Abstract
In 2008, clinical observations in our colony of sooty mangabeys (Cercocebus atys) suggested a high frequency of type 2 diabetes. Postmortem studies of diabetic animals revealed dense amyloid deposits in pancreatic islets. To investigate these findings, we screened our colony (97 male mangabeys; 99 female mangabeys) for the disease from 2008 to 2012. The overall prevalence of diabetes was 11% and of prediabetes was 7%, which is nearly double that reported for other primate species (less than 6%). Fructosamine and triglyceride levels were the best indicators of diabetes; total cholesterol and glycated hemoglobin were not associated with disease. Increasing age was a significant risk factor: prevalence increased from 0% in infants, juveniles, and young adults to 11% in adults and 19% in geriatric mangabeys. Sex, medroxyprogesterone acetate exposure, and SIV status were unrelated to disease. Weight was marginally higher in prediabetics, but body condition did not indicate obesity. Of the 49 mangabeys that were necropsied after clinical euthanasia or death from natural causes, 22 were diabetic; all 22 animals demonstrated pancreatic amyloid, and most had more than 75% of islets replaced with amyloid. We conclude that type 2 diabetes is more common in mangabeys than in other primate species. Diabetes in mangabeys has some unusual pathologic characteristics, including the absence of altered cholesterol levels and glycated hemoglobin but a robust association of pancreatic insular amyloidosis with clinical diabetes. Future research will examine the genetic basis of mangabey diabetes and evaluate additional diagnostic tools using imaging and serum markers.
Abbreviations: HbA1c, glycated hemoglobin; MPA, medroxyprogesterone acetate; YNPRC, Yerkes National Primate Research Center
Sooty mangabeys (Cercocebus atys) are Old World NHP that are native to West Africa. Historically their use in research has been limited to infectious disease studies, leprosy studies, and behavioral research.14,25 Over the past 20 to 30 y, they have been used in HIV–AIDS research. Mangabeys are natural hosts of SIVsmm, which is recognized as the origin of HIV2 infection in humans.7,8,30,36,42 SIV typically is nonpathogenic in mangabeys despite high levels of virus replication, which makes this species a unique and invaluable model in AIDS research.7,30,36,42 Our facility maintains a colony of approximately 200 sooty mangabeys. In 2008 clinical observations of relative hyperglycemia, glucosuria, and weight loss in our colony suggested that type 2 diabetes mellitus occurred at a relatively high frequency in this population. Spontaneous diabetes was found in 10% of the colony, and 5% of animals were prediabetic; this incidence is higher than that typically reported for other NHP species, such as cynomolgus macaques (less than 1% to 2%)22 and chimpanzees (less than 1%).37 The prevalence of spontaneous diabetes in humans is typically 8.3%.2,6,22,37 In addition, necropsies revealed that many affected animals had dense amyloid deposits in pancreatic islet cells. Insular amyloidosis was seen on histology, with a total replacement of islets by amyloid deposition in advanced diabetes. Advanced diabetes was determined by increased weight loss and severity of relative hyperglycemia. The increased clinical prevalence of diabetes in our mangabey colony prompted additional characterization of the clinicopathologic profile, risk factors, and prevalence of diabetes in our mangabey colony.
The form of diabetes in this mangabey colony is characterized as type 2 diabetes mellitus, as they have hyperglycemia, hypertriglyceridemia, and islet amyloidosis. Type 2 diabetes mellitus is the most common of the 3 forms of diabetes, and has been documented in humans and NHP,22,31,37,55 including rhesus macaques (Macaca mulatta), cynomolgus macaques (Macaca fascicularis), Celebes crested macaques (Macaca nigra), bonnet macaques (Macaca radiate), pigtailed macaques (Macaca nemestrina), vervet monkeys (Chlorocebus pygerythrus), squirrel monkeys (Saimiri sciureus), chimpanzees (Pan troglodytes), and woolly monkeys (Lagothrix spp.).1,24,31,52,55 Type 2 diabetes is a chronic metabolic disorder in which insulin resistance occurs in liver, muscle, and adipose tissue. As type 2 diabetes progresses, it also can be characterized as a relative insulin deficiency.1,6,15,22,29,31,37,55 The initial clinical presentation of diabetes in humans and NHP includes polydipsia, polyuria, polyphagia, weight loss, and lethargy.1,6,22,27,31,37,55 Similar presentation was observed in our colony of diabetic mangabeys.
Diagnostic criteria of diabetes in NHP species is similar to that for humans and is based on clinical symptoms and routine lab tests, including serum chemistry panel to evaluate persistent fasting hyperglycemia, hypertriglyceridemia, and hypercholesterolemia.2,6,11,16-18,21,22,29,31,37,48-50,52,55 Hypertriglyceridemia and hypercholesterolemia frequently are elevated due to diabetes and therefore are used as supportive diagnostic markers. In addition, the disease is characterized by transient hyperinsulinemia followed by insulin deficiency subsequent to glucose challenge. Urinalysis is used to evaluate glucosuria and ketonuria. These tests are not exclusive for diagnosing diabetes and can be inconsistent between species, thus making conclusive diagnosis challenging. For example, hyperglycemia can be a transient finding associated with recent food intake or stress associated with restraint for blood sample collection or anesthetic access, whereas hypertriglyceridemia can be seen in obese animals and those with other metabolic diseases such as pancreatitis and hypothyroidism.1,22,37,55
The typical clinical approach to the diagnosis of diabetes in NHP and other veterinary patients includes evaluation of fructosamine and glycated hemoglobin (HbA1c) levels and glucose tolerance testing. These tests are indices of glycemic control and are used in clinical settings primarily to assess prognosis and response to treatment; they are also useful for the initial diagnosis of diabetes when used in parallel with serum chemistry markers. Fructosamine and HbA1c can both provide information on long-term glycemic control, because fructosamine reflects average blood glucose levels over 2 to 3 wk whereas HbA1c reflects average blood glucose over 2 to 3 mo preceding blood collection. HbA1c is the primary test for diabetes in human medicine,6,31,35,37 whereas fructosamine is commonly used in veterinary medicine. Glucose tolerance testing provides an indirect measure of insulin sensitivity, but it is not frequently used clinically in NHP because of the requirement for prolonged physical restraint or sedation.1,21,22,26,27,34,37,55
Prevention and management of diabetes in NHP and humans can be achieved by identifying potential risk factors, including age, weight, sex, genetics, hormone drug exposure, and viral status.1,6,15,22,29,31,37,42,55 Advanced age, obesity, sex, and genetics are associated with diabetes in some species of NHP and humans.1,6,15,22,29,31,37,55 In addition, exposure to drugs such as medroxyprogesterone acetate (MPA) is suspected to be linked to diabetes due to the hormonal effects of progesterone impacting glucoregulatory function.1,6,10,22,23,31,34,55 MPA exposure is of interest, because it is used regularly in our mangabey colony as both a contraceptive and as therapy for endometriosis. In addition, SIV status is being evaluated as a risk factor, because a portion of our colony is SIV positive. Although HIV is not thought to be associated with diabetes in people, SIV pathogenesis in mangabeys differs; therefore it was of interest to explore the possible association of SIV and diabetes in mangabeys.7,30,36,42 Pancreatic insular amyloidosis has been documented to be associated with type 2 diabetes in several species. Amyloidosis is a group of disorders that are caused by extracellular deposition of misfolded proteins that can result in impaired function of any organ.15,20,23,28,32,43,45,48,49 Because a high incidence of pancreatic insular amyloid was noted at necropsy, we sought to document the relationship with clinical diabetes in mangabeys.
Spontaneous type 2 diabetes mellitus has been well documented in several species of NHP. Because the literature contains little information regarding the clinicopathologic features (the ‘profile’), risk factors, and prevalence of spontaneous diabetes mellitus in sooty mangabeys, the primary aims of the current study were 1) to determine whether elevated levels of fasting blood glucose, fructosamine, HbA1c, triglycerides, and total cholesterol levels are reliable diagnostic markers of type 2 diabetes mellitus in this NHP species; 2) to determine whether age, sex, MPA exposure, and SIV status influence the risk of diabetes; 3) to determine whether body weight influences diabetic status; 4) to evaluate the relationship between pancreatic amyloidosis and diabetes mellitus; and 5) to characterize the prevalence of diabetes mellitus in the mangabey population at our institution. To our knowledge, this report is the first to describe the natural occurrence of type 2 diabetes mellitus within a captive colony of sooty mangabeys. We hypothesized that blood glucose, fructosamine, HbA1c, triglyceride, and total cholesterol would be reliable diagnostic markers and that age, sex, and MPA exposure would influence the risk of diabetes in this species.
Materials and Methods
Animals.
A population of 196 sooty mangabeys (male, 97; female, 99) at Yerkes National Primate Research Center was screened for diabetes. Ages ranged from 1 to 32 y, with an average age of 16 y (SE, 0.4 y). The median survival for both female and male mangabeys is about 19 y. All of the mangabeys are related genetically to each other, because they are all descendants from an original founder group consisting of 22 animals. Incomplete pedigree records preclude a conclusive measure of kinship coefficient between subjects, but the allelic frequency of polymorphic microsatellite loci suggest limited genetic diversity within the group.
