Abstract
Introduction
Rotavirus (RV) is a leading cause of acute gastroenteritis (AGE), affecting 95% of children below five years of age.
Methods
In this prospective, multi-center study, children below five years of age who were hospitalized or those who visited the emergency room (ER) due to AGE or who developed AGE at least 48 hours after hospitalization (nosocomial infection) and had a RV-positive stool sample were included (n=1,222). RV-positive samples were genotyped by reverse-transcriptase polymerase chain reaction.
Results
RV test results were available for 1,212 children (hospitalizations [n=677], ER visits [n=398] and nosocomial AGE cases [n=137]). Proportions of rotavirus gastroenteritis (RVGE) hospitalizations and ER visits were 51.70% (350/677; 95%CI: 47.86–55.52) and 36.18% (144/398; 95%CI: 31.45–41.12), respectively. Overall, 45.95% (494/1075) of all community-acquired AGE cases were due to RV. High numbers of RVGE cases were recorded between January and March. Most common genotypes were G9P[8] (34.27%) followed by G4P[8] (25.83%) and G1P[8] (23.02%). Of all community-acquired RVGE cases, the highest number of cases was observed in children aged 12–23 months. Median duration of hospitalization among RV-positive subjects was six days (range: 2–31 days). Incidence of nosocomial RVGE was 0.52 (95%CI: 0.45–0.60) cases per 1,000 child-days hospitalization. Median duration for additional hospitalization due to nosocomial RVGE was five days (range: 1–10). The highest burden of nosocomial RVGE was observed in children aged 12–23 months (42.34%, 58/137). Our findings confirm a high burden of acute RVGE disease in Romania and provide useful data to support the implementation of RV vaccination in Romania.
Trial registration
Keywords: Rotavirus, Romania, epidemiology, nosocomial, incidence, acute gastroenteritis
Introduction
Rotavirus (RV) is the most common cause of acute gastroenteritis (AGE) worldwide, affecting 95% of children by the age of five years.1,2 Globally, it is estimated that RV infection results in 3.6 million episodes of AGE per year.3 The clinical symptoms of RV gastroenteritis (RVGE) include mild to severe diarrhea with fever and vomiting that can result in severe dehydration and even death.2 Recent surveillance data suggests that in Europe nearly half of AGE cases reported are attributed to infection with RV.4–6 RV also plays a major role in the acquisition of nosocomial AGE in children hospitalized for other causes resulting in significant clinical and economic burden in Europe.7
Very few studies describe the epidemiology of RVGE in Central and Eastern Europe.8,9 The proportions of RVGE among all AGE reported for Romania in the last decade ranged between 15.0–50.0% with the highest number of RVGE cases reported between January and May.8–15 The greatest burden of RVGE has been observed in children aged 6–12 months.13,15 The incidence of fatal RVGE disease in Romania between 1998 and 2003 was estimated to be high and similar to that of developing countries.1,8 Precise estimates of RVGE disease burden for Romania are limited due to absence of routine RV testing and the fact that reporting of AGE is not mandatory.
Following availability of two orally administered RV vaccines worldwide – Rotarix™ (GlaxoSmithKline Vaccines, Belgium) and RotaTeq® (Merck and Co., Inc., Whitehouse Station, NJ, USA) – there has been renewed interest in preventing RVGE disease. Both vaccines have demonstrated good safety and efficacy in clinical trials conducted in diverse settings.16–19 The World Health Organization (WHO) recommends RV vaccination of healthy infants.20 In Romania, vaccination against RV is recommended by professional societies, but is not included in the routine immunization program. Up-to date epidemiological data are therefore needed to guide recommendations for RV vaccine use and to help assess the potential benefit and impact of RV vaccination. This study in Romania was conducted to assess RVGE disease burden in children below five years of age, which may provide important information in determining the need for implementation of universal RV vaccination in Romania.
