Abstract
BACKGROUND:
Caffeine use is on the rise among children and adolescents. Previous studies from our laboratory reported gender differences in the effects of caffeine in adolescents. The purpose of this study was to test the hypotheses that gender differences in cardiovascular responses to caffeine emerge after puberty and that cardiovascular responses to caffeine differ across the phases of the menstrual cycle.
METHODS:
To test these hypotheses, we examined heart rate and blood pressure before and after administration of placebo and 2 doses of caffeine (1 and 2 mg/kg) in prepubertal (8- to 9-year-olds; n = 52) and postpubertal (15- to 17-year-olds; n = 49) boys (n = 54) and girls (n = 47) by using a double-blind, placebo-controlled, dose-response design.
RESULTS:
There was an interaction between gender and caffeine dose, with boys having a greater response to caffeine than girls. In addition, we found interactions between pubertal phase, gender, and caffeine dose, with gender differences present in postpubertal, but not in prepubertal, participants. Finally, we found differences in responses to caffeine across the menstrual cycle in post-pubertal girls, with decreases in heart rate greater in the midluteal phase and blood pressure increases greater in the midfollicular phase of the menstrual cycle.
CONCLUSIONS:
These data suggest that gender differences in response to caffeine emerge after puberty. Future research will determine the extent to which these gender differences are mediated by physiological factors, such as steroid hormones, or psychosocial factors, such as more autonomy and control over beverage purchases.
What’s Known on This Subject:
Caffeine has predictable effects on cardiovascular function in both adults and children. Our previous work has shown that there are gender differences in this cardiovascular response, with boys having a greater change in heart rate and blood pressure than girls.
What This Study Adds:
This study shows that the gender differences in cardiovascular response to caffeine emerge after puberty and there are some differences in postpubertal girls across the menstrual cycle.
Caffeinated beverage consumption, such as soda and energy drinks, is on the rise among children and adolescents. Studies in adults and children have shown that acute, moderate doses of caffeine (1–3 mg/kg, which is equivalent to amounts ranging from the caffeine content of a can of soda to that contained in 2 cups of coffee) decrease heart rate and increase blood pressure. 1–5 In adults, there is good evidence that the cardiovascular effects of acute caffeine are inversely related to usual caffeine use,6,7 but results in children are inconsistent.3,4 This finding may be because caffeine use patterns, amounts, and sources tend to differ between children and adults, with children consuming caffeine in beverages to which caffeine has been added and with less regularity and frequency.8
Previous work from our laboratory has shown that acute caffeine administration increases blood pressure and decreases heart rate in a dose-dependent manner in adolescents, and that boys tend to have heightened responses to acute caffeine compared with girls.3,4 We tested the hypotheses that gender differences in cardiovascular responses to caffeine emerge after puberty and that these responses differ across the menstrual cycle.
Methods
Participants and Recruitment
Participants were boys (n = 54) and girls (n = 47) between the ages of 8 to 9 (prepubertal; n = 52) and 15 to 17 (postpubertal; n = 49) years. Eligible participants were nonsmokers, had previous experience with caffeine and had no adverse reactions, were not using hormone-based contraceptives and/or were not pregnant, were not taking any medications affecting caffeine metabolism, and were willing to abstain from regular caffeine use. Only 13 participants reported medication use and, of these, 1 participant was taking an inhaled steroid with potential effects on heart rate and blood pressure.
Experimental Procedures
Participants visited the laboratory on 6 occasions: 3 visits during 1 week with the remaining 3 visits occurring 2 weeks later. For the 15- to 17-year-old girls, 3 of the sessions occurred during the midfollicular phase of the menstrual cycle and 3 occurred during the midluteal phase. Prepubertal participants and postpubertal boys received each caffeine dose on 2 separate visits in order to equalize the number of visits. Participants were randomly assigned to order of caffeine administration by using a random number table. Participants were asked to abstain from all soda and caffeine-containing products for 24 hours before their appointment times, as well as from all food and drink other than water for 2 hours before their appointments.
Upon arrival to the laboratory, participants and parents read and signed consent and assent forms and parents completed a demographic questionnaire. The participants had their height and weight measured, and parents and children completed the Tanner questionnaire (described later). Participants then completed a caffeine use questionnaire.
At each visit, participants provided saliva samples, completed 24-hour food and physical activity recalls, and completed the Behavioral Checklist at baseline and after 60 minutes. Participants then consumed a 300-mL portion of lemon-lime–flavored soda, orange juice, or lemonade containing either placebo or caffeine (1.0 or 2.0 mg/kg; order counterbalanced) and had cardiovascular measurements taken. The participants and parents were not told directly that caffeine was being manipulated, although it was listed as a possibility. At the end of the final visit, participants were debriefed and compensated for their time. These data were collected between August 2011 and October 2012. All procedures described here were approved by the University at Buffalo Social and Behavioral Sciences Institutional Review Board.
