Abstract
Immunological memory is a hallmark of adaptive immunity, a defense mechanism endowed to vertebrates during evolution. However, an autoimmune pathogenic role of memory lymphocytes is also emerging with accumulating evidence, despite reasonable skepticism on their existence in a chronic setting of autoimmune damage. It is conceivable that autoimmune memory would be particularly harmful since memory cells would constantly “remember” and attack the body's healthy tissues. It is even more detrimental given the resistance of memory T cells to immunomodulatory therapies. In this review, we focus on self-antigen-reactive CD4+ effector memory T (TEM) cells, surveying the evidence for the role of the TEM compartment in autoimmune pathogenesis. We will also discuss the role of TEM cells in chronic and acute infectious disease settings and how they compare to their counterparts in autoimmune diseases. With their long-lasting potency, the autoimmune TEM cells could also play a critical role in anti-tumor immunity, which may be largely based on their reactivity to self-antigens. Therefore, although autoimmune TEM cells are “bad” due to their role in relentless perpetration of tissue damage in autoimmune disease settings, they are unlikely a by-product of industrial development along the modern surge of autoimmune disease prevalence. Rather, they may be a product of evolution for their “good” in clearing damaged host cells in chronic infections and malignant cells in cancer settings.
Keywords: Autoimmunity, Tumor, T cells, Memory, CTLA4, Genomic
Introduction
Autoimmune diseases, wherein the body's immune system attacks self-tissues, collectively afflict 5–10 % of the world's developed population. The incidence of many autoimmune diseases, including type 1 diabetes (T1D) [1], system lupus erythematosus (SLE) [2] and multiple sclerosis (MS) [3] have been increasing over the past decade and is estimated to increase further in the coming years [4]. The exact cause of this surge remains unclear, but environmental changes associated with industrialization have long been suspected. Those changes include sanitization from parasitic and microbial agents, as formulated in the hygiene hypothesis [5], and industrial pollutants that may alter the differentiation of immune cells [6–8]. Despite advances in treating autoimmune diseases, many of them involve general immunosuppression, leading to adverse side effects. A better understanding of the immunological causes of autoimmune diseases is needed for developing therapies that specifically target the pathogenic immuno-logical subsets responsible for the autoimmune attack.
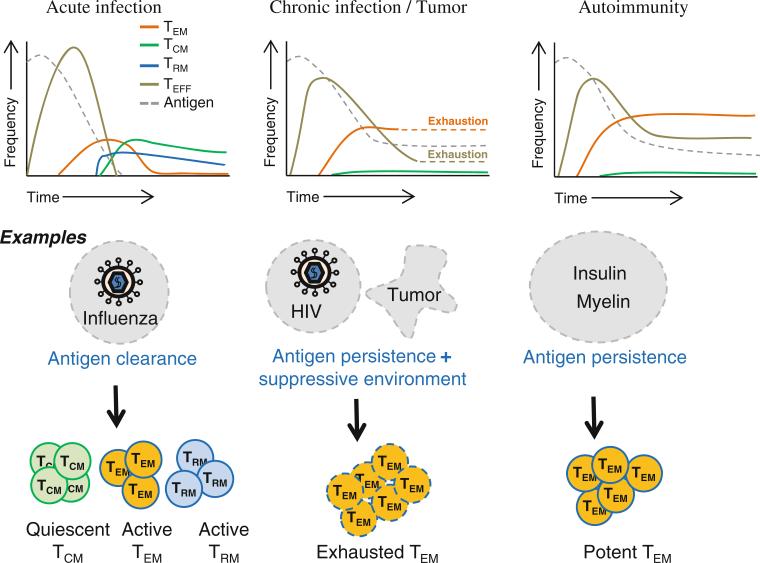
Over the past decade, it has come to light that immunological memory can exist in the context of autoimmunity as well. It represents a “constant-remembrance” of self-antigen that may account for the persistence of autoimmune attack. Combating autoimmune memory has been a challenge not just in autoimmune diseases but also in transplantation, where the autoimmune memory cells attack the transplanted tissue. This review gives a brief overview of the different subsets of memory T cells and discusses in detail the effector memory T (TEM) cell subset that is emerging as a major contributor to autoimmune pathogenicity. We will also explore the prospect that autoimmune memory responses, while pathogenic in autoimmune diseases, could be put to good use in anti-tumor responses (Fig. 1).
Fig. 1.
Predominant subsets of memory T cells in infections, tumors and autoimmune diseases. (1) Persistence of antigens in chronic infections, tumor microenvironment and autoimmune disease settings leads to the increased formation of the effector memory T (TEM) cell subset. (2) TEM cells are exhausted in settings of chronic infections and tumors. (3) In autoimmune diseases, TEM cells are not exhausted, making them highly pathogenic. Their longevity and active functionality perpetuate autoimmune damage. (4) Persistence of antigens in chronic infections, tumors and autoimmune disease settings diminishes the formation of the central memory T (TCM) cells and possibly the tissue-resident memory T (TRM) cell subsets. (5) Immune control genes such as CTLA4 are highly polymorphic, and the polymorphisms that may promote TEM cells are well preserved in human populations despite their deleterious potential in causing autoimmune diseases. These genetic variations suggest that the differentiation of autoimmune TEM cells may have evolved for their beneficial potential in clearing unhealthy cells in chronic infections or boosting anti-tumor immunity. Further studies are needed to bridge the genetic discoveries to immunobiology and pathophysiology
T lymphocytes in autoimmune diseases
The involvement of the adaptive immune system in auto-immune diseases has been extensively characterized. T cells are critical contributors to autoimmune diseases, including T regulatory (Treg) cells that inhibit disease development and conventional T (Tconv) cell subsets that play a role in B cell activation and differentiation, produce various inflammatory cytokines and destroy target cells with direct cytotoxicity. An important characteristic of the adaptive immune response is the formation of immunological memory after initial antigen exposure that helps the immune system learn with experience. Naïve Tconv cells, upon antigen exposure, can differentiate into effector T (TEFF) cells, TEM cells, tissue-resident memory T (TRM) cells and central memory T (TCM) cells. The T cell clones carrying the T cell receptor (TCRs) that recognize antigens most effectively are preserved in the form of long-lived memory T cells. On secondary antigen exposure, these expanded clones help mount a quicker and stronger immune response against invading pathogens. However, immunological memory is a double-edged sword. In an autoimmune response, when memory cells are formed against the “self,” they help mount a highly efficient pathogenic response against the body's own tissues. These memory cells, by virtue of being long lived also become very difficult to eliminate. It is believed that these memory cells play a critical role in making the autoimmune response persistent.