Methods
Study design and population
The present observational, hospital-based surveillance was conducted at nine hospitals in Romania with an estimated coverage of 28% of the pediatric population of Romania. The study protocol was based on the latest generic protocol version available from the Centers for Disease Control and Prevention (CDC) and WHO for hospital-based surveillance to estimate the disease burden of RVGE in children below five years of age.21 The study population included children below five years of age who were being treated at either a hospital or emergency room (ER) for AGE and those children who acquired AGE during hospitalization due to any cause. AGE in this study is defined as occurrence of diarrhea (≥3 loose stools within 24 hours) for less than a duration of 14 days at enrolment. Children below five years of age at the time of enrolment, who were hospitalized, or those who visited the emergency room due to AGE or who developed AGE at least 48 hours after hospitalization (nosocomial RVGE) and had RV-positive stool sample during the study period were included in the analysis. The study involved an interview with the child's parent/guardian and stool sample analysis. Children with AGE that were hospitalized or those who visited the ER previously were enrolled as new subjects at each subsequent hospitalization or ER visit. The study was conducted for a period of 23 months between February 2008 and December 2009. Severity of the GE episode was analyzed using the 20-point Vesikari scale.22
Sample size calculation
A total of 1,445 subjects were planned to be included in the study. The sample size was calculated for each subgroup separately. At a confidence level of 95% and a precision of 3.7%, the ascertained sample size was 540 AGE hospitalized cases (assumption: prevalence of RVGE at 25%) and 665 AGE ER cases (assumption: prevalence of RVGE at 35%). For nosocomial RVGE it was proposed to enroll 240 cases in order to have at least 50 positive samples for genotyping. Additionally, it was assumed that 80% of RVGE cases (n=1,156) would occur during the RV season (December to May).23 The enrolment plan employed in the study was based on the estimated disease burden and seasonality and involved assignment of target enrolment numbers for each center per half-month. Each participating center had an identical target enrolment, however the profile and the number of treated patients per month were different among centers. Subjects were enrolled until the target was reached for each half-month enrolment period. This pattern ensured that RV peaks were captured. Data collection was planned for a year (February 2008 to January 2009), but due to failure in reaching the target enrolment, the study protocol was amended and data collection was extended until December 2009. All children meeting the established inclusion criteria were continuously registered in separate logbooks by specific subgroups. The enrolment started each month on the 1st and 15th day and continued till the bi-monthly target was met. Stool sample results were not available during enrolment, except for nosocomial cases.
Ethical considerations
Prior to enrolment, informed consent was obtained from the parents/guardians. Unique subject numbers were assigned to the children upon enrolment. The study was conducted in accordance with the Declaration of Helsinki, 1996, Good Clinical Practice guidelines and the local regulations of the country. The protocol was reviewed and approved by the local independent ethics committee or institutional review board for each participating center.
Laboratory methods
Stool samples collected from all children enrolled in the study were tested for the presence of RV by using the Rapid testing method (RotaStrip™; Coris BioConcept, Gembloux, Belgium).24 Stool testing was performed locally at each of the study centers and sample genotyping by reverse transcriptase polymerase chain reaction (RT-PCR) was performed at the central laboratory of the National Institute of Infectious Diseases “Prof Dr. Matei Balş”, Bucharest. RV-positive stool samples were prepared as 10% suspensions in balanced salt solutions. This suspension (200 µL) was used for nucleic acid extraction with guanidinium isothiocyanate and silica followed by reverse transcription using random hexamers. The cDNA was used for RV genotyping using a semi-nested PCR.25–27
Statistical analyses
Proportions of RVGE among all AGE hospitalizations and ER visits; the incidence of nosocomial RVGE among all hospitalizations were estimated. Age and seasonal distribution of RVGE cases, genotype distribution of RV, severity of the AGE episode, duration of hospitalization, prolongation of hospitalization due to nosocomial RVGE, treatment, symptoms and outcome of the AGE episode are reported. Risk of acquiring RVGE was assessed, the basis of which was the child's medical history (presence/absence of chronic diseases).
All statistical analyses were done using SPSS 17.0 and Open Epi. All variables are not symmetrically distributed (p<0.05). Scale variables are reported as medians (range). Comparisons of non-symmetric variables by RV status were done using the Mann-Whitney U-test. A p-value <0.05 was considered for statistical significance (two-tailed test). Categorical data (age, gender) are presented as proportions with two decimals. Proportions are compared by using Chi square or Fischer exact test (p<0.05). Odds probabilities of occurrence for nominal variables by RV status are reported with their confidence intervals (CI).
Results
Population characteristics
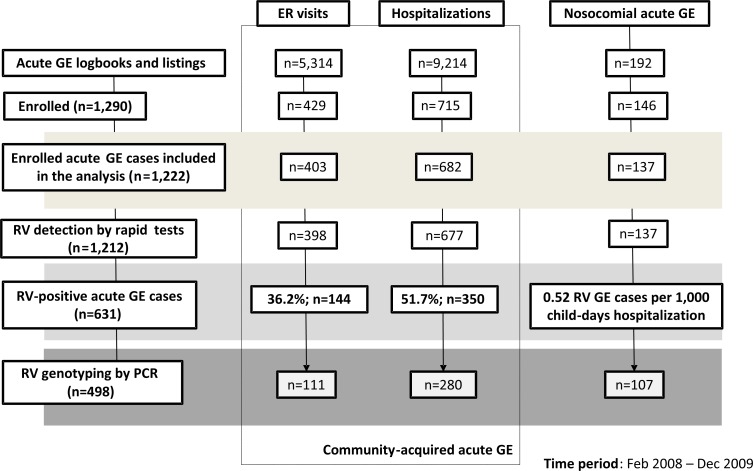
The number of subjects per subgroup retained and analyzed in each stage of the study is shown in Figure 1. The proportions of AGE cases among all hospitalizations and all ER visits were 17.07% (9,124 AGE cases/53,445 hospital admissions) and 5.40% (5,314 AGE cases/98,187 hospital admissions), respectively (Figure 1). Overall, 1,222 children were enrolled and included in the study analysis (84.5% of the proposed target enrolment). The total recruitment between centers varied from 6.06% to 15.22%. A total of 1,290 children with AGE were enrolled into the study initially; 68 children were excluded from the analysis due to missing data or having failed to meet inclusion criteria.
