Abstract
OBJECTIVE:
Indoor tanning with UV radiation–emitting lamps is common among adolescents and young adults. Rising incidence rates of basal cell carcinoma (BCC) have been reported for the United States and elsewhere, particularly among those diagnosed at younger ages. Recent epidemiologic studies have raised concerns that indoor tanning may be contributing to early occurrence of BCC, and younger people may be especially vulnerable to cancer risk associated with this exposure. Therefore, we sought to address these issues in a population-based case–control study from New Hampshire.
METHODS:
Data on indoor tanning were obtained on 657 cases of BCC and 452 controls ≤50 years of age.
RESULTS:
Early-onset BCC was related to indoor tanning, with an adjusted odds ratio (OR) of 1.6 (95% confidence interval, 1.3–2.1). The strongest association was observed for first exposure as an adolescent or young adult, with a 10% increase in the OR with each age younger at first exposure (OR per year of age ≤23 = 1.1; 95% confidence interval, 1.0–1.2). Associations were present for each type of device examined (ie, sunlamps, tanning beds, and tanning booths).
CONCLUSIONS:
Our findings suggest early exposure to indoor tanning increases the risk of early development of BCC. They also underscore the importance of counseling adolescents and young adults about the risks of indoor tanning and for discouraging parents from consenting minors to this practice.
Keywords: early onset, etiology, indoor tanning, adolescents, skin cancer, radiation
What’s Known on This Subject:
Indoor tanning has gained widespread popularity among adolescents and young adults. Incidence rates of early-onset basal cell carcinoma also appear to be rising. Scant evidence exists on the impacts of early exposure and whether it leads to early occurrence of this malignancy.
What This Study Adds:
In a US population-based study, indoor tanning was associated with an elevated risk of basal cell carcinomas occurring at or before the age of 50 years, with an increasing trend in risk with younger age at exposure among adolescents and young adults.
Basal cell carcinoma (BCC) is the most frequently diagnosed malignancy in humans.1 These tumors often occur near vital structures (eg, eyes, nose, ear, and lips) and can cause significant disfigurement if not treated early.2 Until recently, BCC was considered a tumor of late adult onset, occurring more prominently in men.3 However, recent evidence suggests a dramatic rise in the incidence rates of BCCs in younger adults,4–8 particularly among women. Despite having a favorable prognosis and low mortality rate,9 BCCs can recur and be difficult to treat.10 Additionally, those diagnosed with a BCC are at a markedly elevated risk of developing additional BCC tumors.11
UV radiation from the sun and skin sensitivity to sun exposure are among the major etiologic factors for keratinocyte cancers.3 Artificial tanning devices emit UV radiation at wavelengths with known carcinogenic properties.12–15 Use of these devices for cosmetic purposes has gained immense popularity in recent decades, especially among adolescent girls.14,16–19 Previous studies provide consistent evidence about the risks of melanoma and squamous cell carcinomas of the skin related to tanning lamp use.15,20,21 More recent work raises the possibility of an elevated risk of BCC from early exposure,21,22 and that exposure may lead to earlier onset of the disease.23 As part of a population-based case–control study of BCC, we had an opportunity to examine age at first tanning lamp exposure in relation to the incidence of early-onset BCC and to investigate whether effects were specific to the type of device used (eg, sunlamps, tanning beds, or booths) or period of use.
Methods
Study Settings
Study subjects were patients with BCC newly diagnosed between the ages of 25 and 50 years and controls aged 25 to 50 years who participated in the New Hampshire Skin Cancer Study, a population-based case–control study of keratinocyte cancers identified through comprehensive surveillance of dermatology and dermatopathology practices along with pathology laboratories serving New Hampshire.24–27 BCC cases diagnosed from July 1993 to June 1995 and July 1997 to March 2000 were randomly selected (for efficiency) within strata defined by anatomic site, age, and gender to ensure representation of the entire group of BCC diagnoses.26 For diagnoses from July 2001 through July 2002, we sampled all BCC cases diagnosed between ages 25 and 50 years. Controls were randomly selected from lists of New Hampshire residents provided by the New Hampshire Department of Transportation and frequency-matched on age (25–35, 36–45, 46–50, 51–59, 60–64, 65–69, and 70–74 years) and gender to represent the distribution of the full New Hampshire Skin Cancer Study case group of keratinocyte cancers (which included both BCC and squamous cell carcinoma [SCC] diagnoses). Dates matched to the case diagnosis dates were generated as the reference dates for controls. Eligible subjects included New Hampshire residents who spoke English and had a listed telephone number. A small percentage of cases (<1%) were excluded because of physician refusal to contact. Of the 802 cases and 667 controls 25 to 50 years of age confirmed eligible, 622 (84%) cases and 457 (73%) controls were interviewed.
Study Criteria and Data Collection
All participants provided informed consent in accordance with the Committee for the Protection of Human Subjects at Dartmouth College. Study participants completed a structured personal interview, usually at their homes. Questions included detailed sociodemographic and skin cancer risk factors including skin reaction to the sun after first exposure in the summer (ie, tendency to sunburn). To estimate sun exposure, we used a standardized questionnaire adapted from an earlier study28 and tested for its validity in our population27 to elicit information about amount of time spent outdoors on work days and nonwork days (both in the summer and other times of the year) and history of painful and blistering sunburns. For diagnoses beginning with July 1998, we requested a diagnostic slide of the original tumor. These were re-reviewed by a board-certified dermatopathologist (A.E.P.), who determined the severity of solar elastosis in the skin adjacent to each tumor (graded as absent, minimal, moderate, or severe).27 Solar elastosis was categorized as mild if single, scattered, blue-gray elastotic fibers were identified in the papillary dermis; moderate if clumps of elastotic fibers with intervening normal papillary dermis were present; and severe if replacement of the papillary dermis by clumped elastotic fibers or amorphous masses of elastotic material was observed.
