Abstract
Selective serotonin reuptake inhibitors (SSRI) are associated with an increased bleeding risk in humans. This report describes a bleeding event in a cynomolgus macaque (Macaca fascicularis) treated with the SSRI sertraline HCl (Zoloft). During the treatment course, the subject presented with a maculopapular rash, cutaneous bleeding, epistaxis, bleeding from the eye, melena, and a severe thrombocytopenia. To our knowledge, this report is the first description of an SSRI-related adverse event in a nonhuman primate. This report demonstrates that the clinical presentation of SSRI-associated bleeding in cynomolgus macaques is consistent with that reported in humans and that complications from SSRI treatment should be considered as a differential diagnosis for maculopapular dermatitis or spontaneous bleeding in this species.
Abbreviations: SSRI, selective serotonin reuptake inhibitor; NHP, nonhuman primate
Antidepressant use rose 400% between the early 1990s and mid-2000s, making them the third most commonly prescribed drug in America and the most frequently used drug in persons 18 to 44 y of age.7 Selective serotonin reuptake inhibitors (SSRI) are one of the most commonly prescribed antidepressants.8 Despite a favorable safety profile, there is increasing evidence that SSRI may precipitate bleeding events. A 2005 search in VigiBase, the World Health Organization Adverse Drug Reporting database, produced 4847 cases of SSRI-associated bleeding disorders. The most common entries were for bruising, epistaxis, petechiation, prolonged bleeding time, and genitourinary bleeding, but there were also reports of upper gastrointestinal bleeding, cerebral hemorrhage, and death.3 Cutaneous manifestations of a vascular reaction have been noted also and include cutaneous pruritus, urticaria, angioedema, erythema multiforme, and erythema nodosum.6
The mechanism by which SSRI induce bleeding likely is rooted in the blockade of platelet serotonin reuptake.2 SSRI downregulate serotonin transporters on platelets,2 just as they do in neurons, producing a reversible serotonin deficiency in these cells.11 Serotonin plays a crucial role in amplifying platelet activation and promoting aggregation.10 Bleeding results from inadequate platelet plug formation and subsequent dysregulation of primary hemostasis.
The current case report is the first description of an SSRI-related adverse event in a nonhuman primate (NHP), a cynomolgus macaque (Macaca fascicularis). Adult female cynomolgus monkeys are an established nonhuman primate model of depression.12 The present report describes bleeding associated with the administration of the SSRI sertraline HCl in an adult female cynomolgus macaque.
Case Report
The subject of the current case report was a 19-y-old, socially housed, intact female cynomolgus macaque that was part of a randomized study investigating the effect of sertraline, a commonly prescribed SSRI, and depression on cardiovascular health. All monkeys were imported from the Institut Pertanian (Bogor, Bogor, Indonesia). Social groups consisted of 3 to 5 monkeys housed in indoor pens maintained under the following conditions: 12:12-h light:dark cycle and controlled temperature (68 to 84 °F [30.0 to 28.9 °C]); humidity (30% to 70%), and ventilation (10 to 15 air exchanges hourly). All animal manipulations were performed according to the guidelines of state and federal laws, the US Department of Health and Human Services, and the Animal Care and Use Committee of Wake Forest University School of Medicine. Wake Forest University is fully accredited by AAALAC.
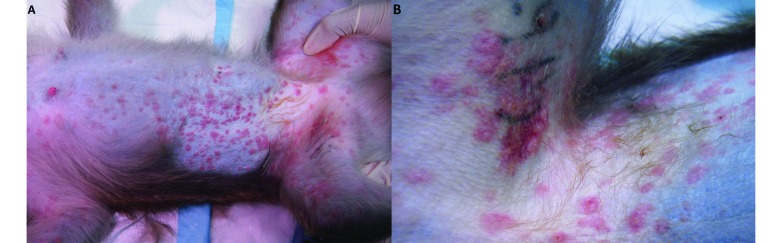
The subject was categorized as nondepressed9 and randomized to the sertraline treatment group. Dosage of oral sertraline began at 5 mg/kg and increased weekly by 5 mg/kg. On day 7 of the 15-mg/kg dose, the subject presented with an erythematous maculopapular rash that affected all areas of body except the feet, hands, and tail (Figure 1). Skin scrapes were negative. A punch biopsy was obtained from the ventral abdomen. Histologic evaluation revealed no evidence of infectious disease, and immunohistochemical staining for measles virus was negative. The subject was treated with a miconazole–chlorhexidine shampoo bath, diphenhydramine, and ketoprofen.