A case of systemic autoimmune disease: systemic lupus erythematosus
System lupus erythematosus is an autoimmune disease characterized by immune tolerance breakdown in both T and B lymphocyte compartments. Extensive studies have implicated both the innate and adaptive immune branches in SLE pathogenesis [9–13], but mechanisms that drive sustained systemic autoimmune damage remain unclear. Decades of research has unequivocally established a major role of B lymphocytes in SLE pathogenesis, culminating in the recent FDA approval of SLE treatment by neutralizing BlyS (BAFF), a B cell survival cytokine [12, 14]. CD4+ T cells are believed to play an important role in helping the activation of autoreactive B cells and their further differentiation into antibody-secreting cells [15–17] which produce an array of self-antigen-specific antibodies, in particular those against cell nuclear materials. Those antibodies cause multi-organ damage in advanced stages of SLE [9–12]. Dysfunction in a special subset of T helper cells, T follicular helper (TFH) cells, which play an important role in B cell differentiation and maturation in the germinal centers, has also been implicated in SLE pathogenesis in both mouse models and human patients [18–21]. Whether T cells continue to play a pathogenic role in late stage SLE remains to be clarified, an issue carrying substantial relevance in SLE treatment. There is evidence suggesting the involvement of CD4+ TEM cells in SLE pathogenesis [22]. Understanding the role of CD4+ TEM cells in advanced SLE could lead to new therapeutic targets.
A case of organ-specific autoimmune disease: type 1 diabetes
Type 1 diabetes is amongst the best studied T cell-driven autoimmune diseases where pathogenic T cells target the insulin-producing beta cells of pancreatic islets. The MHC class II haplotype is the major genetic contributor to T1D susceptibility [23–26] which indicates the importance of the CD4+ T cell compartment in the autoimmune response in T1D. Studies involving MHC class I transgenic mice and CD8-restricted TCR transgenic models have also shown the involvement of CD8+ T cells in the response [27, 28]. CD4+ Treg cells help control the autoimmune pathogenic T cell responses by regulating antigen presentation, direct cell–cell inhibition of T effectors and by production of anti-inflammatory mediators like IL10 and TGF-β. Their dys-function has been observed as a potential contributor to T1D pathogenesis, in experimental mouse models of T1D and in human patients with T1D [29–33]. Recent studies have also shown that CD4+ Treg cells can further be divided functionally into effector and memory subsets [34], showing that regulatory memory T cells in the tissue are more potent at suppressing the autoimmune response when compared to regulatory TEFF cells.
A number of studies suggest that the autoimmune CD4+ memory T cells are pathogenic in T1D [35–37], although the exact contribution of each subset remains to be further studied. These cells have the potential to secrete cytokines like IFN-γ and IL17 which have been shown to promote T1D pathogenesis [38]. Autoimmune memory T cells persist even after complete destruction of pancreatic islets of the patient. They may also be the major cause of transplant rejection in T1D patients with islet transplants [39, 40].
T cell memory
Upon antigen exposure, Naïve T cells undergo clonal expansion to become activated TEFF cells. Most of TEFF cells migrate to the site of infection where they tackle the invading pathogens. Once the infection is cleared, the majority of the TEFF cells generated by clonal expansion die. Along the process, some of the antigen-activated cells become long-lived memory T cells. Whether and to what extent the memory compartment is derived from TEFF cells remain debated.
Memory T cells have been classically delineated into two subsets: TCM and TEM cells. TCM cells are considered to be long-lived memory T cells with greater proliferation potential and are predominant in the secondary lymphoid organs. Compared to TCM cells, TEM cells are relatively short-lived, have lesser proliferation potential but possess a range of effector functions and are predominant in the target tissues. They are in a state of readiness to respond to antigen re-challenge at the tissue much faster than TCM cells. In typical settings of acute infectious diseases, the TEM compartment declines with time after antigen exposure, but a stable quiescent population of TCM can give rise to a secondary immune effector response even decades after initial antigen exposure [41]. On the other hand, in autoimmune disease settings, as in chronic infections, the constant presence of antigen at the tissue may preclude the development of traditional memory response, particularly the formation of TCM compartment [42, 43].
While they are a long-lived subset, quiescent TCM cells need to be re-activated on antigen encounter, in order to produce a TEFF population and migrate to the target tissue to execute their functions. An expedited memory response could be mounted if memory cells are present in lymph nodes that drain the target tissues, or within the target tissue itself, with an effector arsenal ready to function. Such subsets of memory cells indeed exist in the form of TEM and TRM cells.
A well-justified doubt on existence of memory T cells in autoimmune settings: lack of TRM and TCM cells?
TRM cells are a new subset of memory T cells, recently identified as the population of T cells that permanently resides in the tissue even after the infection is cleared [44– 47]. These memory cells have poor proliferation potential and survival when compared to TCM and TEM cells and appear to be terminally differentiated [48]. Besides these differences, the functional difference between TEM and TRM cells at the tissue is unclear. The basic distinction seems to be that while the TEM cells can recirculate between the spleen, blood and tissues, the TRM cells can migrate only within the tissue [44–47]. CD69 and CD103 are the phenotypic markers that define TRM cells. These molecules may also be involved in the formation/persistence of the TRM compartment. Hence, the three different memory subsets are phenotypically defined as—TRM: CD44hiCD62L−CD69+CD103+, TCM: CD44hiCD62L+ CD69−CD103−CD127+, and TEM: CD44hiCD62L−CD69-CD103−CD127+ [48]. TEFF cells generated during the response at the tissue phenotypically (CD44hiCD62L CD69+CD103+CD127−) resemble TRM cells, but it is assumed that antigen-specific cells present in the tissue long after antigen clearance belong to the TRM compartment since TEFF cells are short-lived cells.
One of the first studies identifying CD8+ TRM cells in a HSV infection model showed that the CD8+ TRM antigen-specific cells persisted for more than 100 days after primary infection in the absence of antigen. This TRM subset formed the first line of defense against reinfection and provided site-specific immunity at the particular tissue up to 100 fold better than in a site without previously generated TRM cells [45]. Similar results were obtained in a CD4+ T cell response, where CD4+ TRM cells were far superior in terms of protection from influenza reinfection [47]. Thus, protection mediated by both CD4+ and CD8+ TRM subsets has been shown in various models of infection in various tissues [44–49]. The TRM subset has also been identified in humans [50]. Though there is strong evidence for the existence of TRM cells in many different tissues in different settings of infection, their existence in the context of autoimmune diseases has yet to be clearly demonstrated. Akin to how the constant presence of antigen may preclude the development of TCM cells, constant self-antigen exposure may preclude the formation of TRM cells as well [51].
TEM defined in infectious disease settings
TEM cells are relatively long-lived and have higher proliferation potential when compared to TRM cells. They can undergo self-renewal using IL7 and IL15 with their CD127 and CD122 receptors like TCM cells [52] and also possess immediate effector functionality like the TEFF subset. They circulate between the spleen, blood and peripheral tissue and are thus able to mediate the first line of defense against pathogens at the site of tissue invasion. It has been shown that CD4+ TEM cells that persist in lung tissues and airways after a respiratory virus infection can substantially protect against secondary virus infections [53]. In a model of intranasal parainfluenza virus infection, it was shown that the CD8+ TEM cells played a more prominent role than the CD8+ TCM cells in recall responses in the lung [54]. Another subset that forms a part of the CD8+ TEM compartment, the CD8+CD44+CD27loCD43lo subset, localizes to the tissue compartment. This compartment has been shown to have immediate perforin-dependent effector functionality and is thus able to mediate superior protection against bacterial and viral infections [55]. Thus, even in acute infections where a functional TCM subset is formed, the TEM subset plays an important role at the front line of immune defense until the TCM subset is able to generate a second wave of effectors.
