilis to colonize the liver and the propensity of H. hepaticus to cause hepatitis or hepatocellular carcinoma in susceptible strains of mice. Across all groups of mice, hepatic lesions were rare to nonexistent, with only background levels of inflammation.
Speciation of Helicobacter organisms detected in RP mice.
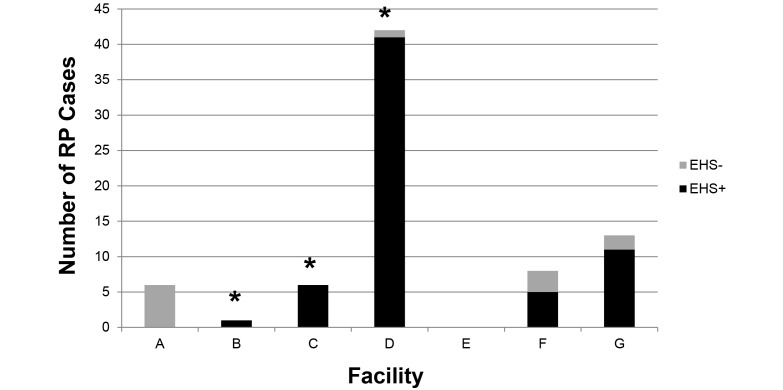
Colon and cecal tissues of all RP mice were initially evaluated for the Helicobacter 16S rRNA gene by PCR analysis. A large majority of all total cases (64 of 76, 84%) was Helicobacter-positive. Figure 4 shows the distribution of these positive cases by each facility. None of the RP cases detected in the barrier facility tested positive for EHS. These cases primarily consisted of the mice in the IBD-prone group of strains, and this area primarily housed breeding colonies of these strains, such as Il10-deficient and Rag-deficient mice. Facility G is not maintained as EHS-free but did house Il10−/− mice that were identified as RP cases in this study.
Figure 4.
EHS infection status of RP mice by facility. Total number of RP cases from each facility are shown by EHS infection status. Of note, none of the mice with RP in facility A were EHS-positive; this finding is expected given that facility A is an SPF barrier for EHS. Mouse colonies of facility D were moved to a newly built vivarium in 2011, but the population of mice is the same. The majority of cases originated from facility D; this finding was not unexpected because this is the largest rodent facility on the MIT campus, it houses primarily immunodeficient or neoplasia-prone strains, and it is home to the Lpd–/– strain which appears to be uniquely susceptible to RP. In addition, many of the colonies kept in this facility predate the rederivation importation policy at MIT; thus EHS have been endemic in these colonies for at least 15 y. No RP cases were reported in facility E; this facility typically has a low prevalence of EHS and was negative in our 2009 surveillance. Facility G is a nearby research institute and not a part of the MIT campus. The majority of RP cases in the remaining facilities were EHS-positive; those that were EHS-negative were transgenic mice with deficiencies in innate immunity known to predispose the mice to intestinal inflammation. Asterisks indicate that this facility was known to be positive for at least one EHS species in our 2009 survey.
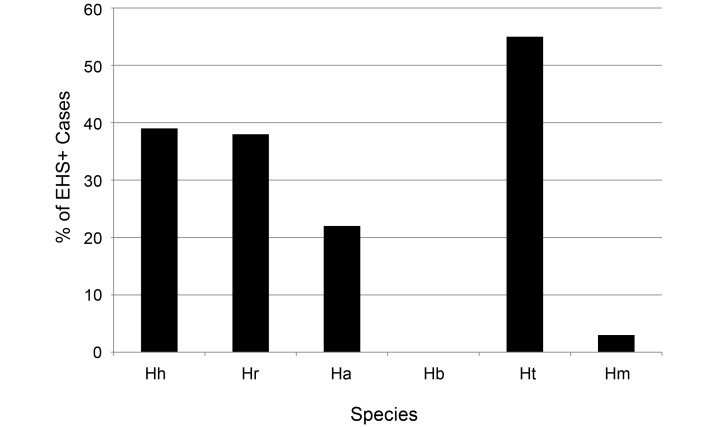
After the initial genus-specific PCR, positive EHS samples were assessed by using RFLP analysis, species-specific PCR assays, microaerobic culture, and 16S rRNA gene sequencing. Results of Helicobacter speciation are presented in Figure 5. Notably, although H. bilis was known to be present in at least 3 of the facilities according to past surveillance, no mice with clinically apparent RP tested positive for H. bilis. The most prevalent species was H. typhlonius. After the identification of 3 potential H. apodemus isolates by 16S rRNA gene sequencing, which is known to crossreact with the primers used in our H. rodentium-specific PCR assay, we designed 2 new sets of primers to distinguish H. rodentium from H. apodemus, and all H. rodentium-positive samples were retested by using the new primers. This revealed a larger percentage of H. apodemus-infected mice than previously known; interestingly only 2 samples were monoinfected with H. apodemus, whereas those remaining were coinfected with other EHS.