Figure 1.
Cutaneous manifestation of a SSRI-associated adverse event in a cynomolgus monkey (Macaca fasicularis). Notice the (A) whole-body erythematous maculopapular dermatitis with (B) ecchymosis over the right inguinal region.
The following day, the rash seemed to have decreased, and sertraline dosing was increased to 20 mg/kg. Three days later, the subject represented with bilateral epistaxis and bruising of the right upper arm. She became lethargic and showed signs of decreased perfusion (pale and tacky mucus membranes; capillary refill time, 4 s). Physical exam revealed ecchymosis on the right arm and inguinal area and blood was present beneath the left eye. Blood work revealed a normochromic anemia with a severe thrombocytopenia (41,000 platelets/μL). The subject received an emergency transfusion of whole blood, diphenhydramine, vitamin B, and iron dextran. Although viral titers were positive for herpes B virus and simian varicella virus, the development of severe bleeding despite improved dermatologic signs suggested an adverse SSRI-related event, and experimental sertraline dosing was discontinued.
After 5 d of continued bleeding (bilateral epistaxis and melena), anemia, and spherocytosis (noted on a peripheral blood smear), the subject was started on an immunosuppressive dose of dexamethasone. Nasal culture results, obtained to rule out Moraxella catarrhalis as a causative agent for the epistaxis, came back positive for β-hemolytic Streptococcus (4+) and coagulase-positive Staphylococcus (4+). A 7-d course of enrofloxacin was added to the treatment protocol, and there were no additional bleeding episodes. At 3 wk after the initial presentation, follow-up blood work showed evidence of a regenerative anemia and a normal platelet count. Secondary hemostasis tests (prothrombin time, activated partial thromboplastin time) remained within normal limits during the treatment period. The subject did not return to sertraline dosing and was followed for an additional year without reoccurrence of clinical signs before termination of the study. As a result, her clinical presentation was attributed to an adverse SSRI-related event.
Discussion
Adult female macaques develop social stress-associated depression that closely resembles human depression in physiologic, neurobiologic, and behavioral characteristics, including altered serotonergic function.12 For this reason, they are used as a NHP model for studying the physiologic effects of depression on human health and evaluating possible therapeutic interventions, such as SSRI use. The present case provides evidence that SSRI-associated bleeding can occur in macaques and has clinical signs similar to those in humans, including cutaneous alterations, spontaneous bleeding, and upper gastrointestinal bleeding.