The mangabeys were housed at 2 locations. The majority of the animals (n = 131) were housed at the field station (Lawrenceville, GA) in indoor–outdoor enclosures containing mixed-sex social groups of as many as 45 animals. All outdoor enclosures have an indoor shelter area, as well as enrichment, such as climbing structures and toys. The enclosures vary in size from runs, which are 120 square feet, to compounds of 10,347 ft2. The remaining mangabeys (n = 65) were housed at the main station (Atlanta, GA), where they were kept in standard NHP cages in a mix of single and pair housing. SIV-positive and SIV-negative animals are physically isolated, in separate housing structures or by strict division within animal rooms. Mangabey at both facilities were fed a standard commercial primate diet (Lab Diet 5037 Monkey Diet Jumbo, Brentwood, MO) and supplemented daily with fresh oranges and approved enrichment (that is seeds or grain, popcorn, oatmeal, applesauce, and vegetable produce). Free access to water was provided through an automatic watering system. All animals at both facilities were assigned to colony protocols and SIV studies for periodic sample collection; several animals at the field station were maintained as part of the breeding colony. Table 1 shows that 32 female mangabeys were pregnant during the study. All animal procedures were approved by Emory University's IACUC. Animals were maintained in accordance with the Guide for the Care and Use of Laboratory Animals.24 The facility and its programs are fully AAALAC accredited.
Table 1.
Total numbers of samples collected for diagnostic tests and risk factors stratified by diabetic status
| Nondiabetic | Prediabetic | Diabetic | |
| No. of mangabeys | 161 | 13 | 22 |
| No. of male/female mangabeys | 82/79 | 7/6 | 8/14 |
| No. SIV positive/negative | 87/62 | 6/7 | 10/12 |
| No. of pregnant/not pregnant female mangabeys | 30/49 | 1/5 | 1/13 |
| No. female magabeys with MPA exposure/without MPA exposure | 48/31 | 4/2 | 9/5 |
| No. of samples collected for | |||
| Blood glucose (glucometer) | 142 | 8 | 15 |
| Serum glucose | 137 | 13 | 22 |
| Triglycerides | 134 | 13 | 22 |
| Total cholesterol | 132 | 13 | 22 |
| Fructosamine | 70 | 9 | 22 |
| Glycated Hgb (HbA1c) | 16 | 2 | 7 |
| Age classification | |||
| Infant (<1 y) | 7 | 0 | 0 |
| Juvenile (1–3 y) | 8 | 0 | 0 |
| Young adult (3–10 y) | 22 | 0 | 0 |
| Adult (10–18 y) | 82 | 9 | 11 |
| Geriatric (>18 y) | 42 | 4 | 11 |
| Adult body weight (kg) | 127 | 13 | 22 |
Samples.
From 2008 to 2012, blood and urine samples were collected from the 196 mangabeys, primarily during annual health exams. A small number of additional samples were obtained opportunistically, during experimental and clinical events (for example, trauma, arthritis). Annual health exams included physical examination, tuberculosis testing, parasite control, and fasted sample collections as needed for colony management and research services. Mangabeys were fasted by receiving their last feeding the afternoon prior to the morning access. Animals were sedated with either ketamine (10 mg/kg IM) or tiletamine–zolazepam (3 to 5 mg/kg IM) for examinations and sample collections. A glucometer (Freestyle Lite, Abbott Park, IL) was used to determine blood glucose levels from a peripheral skin stick. Whole blood was collected via the femoral vein for a chemistry panel, fructosamine concentration, HbA1c measurements, and SIV serology titers. Table 1 illustrates the number of animals for each sample collection. The whole blood was collected into serum-separator and EDTA tubes and then stored at 3.3 °C until assayed. The blood in the serum-separator tubes were centrifuged prior to being assayed. The fasting blood glucose samples included those obtained from the serum chemistry panel as well as the glucometer. Blood serum chemistry panels were analyzed at either the institutional diagnostic laboratory or a commercial laboratory (SmithKline Beecham, Decatur, GA) prior to March 2010. After March 2010, AMS Liasys Chemistry Analyzer (AMS Diagnostics, Weston, FL) and Antech Diagnostics (Olympus, Marietta, GA) were used. Serum samples for fructosamine were analyzed by using a colorimetric assay at Antech Diagnostics, whereas plasma samples for HbA1c were analyzed by at the Emory Medical Lab by using HPLC (Variant II Turbo Hemoglobin Testing System, Bio-Rad, Hercules, CA).13 Serum samples for serology SIV titers were analyzed by using a Luminex system (Austin, TX) at the Yerkes Virology Core. All samples were analyzed within 24 h; glucometer data were analyzed immediately. Urine samples were obtained via catheterization or cystocentesis, and urinalysis was performed at the institutional diagnostic laboratory.
Mangabeys were assigned to 3 categories: nondiabetic, prediabetic, and diabetic. Animals were classified as diabetic according to clinical symptoms (for example, weight loss, lethargy, polyuria), relative hyperglycemia, or glucosuria. Once these animals were identified, their mean fasting hyperglycemia levels were determined to be greater than 175 mg/dL. In light of these findings, diabetes was determined as the presence of 2 of the 3 following criteria: persistent hyperglycemia, glucosuria, and weight loss. Hyperglycemia was defined as a glucose level greater than 175 mg/dL that occurred on more than 2 consecutive readings. Glucosuria was any content of glucose in the urine. Weight loss was defined by loss of greater than 10% of body weight. Animals were classified as prediabetic based on transient elevated fasting glucose levels that were less than diabetic levels but greater than 135 mg/mL. Prediabetics were asymptomatic. Diabetic animals were not treated with glucose-alerting medications, to allow them to remain in large social groups as well as to avoid possible research interactions. Mangabeys were treated for secondary complications and clinical conditions, and weights were monitored closely. Animals were euthanized according to IACUC endpoints.
Table 1 illustrates the number of animals in each potential risk factor category. Animals were categorized into age groups. MPA (Depo-Provera, Wickliffe Veterinary Pharmacy, Lexington, KY) was administered to 61 female sooty mangabeys (age, 8 to 32 y) as a contraceptive or therapy for endometriosis at a dose of 200 mg IM approximately every 3 mo; the frequency of administration depended on cycle length and clinical signs. SIV-negative animals were evaluated annually, and once the animal became SIV positive, SIV status was no longer monitored. The majority of SIV-positive mangabeys evaluated in this study were natural carriers of the virus, although a few animals had been historically infected experimentally. Adult body weight was evaluated as a possible influence of diabetes among the nondiabetic and prediabetic mangabeys. Weight was obtained during annual health exams and collected opportunistically during sample collection or other examinations. Weight was rounded to the nearest 0.1 kg, and intervals between weights ranged from weeks to 1 y.
Pathology.
A comprehensive review of our pathology database was performed for gross and microscopic diagnoses of pancreatic insular amyloidosis in sooty mangabeys between 2008 and 2012. The clinical records, pathology reports, and glass slides were reviewed to clarify the diagnoses of insular amyloidosis. During the study, 49 of the 196 sooty mangabeys died naturally or were euthanized for medical reasons. Because we have an aging population of mangabeys, most causes of death were related to chronic processes (that is, diabetes, neoplasia, cardiovascular disorder, endometriosis, various inflammatory processes). Of the animals known to be diabetic at necropsy, 88% were euthanized secondary to complications of diabetes. All mangabeys underwent a complete necropsy, and tissues from major organs were collected, including pancreatic tissue samples for histopathologic evaluation. Tissue specimens were fixed in 10% neutral buffered formalin, embedded in paraffin, sectioned at 6 µm, and stained with hematoxylin and eosin. Sections of paraffin-embedded tissues were evaluated for amyloid by using Congo red and or sulfated Alcian blue staining.33
Sections of the pancreas were stained with hematoxylin and eosin and evaluated qualitatively for the presence of islet amyloid deposit by a board-certified veterinary pathologist. Characterization of the extent of pancreatic islet amyloid deposit was based on the approximate percentage of islet area replaced by amyloid. The classification scheme recognized grades of amyloidosis of minimal, mild, moderate, and marked (Figure 1).
Figure 1.

Scheme for classification of pancreatic islet amyloid deposition.
Validation of blood glucose measurements.
The validity of the glucometer glucose reading as an index of the serum glucose was evaluated by using linear regression (SPSS, IBM, Armonk, NY). In this analysis, serum glucose was the dependent variable, and the glucometer was the predictor. Residuals were examined for normality, and the significance of the linear term was determined. In addition, the validity of processing time effect on blood glucose measurements was evaluated. A subset of data (n = 61) was used to evaluate the relationship between glucose processed immediately (glucometer) and after delayed processing (serum).
Data analysis.