Figure 1. Subjects per subgroup.
ER emergency room; GE gastroenteritis; RV rotavirus.
The median age of children was 15 months; 56.50% of children were male. Of all AGE cases enrolled, 55.81% (682/1,222) of cases were hospitalized (median age: 13 months; males: 57.62%), 32.98% (403/1,222) of cases visited ERs (median age: 19 months; males: 53.35%) and 11.20% (137/1,222) of cases were identified as nosocomial RVGE (median age: 15 months; males: 60.58%). Rapid RV test results were available for 99.27% (677/682) of AGE hospitalized cases and 98.76% (398/403) of AGE ER visits (Figure 1).
Occurrence of RVGE
Overall, 45.95% of all community-acquired AGE (i.e. both hospitalizations and ER visits) were due to RV. The proportions of RVGE among AGE hospitalizations and ER visits were 51.70% (350/677; 95%CI: 47.86–55.52) and 36.18% (144/398; 95%CI: 31.45–41.12), respectively (Figure 1). Numbers and proportions of RVGE cases among AGE hospitalizations and ER visits stratified by age groups are given in Table 1. The estimated proportions of RVGE among all hospitalizations and ER visits of children below five years of age were 8.83% (95%CI: 8.17–9.48) and 1.96% (95%CI: 1.70–2.22). The incidence of nosocomial RVGE was 0.52 (n=137; 95%CI: 0.50–0.60) RVGE cases per 1,000 child-days hospitalization (Figure 1).
Table 1. Proportion of hospitalized children and emergency room (ER) visits with rotavirus gastroenteritis (RVGE) in Romania, 2008–09.
| Hospitalizations | ER Visits | |||
| Age group | RV+ cases | Proportion of RVGE (95%CI) | RV+ cases | Proportion of RVGE (95%CI) |
| <6 months | 50 | 37.88 (29.58–46.73) | 13 | 22.81 (12.74–35.84) |
| 6–11 months | 74 | 45.96 (38.09–53.98) | 23 | 37.10 (25.15–50.31) |
| 0 to <1 year | 124 | 42.32 (36.60–48.20) | 36 | 30.25 (22.17–39.35) |
| 12–23 months | 130 | 59.09 (52.28–65.65) | 50 | 39.06 (30.56–48.08) |
| 24–35 months | 50 | 57.47 (46.41–68.01) | 33 | 37.50 (27.40–48.46) |
| 36–47 months | 32 | 59.26 (45.03–72.43) | 20 | 50.00 (33.80–66.20) |
| 48–59 months | 14 | 60.87 (38.54–80.29) | 5 | 21.74 (7.46–43.70) |
| 0 to <2 years | 254 | 49.51 (45.10–53.93) | 86 | 34.82 (28.89–41.12) |
| 2 to <5 years | 96 | 58.54 (50.59–66.16) | 58 | 38.41 (30.62–46.67) |
| Overall | 350 | 51.70 (47.86–55.52) | 144 | 36.18 (31.45–41.12) |
Age distribution of RVGE
Among all community-acquired RVGE cases, 32.39% (160/494) occurred in children below one year of age and 68.83% (340/494) occurred in children below two years of age. Among nosocomial RVGE cases, 35.77% (49/137) of cases occurred in children below the age of one year and 78.10% (107/137) occurred in children below two years of age. The age distributions of RVGE cases by subject groups – i.e. AGE, ER visits and hospitalizations, and nosocomial RVGE – are shown in Figure 2.
Figure 2. Age distribution of RVGE cases in community-acquired AGE hospitalizations and ER visits.
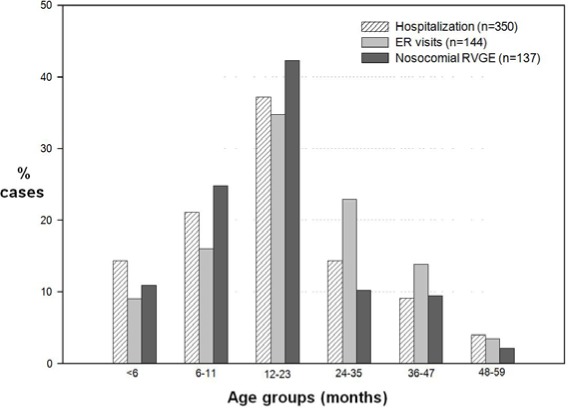
AGE acute gastroenteritis; ER emergency room; RVGE rotavirus gastroenteritis.
Of all community-acquired RVGE cases, the highest number of cases was reported in children aged 12–23 months (36.44%, 180/494) followed by children aged 6–11 months (19.64%, 97/494). Similarly, among nosocomial RVGE cases, the highest number of cases was reported in children of ages 12 and 23 months (42.34%, 58/137) followed by children aged 6–11 months (24.82%, 34/137). The proportions of RVGE in children stratified by age groups are presented in Table 1/Figure 2.