Studies from chronic viral infections like CMV infections have shown that persistence of high levels of antigen favors the development of the TEM cells [42, 56]. These chronic infections are characterized by a life-long persistence of a high frequency of CD4+ and CD8+ TEM cells that prevent disease progression though they cannot completely eliminate the pathogen [56, 57]. This inability to eliminate pathogen has been attributed to the exhaustion of the TEM compartment due to the persistence of antigen in chronic infections, which has lower functionality when compared to TEM cells from acute infections [58]. It has been shown that CD4+ and CD8+ TEM responses help combat HIV so much so that current vaccine strategies aim to specifically increase the HIV-epitope-specific CD4+ TEM and CD8+ TEM cells. This vaccine strategy that elicited continuous TEM immune surveillance has been effective in clearing highly pathogenic SIV infection in 50 % of rhesus macaques [59, 60].
Thus, the TEM population presents itself as a highly effective, antigen-specific memory subset capable of eliciting effector functions on pathogen encounter at the tissue in both chronic and acute infections. The TEM subset represents the balance between the long-lived quiescent TCM subset, which require re-activation despite their superior proliferation potential, and the TEFF subset which posses potent effector function but are short-lived and have poor proliferation potential. These TEM cells form the front line of defense against many invading pathogens at the tissue and thus often determine the final outcome of the immune response.
TEM as the main subset of memory T cells in autoimmune conditions
T cell-mediated pathogenicity in autoimmune diseases is most likely brought about by both TEFF and TEM subsets because the persistence of auto-antigen precludes the formation of autoimmune TCM and TRM cells. Between the TEFF and TEM cells, TEM cells likely drive the persistence of autoimmune diseases because of their ready effector functionality and relative longevity. As shown in studies of chronic infections, persistent antigen increases the pool of TEM cells, which would be the case in autoimmune disease settings as well with persistence of self-antigens.
Recent evidence from gene expression studies suggests substantial contribution of the CD4+ TEM compartment to autoimmune disease pathogenesis. Genome-wide association studies (GWAS) have implicated many candidate immune genes in autoimmune pathogenesis, including CTLA4, BACH2 and PD-1. However, it remains unknown as to which specific cell compartment is affected by the genetic variations. The identified gene may have functions in many different cell types, making it hard to associate the genetic polymorphism identified from the GWAS with a mechanism of disease pathogenesis. In this regard, genome-wide gene expression analyses of distinct cell subsets, like the immunological genome project [61], could offer helpful insights. In particular, studies can be conducted to link the pool of disease-susceptible gene polymorphisms identified with profiles of genes expressed in distinct cell types. One such recent study analyzing gene expression data from pathogenic cell types in auto-immune diseases has been able to show the enrichment of CD4+ TEM-cell-associated genes within SLE loci, Crohn's loci and rheumatoid arthritis (RA) loci [62]. In another study, RA susceptibility loci identified by high-density genetic mapping contained genes that were most significantly expressed in CD4+ TEM cells [63]. When such bioinformatics approaches using large datasets from large populations involving genes expressed in a broad range of cell types converge on a single subset, CD4+ TEM cells, the evidence lends further support to the hypothesis that CD4+ TEM cells play a crucial role in autoimmune disease pathogenesis.
Studies on the involvement of memory cells in autoimmunity have been hindered by technical difficulties in identifying the actual autoimmune memory population. In many infectious disease studies, the memory cells are not necessarily phenotypically defined because their presence long after antigen clearance is sufficient to classify them as memory T cells. After antigen clearance, the CD44hiCD62Llow subset is phenotypically defined as the TEM subset, because the TEFF cells that are also CD44hiCD62Llow are assumed to be short-lived. This method of identifying the TEM cells after antigen clearance is convincing in context of acute infectious diseases. However, in context of persisting self-antigen in autoimmune diseases, the CD44hiCD62Llow subset will include a signifi-cant number of short-lived TEFF cells as well. To resolve the two populations, additional markers like CD69 and CD127 are required.
Evidence gathered from experimental studies in animal models and ex vivo using peripheral blood samples from patients, especially in the past few years, indicates that CD4+ TEM subset is emerging as an important contributor to many T cell-mediated autoimmune diseases. For example, in the experimental autoimmune encephalomyelitis (EAE) model of MS, adoptive transfer experiments showed that autoimmune memory was maintained by TEM cells with intact cytokine production and tissue damage potentials [64]. Another study showed that in autoimmune diabetes, unstable Treg cells converted to CD4+ TEM cells that were highly pathogenic with disease-causing potential [35]. An increased population of CD4+ TEM cells was found in human patients with SLE, even in a disease that is thought to be primarily B cell mediated [22]. The anti-neutrophil cytoplasmic autoantibody associated systemic vasculitis (AAV) disorders have been thought to be caused by autoantibodies against neutrophil proteins. These disorders are characterized by autoimmune damage of blood vessels that leads to vessel occlusion and systemic organ damage. There is increasing evidence that the immuno-pathogenesis in AAV disorders is mediated by CD4+ TEM cells [65]. It was also shown that there was an increase in CD4+ and CD8+ TEM subsets in patients with aplastic anemia [66]. Thus, a large body of evidence from studies encompassing many autoimmune diseases, including T1D, SLE, EAE to AAV and aplastic anemia, strongly suggests that CD4+ TEM cells are a crucial mediators of autoimmune destruction.
Unlike in the chronic infection settings wherein TEM cells are often exhausted and have reduced levels of cytokine production [58, 67], in context of autoimmune diseases, TEM cells are potent producers of cytokines despite persistence of self-antigens. Human CD4+ TEM cells from patients with RA have been shown to produce IFN-γ [68]. In EAE, it has also been shown that autoimmune CD4+ TEM cells produced IFN-γ [64]. Whereas in an acute infectious disease setting, it was shown that long-lived Th17 memory did not develop although the primary effectors had no defect in IL17 production [69], in the EAE autoimmune model it was shown that Th17 autoimmune memory cells were more potent at transferring disease than their non-Th17 counterparts [70]. In autoimmune diabetes settings, TEM cells were shown to produce pathogenic cytokines IL17 and IFN-γ [35]. Thus, in autoimmune disease settings, not only is there an increase in the autoimmune CD4+ TEM compartment correlating with disease pathogenesis, but also these CD4+ TEM cells have the potential to produce likely pathogenic cytokines such as IL17 and IFN-γ. In other words, unlike other settings of persistent antigen such as in chronic infections, the auto-immune TEM cells are not exhausted in their effector functionality in autoimmune disorders.