Individual measures (for example, glucose, fructosamine) were collected intermittently at various time points. Not every measure was evaluated at each blood sample collection. Therefore we obtained the mean value for each measure after the animal attained its final diabetic status as observed in the study. This mean was based on 1 to 5 samples and was used for further analyses. We did not adjust the means for each animal to account for within-animal variability. All statistical analyses were performed by using SPSS (version 20, IBM). ANOVA was used to determine the influence of diabetic status, sex, and the interaction of these factors on serum levels of fructosamine, HbA1c, glucose, triglycerides, and cholesterol. We used the Tukey Highly Significant Difference test after ANOVA to examine differences among means for the 3 categories of diabetic status. The Pearson product-moment correlation was used to compare serum glucose measurements with those obtained with the glucometer.
The relationships between age group, sex, MPA exposure (dichotomous variable: any exposure or no exposure), and positive compared with negative SIV status were evaluated as possible risk factors by using the χ2 procedure within SPSS. In addition, the χ2 procedure was used to evaluate the relationship between necropsied findings of pancreatic islet amyloid deposit and diabetic status. Mangabeys were categorized as infant (younger than 1 y), juvenile (age, 1 to 3 y), young adult (3 to 10 y), adult (10 to 18 y), and geriatric (older than 18 y). The influence of increasing age (an ordered categorical variable) was evaluated by using means of the linear-by-linear association module. The risk factors were analyzed by using frequency tables of each risk factor in relation to diabetic status. The influence of body weight on the 3 categories of diabetic status and sex influence on body weight was evaluated by ANOVA, Tukey Highly Significant Difference test, and covariate analysis.
The relationships of age with measurements of serum glucose and fructosamine were examined by using linear regression within SPSS. In these analyses, serum glucose and fructosamine were the dependent variables and age was the predictor. Statistical significance was defined as a P value of less than 0.05.
Results
Serology.
Antibody titers revealed that 103 mangabeys were SIV positive and 81 were SIV negative.
Glycemic control status.
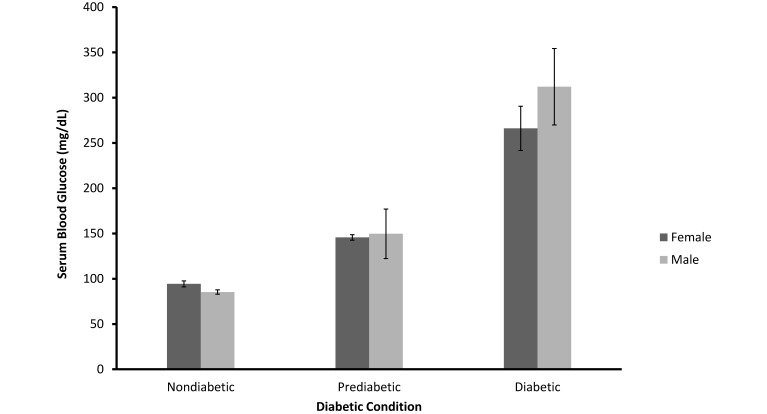
Using the criteria described, we classified 161 (82%) of the mangabeys as nondiabetic, 13 (7%) as prediabetic, and 22 (11%) as diabetic. By definition, fasting blood glucose levels were significantly related to diabetic status (F = 171.28; df = 2, 166; P < 0.001). Mean values for nondiabetic, prediabetic, and diabetic animals were 90, 148, and 283 mg/dL (SEM, 10.43), respectively (Figure 2). Blood glucose levels for nondiabetic and prediabetic animals were statistically equivalent for male and female mangabeys (F = 1.502; df = 1, 166; P > 0.10). However there was a significant interaction in diabetic blood glucose levels between the sexes (F = 3.237; df = 2, 166; P < 0.05). Figure 2 indicates that this interaction reflects a greater elevation of glucose in male than in female diabetic mangabeys.
Figure 2.
Mean (± SEM) of fasting serum blood glucose for nondiabetic (n = 137), prediabetic (n = 13), and diabetic (n = 22) female and male sooty mangabeys (P < 0.001).
Validation of blood glucose measurements.
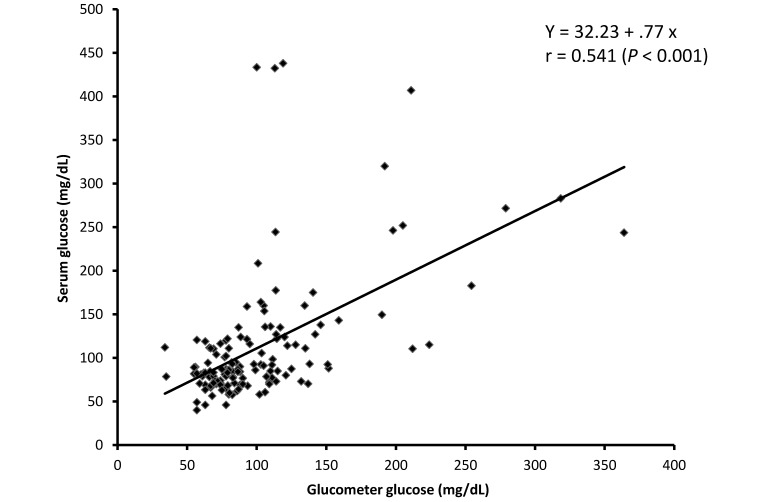
Glucometer readings of glucose were valid as an index of the serum glucose, because the linear correlation between the glucometer and serum glucose was significant (r = 0.54, P < 0.001); the scatter plot and linear regression results for this relationship are presented in Figure 3. It is clear from this plot that the glucometer predicts lower glucose values more accurately than it does higher values. Processing time did not affect glucose immediately processed (glucometer) and delayed processing (serum)—there was no linear association. All samples were processed within 24 h, and the subsets evaluated were processed within 3 h.
Figure 3.
Pearson product-moment correlation was used to compare serum glucose measurements (in mg/dL) with those obtained with the glucometer (in mg/dL). The solid line represents the linear correlation. Pearson correlation, r = 0.541 (P < 0.001).
Diagnostics.
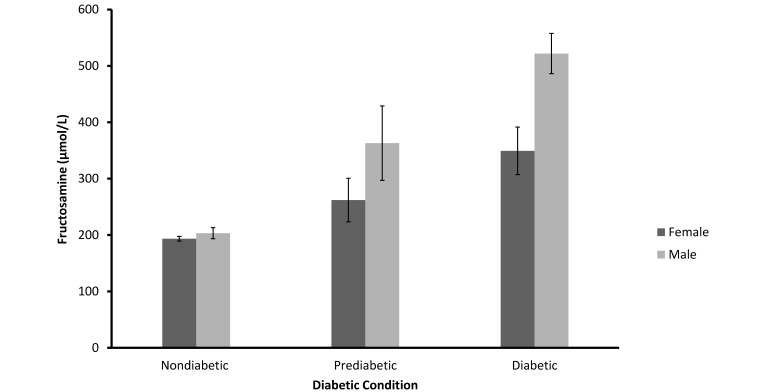
Fructosamine levels clearly were influenced by diabetic status (F = 64.67; df = 2, 96; SEM = 21.12: P < 0.001), with the highest levels in the diabetic animals (Figure 4, Table 2). Overall fructosamine levels were higher in male than in female mangabeys (F = 16.47; df = 1, 95; P < 0.001). In addition, there was a significant interaction of diabetic status with sex (F = 7.77; df= 2, 95; P < 0.001), indicating that diabetic status had a proportionally greater influence on fructosamine in male mangabeys than in female, with little difference between the sexes in the nondiabetic group but with increasing differences in prediabetic and diabetic animals.
Figure 4.
Mean (± SEM) of fructosamine values for nondiabetic (n = 70), prediabetic (n = 9), and diabetic (n = 22) female and male sooty mangabeys (P < 0.001).
Table 2.