Figure 5.
Percentage of EHS-positive RP mice infected with each Helicobacter species. This speciation shows which organisms were most commonly associated with RP; 30% of mice had coinfections with 2 or more EHS species. The most common species to infect these mice was H. typhlonius; this figure may actually be higher than that shown because the Lpd colony had 100% prevalence of this organism, and this mouse strain made up the largest single population of mice in this investigation.
Table 2 indicates the distribution of EHS in each facility included in this study, as well as a comparison of how the data collected in this study differ from 2009 surveillance results. Although facilities A and E remained negative over these 2 periods, facility F appears to have acquired new EHS during these 3 y. However, because only 2 of the 12 holding rooms in that facility were surveyed in 2009, it is certainly plausible that facility F may have been EHS-positive at that time. In addition, note the lack of H. bilis in RP cases housed in facilities where this organism is known to be endemic and the detection of H. apodemus, which previously was unreported at our facilities.
Table 2.
Comparison of EHS species detected in each facility between 2009 surveillance and 2012 clinical cases
| Facility | No. of rooms tested in 2009 | Species detected by surveillance in 2009 | Species detected in RP cases in 2012 |
| A | 14 | none | none |
| B | 5 | Hb, Hr | Hr, Ha |
| C | 19 | Hb, Hr, Ht, Hh | Hr, Ha, Ht, Hh, Hma |
| D | 14 | Hb, Hr, Ht, Hh, Hm | Hr, Ha, Ht, Hh, Hm |
| E | 3 | none | none |
| F | 2 | none | Hr, Ha, Ht |
| G | not applicable | not applicable | Hr, Ha, Ht, Hh |
Ha, H. apodemus; Hb, H. bilis; Hh, H. hepaticus; Hm, H. mastomyrinus; Hr, H. rodentium; Ht, H. typhlonius
Hm was not detected in our clinical RP cases in facility C, but Hm was detected during our 2011–2012 investigation of clincally normal animals from the Lpd colony. Hm detection differed between 2009 and 2012 for this facility, which may represent new infection but also could indicate poor transmission to sentinels and lower overall prevalence.
A comparison of EHS prevalence based on fecal PCR assays of dirty-bedding sentinels in 2009 shows a different EHS distribution from that detected in clinical RP cases in 2012. Facility G was not part of the 2009 survey, but speciation results are included here for comparison of EHS distribution.
Discussion
It is difficult to determine the exact incidence of spontaneous RP on our campus. First, cage census, number of animals housed per cage, and the average duration of a given mouse in a facilities (and thus the time to become infected and develop inflammation) are all highly variable. Second, we relied on the donation of mice for necropsy. Although the majority of mice were donated to or shared with the Division of Comparative Medicine for necropsy purposes, some of the mice identified through our health-check system were valuable animals on study and were required by the researcher. Therefore, a small subset of mice with RP from our campus was not included in this survey. According to the average census and total number of cases of RP per year at our institution, we estimate incidence to be 0.019% of the mouse population. In addition, the severity of RP lesions is difficult to compare from mouse to mouse, because some may have had chronic typhlocolitis prior to RP whereas others may have had a more acute presentation of RP. In addition, as part of our clinical assessment, very mild prolapses typically are monitored, and the mouse is required to be euthanized only once the prolapse is large (0.5 cm or more) or when the mouse begins losing weight or presents with other clinical signs. As a result, the progression of lesions is difficult to compare between mice. Despite these limitations, this survey did reveal interesting trends and provides new insights into naturally occurring EHS-related disease in mouse colonies. Our most noteworthy findings were the identification of the highly susceptible Lpd −/−mouse, the lack of H. bilis-related RP, and the identification of H. apodemus in EHS-associated RP.