Targeting undesirable memories
Autoimmune memory cells have been thought to be a major contributor to the resistance of autoimmune diseases to many immunomodulatory therapies despite that substantial advances have been made in curtailing autoimmune damages. For example, blocking the CD28/B7 co-stimulation pathway [71] or the CD154/CD40 [72] pathway was shown to effectively control autoimmune diseases like EAE [73, 74], T1D [75–78], psoriasis [79] and SLE [80, 81]. However, memory cells are less dependent on co-stimulation than Naïve T cells [82–84], which might account for the limited success of such therapies. Treg infusion therapies have great promise in controlling adverse immune responses [85]. However, some studies have demonstrated that memory cells are also relatively more resistant than both the Naïve and effector compartments to Treg suppression therapies [86, 87].
With the memory compartment being resistant to many of the immunosuppression therapies and with the CD4+ TEM compartment emerging as a crucial contributor to autoimmune diseases, there is a need for therapies that target this specific compartment. Two possibilities along these lines have been shown in studies of the potassium channel Kv1.3 and the TNF family receptor Fas/CD95. The Fas death inducing signaling complex is more efficiently formed and enriched in the lipid raft microdomains in CD4+ TEM cells. This makes these cells specifically susceptible to Fas-induced cell death while the CD4+ TCM and CD4+ Naïve T cells remain unaffected [88]. Kv1.3 is a calcium-activated potassium-gated ion channel expressed by the KCNN4 gene [89]. Pathogenic CD4+ TEM cells have been shown to express much higher levels of Kv1.3 channels compared to the Naïve and central memory subsets. Kv1.3 inhibitors have been shown to ameliorate autoimmune disease in models of T1D, RA and EAE, likely by a specific effect against the pathogenic CD4+ TEM subset [89–91].
Targeting autoimmune TEM cells could benefit from understanding the molecular basis of their formation and function. Twist1 is highly upregulated in CD4+ TEM cells isolated from patients with RA, Crohn's disease and ulcerative colitis. Twist1 is an endogenous regulator of the Th1 compartment. Its expression in response to repeated antigen exposure may lead to CD4+ TEM cell differentiation [92]. It was suggested that Twist1-expressing Th1 cells in patients undergoing immunosuppressive treatment belonged to a refractory CD4+ TEM compartment [93]. Genetically predisposed reduction in CTLA4 splice variant expression in human subjects has been correlated with T1D susceptibility [94]. Genetic polymorphisms in Bach2 have been associated with numerous autoimmune diseases including T1D, MS and Crohn's disease. Bach2 knock-out mice develop autoimmune disease [95]. One of the mechanisms by which Bach2 is thought to prevent auto-immune disease is by regulating the generation of the pathogenic effector memory compartment [96]. Elucidating such molecular pathways of the pathogenic CD4+ TEM compartment will enable the development of new strategies to modulate the function of this compartment without general immunosuppression.
Putting autoimmune effector memory to good use: the anti-tumor response
It is often said that autoimmunity and anti-tumor immunity are the opposite sides of the same “coin.” In essence, however, we suggest that they are actually on the same side of the “coin,” because reactivity to self-antigens underpins both types of immune responses [97, 98]. Our recent studies have shown that autoimmune responses can indeed be potent mediators of anti-tumor immunity [97]. Considering that the CD4+ TEM subset is emerging as a driving force for autoimmune disease pathogenesis, why is an individual not able to mount a potent anti-tumor response mediated by autoimmune TEM cells, despite there being persistent self-antigen like in autoimmune diseases? The answer could lie in a parallel with chronic infection settings where persistent antigen causes exhaustion of the TEM compartment [58] (Fig. 1).
One of the major molecules contributing to exhaustion in chronic infections has been found to be PD-1 [99]. In mice with chronic LCMV infection, treatment with antibodies against PD-L1 (the ligand for PD-1) enhanced clearance of virus [99]. This suggest that PD-L1 blockade somehow restores previously exhausted TEM cells to help clear virus infection. In concordance with this argument, a recent study showed that PD-1 likely prevented the formation of the TEM compartment [100]. Studies have also shown that PD-L1 is expressed by most types of tumor cells, and blockade of this leads to a more potent anti-tumor response [101–103]. However, PD-L1 is expressed by many tissues of the body including non-parenchymal liver cells, lung, cornea, vascular endothelium, pancreatic islets and keratinocytes. The PD-L1/PD-1 interaction is a major factor in controlling autoimmunity [104], which may explain why overall PD-L1 or PD-1 blockade could be accompanied by autoimmune toxicity in clinical trials of cancer immunotherapies [102, 103].
Conceivably, anti-tumor effect can be achieved by potentiating tumor-infiltrating autoimmune TEM cells. Adoptive cell therapy has shown efficacy in this context, in studies where tumor-infiltrating lymphocytes from the patient re-stimulated and expanded were able to mount a potent anti-tumor response when transferred back into the patient [105]. In this regard, the tumor microenvironment could function as an immunoprivileged self [97, 98] and cause exhaustion of self-antigen-reactive TEM cells that infiltrate tumors. However, if these TEM cells could be isolated, re-potentiated and used for adoptive cell therapies against tumors, we suggest that it might elicit a better outcome.
Back to the Future: perspective from evolution of CTLA4 genetic variations to genomic medicine
Studies from various autoimmune diseases have implicated TEM cells in autoimmune pathogenicity. The TEM subset in such a condition is distinct from the memory counterparts generated in chronic infections, acute infections and tumor settings (Fig. 1). Further studies are needed to characterize this autoimmune pathogenic population in terms of phenotypic markers that define it and genes that regulate it. Equipped with this knowledge, it would be possible to develop strategies that specifically target this specific subset. The involvement of the pathogenic effector memory subset in various autoimmune diseases also presents a common target against many autoimmune diseases. In addition, advances in understanding this pathogenic subset may also propel the development of novel strategies using the autoimmune TEM subset to combat tumors.
The genetic basis of TEM differentiation remains to be elucidated. It is curious that heritable genetic polymorphisms, such as those in the CTLA4 gene which predispose an individual to autoimmune diseases, have stood the test of evolutionary selection pressure and been preserved in the human population. As a matter of fact, the frequency of a few autoimmune disease susceptibility alleles may even be increasing in the population [106, 107]. This suggests that the benefits of having an “overactive” immune system balance out the disadvantage of the individual's predisposition to autoimmune diseases. These benefits may include the ability to mount stronger anti-tumor and anti-pathogen responses. In concordance with this thought, recent studies have shown that parasite infections are a driving force for the positive/negative selection of inflammatory bowel disease (IBD)-associated loci [108, 109]. In context of anti-tumor immunity, genetic studies found that CTLA4 polymorphisms that predict reduced CTLA4 expression in mRNA and/or proteins [110–114] were associated with protection from lymphoma, breast cancer and skin cancer in humans [115–119]. Enhancement of anti-tumor immunity by CTLA4 blockade was shown to be associated with increased formation of memory T cells [120]. In our recent study [97], we tested the role of CTLA4 expression levels on autoimmunity-mediated anti-tumor immunity, by using a CTLA4 shRNA transgenic model that was constructed to mimic human CTLA4 genetic variations that predispose to T1D development [121]. Indeed, the modest reduction in CTLA4 overcame tumor-associated immunoprivilege in a lymphoma model and curtailed spontaneous development of breast cancer [97] in a model wherein the cancer development is attributed to immune tolerance [122]. Therefore, one might speculate that this potent CD4+ TEM subset in various autoimmune diseases, as discussed previously, could represent an increasingly potent immune system that may be evolved to combat tumors and infections. Along these lines, in a situation wherein collateral damage to healthy tissue is acceptable, one might envision that autoimmune TEM cells with their lasting potency, could be a valuable arsenal complementing the current momentum of adoptive T cell therapies against cancer [123, 124].