We asked participants whether they ever used tanning lamps that produced UV radiation, for nonmedical reasons, before the reference date. For positive responses, we asked the age at which they first used tanning lamps. Beginning with reference dates of July 1998, we asked participants whether they used specific types of tanning devices including sunlamps (smaller units placed on a desk, table, or floor), tanning beds (horizontal devices with lamps above or both above and below the user), and tanning booths (vertical walk-in devices); and beginning with July 2001, we added visual aids that allowed participants to distinguish between circular desktop lamps, rectangular desktop lamps, lightweight floor lamps, lightweight tilting floor lamps, tanning beds with top lamps only, tanning beds with bottom lamps only, tanning beds with both top and bottom lamps, ceiling fixtures, walk-in booths, and walk-in closets and also asked the location where they tanned. To minimize potential reporting bias, we did not reveal the specific hypotheses of interest to either the interviewer or participant, and we did not inform the interviewers of the case–control status of participants.
Statistical Analysis
We computed the odds ratio (OR) and 95% confidence interval (CI) of BCC associated with the use of UV radiation tanning devices before the reference date using unconditional logistic regression, taking into account multiple confounding factors.29 Aside from age and gender, we assessed the potentially confounding effects of level of education (less than college, college, graduate school), skin reaction to the first hour of sun exposure in summer (blistering, or painful sunburns versus mild sunburn with some tanning, or tanning with no sunburn), lifetime hours of sun exposure (≤11 026, >11 026 hours), number of painful sunburns (0, 1–2, 3–19, ≥20), hours of sun exposure in childhood, that is, age <15 years (≤3737, >3737 hours), and proportion of time spent outdoors during nonworking days in childhood, that is, age <15 years (<1.0, 1.0). Of these factors, only skin reaction to the first hour of sun exposure in summer was included in the final models because no other factors appreciably influenced the results (ie, changed the estimates by 10% or more). Given that our analysis included 3 study phases (1993–1997, 1998–2000, and 2001–2002), we included a variable for study phase in our models.
In addition to any use of tanning lamps, we examined both age at first use (classified as no use, first use <20, 20–35, and ≥36 years old) and time since first use to diagnosis (classified as no use, <15 years, 15–30, and >30 years since first use). We also modeled age at first tanning lamp exposure as a continuous variable by plotting a locally smoothed curve of the proportion of cases30 and comparing it with parametric models. We examined calendar year of first use (<1975, 1975–1986, >1986), with categories reflecting trends in tanning practices, such as introduction of tanning beds in 1975 and US Food and Drug Administration regulations implemented in 1986.
We assessed the potential modifying effects of gender, study phase, and UV radiation–related factors (skin reaction to first hour of sun exposure in summer, number of painful sunburns, total lifetime hours spent outdoors, and hours spent outdoors in childhood and the proportion of time spent outdoors during nonworking days in childhood) and conducted subgroup analyses by anatomic site and severity of solar elastosis. The statistical package SAS version 9.2 (SAS Institute, Inc, Cary, NC) was used for all analyses except for the locally smoothed curve generated in R.
Results
Information on indoor tanning was available on 98% of participants, providing 657 cases and 452 controls for the analysis. Participants with early-onset BCC were more likely to report a greater propensity to sunburn rather than suntan in response to the first hour of sun exposure in summer as compared with controls (Table 1). The cumulative amount of time spent outdoors during the warmer months overall or in childhood did not differ markedly between cases and controls, but the number of reported painful sunburns was higher among cases than controls. For most participants, time spent outdoors during childhood occurred on days off, and this proportion was similar for cases and controls. BCCs were located on head and neck sites in 57% of the cases, and about 50% had histologic evidence of severe solar elastosis.
TABLE 1.
Selected Demographic Characteristics, Sun Exposure, and Tumor Characteristics of Early-Onset BCC Cases and Controls
| Controls, n (%) | Early-Onset BCC Cases, n (%) | |
|---|---|---|
| Age (y) | ||
| <30 | 13 (2.9) | 22 (3.3) |
| 31–40 | 138 (30.5) | 156 (23.7) |
| 41–45 | 123 (27.2) | 191 (29.1) |
| 46–50 | 178 (39.4) | 288 (43.8) |
| Gender | ||
| Men | 184 (40.7) | 251 (38.2) |
| Women | 268 (59.3) | 406 (61.8) |
| Educationa | ||
| Less than college | 128 (28.3) | 142 (21.7) |
| College | 220 (48.7) | 307 (47.0) |
| Graduate or professional school | 104 (23.0) | 204 (31.2) |
| Skin reaction to first hour of sun exposure in summerb | ||
| Tan, or mild burn then tan | 273 (60.5) | 309 (47.1) |
| Painful or blistering sunburn | 178 (39.5) | 347 (52.9) |
| Number of painful sunburnsc | ||
| 0 | 116 (26.8) | 134 (21.9) |
| 1–2 | 95 (21.9) | 89 (14.5) |
| 3–19 | 97 (22.4) | 135 (22.1) |
| ≥20 | 125 (28.9) | 254 (41.5) |
| Hours of sun exposure, warm months, 9 am–5 pmd | ||
| ≤11 026 | 217 (50.0) | 334 (52.8) |
| >11 026 | 217 (50.0) | 298 (47.2) |
| Hours of sun exposure, warm months, 9 am–5 pm in childhoode | ||
| ≤3737 | 221 (50.8) | 336 (52.8) |
| >3737 | 214 (49.2) | 300 (47.2) |
| Proportion of time spent outdoors during nonworking days in childhood | ||
| <1.0 | 44 (10.2) | 81 (12.3) |
| 1.0 | 388 (89.8) | 530 (86.7) |
| Anatomic sitef | ||
| Head or neck | — | 365 (56.9) |
| Trunk | — | 204 (31.8) |
| Upper limb | — | 34 (5.3) |
| Lower limb | — | 30 (4.7) |
| Other sites | — | 8 (1.2) |
| Solar elastosisf | ||
| Absent | — | 4 (1.3) |
| Minimal | — | 57 (17.9) |
| Moderate | — | 97 (30.5) |
| Severe | — | 160 (50.3) |
| Number of BCCsf | ||
| 1 | — | 595 (90.6) |
| >1 | — | 62 (9.4) |
Education missing in 4 cases.