We screened all mice included in this study for pinworms, because several older reports have implicated these parasites in the pathogenesis of RP.39 However, these early reports predated the isolation and characterization of EHS and C. rodentium, and the mice would not have been screened for these agents. As our knowledge of rodent pathogens has grown during the past several decades and because both the bacterial and parasitic agents remain prevalent, characteristics of recent outbreaks have shown that pinworms are unlikely to be associated with RP. In our study, pinworms were identified only in 4 mice from facility G; these mice were also positive for EHS. Importantly, all RP mice housed on our campus were free of pinworms.
We assessed a total of 76 RP cases in mice over a 1-y period. These cases were divided into 4 broad categories: IBD-prone, other immunocompromised mice, Lpd -deficient mice, and miscellaneous strains not known to be immunocompromised. Predictably, the pathology in most of these mice was a diffuse typhlocolitis of varying degrees, with the most severe cases generally in the IBD-prone group. During this study, we identified a transgenic mouse strain, the lamellipodin knockout (Lpd −/−), which had the highest incidence of RP among the strains of mice housed at our institution and which accounted for 25% of the RP cases we report here. Lamellipodin is an Ena/VASP ligand and plays a critical role in regulation of actin dynamics to form membrane projections.18 Since this survey, we have rederived this strain by embryo transfer; the resulting mice do not exhibit RP in the absence of Helicobacter spp.25 This strain is being characterized further; currently we speculate that the absence of Lpd impairs epithelial integrity, which allows EHS to induce a profound inflammatory response with subsequent neoplastic transformation.25
Another observation based on our identification of the RP phenotype in the Lpd −/− mouse strain is that the colony was fairly homogenous in terms of EHS infection. H. typhlonius occurred with 100% prevalence in this group of mice, and approximately half of the mice were coinfected with H. hepaticus. The large number of RP cases from this colony therefore had a profound effect on the overall number of H. typhlonius-positive animals in this survey. In the few other instances of RP occurring in the same strain of mouse (usually from the same investigator), the groups of 2 or 3 also shared the same EHS profile. Interestingly, one additional case of RP was reported from the holding room housing the Lpd−/− mice. The affected mouse belonged to a different investigator and was infected with H. rodentium and H. apodemus. This pattern again implies strength in husbandry practices—that is, endemic organisms appear to have stayed confined to their respective colonies.
RP did not occur exclusively in EHS-infected mice: an etiologic agent was not defined in 12 (16%) mice with RP. However, most of these mice were in the IBD-prone category, and this figure includes all of the RP mice found in our SPF barrier facility. We speculate that given the innate deficiencies, any minor insult that causes a slight dysbiosis can shift the intestinal microenvironment such that an overwhelming inflammatory response occurs and triggers prolapse. Typhlocolitis certainly occurs due to microbes that are more typically considered commensal when alterations in the microbiome occur in the appropriate host.28,33
Although H. bilis is prevalent on our campus, no mice with RP were colonized with this organism, either as a monoinfection or as a coinfection. This result may imply that H. bilis, or specific strains of H. bilis, may be less virulent than are the other common rodent EHS that were detected in many of the mice examined in this study. However, numerous IBD models in the literature indicate that H. bilis is a significant factor in IBD development, and these findings discount this hypothesis in part.23,26,29,41 Perhaps the combination of mutant mouse strain, commensal flora, and exposure to H. bilis has not occurred in mice housed at our institution to implicate H. bilis in RP pathogenesis. Alternatively this finding may be a testament to husbandry and management practices that serve to minimize EHS transmission—that is, although the organism is present in mice in some areas, it does not appear to have been transmitted to any susceptible mice.