Seasonality of RVGE
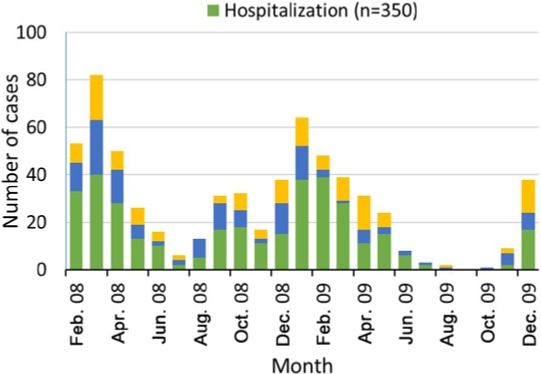
During the RV season (assumed to be between December and May in the present study), 72.44% (878/1212) of children were identified with AGE. The proportions of AGE cases during the RV season were 75.78% (513/677), 63.07% (251/398) and 83.21% (114/137) among AGE hospitalized, ER visits and nosocomially-acquired AGE cases, respectively. Of the total number of RVGE cases, 78.13% (493/631) were reported during the RV season with the proportions of RVGE being 79.14% (277/350), 70.83% (102/144) and 83.21% (114/137) in AGE hospitalizations and ER visits, and nosocomial AGE cases, respectively.
Among AGE hospitalizations, 54.00% of cases tested during the RV season were RV-positive when compared to 44.51% of the RVGE cases recorded in summer-autumn (p=0.042). In AGE ER cases, 40.64% and 28.57% of tested AGE were found to be RV-positive during the RV season and in summer-autumn, respectively (p=0.020). The seasonal distribution stratified by month is shown in Figure 3. Higher proportions of RVGE were recorded between January and March in all subject groups – RVGE hospitalizations, ER visits and nosocomial RVGE.
Figure 3. Occurrence of rotavirus gastroenteritis (RVGE) cases by month in Romania, 2008–09.
Genotype distribution
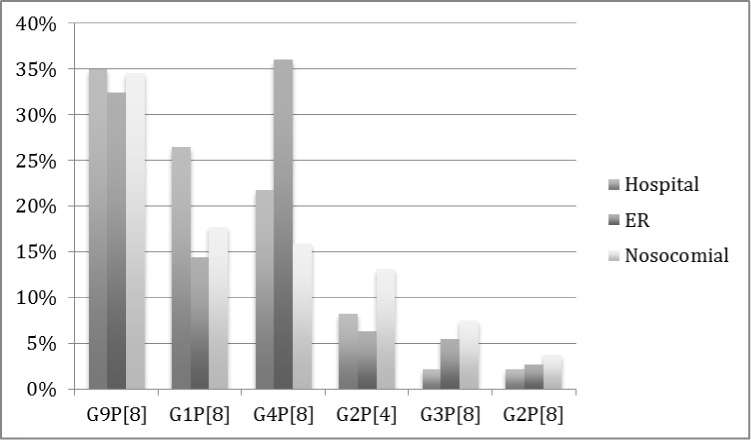
The most commonly detected genotypes in community-acquired AGE cases were G9P[8] (34.27%, 134/391) followed by G4P[8] (25.83%, 101/391) and G1P[8] (23.02%, 90/391). The most common genotypes in nosocomial RVGE cases were G9P[8] (34.58%, 37/107), followed by G1P[8] (17.76%, 19/107) and G4P[8] (15.89%, 17/107). The genotype distributions in RVGE hospitalizations, ER visits and nosocomial RVGE are shown in Figure 4. The most prevalent genotypes observed for all types of cases (community-acquired and nosocomial RVGE) were G9P[8], G1P[8] and G4P[8]. However, G9P[8] was the most prevalent genotype in hospitals (both community-acquired and nosocomial) and G4P[8] was the most prevalent genotype observed in the cases that visited the ER.
Figure 4. Genotype distributions in rotavirus gastroenteritis (RVGE) cases in Romania, 2008–09.
Clinical characteristics of RVGE disease
The clinical characteristics of RVGE disease by AGE hospitalizations and ER visits; and nosocomial RVGE are given in Table 2. Vesikari scores were available for 65.73% (445/677) of hospitalized AGE cases and 87.44% (348/398) of AGE ER visits. Of all community-acquired AGE cases, the proportion of severe cases was 72.75% in RVGE cases as opposed to non-RVGE cases (46.45%) (Table 2). Among nosocomial RVGE, 43.69% of cases reported severe disease. Median duration of hospitalization among RV positive subjects was six days (range: 2–31 days). The median duration for additional hospitalization due to nosocomial RVGE was five days (range: 1–10).