Caffeine Preparation
Caffeine solutions were created by adding caffeine to a flattened, lemon-lime–flavored soda at 2 doses, 1 mg/kg (10 mg/mL caffeine/soda) and 2 mg/kg (20 mg/mL caffeine/soda), and then freezing aliquots.
Measurements
Anthropometric Measurements
Body weight was assessed by the use of a digital scale (Seca Corporation, Hanover, MD). Height was assessed by using a Seca digital stadiometer.
Cardiovascular Measurements
An automated heart rate and blood pressure monitor (NIBP 2400; Welch Allyn, Skaneateles, NY) was used to measure cardiovascular variables. Participants were seated in an upright position and told to minimize movement for the duration of the measurements. Baseline measurements were taken ∼20 to 30 minutes after the participant arrived in the laboratory. Participants then consumed the beverage and watched an age-appropriate video of their choice to help them stay relaxed. Measurements were taken every 10 minutes for 1 hour. The video was paused for 1 minute before each measurement. The 1-minute rest period was chosen because it was sufficient to allow the children to relax without disrupted enjoyment of the video.
Salivary Steroid Hormone Measurements
To assess steroid hormone levels of all participants, a 2.5-mL sample of saliva was collected each day. The participant expectorated a saliva sample into a sterile tube, which was stored at −20°C until analyzed (Salimetrics, State College, PA). Estradiol, progesterone, and testosterone levels were measured in duplicate from each sample by using standard radioimmunologic techniques.
Questionnaires
Demographic Questionnaire
Parents completed a demographic questionnaire that provided information about household/parental income, education, profession, and race/ethnicity of both the parent and child.
Caffeine Use Questionnaire
This questionnaire was designed to assess sources, amounts, and frequency of caffeinated food and beverage intake in our study population. It also assesses reasons why adolescents use and/or do not use caffeine.9
Behavioral Checklist
A questionnaire containing 31 adjectives describing mood and physiological symptoms was presented to the subjects at t = 0 and t = 60 minutes. Subjects rated how each adjective described how they “feel right now” on a 5-point Likert-type scale anchored by “Not at all” (1) and “Extremely” (5). We adapted the adjectives in the checklist for 8- to 9-year-olds. This questionnaire was adapted from the Profile of Mood States bipolar form and the Activation-Deactivation Adjective Checklist. This questionnaire has been used by multiple investigators and is sensitive to caffeine use.10,11
Tanner Stage Evaluation
Boys and girls were given same-gender line drawings of the 5 stages of pubertal development and asked to circle the one that looks most like them. Parents independently completed the same forms, and the average of the 2 scores was used. Self-assessment of pubertal stage has been shown to be accurate and an acceptable substitute when physical examinations are not feasible.12,13
Analytic Plan
Gender and pubertal group differences in participant characteristics were analyzed by using either an analysis of variance (BMI, age, caffeine consumption, and Tanner stage) or χ2 analyses for categorical variables (race, household income, and parental education). After confirming that the data did not differ as a function of visit for prepubertal boys and girls and postpubertal boys, the data from the same-dose sessions were averaged. The pattern of diastolic and systolic blood pressure and heart rate were analyzed by using mixed-effects regression models with gender and pubertal group as the time-invariant predictors, time (from 10 to 60 minutes after caffeine administration) and caffeine dose (0, 1, or 2 mg/kg) as time-variant predictors, and average daily caffeine use (mg/day) and baseline blood pressure and heart rate as covariates. We used unstructured models with the intercept, identifier (ID), and time treated as random variables. The Akaike information criteria (AIC) for these models were as follows: 25 373 (heart rate), 25 026 (systolic blood pressure), and 22 108 (diastolic blood pressure). Answers on the behavioral checklist were analyzed by using a mixed repeated-measures analysis of covariance with gender and pubertal group as between-subject variables, caffeine dose (0, 1, or 2 mg/kg) and pre/post as within-subject variables, and average daily caffeine use (mg/day) as a covariate. To confirm menstrual cycle phase, we analyzed salivary steroid hormone concentrations during the self-reported follicular and luteal phases in postpubertal girls only. For analysis of differences across the menstrual cycle, postpubertal girls were selected and a mixed-effects regression model was applied with caffeine dose (0, 1, or 2 mg/kg) and menstrual cycle phase (luteal versus follicular) as time-variant predictors and baseline blood pressure and heart rate and average daily caffeine use (mg/day) as covariates. The regression analyses were conducted by using SAS 9.3 (SAS Institute, Cary, NC), and the other analyses were conducted by using SYSTAT 11.0 (Systat Software, San Jose, CA).