Another significant observation from this study was the large number of mice infected with the organism known as H. apodemus. Reports on this organism are infrequent; furthermore, it is not a formally named member of the Helicobacter genus. To date, H. apodemus has been named informally on the basis of its isolation from wild striped field mice (Apodemus agrarius) in Korea.15 Interestingly, an organism with this same 16S rRNA sequence has been detected in a laboratory mouse in Sweden.17
Unfortunately, because most of the H. apodemus infections that we detected were in mice coinfected with additional Helicobacterspecies, it is difficult to draw any interpretations about the pathogenicity of H. apodemus. However, we did find this organism in 2 mice with RP in which no other EHS were detected: one mouse in the miscellaneous category, and another in the immunocompromised category. In addition, 6 of the 14 cases infected with H. apodemus occurred in facility G, an institution with less stringent importation requirements than those at our institution. This finding suggests that H. apodemus may be an important emerging disease-causing agent in mouse colonies housed at research institutions.
The significance of the H. apodemus organism in laboratory mice remains to be determined. Isolates of H. apodemus from several laboratory mice will allow further characterization of this organism. Because H. apodemus 16S rRNA anneals to our standard species-specific primers used for H. rodentium, perhaps the former has a significant prevalence on our campus but has been misdiagnosed as H. rodentium. Additional surveillance of mice housed at our institution to attempt detection of H. apodemus, differentiate this organism from H. rodentium, and explore any differences in virulence and host susceptibility is underway.
In conclusion, this study documents the association of various EHS with the clinical presentation of RP. This association is particularly prevalent among mice with known or suspected immune deficiencies. The presence of RP in mice used in research or testing should prompt diagnostic testing to ascertain whether EHS are present in the affected animals.
Acknowledgments
This work was supported by NIH R01 OD011141 (JGF), NIH T32 OD010978 (JGF), and NIH P30 ES002109 (JGF).
References
- 1.Barthold SW, Coleman GL, Jacoby RO, Livestone EM, Jonas AM. 1978. Transmissible murine colonic hyperplasia. Vet Pathol 15:223–236 [DOI] [PubMed] [Google Scholar]
- 2.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, Itzkowitz SH, Groden J, Coffey RJ. 2003. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology 124:762–777 [DOI] [PubMed] [Google Scholar]
- 3.Cahill RJ, Foltz CJ, Fox JG, Dangler CA, Powrie F, Schauer DB. 1997. Inflammatory bowel disease: an immunity-mediated condition triggered by bacterial infection with Helicobacter hepaticus. Infect Immun 65:3126–3131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chichlowski M, Sharp JM, Vanderford DA, Myles MH, Hale LP. 2008. Helicobacter typhlonius and Helicobacter rodentium differentially affect the severity of colon inflammation and inflammation-associated neoplasia in IL10-deficient mice. Comp Med 58:534–541 [PMC free article] [PubMed] [Google Scholar]
- 5.Chin EY, Dangler CA, Fox JG, Schauer DB. 2000. Helicobacter hepaticus infection triggers inflammatory bowel disease in T cell receptor αβ mutant mice. Comp Med 50:586–594 [PubMed] [Google Scholar]
- 6.Ediger RD, Kovatch RM, Rabstein MM. 1974. Colitis in mice with a high incidence of rectal prolapse. Lab Anim Sci 24:488–494 [PubMed] [Google Scholar]
- 7.Engle SJ, Ormsby I, Pawlowski S, Boivin GP, Croft J, Balish E, Doetschman T. 2002. Elimination of colon cancer in germ-free transforming growth factor β1-deficient mice. Cancer Res 62:6362–6366 [PubMed] [Google Scholar]
- 8.Erdman S, Fox JG, Dangler CA, Feldman D, Horwitz BH. 2001. Typhlocolitis in NFκB-deficient mice. J Immunol 166:1443–1447 [DOI] [PubMed] [Google Scholar]