Table 2. Clinical characteristics of community-acquired AGE hospitalizations and ER visits by RV status in Romania, 2008–09.
| Hospitalizations n (%) | ER visits n (%) | Nosocomial RVGE | |||||||
| Characteristic | RV-positive | RV-negative | p-value | OR | RV-positive | RV-negative | p-value | OR | |
| Severity | |||||||||
| Severe (≥11) | 165 (73.66) | 105 (47.51) | <0.001 | 3.08 | 94 (71.21) | 98 (45.37) | <0.001 | 2.97 | 60 (43.69) |
| Median Duration# (days) | - | - | |||||||
| Hospitalization | 6 (range:2–31) | 5.5 (range:1–28) | - | - | - | - | - | - | 5 (range:1–10)a |
| Vomiting | 2 (range:0–8) | 1 (range:0–10) | 1 (range:0–20) | 2 (range:0–14) | 1 (range:0–4) | ||||
| Diarrhea | 5 (range:1–13) | 4 (range: 1–16) | 3 (range:1–14) | 3 (range:1–20) | 3 (range:1–7) | ||||
| Symptoms | |||||||||
| Vomiting | 310 (88.57) | 227 (69.42) | <0.001 | 3.41 | 118 (81.94) | 153 (60.24) | <0.001 | 3.00 | 88 (64.23) |
| Fever | 302 (86.29) | 255 (78.22) | 0.007 | 1.75 | 125 (87.41) | 178 (70.08) | <0.001 | 2.96 | 97 (70.80) |
| Change in behavior | 313 (89.30) | 266 (82.10) | 0.008 | 1.84 | 111 (77.08) | 153 (60.24) | <0.001 | 2.22 | 115 (83.94) |
| Weight loss | 102 (45.13) | 63 (31.98) | 0.007 | 1.75 | 37 (45.68) | 47 (41.23) | 0.637 | 1.20 | 33 (29.73) |
| Treatment received | |||||||||
| Rehydration therapy | 346 (98.86) | 320 (97.86) | 0.471 | 1.89 | 137 (95.14) | 229 (90.16) | 0.111 | 2.13 | 135 (98.54) |
| IC unitb | 23 (6.63) | 13 (4.14) | 0.173 | 1.71 | 10 (7.25) | 6 (2.44) | 0.050 | 3.12 | 14 (10.37) |
| Outcome at discharge | |||||||||
| Died | 0 (0.00) | 0 (0.00) | - | - | 0 (0.00) | 0 (0.00) | - | - | 2 (1.48) |
| Recovered | 339 (96.86) | 305 (93.56) | 51 (35.42) | 80 (31.50) | 127 (94.07) | ||||
| Ongoing GE | 9 (2.57) | 19 (5.83) | 91 (63.19) | 172 (67.72) | 4 (2.96) | ||||
| Transferred | 2 (0.57) | 2 (0.61) | 2 (1.39) | 2 (0.79) | 2 (1.48) | ||||
Comparison between groups using Mann-Whitney U-test, p<0.05;
For nosocomial RVGE, duration was additional hospitalization duration;
Treatment in IC units for ER visits is not relevant for the Romanian health system because only hospitalized cases are usually treated in IC units.
AGE acute gastroenteritis; ER emergency room; GE gastroenteritis; IC intensive care; RV rotavirus; RVGE rotavirus gastroenteritis.
Among nosocomial RVGE, 94.07% of cases (n=127) recovered and 1.48% of cases (n=2) died. The causative association between nosocomial RVGE and death was uncertain in these two cases. No link was found between RV status and patient medical history. Children identified with medical history (prematurity, pulmonary or cardiac diseases, combined) did not seem to have a higher probability of acquiring RV infection neither in hospitalized cases (RV+/RV-: 20.57%/14.67%; p=0.056) nor in ER visiting cases (RV+/RV-: 15.27%/18.89%; p=0.440).
Discussion
This is the first multi-center study in Romania to assess the burden of RVGE in children below five years of age through prospective monitoring. Very few studies have described the burden of RVGE disease in Romania.8–15
However, these available data for Romania were mostly obtained from either retrospective single-center studies or regional surveys, or are now outdated.10–14
The data from the present study confirms that RV is a major cause of community-acquired AGE (overall proportion: 45.95%), which is consistent with findings from Europe (43.4%) and more specifically from Central and Eastern Europe, where RV is responsible for 22–55% of community-acquired AGE cases.4,5,8,9,28–30 Among all RVGE cases, RV was responsible for 51.70% of hospitalized AGE cases and 36.18% of AGE cases visiting the ER; these data are in-line with data reported by Forster et al. (RV hospitalization: 56.2% and RV emergency department visits: 32.18%) and other studies conducted in Europe.4,28–30 These proportions are also consistent with two recent studies conducted in Romania.14,15 Comparisons of data available for Romania with findings from the present study are limited due to differences in methodology between studies conducted in the region.