Results
Participant Characteristics
A total of 101 participants began the study, but 5 participants were removed from the analyses for having incomplete or missing data (n = 3) or for not meeting Tanner stage requirements (n = 2), which left 96 participants. Male (n = 26) and female (n = 24) participants ages 8 to 9 years and boys (n = 26) and girls (n = 20) ages 15 to 17 years were included in the analyses. In postpubertal girls, the mean ± SEM concentrations of estradiol and progesterone in the midfollicular phase were 1.5 ± 0.3 and 30.2 ± 9.5 pg/mL, respectively, and in the midluteal phase were 1.9 ± 0.3 and 55.1 ± 10.9 pg/mL, respectively. Table 1 shows the mean ± SEM age (years), BMI (mg/kg), and average daily caffeine consumption (mg/day) of participants in each group. As expected, the postpubertal group was significantly older, had a higher BMI, and consumed more daily caffeine than the prepubertal group (all P < .0001). Postpubertal participants were significantly more likely than prepubertal participants to drink soda (93% vs 78%; P = .03), tea (87% vs 64%; P = .009), coffee (35% vs 8%; P = .001), and energy drinks (28% vs 6%; P = .003). Among consumers, there were differences in consumption frequency as a function of pubertal group for soda (F[1, 79] = 11.9; P = .001) and tea (F[1, 69] = 10.9; P = .002) but not for coffee (F[1, 20] = 3.3; P = .08) or energy drinks (F[1, 17] = 1.9; P = 0.19). There was a trend for postpubertal boys to consume more caffeine than postpubertal girls (P = .10).
TABLE 1.
Participant Characteristics
| Category | Boys | Girls | P | ||
|---|---|---|---|---|---|
| Prepubertal (n = 26) | Postpubertal (n = 26) | Prepubertal (n = 24) | Postpubertal (n = 20) | ||
| Age, mean ± SEM, y | 8.62 ± 0.12a | 16.08 ± 0.12 | 8.38 ± 0.12a | 15.75 ± 0.14 | <.0001 |
| BMI, mean ± SEM | 19.19 ± 0.94a | 24.57 ± 0.94 | 17.12 ± 0.98a | 22.95 ± 1.07 | <.0001 |
| Average daily caffeine consumption, mean ± SEM, mg/d | 26.84 ± 6.83a | 92.34 ± 14.56 | 28.00 ± 7.48a | 61.91 ± 9.06 | <.0001 |
| Socioeconomic status, mean ± SEM | 50.9 ± 1.5b | 49.6 ± 1.9b | 45.1 ± 2.5 | 43.4 ± 2.3 | .004 |
| Child race/ethnicity, n (%) | .48 | ||||
| Asian | 1 (4) | 0 (0) | 1 (4) | 0 (0) | |
| Black or African American | 3 (13) | 5 (19) | 2 (9) | 6 (30) | |
| White | 20 (83) | 21 (81) | 19 (83) | 14 (70) | |
| Other | 0 (0) | 0 (0) | 1 (4) | 0 (0) | |
| Parental education, n (%) | .48 | ||||
| High school | 1 (4) | 3 (12) | 2 (8) | 0 (0) | |
| Some college | 3 (12) | 6 (23) | 5 (21) | 6 (30) | |
| Completed college | 13 (52) | 11 (42) | 7 (29) | 10 (50) | |
| Graduate school | 8 (32) | 6 (23) | 10 (42) | 4 (20) | |
| Household income, n (%) | .99 | ||||
| <$30 000 | 1 (4) | 2 (8) | 2 (10) | 3 (17) | |
| $30 000–$50 000 | 4 (17) | 5 (20) | 2 (10) | 3 (17) | |
| $50 000–$70 000 | 5 (22) | 2 (8) | 2 (10) | 1 (6) | |
| $70 000–$110 000 | 9 (39) | 11 (44) | 10 (48) | 8 (44) | |
| >$110 000 | 4 (17) | 5 (20) | 5 (21) | 2 (11) | |
| Socioeconomic status category, n (%) | .11 | ||||
| I | 0 (0) | 0 (0) | 1 (4) | 0 (0) | |
| II | 0 (0) | 2 (8) | 2 (8) | 4 (20) | |
| III | 9 (36) | 7 (27) | 9 (38) | 9 (45) | |
| IV | 15 (60) | 16 (62) | 12 (60) | 7 (35) | |
| V | 1 (4) | 1 (3) | 0 (0%) | 0 (0%) | |
Significant difference by pubertal group (P < .0001).