- 9.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. 2003. CD4+CD25+ regulatory T lymphocytes inhibit microbially induced colon cancer in Rag2-deficient mice. Am J Pathol 162:691–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng S, Ku K, Hodzic E, Lorenzana E, Freet K, Barthold SW. 2005. Differential detection of 5 mouse-infecting Helicobacter species by multiplex PCR. Clin Diagn Lab Immunol 12:531–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foltz CJ, Fox JG, Cahill R, Murphy JC, Yan L, Shames B, Schauer DB. 1998. Spontaneous inflammatory bowel disease in multiple mutant mouse lines: association with colonization by Helicobacter hepaticus. Helicobacter 3:69–78 [DOI] [PubMed] [Google Scholar]
- 12.Fox JG, Dewhirst FE, Shen Z, Feng Y, Taylor NS, Paster BJ, Ericson RL, Lau CN, Correa P, Araya JC, Roa I. 1998. Hepatic Helicobacter species identified in bile and gallbladder tissue from Chileans with chronic cholecystitis. Gastroenterology 114:755–763 [DOI] [PubMed] [Google Scholar]
- 13.Fox JG, Gorelick PL, Kullberg MC, Ge Z, Dewhirst FE, Ward JM. 1999. A novel urease-negative Helicobacter species associated with colitis and typhlitis in IL10-deficient mice. Infect Immun 67:1757–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fox JG, Yan LL, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN. 1995. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol 33:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeon WJ, Yun YM, Roh KJ, Lee YC, Choi YK, Hyun BH, Kim CK, Cho JS, Seong JK. 2001. Helicobacter apodemus sp. nov., isolated from intestine of the striped field mouse (Apodemus agrarius). Exp Anim 50: S79. [Google Scholar]
- 16.Jergens AE, Wilson-Welder JH, Dorn A, Henderson A, Liu Z, Evans RB, Hostetter J, Wannemuehler MJ. 2007. Helicobacter bilis triggers persistent immune reactivity to antigens derived from the commensal bacteria in gnotobiotic C3H/HeN mice. Gut 56:934–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson SK, Feinstein RE, Johansson KE, Lindberg AV. 2006. Occurrence of Helicobacter species other than H. hepaticus in laboratory mice and rats in Sweden. Comp Med 56:110–113 [PubMed] [Google Scholar]
- 18.Krause M, Leslie JD, Stewart M, Lafuente EM, Valderrama F, Jagannathan R, Strasser GA, Rubinson DA, Liu H, Way M, Yaffe MB, Boussiotis VA, Gertler FB. 2004. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev Cell 7:571–583 [DOI] [PubMed] [Google Scholar]
- 19.Kuhn R, Lohler J, Rennick D, Rajewsky K, Muller W. 1993. Interleukin-10-deficient mice develop chronic enterocolitis. Cell 75:263–274 [DOI] [PubMed] [Google Scholar]
- 20.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. 1998. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL10)-deficient mice through an IL12- and γ-interferon-dependent mechanism. Infect Immun 66:5157–5166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lofgren JL, Esmail M, Mobley M, McCabe A, Taylor NS, Shen Z, Erdman S, Hewes C, Whary MT, Fox JG. 2012. Prevalence of murine Helicobacter spp. infection is reduced by restocking research colonies with Helicobacter-free mice. J Am Assoc Lab Anim Sci 51:436–442 [PMC free article] [PubMed] [Google Scholar]
- 22.Maggio-Price L, Treuting P, Bielefeldt-Ohmann H, Seamons A, Drivdahl R, Zeng W, Lai L, Huycke M, Phelps S, Brabb T, Iritani BM. 2009. Bacterial infection of Smad3/Rag2 double-null mice with transforming growth factor β dysregulation as a model for studying inflammation-associated colon cancer. Am J Pathol 174:317–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maggio-Price L, Treuting P, Zeng W, Tsang M, Bielefeldt-Ohmann H, Iritani BM. 2006. Helicobacter infection is required for inflammation and colon cancer in SMAD3-deficient mice. Cancer Res 66:828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangerich A, Knutson CG, Parry NM, Muthupalani S, Ye W, Prestwich E, Cui L, McFaline JL, Mobley M, Ge Z, Taghizadeh K, Wishnok JS, Wogan GN, Fox JG, Tannenbaum SR, Dedon PC. 2012. Infection-induced colitis in mice causes dynamic and tissue-specific changes in stress response and DNA damage leading to colon cancer. Proc Natl Acad Sci USA 109:E1820–E1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller CL, Muthupalani S, Drees F, Gertler FB, Fox JG. 2013. The lamellipodin knockout mouse as a model of rectal carcinoma. Gastroenterology 144:S832 [Google Scholar]