In Europe, the significance of RV in nosocomial AGE has been previously documented.7 The incidence of nosocomial RVGE reported in this study was 0.52 per 1,000 days of hospitalization, which is well within the range (0.0–1.87 per 1,000 days of hospitalization) reported for Europe in children below five years of age.4 Most community-acquired cases occurred in children below two years of age (68.83%). Nosocomial RVGE cases were also reportedly higher in children below two years of age (78.10%). The age-distributions of community-acquired RVGE and nosocomial RVGE cases are similar to those observed for Europe.8,9,28 The proportions of RVGE in infants below six months of age among community-acquired cases (12.75%) and nosocomial RVGE (10.95%) are also similar to age-distribution data observed for Europe.4
Five RV genotypes are most prevalent worldwide, however, substantial variation in their incidence has been observed.31 This study identified G9P[8] (34.27%), G4P[8] (25.83%) and G1P[8] (23.02%) to be the most common RV strains. This distribution is comparable to strain surveillance reports from different countries in Europe and more specifically Central and Eastern Europe.8,29,32 In the present study, circulation of G2P[4] was low, accounting for only 7.67% of all cases. This was similar to what has been reported previously in Europe.29 While strain surveillance reports across Europe have remained fairly consistent, some fluctuations based on season and vaccination status are expected and have been reported.4,32,33 RV infections are more prevalent during the cooler months of the year in Europe.4,29,34 Data from our study confirm the peak season of RV infection in Romania which was observed between January and March.15 The seasonal distribution from our study also confirm findings from Europe 8,9,29 The peak of RVGE coincides with the peak incidence of other childhood diseases (seasonal influenza, etc.) that also require medical attention, thereby adding pressure on health care services.
Overall, the proportion of severe episodes was higher in RV-positive cases (72.75%) when compared to RV-negative cases (46.45%). Similar findings were observed in a recent study in Romania (severe AGE in RV-positive cases: 65.2%).15 Among hospitalizations, AGE was more severe in RV-positive subjects than RV-negative subjects, resulting in longer durations of vomiting and diarrhea. Among ER visits, AGE was more severe; however, durations of vomiting and diarrhea were comparable to those observed in RV-negative cases. Duration of hospitalization among RV-positive subjects was six days. Additional hospitalization duration due to nosocomial RVGE was five days, almost doubling the total in-hospital time span, and placing high demands on the healthcare systems.
RVGE disease worldwide is currently managed by fluid and electrolyte replacement, improvements in sanitation and water supply. Improvements in hygiene and provision of safe water supply may not be as effective in the prevention of RVGE. Vaccination against RV is considered as the most effective primary public health intervention for RVGE disease.20 Results of observational studies conducted in different countries worldwide where RV vaccines have been included into the national immunization programs have demonstrated a significant reduction in the burden of RVGE.35–37 The data from this study provides evidence of a high burden of RVGE disease in Romania. RV vaccines have been available in Romania since 2008. However, these vaccines are not offered through the national immunization program. Consideration from the decision-making level is needed to recommend vaccination or implement RV vaccination through the national immunization program in Romania.
Merits of this study include comparison of study results to the data obtained from other areas in Europe due to use of a common generic protocol to assess the burden of RVGE disease using hospital-based surveillance.21 It is however important to note that this study was not designed to evaluate vaccination specifically, as the WHO generic protocol specifies. However, the baseline assessments recommended by WHO were applied.
The novelty of this research lies in the fact that this study in Romania is the first of its kind wherein RV burden was analyzed prospectively in such a large number of subjects, and a single standardized and validated method for RV detection was used at all the study centers. Additionally, the genotyping of all samples was performed at one central laboratory that allowed comparability and consistency of data obtained. The main limitation of the study is that the findings from this analysis cannot be generalized to the Romanian population aged below five years since the selected centers covered only an estimated 28% of the pediatric population. This study's generalizability was limited by feasibility issues surrounding the national surveillance system in the sense that the WHO Generic Protocol for hospital-based surveillance to estimate the RVGE disease burden in children could not be implemented due to the fact that in Romania there are more than 300 hospitals treating children and the health system is based on free choice of the medical provider. Potential sources of bias include the fact that equal targets were established for each center even though the centers were different in terms of access. Results may have also been influenced by variation in clinical practice, healthcare seeking behavior and the local rules of re-imbursement with the Romanian social health insurance system.
Conclusion
The findings from this study provide evidence of a significant burden due to RVGE disease on healthcare services, and warrant the need for consideration of introduction of RV vaccine through routine immunization of infants in Romania. RVGE prevention through routine childhood vaccination would be expected to substantially reduce the burden of AGE in infants and young children in Romania.
Acknowledgements
The authors are grateful to Roeland Van Kerckhoven (Consultant for Keyrus Biopharma) for editorial assistance and publication coordination on behalf of GlaxoSmithKline group of companies and Amrita Ostawal (consultant publications writer to GlaxoSmithKline group of companies) for medical writing.
Footnotes
Authors' contributing statements FLF, DP, ASC, SR and KH made substantial contributions to either the interpretation or the collection of data. FLF and SR either performed or supervised the statistical data analyses. All authors reviewed and commented on the initial draft of the manuscript and all authors read and approved the final version of the manuscript.
Trial registration NCT01253967
Trademark Rotarix is a trademark of GlaxoSmithKline group of companies. RotaTeq is a registered trademark of Merck and Co., Inc., USA. RotaStrip is a trademark of Coris BioConcept, Gembloux, Belgium.