Skin reaction acute sun missing in 1 control, 1 BCC case.
Number of painful sunburns missing in 19 controls and 45 BCC cases.
Number of lifetime hours of sun exposure in warm months missing in 18 controls and 25 BCC cases and categorized based on the median value of the control participants.
Number of lifetime hours of sun exposure in childhood during warm months is missing in 17 controls and 21 BCC cases and categorized based on the median value of the control participants.
Tumors of cases were classified according to their anatomic site of occurrence, the degree of solar elastosis according to the study pathologist's re-review of the diagnostic tumor, and presence of multiple BCC tumors.
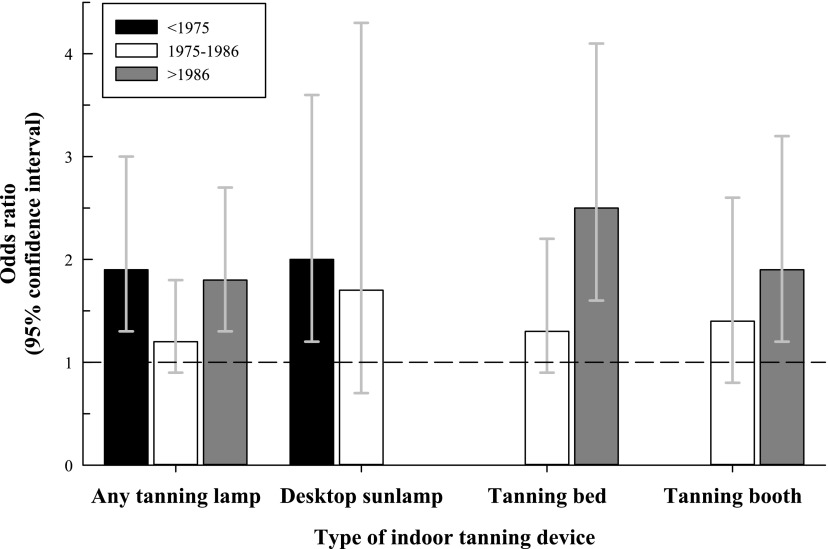
A higher proportion of participants with early-onset BCC reported indoor tanning with a tanning lamp as compared with controls (OR = 1.6; 95% CI, 1.3–2.1), and the association was present for all types of tanning devices (Table 2). Elevated ORs were found for both early (<1975) and late (>1986) calendar periods of first exposure (Fig 1). Before 1975, desktop sunlamps were the most common indoor tanning devices, and first use in this period was associated with a somewhat higher OR of early-onset BCC than in 1975 to 1986. After 1986, desktop sunlamps were infrequently used. In contrast, tanning beds and booths became more common after 1975, and the OR associated with first use of tanning beds and booths was highest in the most recent time period (eg, after 1986). Based on information available in the third study phase, desktop sunlamps were used almost exclusively at home (>95% of reported use), and tanning beds or booths were almost always used at business such as tanning salons, beauty parlors, and health spas (>99% of reported use).
TABLE 2.
ORs of Early-Onset BCC and Indoor Tanning Overall and by Type of Tanning Device, Age, and Time Since First Use
| Controls | Early-Onset BCC Cases | OR (95% CI)a | |
|---|---|---|---|
| n (%) | n (%) | ||
| Any tanning lamp useb | |||
| No | 290 (64.2) | 354 (53.9) | Referent |
| Yes | 162 (35.8) | 303 (46.1) | 1.6 (1.3–2.1) |
| Device typec | |||
| None | 214 (62.9) | 249 (51.1) | Referent |
| Sunlamp | 30 (8.8) | 66 (13.6) | 1.9 (1.2–3.1) |
| Tanning bed | 71 (20.9) | 154 (31.6) | 2.1 (1.5–3.0) |
| Tanning booth | 59 (17.4) | 113 (23.2) | 1.8 (1.3–2.7) |
| Specific tanning equipmentd | |||
| None | 54 (61.6) | 164 (45.8) | Referent |
| Circular desk lamp | 12 (4.8) | 26 (7.3) | 2.0 (1.0–4.2) |
| Rectangular desk lamp | 5 (2.0) | 15 (4.2) | 3.0 (1.0–8.5) |
| Lightweight floor lamp | 6 (2.4) | 10 (2.8) | 1.6 (0.5–4.5) |
| Tanning bed, top and bottom radiance | 59 (23.6) | 133 (37.2) | 2.4 (1.6–3.6) |
| Walk-in closet | 35 (14.0) | 85 (23.7) | 2.7 (1.7–4.4) |
| Walk-in booth | 11 (4.4) | 17 (4.8) | 1.6 (0.7–3.7) |
| Age at first tanning device use, yb | |||
| No device use | 290 (64.3) | 354 (54.1) | Referent |
| >36 | 31 (6.9) | 61 (9.3) | 1.6 (1.0–2.6) |
| 20–35 | 82 (18.2) | 129 (19.7) | 1.4 (1.0–2.0) |
| <20 | 48 (10.6) | 110 (16.8) | 2.0 (1.4–3.0) |
| Time since first tanning device use, yb | |||
| No device use | 290 (64.3) | 354 (54.1) | Referent |
| <15 | 82 (18.2) | 152 (23.2) | 1.6 (1.2–2.3) |
| 16–30 | 70 (15.5) | 112 (17.1) | 1.4 (1.0–2.0) |
| >30 | 9 (2.0) | 36 (5.5) | 3.3 (1.5–7.1) |
All models adjusted for age, gender, skin reaction to first hour of sun exposure in summer, and study phase.
All 3 phases included.
Phase II and III.
Phase III only.
FIGURE 1.
ORs for early-onset BCC by year of first use of any tanning device, desktop sunlamps, tanning beds, and tanning booths. The dotted line represents the referent group.