Undoubtedly, translating the advances from various model systems of TEM differentiation and function to human disease settings in patients remains a daunting challenge, because of limitations in clinical feasibility and ethical considerations in studying the pathophysiology of human disease development in most cases. For example, during the development of T1D, the autoimmune damage in the pancreas typically remains undetected until most of the pancreatic β cells are destroyed, unless at-risk patients are actively monitored in research settings. Furthermore, peripheral blood is often the sole access for analyses of immune cell activity in human patients, and questions often arise concerning how much the activity and profiles of peripheral blood immune cells reflect immune damage in the pancreas. In this regard, it is worth noting that TEM cells are thought to traffic between target tissue and systemic circulation [48], although such an analysis of one subset of cells from one site is still no better than a “blind man's” effort to approach a disease “elephant.”
One can envision that the function of TEM cells is orchestrated by interactions with various types of innate and adaptive immune cells, through both yet-to-be identified and well-known molecular “bridges,” such as CTLA4-B7 [125]. Much akin to cellular networks, functionally related genes may also be organized in networks of coordinated expression and activity. In our study of gene expression profiles of peripheral blood samples from patients with autoimmune diabetes, intriguing patterns of innate and adaptive gene expression were identified in samples from patients at different stages of disease development [126, 127]. Of particular interest, CTLA4 gene expression differentially networked with a set of other adaptive and innate immune genes as the disease progressed from at-risk, to new-onset and to long-term diabetic stages [127]. It should be noted, however, that these studies were done with small subsets of selected innate and adaptive immune genes, with then-available technologies. The rapid development of genome technologies, such as next-generation sequencing and computational methods for data mining, enables the research community to access genome-wide studies of biology and pathophysiology of human diseases that have been beyond the reach of most biomedical researchers. In the case of TEM cells, for example, one can expect the unfolding of its genome biology, in terms of both the genomic underpinning of its differentiation and genome-wide impact if altered. Akin to “blood work” checkups routinely used in clinics, such genomic datasets could potentially serve to provide not only specific indicators for a particular disease activity, but also could help gauge a patient's overall genomic wellness. This could lead to better clinical management both before and after frank disease development, in the emerging era of genomic medicine.
Acknowledgments
This work described in the Chen laboratory was supported by grants from the National Institute of Health (Grant #DP3DK085696) and the Bankhead-Coley Cancer Research Program, DOH, Florida (Grant #09BN-05). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding institutions. We thank Jen Bon Lui and Jason Miska for their time and effort in reading the manuscript and providing critical feedback.
Biography

Zhibin Chen
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
References
- 1.Hummel K, McFann KK, Realsen J, Messer LH, Klingensmith GJ, Chase HP. The increasing onset of type 1 diabetes in children. J Pediatr. 2012;161:652–657. e651. doi: 10.1016/j.jpeds.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 2.Furst DE, Clarke AE, Fernandes AW, Bancroft T, Greth W, Iorga SR. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2013;22:99–105. doi: 10.1177/0961203312463110. [DOI] [PubMed] [Google Scholar]
- 3.Koch-Henriksen N, Sorensen PS. The changing demographic pattern of multiple sclerosis epidemiology. Lancet Neurol. 2010;9:520–32. doi: 10.1016/S1474-4422(10)70064-8. [DOI] [PubMed] [Google Scholar]
- 4.Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G, Group ES. Incidence trends for childhood type 1 diabetes in Europe during 1989-2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373:2027–33. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- 5.Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259–60. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, et al. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–9. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 7.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 8.Stevens EA, Bradfield CA. Immunology: T cells hang in the balance. Nature. 2008;453:46–7. doi: 10.1038/453046a. [DOI] [PubMed] [Google Scholar]
- 9.Ardoin SP, Pisetsky DS. Developments in the scientific understanding of lupus. Arthritis Res Ther. 2008;10:218. doi: 10.1186/ar2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi J, Kim ST, Craft J. The pathogenesis of systemic lupus erythematosus-an update. Curr Opin Immunol. 2012;24:651–7. doi: 10.1016/j.coi.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perl A. Pathogenic mechanisms in systemic lupus erythematosus. Autoimmunity. 2010;43:1–6. doi: 10.3109/08916930903374741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu Z, Davidson A. Taming lupus-a new understanding of pathogenesis is leading to clinical advances. Nat Med. 2012;18:871–82. doi: 10.1038/nm.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Z, Koralov SB, Kelsoe G. Complement C4 inhibits systemic autoimmunity through a mechanism independent of complement receptors CR1 and CR2. J Exp Med. 2000;192:1339–52. doi: 10.1084/jem.192.9.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS-lupus connection. Nat Biotechnol. 2011;30:69–77. doi: 10.1038/nbt.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busser BW, Adair BS, Erikson J, Laufer TM. Activation of diverse repertoires of autoreactive T cells enhances the loss of anti-dsDNA B cell tolerance. J Clin Invest. 2003;112:1361–71. doi: 10.1172/JCI18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas PS, Gupta S, Stirzaker RA, Kumar V, Jessberger R, Lu TT, et al. Dual regulation of IRF4 function in T and B cells is required for the coordination of T-B cell interactions and the prevention of autoimmunity. J Exp Med. 2012;209:581–96. doi: 10.1084/jem.20111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu J, Liu X, Xie C, Yan M, Yu Y, Sobel ES, et al. T cell hyperactivity in lupus as a consequence of hyperstimulatory antigen-presenting cells. J Clin Invest. 2005;115:1869–78. doi: 10.1172/JCI23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, et al. Human blood CXCR5+CD4+ T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108–21. doi: 10.1016/j.immuni.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, et al. Follicular helper T cells are required for systemic autoimmunity. J Exp Med. 2009;206:561–76. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, et al. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–86. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simpson N, Gatenby PA, Wilson A, Malik S, Fulcher DA, Tangye SG, et al. Expansion of circulating T cells resembling follicular helper T cells is a fixed phenotype that identifies a subset of severe systemic lupus erythematosus. Arthritis Rheum. 2010;62:234–44. doi: 10.1002/art.25032. [DOI] [PubMed] [Google Scholar]