The initial clinical sign in this case was an erythematous maculopapular rash. In humans, the most commonly reported cutaneous adverse reaction associated with SSRI is cutaneous bleeding, but other vascular reactions have been reported.6 A mild to moderate macular or exanthematic rash, similar to that observed in our subject, has been described in human subjects during the first weeks of SSRI treatment.6 Ecchymosis and bruising, both manifestations of cutaneous bleeding, were present in our macaque as well. Other commonly reported forms of spontaneous bleeding include epistaxis, gastrointestinal bleeding, and cerebral bleeding.3 The case reported here included a 5-d history of epistaxis, melena, and potential bleeding from the left eye. Subhyaloid hemorrhage has been linked anecdotally to SSRI use in humans.13 Like those in people, all clinical signs associated with our case subsided after discontinuation of the SSRI.6
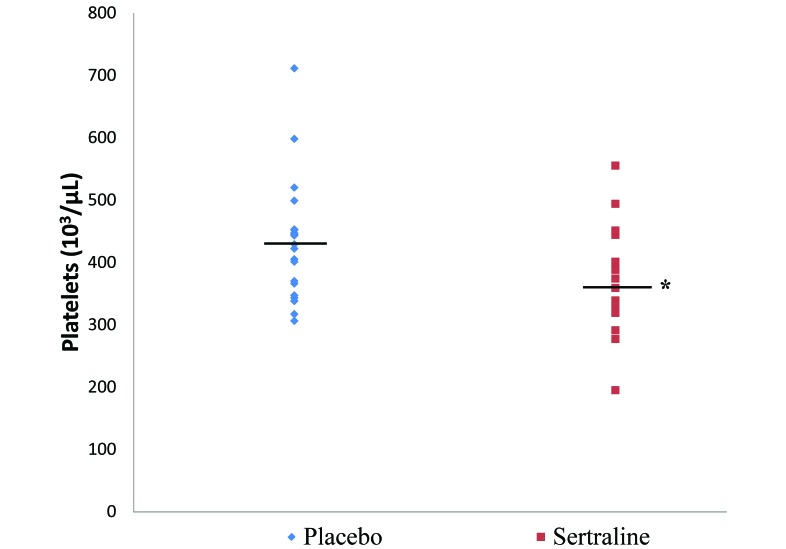
With regard to laboratory assessment of hemostasis, the most common aberrations in human cases of SSRI-associated bleeding are decreased platelet aggregability and activity and prolonged bleeding time. We did not perform bleeding time or platelet aggregation tests in this macaque. The most severe hemostatic aberration in our subject was a decreased platelet count (41,000 platelets/μL), which was evident after 1 wk of sertraline dosing at 20 mg/kg. On average, platelet counts in the sertraline treatment group were increased by 8% (compared with values obtained prior to drug treatment) 1 wk into the 20 mg/kg dose. This increase may represent a stress-reactive thrombocytosis. At the start of treatment, all of the macaques on study were still becoming acclimated to running into dosing cages for daily dosing; this period is presumed to be a stressful adjustment period. Unfortunately, CBC analyses were not done in macaques that received a placebo, and it is not possible to confirm that the thrombocytotic effect was independent of any drug effects. After 1 y of treatment, the sertraline treatment group displayed significantly reduced platelet counts when compared with placebo controls (sertraline group mean ± SE, 360,000 ± 22 platelets/μL; placebo group mean ± SE, 432,000 ± 20 platelets/μL; P = 0.02; Figure 2). A decrease in platelet count is reported occasionally in human cases of SSRI-related bleeding.5 Drug-induced immune thrombocytopenia has been proposed as a mechanism for bleeding in SSRI-treated patients,1 and the spherocytosis noted suggests that there may have been an immune-mediated component to this case. However, spherocytes have not been reported in the human literature and may have been transfusion-related in our macaque. Similar to the case reported here, secondary markers of hemostasis are generally unaltered in human cases.5
Figure 2.
Platelet counts after 1 y of treatment with 20 mg/kg sertraline HCl (Zoloft; n = 21) or placebo (n = 22) treatment. Horizontal bar indicates group mean; *, P = 0.02 (Student t test) compared with placebotreatment group.
It is commonly accepted that SSRI use is accompanied by an increased risk of bleeding due to blockage of serotonin reuptake in platelets and subsequent platelet dysfunction. Although SSRI-associated bleeding events are becoming more frequent, they still are considered rare.8 Voluntary reporting of SSRI-related adverse events from healthcare professionals and consumers results in underreporting of bleeding episodes. In addition, mild reactions, such as cutaneous or upper gastrointestinal bleeding, may not be attributed to SSRI use. The overall effect may be an underestimation of the risk for serious bleeding associated with not just SSRI use but also any antidepressant that modifies serotonin reuptake.5 The case we present here illustrates the complex clinical profile associated with SSRI use and the need for awareness of SSRI-induced bleeding in both human and veterinary medicine.