Reference values for fasting blood glucose (mg/dL), HbA1c (%), and fructosamine (mean; µmol/L) in nondiabetic, prediabetic, and diabetic primates
| Sooty mangabeys | Rhesus macaques | Cynomolgus macaques | Celebes crested macaques | Chimpanzees | Humans | |
| Fasting blood glucose | ||||||
| Nondiabetic | ≤ 135 | ≤ 80 | ≤ 70 | na | ≤ 105 | < 100 |
| Prediabetic | 135–175 | 81–100 | 71–99 | na | 106–119 | 100–125 |
| Diabetic | ≥ 175 | ≥ 100 | ≥ 100 | ≥ 140 | ≥ 120 | ≥ 126 |
| HbA1c | ||||||
| Nondiabetic | ≤ 5.3 | ≤ 4.7 | ≤ 5.0 | ≤ 3.1 | ≤ 5.0 | ≤ 5.6 |
| Prediabetic | 4.0–12.0 | 4.7–5.0 | 5.1–8.1 | 3.1–4.0 | 5.1–5.2 | 5.7–6.4 |
| Diabetic | ≥ 6.6 | ≥ 5.0 | ≥ 8.2 | ≥ 7.1 | ≥ 5.3 | ≥ 6.5 |
| Fructosamine | ||||||
| Nondiabetic | 197 | 193 | 185 | na | na | 227 |
| Prediabetic | 318 | na | na | na | na | na |
| Diabetic | 412 | na | 434 | na | na | 277 |
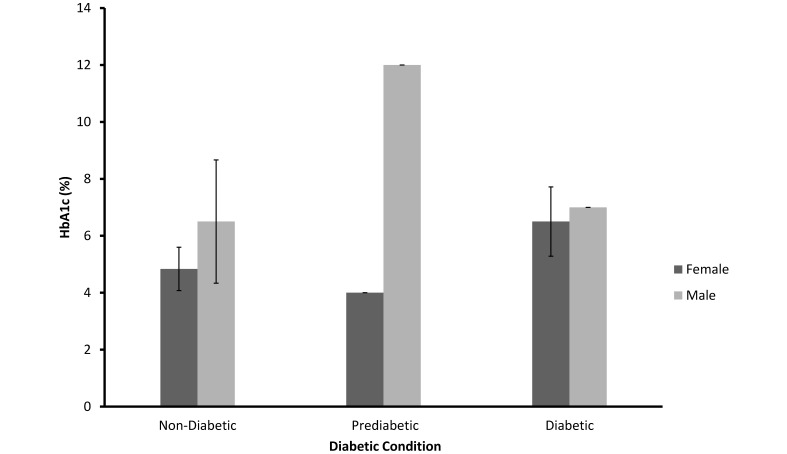
As shown in Figure 5, HbA1c was not significantly related to diabetic status (F = 0.49; df = 2, 19; SEM = 1.47, P > 0.10) or sex (F = 2.65; df = 1, 19; P > 0.10).
Figure 5.
Mean (± SEM) of HbA1c values for nondiabetic (n = 16), prediabetic (n = 2), and diabetic (n = 7) female and male sooty mangabeys (P > 0.10).
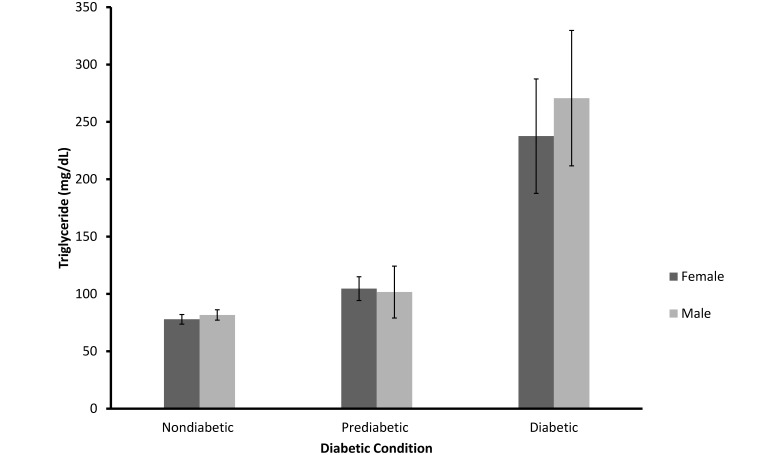
However, as shown in Figure 6, triglyceride levels were strongly related to diabetic status, with increasing values in the prediabetic and diabetic mangabeys as compared with nondiabetic animals (F = 48.09; df = 2, 163; SEM = 17.47; P < 0.001). The relationship between triglycerides and diabetic status did not differ between male and female mangabeys (F = 0.38; df = 2, 136; P > 0.10). The interaction between diabetic status and sex was nonsignificant also (F = 0.37; df = 2, 136; P > 0.10).
Figure 6.
Mean (± SEM) of triglyceride values for nondiabetic (n = 134), prediabetic (n = 13), and diabetic (n = 22) female and male sooty mangabeys (P < 0.001).
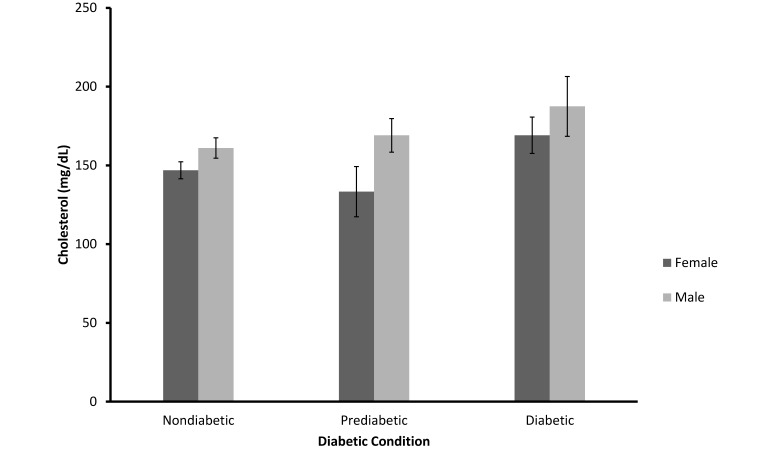
Total cholesterol values were equivalent among the 3 categories of diabetic status (F = 2.39; df = 2, 161; SE = 11.20), P > 0.05). However, male mangabeys had significantly higher values on average than did female ( F = 3.82; df = 1, 161; P = 0.05; Figure 7).
Figure 7.
Mean (± SEM) of total cholesterol values for Nondiabetic (n = 132), Prediabetic (n = 13), and Diabetic (n = 22) female and male sooty mangabeys (P = 0.05).
Risk factors.
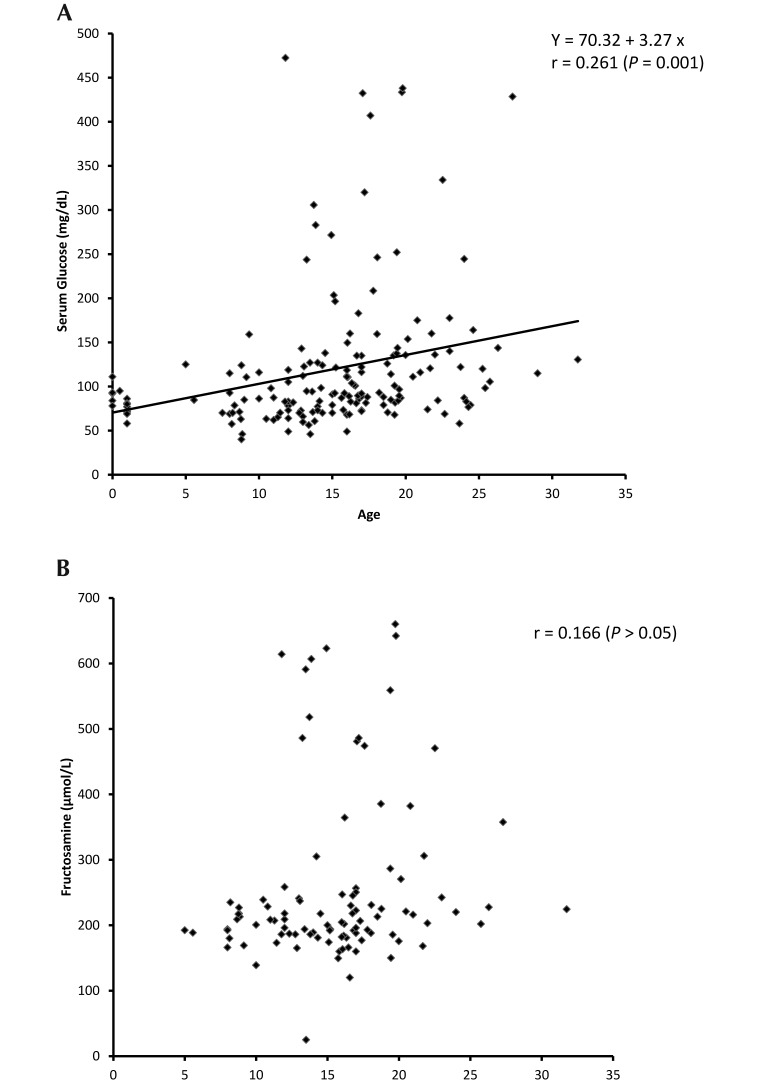
As shown in Table 3, the prevalence of diabetes mellitus was highest among the group of geriatric (19%) mangabeys, and the prevalence of prediabetes was highest among adult (9%) and geriatric (7%) mangabeys. The increasing prevalence of prediabetic and diabetic status with increasing age is significant (P < 0.01, linear-by-linear association). Age also affected serum glucose levels. As shown in Figure 8 A, glucose levels increased significantly with age (r = 0.541, P < 0.001). The variability of glucose levels also increase with age. The scatter plot of fructosamine as a function of age resembles that of glucose, but the linear relationship is not significant in this case (r = 0.166, P > 0.05; Figure 8 B).
Table 3.
Prevalence of prediabetic and diabetic status in infant (< 1 y), juvenile (1–3 y), young adult (3–10 y), middle-aged adult (1—18 y), and geriatric (>18 y) mangabeys
| Infant | Juvenile | Young adult | Adult | Geriatric | |
| Total no. (no. male/no. female) | 7 (3,4) | 8 (4,4) | 22 (8,14) | 102 (53,49) | 57 (29,28) |
| Diabetic status | |||||
| Nondiabetic (n = 161) | 100% | 100% | 100% | 80% | 74% |
| Prediabetic (n = 13) | 0% | 0% | 0% | 9% | 7% |
| Diabetic (n = 22) | 0% | 0% | 0% | 11% | 19% |
Linear-by-linear association, P = 0.003.