Age at first exposure to indoor tanning ranged from 10 to 49 years (mean = 26.0 years, SD = 9.8 years). ORs were elevated among those whose first exposure was before age 20 (OR = 2.0; 95% CI, 1.4–3.0) and those who began later in life but to a lesser extent (OR for first use at 20–35 years = 1.4; 95% CI, 1.0–2.0; and OR for first use at ≥36 years = 1.6; 95% CI, 1.0–2.6) (Table 2). On a continuous scale our data fit a 2-segment model31 with a change point at 23 years of age at first use of tanning lamps (95% CI, 22–25 years). In this model, the ORs remained fairly constant by age for first use after 23 years (OR for each year of age younger =1.0; 95% CI, 0.9–1.0) and increased with each age younger at first exposure at or before 23 years (OR for each year of age younger = 1.1; 95% CI, 1.0–1.2).
A more pronounced OR of early-onset BCC was observed with a longer time interval from initial use of tanning lamps to diagnosis (OR for >30 years since first use = 3.3; 95% CI, 1.5–7.1; versus an OR for ≤15 years since first use = 1.6; 95% CI, 1.2–2.3; and an OR for 16–30 years since first use = 1.4; 95% CI, 1.0–2.0) (Table 2). Among those younger at first exposure (ie, before age 20) even a shorter time period between first exposure and diagnosis (eg, <20 years since first use) was associated with an elevated OR of early-onset BCC (OR = 2.5; 95% CI, 1.1–5.7).
We found positive associations between tanning lamp use and early-onset BCC in all categories of skin types, sunburn history, and hours of outdoor exposure (Table 3). In subgroup analyses, ORs were higher for tumors on the trunk (OR = 2.1; 95% CI, 1.5–3.1) and upper limbs (OR = 2.0; 95% CI, 1.0–4.3) than on the head and neck (OR = 1.4; 95% CI, 1.1–1.9) (Table 3).
TABLE 3.
ORs of Early-Onset BCC and Tanning Lamp Use by Subgroups According to Gender, UV Radiation–Related Factors, Anatomic Site, and Multiplicity
| Subgroup | Tanning Lamp Use | Controls | Early-Onset BCC Cases | OR (95% CI) |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Gender | ||||
| Men | No | 151 (82.0) | 170 (67.7) | Referent |
| Yes | 33 (17.9) | 81 (32.2) | 2.2 (1.4–3.6) | |
| Women | No | 139 (51.8) | 184 (45.3) | Referent |
| Yes | 129 (48.1) | 222 (54.6) | 1.4 (1.0–2.0) | |
| Skin reaction to first hour of sun exposure in summera,b | ||||
| Tan, or mild burn then tan | No | 171 (62.6) | 157 (50.8) | Referent |
| Yes | 102 (37.3) | 152 (49.1) | 1.7 (1.2–2.5) | |
| Painful or blistering sunburn | No | 118 (66.2) | 196 (56.4) | Referent |
| Yes | 60 (33.7) | 151 (43.5) | 1.5 (1.0–2.3) | |
| Lifetime number of painful sunburnsb,c | ||||
| None | No | 82 (70.6) | 76 (56.7) | Referent |
| Yes | 34 (29.3) | 58 (43.2) | 2.5 (1.4–4.5) | |
| 1–2 | No | 61 (64.2) | 44 (49.4) | Referent |
| Yes | 34 (35.7) | 45 (50.5) | 2.0 (1.0–4.0) | |
| 3–19 | No | 58 (61.0) | 80 (64.5) | Referent |
| Yes | 37 (38.9) | 44 (35.4) | 0.8 (0.05–1.5) | |
| ≥20 | No | 74 (59.2) | 125 (49.2) | Referent |
| Yes | 51 (40.8) | 129 (50.7) | 1.6 (1.0–2.5) | |
| Hours of sun exposure, warmer months, 9 am–5 pmb,c | ||||
| ≤11 026 h | No | 134 (61.7) | 169 (50.6) | Referent |
| Yes | 83 (38.2) | 165 (49.4) | 1.6 (1.2–2.4) | |
| >11 026 h | No | 141 (64.9) | 172 (57.7) | Referent |
| Yes | 76 (35.0) | 126 (42.2) | 1.4 (1.0–2.1) | |
| Hours sun exposure, warm months, 9 am–5 pm in childhoodb,c | ||||
| ≤3737 h | No | 139 (62.9) | 182 (54.2) | Referent |
| Yes | 82 (37.1) | 154 (45.8) | 1.5 (1.0–2.2) | |
| >3737 h | No | 136 (63.6) | 159 (53.0) | Referent |
| Yes | 78 (36.5) | 141 (47.0) | 1.7 (1.2–2.5) | |
| Proportion of time spent outdoors during nonworking days in childhoodb,c | ||||
| <1.0 | No | 30 (68.2) | 60 (74.1) | Referent |
| Yes | 14 (31.8) | 21 (25.9) | 0.8 (0.4–1.9) | |
| 1.0 | No | 243 (62.6) | 271 (51.1) | Referent |
| Yes | 145 (37.4) | 259 (48.9) | 1.7 (1.3–2.2) | |
| Solar elastosesc,d | ||||
| Absent, minimal, or moderate | No | 214 (62.9) | 80 (50.6) | Referent |
| Yes | 126 (37.1) | 78 (49.4) | 1.7 (1.1–2.6) | |
| Severe | No | 214 (62.9) | 80 (50.0) | Referent |
| Yes | 126 (37.1) | 80 (50.0) | 2.0 (1.3–3.0) | |
| Anatomic siteb,c | ||||
| Head and neck | No | 290 (64.2) | 209 (57.3) | Referent |
| Yes | 162 (35.9) | 156 (42.7) | 1.4 (1.1–1.9) | |
| All non–head and neck sites | No | 290 (64.2) | 135 (49.3) | Referent |
| Yes | 162 (35.8) | 139 (50.7) | 2.0 (1.5–2.8) | |
| Trunk | No | 290 (64.1) | 97 (47.5) | Referent |
| Yes | 162 (35.8) | 107 (52.4) | 2.1 (1.5–3.1) | |
| Upper limbs | No | 290 (64.1) | 18 (52.9) | Referent |
| Yes | 162 (35.8) | 16 (47.0) | 2.0 (1.0–4.3) | |
| Lower limbs | No | 290 (64.1) | 17 (56.6) | Referent |
| Yes | 162 (35.8) | 13 (43.3) | 1.3 (0.6–2.9) | |
| Number of BCCsb,c | ||||
| 1 BCC | No | 290 (64.1) | 321 (53.9) | Referent |
| Yes | 162 (35.8) | 274 (46.0) | 1.6 (1.2–2.1) | |
| >1 BCC | No | 290 (64.1) | 33 (53.2) | Referent |
| Yes | 162 (35.8) | 29 (46.7) | 1.8 (1.0–3.3) | |
Model adjusted for age, gender, and study phase.