Conflicts of interest Ioana Alina Anca has been the principal investigator in clinical studies supported by GlaxoSmithKline (GSK) group of companies, Ferring, Apogepha and Institut de Recherche Pierre Fabre, and scientific consultant to GSK group of companies, Wyeth, Teva, Reckitt-Benckiser, Astra Zeneca and Nestle. She received sponsorship from GSK group of companies, Wyeth and Nestle to attend scientific meetings. Florentina Ligia Furtunescu received funds from GlaxoSmithKline group of companies for organizing the e-database, data analysis and writing of the study report. All other authors, except for Katsiaryna Holl who is an employee of GlaxoSmithKline group of companies, have declared no conflicts of interest.
Role of the funding source GlaxoSmithKline Biologicals SA was the funding source and was involved in all stages of the study conduct and analysis. GSK Biologicals SA also funded all costs associated with the development of the present manuscript. All authors had full access to the data and agreed with the submission of the manuscript for publication.
References
- 1.Parashar UD, Bresee JS, Gentsch JR, Glass RI. Rotavirus. Emerg Infect Dis. 1998;4:561–70. doi: 10.3201/eid0404.980406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–6. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soriano-Gabarró M, Mrukowicz J, Vesikari T, Verstraeten T. Burden of rotavirus disease in European Union countries. Pediatr Infect Dis J. 2006;25(1Suppl):S7–11. doi: 10.1097/01.inf.0000197622.98559.01. [DOI] [PubMed] [Google Scholar]
- 4.Forster J, Guarino A, Parez N, et al. Hospital-based surveillance to estimate the burden of rotavirus gastroenteritis among European children younger than 5 years of age. Pediatrics. 2009;123:e393–e400. doi: 10.1542/peds.2008-2088. [DOI] [PubMed] [Google Scholar]
- 5.Giaquinto C, Van Damme P, Huet F, et al. Clinical consequences of rotavirus acute gastroenteritis in Europe, 2004–2005: the REVEAL study. J Infect Dis. 2007;195(Suppl 1):S26–35. doi: 10.1086/516717. [DOI] [PubMed] [Google Scholar]
- 6.Ogilvie I, Khoury H, Goetghebeur MM, El Khoury AC, Giaquinto C. Burden of community-acquired and nosocomial rotavirus gastroenteritis in the pediatric population of Western Europe: a scoping review. BMC Infect Dis. 2012;12:62. doi: 10.1186/1471-2334-12-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gleizes O, Desselberger U, Tatochenko V, et al. Nosocomial rotavirus infection in European countries; a review of the epidemiology, severity and economic burden of hospital-acquired rotavirus disease. Pediatr Infect Dis J. 2006;25(1 Suppl):S12–21. doi: 10.1097/01.inf.0000197563.03895.91. [DOI] [PubMed] [Google Scholar]
- 8.Ogilvie I, Khoury H, El Khoury AC, Goetghebeur MM. Burden of rotavirus gastroenteritis in the pediatric population in Central and Eastern Europe: serotype distribution and burden of illness. Hum Vaccin. 2011;7(5):523–33. doi: 10.4161/hv.7.5.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meszner Z, Balogh A, Banyai K, et al. The clinical burden of rotavirus disease: Retrospective analysis of infant and childhood gastroenteritis in seven countries in central and eastern Europe. Pediatr Infect Dis J. 2008;27:S33–41. [Google Scholar]
- 10.Serban D, Jebeleanu L, Andronescu D, et al. [The annual and seasonal variation in the bacteria and rotaviruses implicated in the etiology of diarrheal diseases in children]. Bacteriol Virusol Parazitol Epidemiol. 1992;37:58–66. [PubMed] [Google Scholar]
- 11.Ulmeanu C, Nistor I, Crăciun MD, Ion-Nedelcu N. [Frequency and severity of rotavirus acute gastroenteritis hospitalized in Bucharest, Romania. Results of a case-control study]. Bacteriol Virusol Parazitol Epidemiol. 2009;54:41–6. [PubMed] [Google Scholar]
- 12.Strat E, Tansanu I, Goţia S, et al. Detection of group rotavirus antigen with Romanian ELISA kits in the acute gastroenteritis of the infant and young child. Rev Med Chir Soc Med Nat Iasi. 1986;90:323–5. [PubMed] [Google Scholar]
- 13.Avram G, Zavate O, Combiescu AA, et al. [Detection of the rotavirus group antigen by a screening test using the ELISA-IC kit in subjects with acute gastroenteritis, at the pediatric services of Moldavia]. Virologie. 1987;38:169–75. [PubMed] [Google Scholar]
- 14.Mihalache D, Fîntînaru R, Iacob M, Simonca C. [Clinical study of acute diarrhea caused by rotavirus]. Rev Med Chir Soc Med Nat Iasi. 2005;109:488–91. [PubMed] [Google Scholar]
- 15.Lesanu G, Becheanu CA, Vlad RM, Pacurar D, Tincu IF, Smadeanu RE. Clinical characteristics of rotavirus diarrhea in hospitalized Romanian infants. Pediatr Infect Dis J. 2013;32:89–91. doi: 10.1097/INF.0b013e3182706152. [DOI] [PubMed] [Google Scholar]
- 16.Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. [DOI] [PubMed] [Google Scholar]