- 22.Fritsch RD, Shen X, Illei GG, Yarboro CH, Prussin C, Hathcock KS, et al. Abnormal differentiation of memory T cells in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2184–97. doi: 10.1002/art.21943. [DOI] [PubMed] [Google Scholar]
- 23.Todd JA, Bell JI, McDevitt HO. HLA-DQ beta gene contributes to susceptibility and resistance to insulin-dependent diabetes mellitus. Nature. 1987;329:599–604. doi: 10.1038/329599a0. [DOI] [PubMed] [Google Scholar]
- 24.Cucca F, Lampis R, Congia M, Angius E, Nutland S, Bain SC, et al. A correlation between the relative predisposition of MHC class II alleles to type 1 diabetes and the structure of their proteins. Hum Mol Genet. 2001;10:2025–37. doi: 10.1093/hmg/10.19.2025. [DOI] [PubMed] [Google Scholar]
- 25.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, et al. Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet. 2007;39:857–64. doi: 10.1038/ng2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herr M, Dudbridge F, Zavattari P, Cucca F, Guja C, March R, et al. Evaluation of fine mapping strategies for a multifactorial disease locus: systematic linkage and association analysis of IDDM1 in the HLA region on chromosome 6p21. Hum Mol Genet. 2000;9:1291–301. doi: 10.1093/hmg/9.9.1291. [DOI] [PubMed] [Google Scholar]
- 27.DiLorenzo TP, Serreze DV. The good turned ugly: immunopathogenic basis for diabetogenic CD8+ T cells in NOD mice. Immunol Rev. 2005;204:250–63. doi: 10.1111/j.0105-2896.2005.00244.x. [DOI] [PubMed] [Google Scholar]
- 28.Santamaria P. Effector lymphocytes in autoimmunity. Curr Opin Immunol. 2001;13:663–9. doi: 10.1016/s0952-7915(01)00276-x. [DOI] [PubMed] [Google Scholar]
- 29.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–97. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long SA, Cerosaletti K, Bollyky PL, Tatum M, Shilling H, Zhang S, et al. Defects in IL-2R signaling contribute to diminished maintenance of FOXP3 expression in CD4+ CD25+ regulatory T-cells of type 1 diabetic subjects. Diabetes. 2010;59:407–15. doi: 10.2337/db09-0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tree TI, Roep BO, Peakman M. A mini meta-analysis of studies on CD4+ CD25+ T cells in human type 1 diabetes: report of the immunology of diabetes society T cell workshop. Ann N Y Acad Sci. 2006;1079:9–18. doi: 10.1196/annals.1375.002. [DOI] [PubMed] [Google Scholar]
- 32.Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol. 2009;155:173–81. doi: 10.1111/j.1365-2249.2008.03860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4+ CD25+ T-cells from patients with type 1 diabetes. Diabetes. 2005;54:92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 34.Rosenblum MD, Gratz IK, Paw JS, Lee K, Marshak-Rothstein A, Abbas AK. Response to self antigen imprints regulatory memory in tissues. Nature. 2011;480:538–42. doi: 10.1038/nature10664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou X, Bailey-Bucktrout SL, Jeker LT, Penaranda C, Martinez-Llordella M, Ashby M, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–7. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oling V, Reijonen H, Simell O, Knip M, Ilonen J. Autoantigen-specific memory CD4+ T cells are prevalent early in progression to type 1 diabetes. Cell Immunol. 2012;273:133–9. doi: 10.1016/j.cellimm.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65-specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25:303–11. doi: 10.1016/j.jaut.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Bending D, Zaccone P, Cooke A. Inflammation and type one diabetes. Int Immunol. 2012;24:339–46. doi: 10.1093/intimm/dxs049. [DOI] [PubMed] [Google Scholar]
- 39.Alkemade GM, Clemente-Casares X, Yu Z, Xu BY, Wang J, Tsai S, et al. Local autoantigen expression as essential gate-keeper of memory T-cell recruitment to islet grafts in diabetic hosts. Diabetes. 2013;62:905–11. doi: 10.2337/db12-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bingaman AW, Farber DL. Memory T cells in transplantation: generation, function, and potential role in rejection. Am J Transpl. 2004;4:846–52. doi: 10.1111/j.1600-6143.2004.00453.x. [DOI] [PubMed] [Google Scholar]
- 41.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 42.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 43.van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174:5341–50. doi: 10.4049/jimmunol.174.9.5341. [DOI] [PubMed] [Google Scholar]
- 44.Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–64. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–30. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 46.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA. 2010;107:17872–9. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187:5510–4. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–61. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 49.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci USA. 2011;108:16741–6. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38:187–97. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–75. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 53.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4+ T cells that persist in the lungs. J Exp Med. 2001;193:981–6. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roberts AD, Woodland DL. Cutting edge: effector memory CD8+ T cells play a prominent role in recall responses to secondary viral infection in the lung. J Immunol. 2004;172:6533–7. doi: 10.4049/jimmunol.172.11.6533. [DOI] [PubMed] [Google Scholar]
- 55.Olson JA, McDonald-Hyman C, Jameson SC, Hamilton SE. Effector-like CD8+ T cells in the memory population mediate potent protective immunity. Immunity. 2013;38:1250–60. doi: 10.1016/j.immuni.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pitcher CJ, Hagen SI, Walker JM, Lum R, Mitchell BL, Maino VC, et al. Development and homeostasis of T cell memory in rhesus macaque. J Immunol. 2002;168:29–43. doi: 10.4049/jimmunol.168.1.29. [DOI] [PubMed] [Google Scholar]