All 3 phases included.
Models adjusted for age, gender, skin reaction to first hour of sun exposure in summer, and study phase.
Phases II and III only included.
Discussion
Our findings provide additional evidence of an association between indoor tanning and BCCs, particularly among those with a young age at onset. The results of our population-based study support an earlier case–control study derived from a dermatopathology database at Yale University involving 376 cases of early-onset BCCs and 390 controls with benign skin conditions.23 Despite differences between our study designs, the results are remarkably similar: an OR of 1.7 associated with tanning lamp use in the Yale study and 1.6 in ours. Both studies observed stronger associations for early-onset BCCs occurring on the trunk and extremities, sites less likely to receive UV radiation from natural sunlight. Other studies examining indoor tanning and early-onset BCC tended to be very small (<50 cases and <50 controls)32,33 and thus lacked statistical power. Our study, based on 657 cases and 452 controls, had a minimum detectable OR of 1.5 with an α of 0.05 and 80% power.34–36 Nonetheless, we recognize that our estimates for certain analyses (eg, the combined age and latency effects) tended to be less precise.
Importantly, our findings suggest that the risk of developing a BCC at a young age is inversely related to age at exposure. Two studies other than our earlier study investigated BCC occurrence in relation to age when exposed to indoor tanning.22 The Nurse’s Health Study studied 73 494 female married nurses, of whom 5506 developed a BCC. In this study, number of times per year participants reported using a tanning bed was associated with risk of BCC, particularly among those who used tanning beds at a younger age, such as during high school or college; the relative risk for 4 times per year compared with no use in high school or college was 1.40, and for 25 to 35 years of age it was 1.19.21 However, this report did not specifically evaluate early-onset tumors. Likewise, in our previous report, based on only the first phase of accrual,25 we were unable to examine risk of developing an early-onset tumor or the impacts of early age at exposure in much detail because of the limited sample size. In the current study, we noted an inverse relationship between age at first exposure and early-onset BCC tumors, particularly among those who started indoor tanning at ≤23 years of age. Of interest, our findings parallel epidemiologic evidence on outdoor sun exposure; for example, those who migrated to Australia at a young age adopted the BCC risk of Australian-born people, whereas those who migrated later did not.37 Thus, overall the available data suggest that early exposures may render people more susceptible to BCCs independent of cumulative dose of exposure.
At least 3 other case–control studies, 2 from Europe and 1 from Canada of men only, did not find clear evidence of an association with indoor tanning and BCC at any age.38–41 These smaller studies had lower prevalences of use. Our larger study of younger and more recently diagnosed people with BCCs had a 40% prevalence of tanning lamp exposure (among controls), and associations were observed among both women and men.
By design, our study relied on the participant’s recall of tanning lamp use. We do not suspect that this led to differential misclassification or lack of generalizability because excess risks were present in all strata of skin types, time spent outdoors, and history of sunburns. Although our risk estimates were virtually unaltered by adjustment for sun exposure–related variables, we cannot rule out the possibility of residual confounding by these or other factors. Questions about indoor tanning tend to be reproducible,42 and risk factors for skin cancer do not typically show evidence of recall bias.43 Additionally, there was no indication of selection bias, because participants and nonparticipants did not markedly differ by age, gender, or urban versus rural county of residence (data not shown). Moreover, we considered use of driver’s license records for control selection to be representative of the case group because 99% of the cases held a valid driver’s license. Overall, our study had a distinct advantage of ascertaining a large number of early-onset BCC cases and controls from a geographically defined population of the United States along with detailed information on potentially confounding factors.
Indoor tanning can produce 10 to 15 times as much UV radiation of midday sun15 and often induces an erythemal response analogous to a sunburn. In a recent study of college students, the majority of those who reported indoor tanning on a bimonthly diary experienced an erythema response at least once.44 Sunburns are among the strongest risk factors for BCC, and an intermittent pattern of exposure, especially early in life, has been associated with BCC risk.45 Thus, based on our knowledge of the effects of natural sunlight on BCC occurrence, we might expect that young, intermittent exposure to UV radiation from indoor tanning would elevate the risk of BCC. Tanning lamps emit both UV-B, absorbed primarily by the superficial epidermis, and UV-A, which penetrates deeper into the dermis.46,47 As in natural sunlight, UV-B typically represents only a small fraction of the UV radiation output of tanning lamps (ie, <5%) compared with UV-A.15 The carcinogenic properties of UV-B are well known15 and include formation of pyrimidine dimers and thymine–cytosine, 6–4 photoproducts with resultant C→T and tandem CC→TT signature mutations on the transcribed strand of the p53 tumor suppressor gene.46 UV-A also induces mutations, including T→G transversions, as well as 8-hydroxy-2′-deoxyguanosine damage, oxidative signaling,47 and immunosuppression. In epidemiologic studies, high exposure to UV-A increases the incidence of BCC, as evidenced by follow-up studies of patients who have undergone psoralen and UV-A therapy.48
Our study assessed a variety of tanning devices and detected associations with each type of device. The pronounced association we observed with desktop lamps used before 1975 could reflect higher UV-B output of earlier sunlamps.49 In the Yale study, ORs for early-onset BCC were highest among users of the more modern high-speed, high-intensity lamps with higher UV-B output and high-pressure lamps with higher UV-A output. Also, some newer model beds are designed with high-pressure (UV-A) lamps for the face and head and low-pressure (combined UV-A and UV-B) lamps for the rest of the body. Although we did not collect information on the type of bulb, we did note that tanning beds and booths used after 1986 were more strongly related to early-onset BCC than those used earlier. In aggregate, the data suggest that contemporary tanning devices pose at least as great a risk of BCC as previous models and perhaps even more, but this remains to be confirmed.