- 26.Muthupalani S, Ge Z, Feng Y, Rickman B, Mobley M, McCabe A, Van Rooijen N, Fox JG. 2012. Systemic macrophage depletion inhibits Helicobacter bilis-induced proinflammatory cytokine-mediated typhlocolitis and impairs bacterial colonization dynamics in a BALB/c Rag2−/− mouse model of inflammatory bowel disease. Infect Immun 80:4388–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagamine CM, Sohn JJ, Rickman BH, Rogers AB, Fox JG, Schauer DB. 2008. Helicobacter hepaticus infection promotes colon tumorigenesis in the BALB/c-Rag2(−/−)Apc(Min/+) mouse. Infect Immun 76:2758–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nell S, Suerbaum S, Josenhans C. 2010. The impact of the microbiota on the pathogenesis of IBD— lessons from mouse infection models. Nat Rev Microbiol 8:564–577 [DOI] [PubMed] [Google Scholar]
- 29.Nguyen DD, Muthupalani S, Goettel JA, Eston MA, Mobley M, Taylor NS, McCabe A, Marin R, Snapper SB, Fox JG. 2013. Colitis and colon cancer in WASP-deficient mice require Helicobacter species. Inflamm Bowel Dis 19:2041–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Percy DH, Barthold SW. 2007. Pathology of laboratory rodents and rabbits. Ames (IA): Blackwell Publishing. [Google Scholar]
- 31.Rogers AB, Fox JG. 2004. Inflammation and cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol 286:G361–G366 [DOI] [PubMed] [Google Scholar]
- 32.Rogers AB, Houghton J. 2009. Helicobacter-based mouse models of digestive system carcinogenesis. Methods Mol Biol 511:267–295 [DOI] [PubMed] [Google Scholar]
- 33.Sellon RK, Tonkonogy S, Schultz M, Dieleman LA, Grenther W, Balish E, Rennick DM, Sartor RB. 1998. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect Immun 66:5224–5231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shames B, Fox JG, Dewhirst F, Yan L, Shen Z, Taylor NS. 1995. Identification of widespread Helicobacter hepaticus infection in feces in commercial mouse colonies by culture and PCR assay. J Clin Microbiol 33:2968–2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Z, Feng Y, Fox JG. 2000. Identification of enterohepatic Helicobacter species by restriction fragment-length polymorphism analysis of the 16S rRNA gene. Helicobacter 5:121–128 [DOI] [PubMed] [Google Scholar]
- 36.Shen Z, Fox JG, Dewhirst FE, Paster BJ, Foltz CJ, Yan L, Shames B, Perry L. 1997. Helicobacter rodentiumsp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol 47:627–634 [DOI] [PubMed] [Google Scholar]
- 37.Shen Z, Xu S, Dewhirst FE, Paster BJ, Pena JA, Modlin IM, Kidd M, Fox JG. 2005. A novel enterohepatic Helicobacter species ‘Helicobacter mastomyrinus’ isolated from the liver and intestine of rodents. Helicobacter 10:59–70 [DOI] [PubMed] [Google Scholar]
- 38.Shomer NH, Dangler CA, Marini RP, Fox JG. 1998. Helicobacter bilis–Helicobacter rodentium co-infection associated with diarrhea in a colony of SCID mice. Lab Anim Sci 48:455–459 [PubMed] [Google Scholar]
- 39.Taffs LF. 1976. Pinworm infections in laboratory rodents: a review. Lab Anim 10:1–13 [DOI] [PubMed] [Google Scholar]
- 40.Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. 1996. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci 46:15–20 [PubMed] [Google Scholar]
- 41.Willis CR, Seamons A, Maxwell J, Treuting PM, Nelson L, Chen G, Phelps S, Smith CL, Brabb T, Iritani BM, Maggio-Price L. 2012. Interleukin 7 receptor blockade suppresses adaptive and innate inflammatory responses in experimental colitis. J Inflamm (Lond) 9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]