Figure 8.
(A) Pearson product-moment correlation was used to compare serum glucose measurements (in mg/dL) with age (in y). The solid line represents the linear correlation. Pearson correlation, r = 0.261 (P = 0.001). (B) Pearson product-moment correlation was used to compare fructosamine (in µmol/L) with age (in y). There was no significant linear correlation (Pearson correlation, r = 0.166 [P > 0.05]).
Prevalence of diabetes was equivalent in male and female (df = 2, P > 0.10) mangabeys. In addition, equivalent prevalence of diabetes was obtained in SIV-positive and SIV-negative mangabeys (df = 2, P > 0.10) and in female mangabeys regardless of prior exposure (or not) to MPA (df = 2, P > 0.10).
Adult body weights ranged from 5 to 14 kg. Diabetic status was strongly related to body weight (F = 5.21; df = 2, 157; SE = 0.30), with prediabetic mangabeys (mean, 11.6 kg) weighing more than nondiabetics (9.5 kg, P < 0.01). Diabetic animals (9. 5 kg) weighed less than did prediabetics. Higher weight of prediabetic animals also was present when age was controlled as a covariate in ANOVA. Furthermore, sex was strongly related to weight (F = 5.21; df = 1, 157; P < 0.001).
Pathology.
Pancreatic insular amyloidosis was detected in 28 of the 49 sooty mangabeys that were necropsied. Marked insular amyloidosis was present in 14 diabetic and 3 prediabetic mangabeys. Minimum amyloidosis was found in one prediabetic animal and 6 nondiabetic animals. As shown in Table 4, insular amyloidosis was strongly associated with clinical diabetes, because all 16 of the diabetic animals had amyloidosis (P < 0.001).
Table 4.
Prevalence of nondiabetic, prediabetic, and diabetic status in relation to pancreatic insular amyloidosis in sooty mangabeys
| Nondiabetic (n = 26) | Prediabetic (n = 7) | Diabetic (n = 16) | |
| Amyloidosis present (n = 28) | 23% | 86% | 100% |
| Amyloidosis not present (n = 21) | 77% | 14% | 0% |
Pearson χ2, P < 0.001
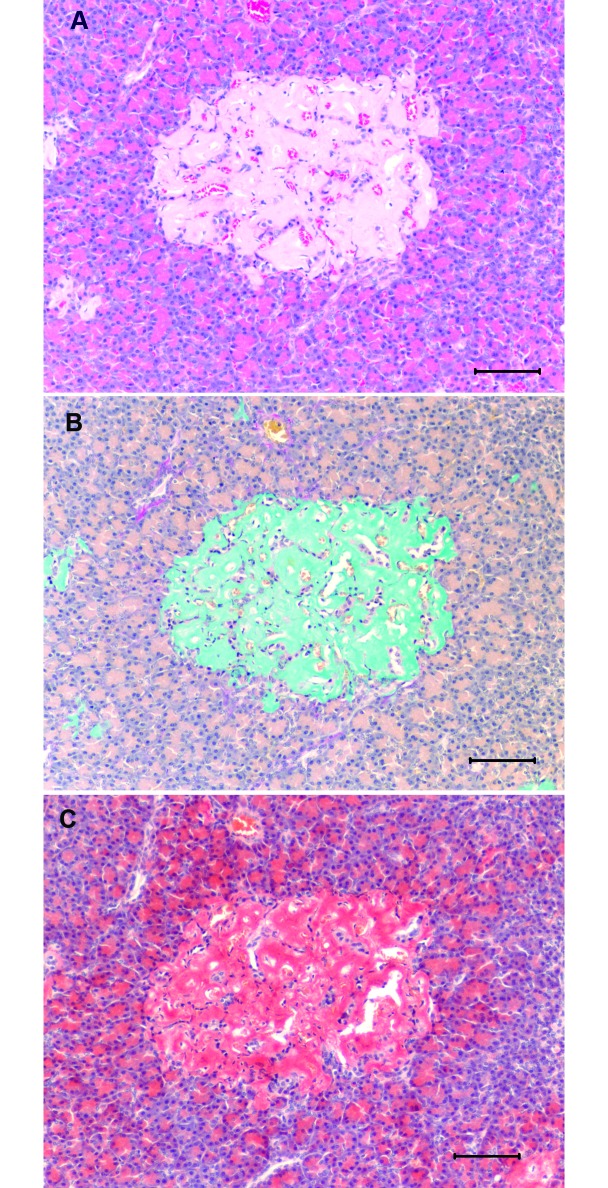
The extent and distribution of amyloid deposition ranged from mild, multifocal deposition around islet capillaries with little or no loss of islet β cells, to moderate deposition in a majority of islets (Figures 9 and 10). Severe cases showed marked, diffuse amyloid deposition; severe atrophy and loss of β cells; and often mineralization within the insular amyloid deposits. Microscopically, amyloid deposition within pancreatic islets appeared as variable amounts of amorphous, acellular, homogeneous to finely fibrillar, lightly eosinophilic extracellular material. Amyloid deposits stained light orange with the Congo red histochemical method and demonstrated green birefringence under polarized light; with sulfated Alcian blue stain, amyloid appeared greenish blue (Figure 11).
Figure 9.
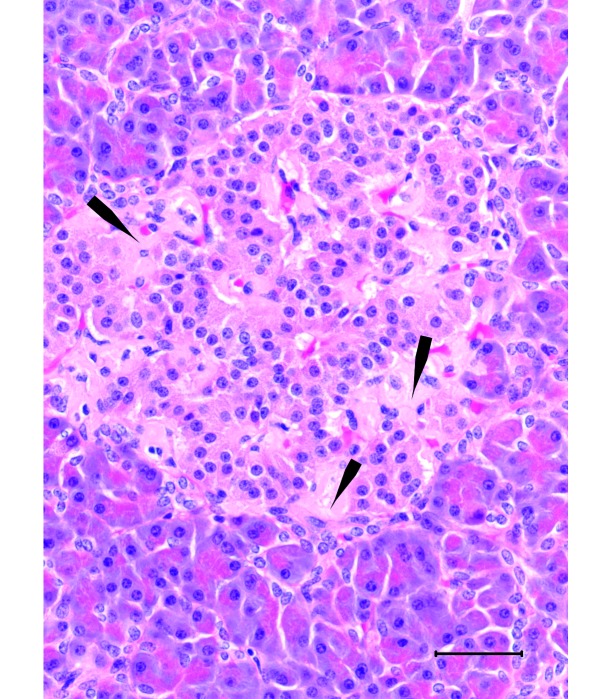
Pancreas from a nondiabetic 17-y-old male sooty mangabey. Islets contain mild amyloid deposits (arrows) and numerous β cells. Hematoxylin and eosin stain; bar, 50 µm.
Figure 10.
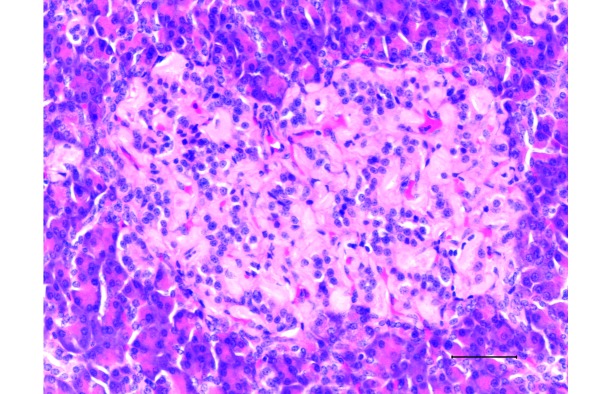
Pancreas from a diabetic 19-y-old male sooty mangabey with a terminal blood glucose concentration of 311 mg/dL. The islet contains moderate amyloid deposits. The remaining β cells appear as small nuclei surrounded by amyloid. There are a few β cells remaining. Hematoxylin and eosin stain; bar, 50 µm.
Figure 11.
Pancreas from a diabetic 23-y-old female sooty mangabey with a terminal blood glucose of 230 mg/dL. (A) Islets have been totally replaced by marked amyloid deposits. Hematoxylin and eosin stain; bar, 100 µm. (B) Insular deposits stain positive for amyloid. Sulfated Alcian blue stain; bar, 100 µm. (C) Insular deposits stain positive for amyloid. Congo red stain; bar, 100 µm.