Indoor tanning has become immensely popular, especially among adolescents and young adults in the United States.16–19 As with tobacco,50–52 advertising campaigns successfully target these age groups. A recent survey in New Hampshire, where our study was conducted, found that 74% of high schools have ≥1 tanning salon within 2 miles, and an additional 22% have easy access to a tanning salon.53 In addition to the desire for a tanned appearance, a growing number of studies are uncovering tanning addiction or dependence as a potential driver of this behavior.54–57 Laws that ban or limit access to commercial tanning facilities by minors do not universally exist. The Affordable Care Act includes a 10% excise tax for providers of indoor tanning, but this along with current Food and Drug Administration regulations is unlikely to sufficiently dissuade usage.58–60 Indoor tanning with UV radiation lamps is now banned in Brazil, and laws restricting minors exist in several countries including in the United Kingdom, Germany, Scotland, Spain, France, Belgium, regions of Australia, and Canada.52 In the United States, indoor tanning is banned for minors in several states, and in some states minors are permitted to engage in indoor tanning with parental consent if they are above a certain age, such as age 13, 14, or 16 years.61,62 As of January 2014, 14 states did not have statewide tanning restrictions for minors in place.61 A US survey found that most salons would allow minors to tan twice the number of times recommended by the US Food and Drug Administration.63 To prevent skin cancers, in 2012 the US Preventive Services Task Force recommended that children, adolescents, and young adults (10–24 years) with fair skin be counseled to minimize UV radiation exposure, including from indoor tanning (a Grade B recommendation).64 Yet pediatric surveys do not indicate a high rate of this practice, despite evidence that pediatricians consider counseling on indoor tanning important65 and that it is likely to be effective.59,60 Thus, although the medical community, including the American Academy of Pediatrics,14 has urged the reduction of exposure to indoor tanning among minors, opportunities remain for pediatricians to directly advise their patients and to discourage caregivers from providing consent for minors. In conclusion, our findings suggest that teens and young adults who seek indoor tanning may be especially vulnerable to developing BCC at a young age.
Acknowledgments
The authors thank the study investigators of the New Hampshire Skin Cancer Study Group, the New Hampshire Society of Dermatology, staff of the New Hampshire Health Study, and study participants.
Glossary
- BCC
basal cell carcinoma
- CI
confidence interval
- OR
odds ratio
Footnotes
Dr Karagas conceptualized and designed the study, directed the study execution, designed the analysis plan, and drafted the manuscript; Drs Zens and Li designed the analysis plan, analyzed the data, and drafted the manuscript; Dr Stukel designed the study and case–control sampling plan for the study; Dr Perry executed the study and drafted the manuscript; Drs Gilbert-Diamond, Barton, and Nelson and Ms Stephenson drafted the manuscript; Ms. Sayarath managed the study execution and critically reviewed the manuscript; Dr Spencer directed the study execution and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health, National Cancer Institute grant R01 CA57494. Drs Karagas, Gilbert-Diamond, and Li are supported by grants P01 ES022832 from the National Institute of Environmental Health Sciences and RD-83544201 from the US Environmental Protection Agency. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Rubin AI, Chen EH, Ratner D. Basal-cell carcinoma. N Engl J Med. 2005;353(21):2262–2269 [DOI] [PubMed] [Google Scholar]
- 2.Shingler SL, Garside J, Samanta K, Lear JT, Keohane S, Lloyd AJ. Utilities for advanced basal cell carcinoma. J Media Econ. 2013;16(6):777–783 [DOI] [PubMed] [Google Scholar]
- 3.Karagas M, Weinstock MA, Nelson HH. Keratinocyte cancers (basal cell and squamous cell carcinomas of the skin). In: Schottenfeld D, Fraumeni JF, eds. Cancer Epidemiology and Prevention. 3rd ed. New York, NY: Oxford University Press; 2006 [Google Scholar]
- 4.Bath-Hextall F, Leonardi-Bee J, Smith C, Meal A, Hubbard R. Trends in incidence of skin basal cell carcinoma. Additional evidence from a UK primary care database study. Int J Cancer. 2007;121(9):2105–2108 [DOI] [PubMed] [Google Scholar]
- 5.Birch-Johansen F, Jensen A, Mortensen L, Olesen AB, Kjær SK. Trends in the incidence of nonmelanoma skin cancer in Denmark 1978–2007: rapid incidence increase among young Danish women. Int J Cancer. 2010;127(9):2190–2198 [DOI] [PubMed] [Google Scholar]
- 6.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294(6):681–690 [DOI] [PubMed] [Google Scholar]
- 7.de Vries E, Louwman M, Bastiaens M, de Gruijl F, Coebergh JW. Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973. J Invest Dermatol. 2004;123(4):634–638 [DOI] [PubMed] [Google Scholar]
- 8.Demers AA, Nugent Z, Mihalcioiu C, Wiseman MC, Kliewer EV. Trends of nonmelanoma skin cancer from 1960 through 2000 in a Canadian population. J Am Acad Dermatol. 2005;53(2):320–328 [DOI] [PubMed] [Google Scholar]
- 9.Jensen AO, Lamberg AL, Jacobsen JB, Braae Olesen A, Sørensen HT. Non-melanoma skin cancer and ten-year all-cause mortality: a population-based cohort study. Acta Derm Venereol. 2010;90(4):362–367 [DOI] [PubMed] [Google Scholar]
- 10.Kyrgidis A, Vahtsevanos K, Tzellos TG, et al. Clinical, histological and demographic predictors for recurrence and second primary tumours of head and neck basal cell carcinoma. A 1062 patient-cohort study from a tertiary cancer referral hospital. Eur J Dermatol. 2010;20(3):276–282 [DOI] [PubMed] [Google Scholar]