- 17.Linhares AC, Velázquez FR, Pérez-Schael I, et al. Efficacy and safety of an oral live attenuated human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in Latin American infants: a randomised, double-blind, placebo-controlled phase III study. Lancet. 2008;371:1181–9. doi: 10.1016/S0140-6736(08)60524-3. [DOI] [PubMed] [Google Scholar]
- 18.Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortant rotavirus vaccine. N Engl J Med. 2006;354:23–33. doi: 10.1056/NEJMoa052664. [DOI] [PubMed] [Google Scholar]
- 19.Vesikari T, Karvonen A, Prymula R, et al. Efficacy of human rotavirus vaccine against rotavirus gastroenteritis during the first 2 years of life in European infants: randomised, double-blind controlled study. Lancet. 2007;370:1757–63. doi: 10.1016/S0140-6736(07)61744-9. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization. Meeting of the Strategic Advisory Group of Experts on immunization, October 2009 - conclusions and recommendations. Wkly Epidemiol Rec. 2009;84:517–32. [PubMed] [Google Scholar]
- 21.Bresee J, Parashar U, Holman R, et al. Generic protocol for hospital-based surveillance to estimate the burden of rotavirus gastroenteritis in children under 5 years of age.Field test version. 2002. [Accessed on: September 24, 2013]. Available at: http://whqlibdoc.who.int/hq/2002/WHO_V&B_02.15.pdf.
- 22.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–67. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 23.Frühwirth M, Heininger U, Ehlken B, et al. International variation in disease burden of rotavirus gastroenteritis in children with community- and nosocomially acquired infection. Pediatr Infect Dis J. 2001;20:784–91. doi: 10.1097/00006454-200108000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Coris BioConcept. RotaStrip™. Rapid diagnostic test for in vitro detection of rotavirus in stool specimens. 2012. [Accessed on: September 24, 2013]. Available at: http://www.corisbio.com/Products/Human-Field/Rota.php.
- 25.Iturriza-Gómara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–65. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 26.Gentsch JR, Glass RI, Woods P, et al. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–73. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Doorn LJ, Kleter B, Hoefnagel E, et al. Detection and genotyping of human rotavirus VP4 and VP7 genes by reverse transcriptase PCR and reverse hybridization. J Clin Microbiol. 2009;47:2704–12. doi: 10.1128/JCM.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giaquinto C, van Damme P, REVEAL Study Group Age distribution of pediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis. 2010;42:142–7. doi: 10.3109/00365540903380495. [DOI] [PubMed] [Google Scholar]
- 29.Diez-Domingo J, Baldo JM, Patrzalek M, et al. Primary care-based surveillance to estimate the burden of rotavirus gastroenteritis among children aged less than 5 years in six European countries. Eur J Pediatr. 2011;170:213–22. doi: 10.1007/s00431-010-1289-1. [DOI] [PubMed] [Google Scholar]
- 30.Williams CJ, Lobanov A, Pebody RG. Estimated mortality and hospital admission due to rotavirus infection in the WHO European region. Epidemiol Infect. 2009;137:607–16. doi: 10.1017/S0950268808001714. [DOI] [PubMed] [Google Scholar]
- 31.O'Ryan M. The ever-changing landscape of rotavirus serotypes. Pediatr Infect Dis J. 2009;28(3 Suppl):S60–2. doi: 10.1097/INF.0b013e3181967c29. [DOI] [PubMed] [Google Scholar]
- 32.Van Damme P, Giaquinto C, Maxwell M, Todd P, Van der Wielen M, REVEAL Study Group Distribution of rotavirus genotypes in Europe, 2004–2005: the REVEAL Study. J Infect Dis. 2007;195(Suppl. 1):S17–25. doi: 10.1086/516715. [DOI] [PubMed] [Google Scholar]
- 33.Antunes H, Afonso A, Iturriza M, et al. G2P[4] the most prevalent rotavirus genotype in 2007 winter season in a European non-vaccinated population. J Clin Virol. 2009;45:76–8. doi: 10.1016/j.jcv.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 34.D'Souza RM, Hall G, Becker NG. Climatic factors associated with hospitalization for rotavirus diarrhoea in children under 5 years of age. Epidemiol Infect. 2008;136:56–64. doi: 10.1017/S0950268807008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention (CDC) Reduction in rotavirus after vaccine introduction - United States, 2000–2009. MMWR Morb Mortal Wkly Rep. 2009;58:1146–9. [PubMed] [Google Scholar]
- 36.Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in austrian children. Pediatr Infect Dis J. 2010;29:319–23. doi: 10.1097/INF.0b013e3181c18434. [DOI] [PubMed] [Google Scholar]
- 37.Lambert SB, Faux CE, Hall L, et al. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med J Aust. 2009;191:157–60. doi: 10.5694/j.1326-5377.2009.tb02727.x. [DOI] [PubMed] [Google Scholar]