Discussion
This report is the first detailed description of spontaneous type 2 diabetes mellitus in sooty mangabeys. In addition to documenting the disease within the colony, we validated routine diagnostic tests in this species and provided a basic description of the epidemiology of the disease, including its prevalence and the relationship of several risk factors to the occurrence of disease. We furthermore demonstrated a relationship between pancreatic insular amyloidosis and clinical diabetes in the mangabeys. The overall prevalence of diabetes in our colony of mangabeys was 11%, which is higher than that reported in other NHP species; for example, cynomolgus macaques have a prevalence of less than 1% to 2%,22 and chimpanzees show less than 1% prevalence.37 The higher prevalence in mangabeys is suspected to be secondary to the longevity of the colony and perhaps to a possible genetic component or a dietary influence; we are unable to rule out these factors at this time.
Hyperglycemia is one of the 3 criteria used to diagnosis diabetes in our colony. The blood glucose reading was obtained from either serum or whole blood (from a glucometer); these measures yielded comparable values. Table 2 illustrates the higher glucose levels in our prediabetic and diabetic mangabeys when compared with humans and other NHP, such as Celebes crested macaques, rhesus macaques, and chimpanzees.2,6,22,31,37,50 This table compares fructosamine and HbA1c levels among our colony of mangabeys, humans, and other NHP. The higher levels of hyperglycemia in our colony of mangabeys compared with other NHP might be secondary to genetics, because the mangabeys are relatively inbred. Stress of access for obtaining samples could be another cause of higher hyperglycemia levels; perhaps the mangabeys might release more cortisol than other species. Hyperglycemia alone is not a consistent or exclusive test for diabetes mellitus; therefore, it is necessary to evaluate the relationship of other diagnostic tests for diabetes.
HbA1c, fructosamine, triglyceride, and cholesterol were evaluated as diagnostics in the mangabeys, because these parameters are commonly used to diagnosis or aide in the diagnosis of diabetes in other animal species as well as humans.6,31,34,37,38,54,55 HbA1c and fructosamine tests provide us with practical methods for monitoring long-term glycemic control in our large outdoor colony of sooty mangabeys; HbA1c indicates the average blood glucose over 2 to 3 mo and fructosamine represents a 2- to 3-wk period.1,21,22,26,27,34,37,55 Both tests can be evaluated without the variables of transient stress, activity, or any other condition that can influence glucose levels.1,6,21,31,34,35,37,38,43,50,55
Although HbA1c is considered the primary test in human medicine6,31,35,37 and is used in several other species of NHP, HbA1c levels did not correlate with clinical diabetes in our colony of mangabeys. These results could be secondary to species-specific variations in hemoglobin. As shown in Table 2, different species of NHP have different HbA1c values. Therefore different methodologies of HbA1c assays could yield different results. There are 3 technologies for HbA1c assays: charge differences (HPLC), structure (boronate affinity or immunoassay combined with general chemistry), and point-of-care.3 HPLC technology was used for our study. Perhaps using another assay might yield different results. In addition, the sample size was small (n = 25), due to the inconsistent test results. A larger sample size might prove to be a more reliable predicator of diabetes.
Fructosamine levels for our mangabeys were associated with clinical diabetes. These results were expected, because fructosamine is a standard test in companion animal medicine and has been used in several species of NHP and in humans.1,26,27,34,38,40,46,54,55 Table 2 illustrates that fructosamine values in diabetic mangabeys are comparable to those in diabetic cynomolgus macaques but higher than those in humans. The different values between these species may indicate species-specific features.
Diabetic male mangabeys had significantly higher levels of fructosamine than did diabetic female mangabeys (Figure 4). This finding is similar to those in humans and rhesus macaques, which show sex-associated differences in fructosamine levels. The male–female distinction might be a consequence of higher concentrations of serum protein or a longer half-life in male mangabeys when compared with female.1,27,34,50,55
Although fructosamine measurement seems promising as a marker of diabetes in mangabeys, it has several potential limitations. Fructosamine levels can be artifactually low during hypoproteinemia, hypoalbuminemia, hypervitaminosis C, hyperthyroidism, and hemolyzed samples. Conversely, fructosamine can be falsely elevated during azotemia. These limitations reflect that the measurement of fructosamine is dependent on the binding of glucose to serum protein via a nonenzymatic glycation reaction. Therefore any alteration that affects the serum will influence fructosamine levels.26,38,54,55 Although the influences of protein, vitamin C markers, and metabolic disorders were not analyzed in the current study, we evaluated the clinical presentation of the animals and all serum chemistry markers. Future analyses of fructosamine levels in our mangabeys will take these limitations into consideration.
Triglyceride levels were elevated in diabetic mangabeys compared with nondiabetic and prediabetic animals (Figure 6). Hypertriglyceridemia in diabetic mangabeys was predicted, because it has been reported to occur in diabetic humans12,16 and diabetic cynomolgus macaques.50 Increased triglyceride concentrations likely are attributable to impaired lipoprotein lipase activity, which prevents the breakdown of triglyceride-rich particles to free fatty acids. Lipoprotein lipase activity is insulin-dependent, and most diabetic NHP are relatively insulin deficient.11,49,50,55
Total cholesterol levels were not associated with diabetic status in our mangabeys (Figure 7). because there is typically a correlation of hypercholesterolemia (LDL and VLDL cholesterol) with diabetes in humans and some NHP, specifically cynomolgus macaques.1,16,50,55 The lack of correlation between total cholesterol levels and diabetes in our mangabeys may be secondary to the fact that the majority of the diabetic population was geriatric (older than 18 y). Humans older than 55 y have increased cholesterol levels; perhaps we're seeing a similar effect seen in the geriatric mangabeys.39 In addition, mangabeys are fed a standard commercial primate diet (Lab Diet 5037) that contains isoflavones and soy, are lipid-lowering compounds; therefore the animals are not exposed to an overabundance of LDL cholesterol.50
We attempted to identify risk factors for type 2 diabetes mellitus in our colony of mangabeys because such knowledge would assist us in predicting and identifying diabetic animals, achieving management decisions, and instituting preventive practices for diabetes. Advanced age correlated with increased prevalence of diabetes in our colony of mangabeys, given that about 50% of the prediabetic and diabetic animals were geriatric (Table 3). In addition, age affected serum glucose levels, in that old animals had increased serum glucose; there was no such correlation with fructosamine. The correlation among advanced age, diabetes, and serum glucose was anticipated, because similar old-age effects have been documented to occur in diabetic cynomolgus macaques, rhesus macaques, and humans.1,2,6,9,22,50,55 The literature reports that diabetic cynomolgus macaques and rhesus macaques typically are older than 15 y,1,22,50 and diabetic humans typically are older than 65 y.6,55 Increase serum glucose was expected, because it is a criterion for diabetes. Fructosamine levels were expected to be elevated with increased age, as documented in rhesus macaques and humans.55 Our colony is skewed toward older animals, with ages ranging from less than 1 to 32 y and a mean age of 16 y. As shown in Table 3, more than one-fourth of the colony is geriatric, with longevity greater than the maximum for sooty mangabeys in the wild.19 Our current results will aide in the management of geriatric mangabeys, because these animals should be examined and blood glucose obtained at increased frequencies to evaluate the progression or initiation of diabetes.
In our mangabeys, sex is not associated with the prevalence of diabetes. Our results contrast with those in humans, given that a recent literature report suggests that men older than 20 y are more likely to have diabetes than are women older than 20 y.6 Species variations and pregnancy status might have influenced the association of sex with diabetes of our mangabeys.2,6,50 Pregnancy is unlikely to be a confounding factor because only 32% (n = 32) of the female mangabeys were pregnant during the study and 30 of the 32 pregnant animals were nondiabetic, one animal was prediabetic, and one was diabetic.
MPA exposure in our mangabeys did not correlate with increased prevalence of diabetes. We postulated that MPA exposure could be a risk factor for diabetes mellitus in mangabeys, in light of the correlation between MPA exposure and diabetes prevalence in rhesus macaques and humans.9,10,29,47,51 MPA is administered to various female mangabeys in our colony for contraceptive purposes as well as therapy for endometriosis, because the drug is a synthetic progesterone derivative. The pathogenesis of progesterone-induced diabetes in rhesus macaques and humans is unknown. Studies have shown that progesterone leads to reduced glucoregulatory function, and MPA has increased affinity for progesterone receptors as well as glucocorticoid and androgen receptors.10,51 MPA might be metabolized differently in mangabeys and thus could explain the lack of increased prevalence of diabetes in female animals that were exposed to MPA.
SIV status was not correlated with prevalence of diabetes in our mangabeys, similar to the situation with HIV status in humans.4,5 With regard to humans, one study suggested that absence of a relationship was likely secondary to the fact that HIV-infected people tend to be younger and have a lower body mass index, which can actually decrease the risk of diabetes mellitus.5 The natural occurrence of SIV in our colony of mangabeys is likely the reason there is no correlation of diabetes with SIV. Although SIV is typically nonpathogenic in mangabeys,7,30,36,42 diabetes mellitus has the potential to be immunosuppressive, which could be a confounding factor for SIV research in these animals. Therefore, it was important to evaluate the relationship of SIV status and diabetes.