- 11.Karagas MR, Stukel TA, Greenberg ER, Baron JA, Mott LA, Stern RS, Skin Cancer Prevention Study Group . Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. JAMA. 1992;267(24):3305–3310 [PubMed] [Google Scholar]
- 12.Whitmore SE, Morison WL, Potten CS, Chadwick C. Tanning salon exposure and molecular alterations. J Am Acad Dermatol. 2001;44(5):775–780 [DOI] [PubMed] [Google Scholar]
- 13.Woollons A, Clingen PH, Price ML, Arlett CF, Green MH. Induction of mutagenic DNA damage in human fibroblasts after exposure to artificial tanning lamps. Br J Dermatol. 1997;137(5):687–692 [PubMed] [Google Scholar]
- 14.Balk SJ, Council on Environmental Health. Section on Dermatology . Ultraviolet radiation: a hazard to children and adolescents. Pediatrics. 2011;127(3). Available at: www.pediatrics.org/cgi/content/full/127/3/e791 [DOI] [PubMed] [Google Scholar]
- 15.The International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 1:Exposure to Artificial UV Radiation and Skin Cancer. Lyon, France: World Health Organization; 2005. Available at: www.iarc.fr/en/publications/pdfs-online/wrk/wrk1/ArtificialUVRad&SkinCancer.pdf
- 16.Choi K, Lazovich D, Southwell B, Forster J, Rolnick SJ, Jackson J. Prevalence and characteristics of indoor tanning use among men and women in the United States. Arch Dermatol. 2010;146(12):1356–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Demko CA, Borawski EA, Debanne SM, Cooper KD, Stange KC. Use of indoor tanning facilities by white adolescents in the United States. Arch Pediatr Adolesc Med. 2003;157(9):854–860 [DOI] [PubMed] [Google Scholar]
- 18.Lazovich D, Forster J. Indoor tanning by adolescents: prevalence, practices and policies. Eur J Cancer. 2005;41(1):20–27 [DOI] [PubMed] [Google Scholar]
- 19.Wehner MR, Chren MM, Nameth D, et al. International prevalence of indoor tanning: a systematic review and meta-analysis. JAMA Dermatol. 2014;150(4):390–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazovich D, Vogel RI, Berwick M, Weinstock MA, Anderson KE, Warshaw EM. Indoor tanning and risk of melanoma: a case–control study in a highly exposed population. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang M, Qureshi AA, Geller AC, Frazier L, Hunter DJ, Han J. Use of tanning beds and incidence of skin cancer. J Clin Oncol. 2012;30(14):1588–1593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wehner MR, Shive ML, Chren MM, Han J, Qureshi AA, Linos E. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrucci LM, Cartmel B, Molinaro AM, Leffell DJ, Bale AE, Mayne ST. Indoor tanning and risk of early-onset basal cell carcinoma. J Am Acad Dermatol. 2012;67(4):552–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karagas MR, Greenberg ER, Spencer SK, Stukel TA, Mott LA, New Hampshire Skin Cancer Study Group . Increase in incidence rates of basal cell and squamous cell skin cancer in New Hampshire, USA. Int J Cancer. 1999;81(4):555–559 [DOI] [PubMed] [Google Scholar]
- 25.Karagas MR, Stannard VA, Mott LA, Slattery MJ, Spencer SK, Weinstock MA. Use of tanning devices and risk of basal cell and squamous cell skin cancers. J Natl Cancer Inst. 2002;94(3):224–226 [DOI] [PubMed] [Google Scholar]
- 26.Karagas MR, Waterboer T, Li Z, et al. New Hampshire Skin Cancer Study Group . Genus beta human papillomaviruses and incidence of basal cell and squamous cell carcinomas of skin: population based case–control study. BMJ. 2010;341:c2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karagas MR, Zens MS, Nelson HH, et al. Measures of cumulative exposure from a standardized sun exposure history questionnaire: a comparison with histologic assessment of solar skin damage. Am J Epidemiol. 2007;165(6):719–726 [DOI] [PubMed] [Google Scholar]
- 28.Kricker A, Armstrong BK, English DR, Heenan PJ. Pigmentary and cutaneous risk factors for non-melanocytic skin cancer: a case–control study. Int J Cancer. 1991;48(5):650–662 [DOI] [PubMed] [Google Scholar]
- 29.Breslow NE, Day NE. Statistical methods in cancer research. Volume I: The analysis of case–control studies. IARC Sci Publ. 1980; 1(32):5–338 [PubMed] [Google Scholar]
- 30.Friedman JH. A variable span smoother. Laboratory for Computational Statistics Stanford University Technical Report. 1984. No. 5
- 31.Pastor R, Guallar E. Use of two-segmented logistic regression to estimate change-points in epidemiologic studies. Am J Epidemiol. 1998;148(7):631–642 [DOI] [PubMed] [Google Scholar]
- 32.Bakos RM, Kriz M, Mühlstädt M, Kunte C, Ruzicka T, Berking C. Risk factors for early-onset basal cell carcinoma in a German institution. Eur J Dermatol. 2011;21(5):705–709 [DOI] [PubMed] [Google Scholar]
- 33.Boyd AS, Shyr Y, King LE, Jr. Basal cell carcinoma in young women: an evaluation of the association of tanning bed use and smoking. J Am Acad Dermatol. 2002;46(5):706–709 [DOI] [PubMed] [Google Scholar]
- 34.Power/sample size calculation for logistic regression with binary covariate(s). Available at: www.dartmouth.edu/∼eugened/power-samplesize.php
- 35.Demidenko E. Sample size determination for logistic regression revisited. Stat Med. 2007;26(18):3385–3397 [DOI] [PubMed] [Google Scholar]
- 36.Demidenko E. Sample size and optimal design for logistic regression with binary interaction. Stat Med. 2008;27(1):36–46 [DOI] [PubMed] [Google Scholar]
- 37.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63(1–3):8–18 [DOI] [PubMed] [Google Scholar]