The current case also highlights the importance of including SSRI-related adverse reactions as a differential diagnoses for maculopapular dermatitis or spontaneous bleeding in NHP. Other differentials for our subject's presentation included a contact dermatitis, Staphlococcus dermatitis, measles virus, simian varicella virus, herpes B virus, Moraxella catarrhalis, gastric ulceration, diverticulitis, and gastrointestinal adenocarcinoma in light of the dermatitis, epistaxis, and melena. Although this case report is the first description of an SSRI-related adverse reaction in any veterinary species, the number of NHP at risk is on the rise. Drugs that affect the serotonin system are being evaluated experimentally more frequently in NHP. In addition, SSRI are increasingly used to treat behavioral disorders (for example, self-injurious behavior) in captive NHP.4 Male macaques treated with the SSRI fluoxetine demonstrate reductions in self-injurious behavior and platelet serotonin.4 Furthermore, drugs that affect the serotonin system are commonly used to treat compulsive behaviors in other veterinary species; therefore SSRI-related adverse reactions must be considered.
Acknowledgments
We acknowledge the following people for their technical support: Beth Uberseder, Dana Morgan, Amanda Gogolak, Stephen Loiacono, and Stephanie Willard. This work was supported in part by NIH grants ROIHL87103, R21MH086731, and T32 OD 010957. The contents are solely the responsibility of the authors and do not necessarily represent the view of the NIH.
References
- 1.Andersohn F, Konzen C, Bronder E, Klimpel A, Garbe E. 2009. Citalopram-induced bleeding due to severe thrombocytopenia. Psychosomatics 50:297–298 [DOI] [PubMed] [Google Scholar]
- 2.Bakish D, Cavazzoni P, Chudzik J, Ravindran A, Hrdina PD. 1997. Effects of selective serotonin reuptake inhibitors on platelet serotonin parameters in major depressive disorder. Biol Psychiatry 41:184–190 [DOI] [PubMed] [Google Scholar]
- 3.De Abajo FJ, Montero D, Rodríguez LA, Madurga M. 2006. Antidepressants and risk of upper gastrointestinal bleeding. Basic Clin Pharmacol Toxicol 98:304–310 [DOI] [PubMed] [Google Scholar]
- 4.Fontenot MB, Musso MW, McFatter RM, Anderson GM. 2009. Dose-finding study of fluoxetine and venlafaxine for the treatment of self-injurious and stereotypic behavior in rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 48:176–184 [PMC free article] [PubMed] [Google Scholar]
- 5.Halperin D, Reber G. 2007. Influence of antidepressants on hemostasis. Dialogues Clin Neurosci 9:47–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krasowska D, Szymanek M, Schwartz RA, Myśliński W. 2007. Cutaneous effects of the most commonly used antidepressant medication, the selective serotonin reuptake inhibitors. J Am Acad Dermatol 56:848–853 [DOI] [PubMed] [Google Scholar]
- 7.National Center for Health Statistics. 2011. Health, United States, 2010: with special feature on death and dying. Hyattsville (MD): National Center for Health Statistics; [PubMed] [Google Scholar]
- 8.Serebruany VL. 2006. Selective serotonin reuptake inhibitors and increased bleeding risk: are we missing something? Am J Med 119:113–116 [DOI] [PubMed] [Google Scholar]
- 9.Shively CA, Laber-Laird K, Anton RF. 1997. Behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry 41:871–882 [DOI] [PubMed] [Google Scholar]
- 10.Skop BP, Brown T. 1996. Potential vascular and bleeding complications of treatment with selective serotonin reuptake inhibitors. Psychosomatics 37:12–16 [DOI] [PubMed] [Google Scholar]
- 11.Wagner A, Montero D, Martensson B, Siwers B, Asberg M. 1990. Effects of fluoxetine treatment of platelet 3H-imipramine binding, 5-HT uptake, and 5-HT content in major depressive disorder. J Affect Disord 20:101–113 [DOI] [PubMed] [Google Scholar]
- 12.Willard SL, Shively CA. 2012. Modeling depression in adult female cynomolgus monkeys (Macaca fascicularis). Am J Primatol 74:528–542 [DOI] [PubMed] [Google Scholar]
- 13.Wilmshurst PT, Kumar AV. 1996. Subhyaloid haemorrhage with fluoxetine. Eye (Lond) 10:141. [DOI] [PubMed] [Google Scholar]