Body weight correlated with the diabetic status of the animals, and prediabetics weighed more than did nondiabetics. Similar results have been observed in prediabetic humans and prediabetic cynomolgus and rhesus macaques, as they were described as weighing more than did nondiabetics. In addition, prediabetic humans, cynomolgus macaques, and rhesus macaques were also described as being overweight compared with nondiabetic subjects.2,6,9,31,40,49,50 Although, prediabetic mangabeys did weigh more than nondiabetics, none of the animals were clinically overweight or obese, because the body condition scores of the animals evaluated never exceeded 3.5 (maximum, 5). In general, obesity is not a clinical problem in this mangabey colony and therefore is not suspected to be a risk factor for diabetes in these animals. As expected, diabetic mangabeys weighed less than did the others, given that weight loss was one of the 3 criteria of clinical diabetes. Also as expected with sexual dimorphism, male mangabeys weighed more than did female.
Pancreatic insular amyloidosis correlated with the clinical diagnosis of diabetes and with prediabetes (Table 4). A total of 16 animals necropsied had diabetes at the time of death. All had pancreatic amyloid, and 95% had more than 75% of their islets replaced with amyloid. In comparison, prediabetic mangabeys had less than 25% of the islet area replaced by amyloid, and 6 nondiabetics had detectable islet amyloid. Type 2 diabetes is suspected to occur in association with pancreatic insular amyloidosis, which may arise secondary to early replacement of islets with amyloid. Loss of β cells reduces insulin secretion and leads to hyperglycemia, which contributes to the progression of diabetes, concurrent with amyloidosis.15,20,23,28,49 Insular amyloidosis association with diabetes has been documented in cats, humans, and a few NHP15,23,49 and now in our colony of mangabeys. In cats, amyloidosis typically is reported as the sole contributor to diabetes,20,23,49 whereas in humans, amyloidosis is usually not the only cause of diabetes.15,28,53 Insular amyloidosis can be seen in as many as 90% of diabetic humans and as many as 33% of nondiabetic humans.15,28,45,53
Insular amyloidosis is more extensive in diabetic humans and diabetic animals than in nondiabetic subjects.15,20,23,28,32,43,48,49,52,53 For instance, some cynomolgus macaques with experimentally induced diabetes have been reported with severe insular amyloidosis. In contrast, nondiabetic cynomolgus macaques have minimal to no amyloidosis,49 similar to findings in our colony. Moreover, in our mangabeys amyloidosis was detected during postmortem examinations of all animals with clinical diabetes (Table 4). The severity of diabetes (that is, increased weight loss, relative hyperglycemia) was associated with the severity of amyloidosis. The uniform association of amyloidosis with diabetes in these mangabeys is unique and may account for some of the disease differences described in this population relative to other populations and species. The influence of amyloidosis on diabetes may be related to the lack of association between diabetes and sex, MPA exposure, and SIV status in our colony.
Mangabeys are a valuable resource. Because information from our colony can ultimately be applied to both conservation efforts and research, characterizing diabetes n our colony was a useful goal. The ability to accurately diagnose diabetic and prediabetic animals is essential for managing clinical care and aids in the selection of healthy animals for research studies, thus helping to avoid confounding variables. Identifying diabetic animals is a key component in the management of the breeding population for diabetes-associated pregnancy complications. Genetics have the potential to influence the high prevalence of diabetes because it can be linked to pancreatic insular amyloidosis and diabetes mellitus. Therefore identifying a link between genetics and these conditions in our mangabey colony might be necessary to minimize the breeding of diabetic animals.
In conclusion, this current study reveals that type 2 diabetes is a common finding in the mangabey population at our institution and appears to have a higher prevalence than that reported for other captive NHP species. In addition, the disease in mangabeys appears to have some unusual pathologic characteristics. Whereas fructosamine and triglyceride concentrations are significantly associated with diabetes, cholesterol and HbA1c are not. As expected, older age is a risk factor for an increased prevalence of diabetes. Being overweight did not influence the prevalence of diabetes. The uniform association of pancreatic insular amyloidosis and spontaneous clinical diabetes is a unique relationship that has not been reported in other NHP species. Future studies will explore pancreatic amyloid as an element of the etiology of the disease and evaluate potential diagnostic tools based on serum values such as serum amyloid A45 or on amyloid-sensitive imaging techniques.41,44 Genetic analyses of diabetes and amyloidosis as well as additional assessment of HbA1c and hemoglobin structure are planned also. Lipid panel evaluation to continue exploration of relationship between cholesterol diabetes, dietary analysis, and exploration of the associations between diabetes and long-term MPA exposure or pregnancy status are other possible future studies.
Acknowledgments
We thank the Division of Animal Resources at Yerkes National Primate Research Center, especially Veterinary Medicine, Colony Management and Pathology for assistance with sample collections and sample processing. This work was funded by the National Center for Research Resources (grant P51RR000165) and is currently supported by the Office of Research Infrastructure Programs (grant OD P51OD11132).
References
- 1.Ange-van Heugten KD, Burns R, Verstegen MW, Jansen WL, Ferket PR, van Heugten E. 2007. Evaluation of diabetes determinants in woolly monkeys (Lagothrix lagotricha). J Anim Physiol Anim Nutr (Berl) 91:481–491 [DOI] [PubMed] [Google Scholar]
- 2.Bauer SA, Leslie KE, Pearl DL, Fournier J, Turner PV. 2010. Retrospective case-control study of hyperglycemia in group-housed, mature female cynomolgus macaques (Macaca fascicularis). J Med Primatol 39:408–416 [DOI] [PubMed] [Google Scholar]
- 3.Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL. 2007. Advances in hemoglobin A1c point-of-care technology. J Diabetes Sci Technol 1:405–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brar I, Shuter J, Thomas A, Daniels E, Absalon J. 2007. A comparison of factors associated with prevalent diabetes mellitus among HIV-infected antiretroviral-naive individuals versus individuals in the National Health and Nutritional Examination Survey cohort. J Acquir Immune Defic Syndr 45:66–71 [DOI] [PubMed] [Google Scholar]
- 5.Butt AA, McGinnis K, Rodriguez-Barradas MC, Crystal S, Simberkoff M, Goetz MB, Leaf D, Justice AC. 2009. HIV infection and the risk of diabetes mellitus. AIDS 23:1227–1234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention. [Internet]. 2011. 2011 National diabetes fact sheet. [Cited 10 October 2012]. Available at: http://www.cdc.gov/diabetes/pubs/factsheet11.htm.
- 7.Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. 2012. Natural SIV hosts: showing AIDS the door. Science 335:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chahroudi A, Meeker T, Lawson B, Ratcliffe S, Else J, Silvestri G. 2011. Mother-to-infant transmission of simian immunodeficiency virus is rare in sooty mangabeys and is associated with low viremia. J Virol 85:5757–5763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science 325:201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cruzen CL, Baum ST, Colman RJ. 2011. Glucoregulatory function in adult rhesus macaques (Macaca mulatta) undergoing treatment with medroxyprogesterone acetate for endometriosis. J Am Assoc Lab Anim Sci 50:921–925 [PMC free article] [PubMed] [Google Scholar]
- 11.Davidson MB, Hu T, Sain G, Hoar B, Stevenson C, Hoogwerf BJ. 2009. The relationship of glycaemic control and triglycerides in patients with diabetes mellitus: a PreCIS database study. Diabetes Obes Metab 11:118–122 [DOI] [PubMed] [Google Scholar]
- 12.de la Hera JM, Garcia-Ruiz JM, Martinez-Camblor P, Martin M, Telleria AL, Corros C, Torres F, Fernandez-Cimadevilla OC, Alvarez-Pichel I, Capin E, Avanzas P, Delgado E. 2013. Real incidence of diabetes mellitus in a coronary disease population. Am J Cardiol 111:333–338. [DOI] [PubMed] [Google Scholar]
- 13.Genc S, Omer B, Aycan-Ustyol E, Ince N, Bal F, Gurdol F. 2012. Evaluation of turbidimetric inhibition immunoassay (TINIA) and HPLC methods for glycated haemoglobin determination. J Clin Lab Anal 26:481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gormus BJ, Wolf RH, Baskin GB, Ohkawa S, Gerone PJ, Walsh GP, Meyers WM, Binford CH, Greer WE. 1988. A second sooty mangabey monkey with naturally acquired leprosy: first reported possible monkey-to-monkey transmission. Int J Lepr Other Mycobact Dis 56:61–65 [PubMed] [Google Scholar]
- 15.Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, Tene-Perez CE, Goldschmidt L, Hart J, Perego C, Comuzzie AG, Tejero ME, Finzi G, Placidi C, La Rosa S, Capella C, Halff G, Gastaldelli A, DeFronzo RA, Folli F. 2009. Pancreatic islet amyloidosis, β-cell apoptosis, and α-cell proliferation are determinants of islet remodeling in type 2 diabetic baboons. Proc Natl Acad Sci USA 106:13992–13997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haffner SMAmerican Diabetes Association. 2004. Dyslipidemia management in adults with diabetes. Diabetes Care 27 Suppl 1:S68–S71 [DOI] [PubMed] [Google Scholar]
- 17.Hansen BC. 2010. The evolution of diabetes in nonhuman primates: comparative physiology implications for human type 2 diabetes mellitus (T2DM). FASEB J 24:1055 [Google Scholar]
- 18.Hansen BC, Bodkin NL. 1993. Primary prevention of diabetes mellitus by prevention of obesity in monkeys. Diabetes 42:1809–1814 [DOI] [PubMed] [Google Scholar]
- 19.Harvey PH, Clutton-Brock TH. 1985. Life history variation in primates. Evolution 39:559–581 [DOI] [PubMed] [Google Scholar]
- 20.Howard CF., Jr 1978. Insular amyloidosis and diabetes mellitus in Macaca nigra. Diabetes 27:357–364 [DOI] [PubMed] [Google Scholar]
- 21.Howard CF., Jr 1982. Correlations of hemoglobin A1c and metabolic status in nondiabetic, borderline diabetic, and diabetic Macaca nigra. Diabetes 31:1105–1108 [DOI] [PubMed] [Google Scholar]
- 22.Howard CF., Jr 1982. Nonhuman primates as models for the study of human diabetes mellitus. Diabetes 31:37–42 [DOI] [PubMed] [Google Scholar]
- 23.Hubbard GB, Steele KE, Davis KJ, 3rd, Leland MM. 2002. Spontaneous pancreatic islet amyloidosis in 40 baboons. J Med Primatol 31:84–90 [DOI] [PubMed] [Google Scholar]
- 24. Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press.