- 38.Bajdik CD, Gallagher RP, Astrakianakis G, Hill GB, Fincham S, McLean DI. Non-solar ultraviolet radiation and the risk of basal and squamous cell skin cancer. Br J Cancer. 1996;73(12):1612–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Corona R, Dogliotti E, D’Errico M, et al. Risk factors for basal cell carcinoma in a Mediterranean population: role of recreational sun exposure early in life. Arch Dermatol. 2001;137(9):1162–1168 [DOI] [PubMed] [Google Scholar]
- 40.Rosso S, Joris F, Zanetti R. Risk of basal and squamous cell carcinomas of the skin in Sion, Switzerland: a case–control study. Tumori. 1999;85(6):435–442 [DOI] [PubMed] [Google Scholar]
- 41.Walther U, Kron M, Sander S, et al. Risk and protective factors for sporadic basal cell carcinoma: results of a two-centre case–control study in southern Germany. Clinical actinic elastosis may be a protective factor. Br J Dermatol. 2004;151(1):170–178 [DOI] [PubMed] [Google Scholar]
- 42.Beane Freeman LE, Dennis LK, Lynch CF, Lowe JB, Clarke WR. Test–retest of self-reported exposure to artificial tanning devices, self-tanning creams, and sun sensitivity showed consistency. J Clin Epidemiol. 2005;58(4):430–432 [DOI] [PubMed] [Google Scholar]
- 43.Han J, Colditz GA, Hunter DJ. Risk factors for skin cancers: a nested case–control study within the Nurses’ Health Study. Int J Epidemiol. 2006;35(6):1514–1521 [DOI] [PubMed] [Google Scholar]
- 44.Stapleton JL, Hillhouse J, Turrisi R, et al. Erythema and ultraviolet indoor tanning: findings from a diary study. Transl Behav Med. 2013;3(1):10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kricker A, Armstrong BK, English DR, Heenan PJ. Does intermittent sun exposure cause basal cell carcinoma? A case–control study in Western Australia. Int J Cancer. 1995;60(4):489–494 [DOI] [PubMed] [Google Scholar]
- 46.Rass K, Reichrath J. UV damage and DNA repair in malignant melanoma and nonmelanoma skin cancer. Adv Exp Med Biol. 2008;624:162–178 [DOI] [PubMed] [Google Scholar]
- 47.Agar NS, Halliday GM, Barnetson RS, Ananthaswamy HN, Wheeler M, Jones AM. The basal layer in human squamous tumors harbors more UVA than UVB fingerprint mutations: a role for UVA in human skin carcinogenesis. Proc Natl Acad Sci USA. 2004;101(14):4954–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stern RS, PUVA Follow-Up Study . The risk of squamous cell and basal cell cancer associated with psoralen and ultraviolet A therapy: a 30-year prospective study. J Am Acad Dermatol. 2012;66(4):553–562 [DOI] [PubMed] [Google Scholar]
- 49.Spencer JM, Amonette R. Tanning beds and skin cancer: artificial sun + old sol = real risk. Clin Dermatol. 1998;16(4):487–501 [DOI] [PubMed] [Google Scholar]
- 50.Greenman J, Jones DA. Comparison of advertising strategies between the indoor tanning and tobacco industries. J Am Acad Dermatol. 2010;62(4):685, e681–618 [DOI] [PubMed] [Google Scholar]
- 51.Zeller S, Lazovich D, Forster J, Widome R. Do adolescent indoor tanners exhibit dependency? J Am Acad Dermatol. 2006;54(4):589–596 [DOI] [PubMed] [Google Scholar]
- 52.Sinclair C, Makin JK. Implications of lessons learned from tobacco control for tanning bed reform. Prev Chronic Dis. 2013;10:E28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olson AL, Carlos HA, Sarnoff RA. Community variation in adolescent access to indoor tanning facilities. J Community Health. 2013;38(2):221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cartmel B, Ferrucci LM, Spain P, et al. Indoor tanning and tanning dependence in young people after a diagnosis of basal cell carcinoma. JAMA Dermatol. 2013;149(9):1110–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heckman CJ, Darlow S, Kloss JD, et al. Measurement of tanning dependence [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 10.1111/jdv.12243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Petit A, Lejoyeux M, Reynaud M, Karila L. Excessive indoor tanning as a behavioral addiction: a literature review [published online ahead of print August 29, 2013] Curr Pharm Des. [DOI] [PubMed] [Google Scholar]
- 57.Heckman CJ, Darlow S, Manne SL, Kashy DA, Munshi T. Correspondence and correlates of couples’ skin cancer screening. JAMA Dermatol. 2013;149(7):825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Balaraman B, Biesbroeck LK, Lickerman SH, Cornelius LA, Jeffe DB. Practices of unregulated tanning facilities in Missouri: implications for statewide legislation. Pediatrics. 2013;131(3):415–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Balk SJ, Fisher DE, Geller AC. Stronger laws are needed to protect teens from indoor tanning. Pediatrics. 2013;131(3):586–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Balk SJ, Fisher DE, Geller AC. Teens and indoor tanning: a cancer prevention opportunity for pediatricians. Pediatrics. 2013;131(4):772–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.National Conference of State Legislatures. Indoor tanning restrictions for minors: a state-by-state comparison. 2014. Available at: www.ncsl.org/research/health/indoor-tanning-restrictions.aspx
- 62.Gosis B, Sampson BP, Seidenberg AB, Balk SJ, Gottlieb M, Geller AC. Comprehensive evaluation of indoor tanning regulations: a 50-state analysis, 2012. J Invest Dermatol. 2014;134(3):620–627 [DOI] [PubMed] [Google Scholar]
- 63.Pichon LC, Mayer JA, Hoerster KD, et al. Youth access to artificial UV radiation exposure: practices of 3647 US indoor tanning facilities. Arch Dermatol. 2009;145(9):997–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moyer VA, U.S. Preventive Services Task Force . Behavioral counseling to prevent skin cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(1):59–65 [DOI] [PubMed] [Google Scholar]
- 65.Davy L, Boyett T, Weathers L, Campbell RJ, Roetzheim RG. Sun protection counseling by pediatricians. Ambul Pediatr. 2002;2(3):207–211 [DOI] [PubMed] [Google Scholar]