- 57.Torti N, Oxenius A. T cell memory in the context of persistent herpes viral infections. Viruses. 2012;4:1116–43. doi: 10.3390/v4071116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wherry EJ, Ahmed R. Memory CD8 T-cell differentiation during viral infection. J Virol. 2004;78:5535–45. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hansen SG, Vieville C, Whizin N, Coyne-Johnson L, Siess DC, Drummond DD, et al. Effector memory T cell responses are associated with protection of rhesus monkeys from mucosal simian immunodeficiency virus challenge. Nat Med. 2009;15:293–9. doi: 10.1038/nm.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hansen SG, Ford JC, Lewis MS, Ventura AB, Hughes CM, Coyne-Johnson L, et al. Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature. 2011;473:523–7. doi: 10.1038/nature10003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Heng TS, Painter MW. Immunological genome project C. The immunological genome project: networks of gene expression in immune cells. Nat Immunol. 2008;9:1091–4. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 62.Hu X, Kim H, Stahl E, Plenge R, Daly M, Raychaudhuri S. Integrating autoimmune risk loci with gene-expression data identifies specific pathogenic immune cell subsets. Am J Hum Genet. 2011;89:496–506. doi: 10.1016/j.ajhg.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Eyre S, Bowes J, Diogo D, Lee A, Barton A, Martin P, et al. High-density genetic mapping identifies new susceptibility loci for rheumatoid arthritis. Nat Genet. 2012;44:1336–40. doi: 10.1038/ng.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kawakami N, Odoardi F, Ziemssen T, Bradl M, Ritter T, Neuhaus O, et al. Autoimmune CD4+ T cell memory: lifelong persistence of encephalitogenic T cell clones in healthy immune repertoires. J Immunol. 2005;175:69–81. doi: 10.4049/jimmunol.175.1.69. [DOI] [PubMed] [Google Scholar]
- 65.Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CG. CD4-positive effector memory T cells participate in disease expression in ANCA-associated vasculitis. Ann N Y Acad Sci. 2007;1107:22–31. doi: 10.1196/annals.1381.003. [DOI] [PubMed] [Google Scholar]
- 66.Hu X, Gu Y, Wang Y, Cong Y, Qu X, Xu C. Increased CD4+ and CD8+ effector memory T cells in patients with aplastic anemia. Haematologica. 2009;94:428–9. doi: 10.3324/haematol.13412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Angelosanto JM, Blackburn SD, Crawford A, Wherry EJ. Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol. 2012;86:8161–70. doi: 10.1128/JVI.00889-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sattler A, Wagner U, Rossol M, Sieper J, Wu P, Krause A, et al. Cytokine-induced human IFN-gamma-secreting effector-memory Th cells in chronic autoimmune inflammation. Blood. 2009;113:1948–56. doi: 10.1182/blood-2008-02-139147. [DOI] [PubMed] [Google Scholar]
- 69.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010;11:83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Haines CJ, Chen Y, Blumenschein WM, Jain R, Chang C, Joyce-Shaikh B, et al. Autoimmune memory T helper 17 cell function and expansion are dependent on interleukin-23. Cell Rep. 2013;3:1378–88. doi: 10.1016/j.celrep.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 71.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001;19:225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 72.Yamada A, Salama AD, Sayegh MH. The role of novel T cell costimulatory pathways in autoimmunity and transplantation. J Am Soc Nephrol. 2002;13:559–75. doi: 10.1681/ASN.V132559. [DOI] [PubMed] [Google Scholar]
- 73.Schaub M, Issazadeh S, Stadlbauer TH, Peach R, Sayegh MH, Khoury SJ. Costimulatory signal blockade in murine relapsing experimental autoimmune encephalomyelitis. J Neuroimmunol. 1999;96:158–66. doi: 10.1016/s0165-5728(99)00022-3. [DOI] [PubMed] [Google Scholar]
- 74.Becher B, Durell BG, Miga AV, Hickey WF, Noelle RJ. The clinical course of experimental autoimmune encephalomyelitis and inflammation is controlled by the expression of CD40 within the central nervous system. J Exp Med. 2001;193:967–74. doi: 10.1084/jem.193.8.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray G, Nabavi N, et al. Differential effects of anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the development of diabetes in the non-obese diabetic mouse. J Exp Med. 1995;181:1145–55. doi: 10.1084/jem.181.3.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Balasa B, Krahl T, Patstone G, Lee J, Tisch R, McDevitt HO, et al. CD40 ligand-CD40 interactions are necessary for the initiation of insulitis and diabetes in nonobese diabetic mice. J Immunol. 1997;159:4620–7. [PubMed] [Google Scholar]
- 77.Kover KL, Geng Z, Hess DM, Benjamin CD, Moore WV. Anti-CD154 (CD40L) prevents recurrence of diabetes in islet isografts in the DR-BB rat. Diabetes. 2000;49:1666–70. doi: 10.2337/diabetes.49.10.1666. [DOI] [PubMed] [Google Scholar]
- 78.Molano RD, Berney T, Li H, Cattan P, Pileggi A, Vizzardelli C, et al. Prolonged islet graft survival in NOD mice by blockade of the CD40-CD154 pathway of T-cell costimulation. Diabetes. 2001;50:270–6. doi: 10.2337/diabetes.50.2.270. [DOI] [PubMed] [Google Scholar]
- 79.Abrams JR, Lebwohl MG, Guzzo CA, Jegasothy BV, Goldfarb MT, Goffe BS, et al. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J Clin Invest. 1999;103:1243–52. doi: 10.1172/JCI5857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liang B, Kashgarian MJ, Sharpe AH, Mamula MJ. Autoantibody responses and pathology regulated by B7-1 and B7-2 costimulation in MRL/lpr lupus. J Immunol. 2000;165:3436–43. doi: 10.4049/jimmunol.165.6.3436. [DOI] [PubMed] [Google Scholar]
- 81.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–8. [PubMed] [Google Scholar]
- 82.Croft M, Bradley LM, Swain SL. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994;152:2675–85. [PubMed] [Google Scholar]
- 83.London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265–72. doi: 10.4049/jimmunol.164.1.265. [DOI] [PubMed] [Google Scholar]
- 84.Lovett-Racke AE, Trotter JL, Lauber J, Perrin PJ, June CH, Racke MK. Decreased dependence of myelin basic protein-reactive T cells on CD28-mediated costimulation in multiple sclerosis patients. A marker of activated/memory T cells. J Clin Invest. 1998;101:725–30. doi: 10.1172/JCI1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Riley JL, June CH, Blazar BR. Human T regulatory cell therapy: take a billion or so and call me in the morning. Immunity. 2009;30:656–65. doi: 10.1016/j.immuni.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, et al. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954–9. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Afzali B, Mitchell PJ, Scotta C, Canavan J, Edozie FC, Fazekasova H, et al. Relative resistance of human CD4+ memory T cells to suppression by CD4+ CD25+ regulatory T cells. Am J Transpl. 2011;11:1734–42. doi: 10.1111/j.1600-6143.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ramaswamy M, Cruz AC, Cleland SY, Deng M, Price S, Rao VK, et al. Specific elimination of effector memory CD4+ T cells due to enhanced Fas signaling complex formation and association with lipid raft microdomains. Cell Death Differ. 2011;18:712–20. doi: 10.1038/cdd.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. K+ channels as targets for specific immunomodulation. Trends Pharmacol Sci. 2004;25:280–9. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, et al. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J Clin Invest. 2003;111:1703–13. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, et al. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc Natl Acad Sci USA. 2006;103:17414–9. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Niesner U, Albrecht I, Janke M, Doebis C, Loddenkemper C, Lexberg MH, et al. Autoregulation of Th1-mediated inflammation by twist1. J Exp Med. 2008;205:1889–901. doi: 10.1084/jem.20072468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang HD, Radbruch A. Targeting pathogenic T helper cell memory. Ann Rheum Dis. 2011;70(Suppl 1):i85–7. doi: 10.1136/ard.2010.140954. [DOI] [PubMed] [Google Scholar]