- 25.Janmaat KRL, Byrne RW, Zuberbühler K. 2006. Evidence for a spatial memory of fruiting states of rainforest trees in wild mangabeys. Anim Behav 72:797–807 [Google Scholar]
- 26.Johnson RN, Metcalf PA, Baker JR. 1983. Fructosamine: a new approach to the estimation of serum glycosylprotein. An index of diabetic control. Clin Chim Acta 127:87–95 [DOI] [PubMed] [Google Scholar]
- 27.Juraschek SP, Steffes MW, Selvin E. 2012. Associations of alternative markers of glycemia with hemoglobin A1c and fasting glucose. Clin Chem 58:1648–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kahn SE, Andrikopoulos S, Verchere CB. 1999. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes 48:241–253 [DOI] [PubMed] [Google Scholar]
- 29.Kaplan JR, Wagner JD. 2006. Type 2 diabetes—an introduction to the development and use of animal models. ILAR J 47:181–185 [DOI] [PubMed] [Google Scholar]
- 30.Klatt NR, Silvestri G, Hirsch V. 2012. Nonpathogenic simian immunodeficiency virus infections. Cold Spring Harb Perspect Med 2:a007153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuzuya T, Nakagawa S, Satoh J, Kanazawa Y, Iwamoto Y, Kobayashi M, Nanjo K, Sasaki A, Seino Y, Ito C, Shima K, Nonaka K, Kadowaki T. 2002. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. Diabetes Res Clin Pract 55:65–85 [DOI] [PubMed] [Google Scholar]
- 32.Kwon HJ, Park MS, Shin NS, Cho DY, Kim DY, Choi YK. 2005. Spontaneous pancreatic islet amyloidosis in a greater white-nosed guenon (Ceropithecus nictitans). J Toxicol Pathol 18:61–63 [Google Scholar]
- 33.Lendrum AC, Slidders W, Fraser DS. 1972. Renal hyalin. A study of amyloidosis and diabetic fibrinous vasculosis with new staining methods. J Clin Pathol 25:373–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macdonald DR, Hanson AM, Holland MR, Singh BM. 2008. Clinical impact of variability in HbA1c as assessed by simultaneously measuring fructosamine and use of error-grid analysis. Ann Clin Biochem 45:421–425 [DOI] [PubMed] [Google Scholar]
- 35.Marigliano M, Casu A, Bertera S, Trucco M, Bottino R. 2011. Hemoglobin A1C percentage in nonhuman primates: a useful tool to monitor diabetes before and after porcine pancreatic islet xenotransplantation. J Transplant 2011:965605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinot AJ, Meythaler M, Pozzi LA, Dalecki Boisvert K, Knight H, Walsh D, Westmoreland S, Anderson DC, Kaur A, O'Neil SP. 2013. Acute SIV infection in sooty mangabey monkeys is characterized by rapid virus clearance from lymph nodes and absence of productive infection in germinal centers. PLoS ONE 8:e57785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McTighe MS, Hansen BC, Ely JJ, Lee DR. 2011. Determination of hemoglobin A1c and fasting blood glucose reference intervals in captive chimpanzees (Pan troglodytes). J Am Assoc Lab Anim Sci 50:165–170 [PMC free article] [PubMed] [Google Scholar]
- 38.Mittman N, Desiraju B, Fazil I, Kapupara H, Chattopadhyay J, Jani CM, Avram MM. 2010. Serum fructosamine versus glycosylated hemoglobin as an index of glycemic control, hospitalization, and infection in diabetic hemodialysis patients. Kidney Int Suppl (117):S41–S45 [DOI] [PubMed] [Google Scholar]
- 39.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III)—final report. Circulation 106:3143–3421 [PubMed] [Google Scholar]
- 40.Neeland IJ, Turer AT, Ayers CR, Powell-Wiley TM, Vega GL, Farzaneh-Far R, Grundy SM, Khera A, McGuire DK, de Lemos JA. 2012. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308:1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Onur MR, Yalniz M, Poyraz AK, Ozercan IH, Ozkan Y. 2012. Pancreatic islet cell amyloidosis manifesting as a large pancreas. Korean J Radiol 13:94 –97. [DOI] [PMC free article] [PubMed]
- 42.Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, Vinton C, Gordon SN, Bosinger SE, Francella N, Hallberg PL, Cramer E, Schlub T, Chan ML, Riddick NE, Collman RG, Apetrei C, Pandrea I, Else J, Munch J, Kirchhoff F, Davenport MP, Brenchley JM, Silvestri G. 2011. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat Med 17:830–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palotay JL, Howard CF., Jr 1982. Insular amyloidosis in spontaneously diabetic nonhuman primates. Vet Pathol Suppl 7:181–192 [PubMed] [Google Scholar]
- 44.Rabinovici GD, Jagust WJ. 2009. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol 21:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rice KA, Chen ES, Metcalf Pate KA, Hutchinson EK, Adams RJ. 2013. Diagnosis of amyloidosis and differentiation from chronic, idiopathic enterocolitis in rhesus (Macaca mulatta) and pig-tailed (M. nemestrina) macaques. Comp Med 63:262–271 [PMC free article] [PubMed] [Google Scholar]
- 46.Tharavanij T, Froud T, Leitao CB, Baidal DA, Paz-Pabon CN, Shari M, Cure P, Bernetti K, Ricordi C, Alejandro R. 2009. Clinical use of fructosamine in islet transplantation. Cell Transplant 18:453–458 [DOI] [PubMed] [Google Scholar]
- 47.Verhaeghe J. 2010. Hormonal contraception in women with the metabolic syndrome: a narrative review. Eur J Contracept Reprod Health Care 15:305–313 [DOI] [PubMed] [Google Scholar]
- 48.Wagner JD, Carlson CS, O'Brien TD, Anthony MS, Bullock BC, Cefalu WT. 1996. Diabetes mellitus and islet amyloidosis in cynomolgus monkeys. Lab Anim Sci 46:36–41 [PubMed] [Google Scholar]
- 49.Wagner JD, Cline JM, Shadoan MK, Bullock BC, Rankin SE, Cefalu WT. 2001. Naturally occurring and experimental diabetes in cynomolgus monkeys: a comparison of carbohydrate and lipid metabolism and islet pathology. Toxicol Pathol 29:142–148 [DOI] [PubMed] [Google Scholar]
- 50.Wagner JE, Kavanagh K, Ward GM, Auerbach BJ, Harwood HJJ, Kaplan JR. 2006. Old world nonhuman primate models of type 2 diabetes mellitus. ILAR J 47:259–271 [DOI] [PubMed] [Google Scholar]
- 51.Wejdmark AK, Bonnett B, Hedhammar A, Fall T. 2011. Lifestyle risk factors for progesterone-related diabetes mellitus in elkhounds— a case-control study. J Small Anim Pract 52:240–245 [DOI] [PubMed] [Google Scholar]
- 52.Westermark P, Andersson A, Westermark GT. 2011. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev 91:795–826 [DOI] [PubMed] [Google Scholar]
- 53.Westermark P, Grimelius L. 1973. The pancreatic islet cells in insular amyloidosis in human diabetic and nondiabetic adults. Acta Pathol Microbiol Scand [A] 81:291–300 [DOI] [PubMed] [Google Scholar]
- 54.Wiedmeyer CE, DeClue AE. 2011. Glucose monitoring in diabetic dogs and cats: adapting new technology for home and hospital care. Clin Lab Med 31:41–50 [DOI] [PubMed] [Google Scholar]
- 55.Williams-Fritze MJ, Smith PC, Zelterman D, Scholz JA. 2011. Fructosamine reference ranges in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 50:462–465 [PMC free article] [PubMed] [Google Scholar]