- 94.Ueda H, Howson JM, Esposito L, Heward J, Snook H, Chamberlain G, et al. Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature. 2003;423:506–11. doi: 10.1038/nature01621. [DOI] [PubMed] [Google Scholar]
- 95.Roychoudhuri R, Hirahara K, Mousavi K, Clever D, Klebanoff CA, Bonelli M, et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature. 2013;498:506–10. doi: 10.1038/nature12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsukumo S, Unno M, Muto A, Takeuchi A, Kometani K, Kurosaki T, et al. Bach2 maintains T cells in a naive state by suppressing effector memory-related genes. Proc Natl Acad Sci USA. 2013;110:10735–40. doi: 10.1073/pnas.1306691110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Miska J, Bas E, Devarajan P, Chen Z. Autoimmunity-mediated antitumor immunity: tumor as an immunoprivileged self. Eur J Immunol. 2012;42:2584–96. doi: 10.1002/eji.201242590. [DOI] [PubMed] [Google Scholar]
- 98.Miska J, Devarajan P, Chen Z. The immunological identity of tumor: self implications. Oncoimmunology. 2013;2:e23794. doi: 10.4161/onci.23794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–7. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 100.Charlton JJ, Chatzidakis I, Tsoukatou D, Boumpas DT, Garinis GA, Mamalaki C. Programmed death-1 shapes memory phenotype CD8 T cell subsets in a cell-intrinsic manner. J Immunol. 2013;190:6104–14. doi: 10.4049/jimmunol.1201617. [DOI] [PubMed] [Google Scholar]
- 101.Blank C, Mackensen A. Contribution of the PD-L1/PD-1 pathway to T-cell exhaustion: an update on implications for chronic infections and tumor evasion. Cancer Immunol Immunother. 2007;56:739–45. doi: 10.1007/s00262-006-0272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Corona E, Dudley JT, Butte AJ. Extreme evolutionary disparities seen in positive selection across seven complex diseases. PLoS One. 2010;5:e12236. doi: 10.1371/journal.pone.0012236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Casto AM, Feldman MW. Genome-wide association study SNPs in the human genome diversity project populations: does selection affect unlinked SNPs with shared trait associations? PLoS Genet. 2011;7:e1001266. doi: 10.1371/journal.pgen.1001266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206:1395–408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cagliani R, Pozzoli U, Forni D, Cassinotti A, Fumagalli M, Giani M, et al. Crohn's disease loci are common targets of protozoa-driven selection. Mol Biol Evol. 2013;30:1077–87. doi: 10.1093/molbev/mst020. [DOI] [PubMed] [Google Scholar]
- 110.Ligers A, Teleshova N, Masterman T, Huang WX, Hillert J. CTLA-4 gene expression is influenced by promoter and exon 1 polymorphisms. Genes Immun. 2001;2:145–52. doi: 10.1038/sj.gene.6363752. [DOI] [PubMed] [Google Scholar]
- 111.Wang XB, Zhao X, Giscombe R, Lefvert AK. A CTLA-4 gene polymorphism at position—318 in the promoter region affects the expression of protein. Genes Immun. 2002;3:233–4. doi: 10.1038/sj.gene.6363869. [DOI] [PubMed] [Google Scholar]
- 112.Anjos S, Nguyen A, Ounissi-Benkalha H, Tessier MC, Polychronakos C. A common autoimmunity predisposing signal peptide variant of the cytotoxic T-lymphocyte antigen 4 results in inefficient glycosylation of the susceptibility allele. J Biol Chem. 2002;277:46478–86. doi: 10.1074/jbc.M206894200. [DOI] [PubMed] [Google Scholar]
- 113.Kristiansen OP, Larsen ZM, Pociot F. CTLA-4 in autoimmune diseases—a general susceptibility gene to autoimmunity? Genes Immun. 2000;1:170–84. doi: 10.1038/sj.gene.6363655. [DOI] [PubMed] [Google Scholar]
- 114.Teft WA, Kirchhof MG, Madrenas J. A molecular perspective of CTLA-4 function. Annu Rev Immunol. 2006;24:65–97. doi: 10.1146/annurev.immunol.24.021605.090535. [DOI] [PubMed] [Google Scholar]
- 115.Sun T, Zhou Y, Yang M, Hu Z, Tan W, Han X, et al. Functional genetic variations in cytotoxic T-lymphocyte antigen 4 and susceptibility to multiple types of cancer. Cancer Res. 2008;68:7025–34. doi: 10.1158/0008-5472.CAN-08-0806. [DOI] [PubMed] [Google Scholar]
- 116.Monne M, Piras G, Palmas A, Arru L, Murineddu M, Latte G, et al. Cytotoxic T-lymphocyte antigen-4 (CTLA-4) gene polymorphism and susceptibility to non-Hodgkin's lymphoma. Am J Hematol. 2004;76:14–8. doi: 10.1002/ajh.20045. [DOI] [PubMed] [Google Scholar]
- 117.Welsh MM, Applebaum KM, Spencer SK, Perry AE, Karagas MR, Nelson HH. CTLA4 variants, UV-induced tolerance, and risk of non-melanoma skin cancer. Cancer Res. 2009;69:6158–63. doi: 10.1158/0008-5472.CAN-09-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erfani N, Razmkhah M, Talei AR, Pezeshki AM, Doroudchi M, Monabati A, et al. Cytotoxic T lymphocyte antigen-4 promoter variants in breast cancer. Cancer Genet Cytogenet. 2006;165:114–20. doi: 10.1016/j.cancergencyto.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 119.Wang L, Li D, Fu Z, Li H, Jiang W, Li D. Association of CTLA-4 gene polymorphisms with sporadic breast cancer in Chinese Han population. BMC Cancer. 2007;7:173. doi: 10.1186/1471-2407-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pedicord VA, Montalvo W, Leiner IM, Allison JP. Single dose of anti-CTLA-4 enhances CD8+ T-cell memory formation, function, and maintenance. Proc Natl Acad Sci USA. 2011;108:266–71. doi: 10.1073/pnas.1016791108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Chen Z, Stockton J, Mathis D, Benoist C. Modeling CTLA4-linked autoimmunity with RNA interference in mice. Proc Natl Acad Sci USA. 2006;103:16400–5. doi: 10.1073/pnas.0607854103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, et al. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–40. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 123.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vatakis DN, Koya RC, Nixon CC, Wei L, Kim SG, Avancena P, et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc Natl Acad Sci USA. 2011;108:E1408–16. doi: 10.1073/pnas.1115050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Suzuki J, Ricordi C, Chen Z. Immune tolerance induction by integrating innate and adaptive immune regulators. Cell Transpl. 2010;19:253–68. doi: 10.3727/096368909X480314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Han D, Cai X, Wen J, Matheson D, Skyler JS, Kenyon NS, et al. Innate and adaptive immune gene expression profiles as bio-markers in human type 1 diabetes. Clin Exp Immunol. 2012;170:131–8. doi: 10.1111/j.1365-2249.2012.04650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Han D, Cai X, Wen J, Kenyon NS, Chen Z. From biomarkers to a clue of biology: a computation-aided perspective of immune gene expression profiles in human type 1 diabetes. Front Immunol. 2012;3:320. doi: 10.3389/fimmu.2012.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]