Significant difference by gender (P < .05).
Effects of Caffeine on Heart Rate
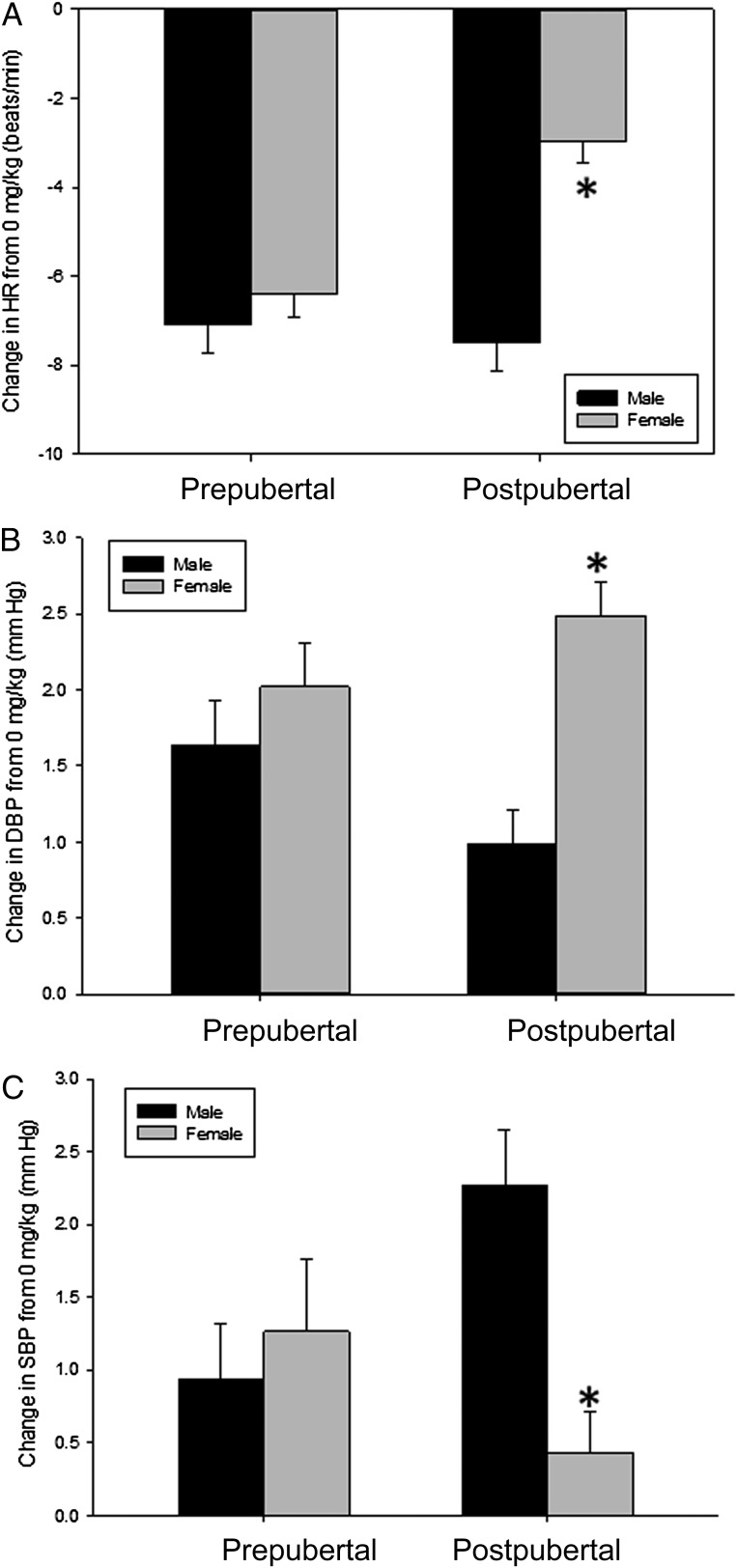
We found a main effect of time on heart rate (z = 12.1; P < .0001), but no interactions between time and any of the other factors. There was also a main effect of caffeine dose on heart rate (z = −2.71; P = .007), with both the 1-mg/kg and 2-mg/kg doses significantly reducing heart rate compared with placebo (z = −2.1 [P = .04] and −2.7 [P = .007], respectively). There was a significant interaction between gender and caffeine dose (z = −2.6; P = .009) and a 3-way interaction between pubertal group, gender, and caffeine dose (z = 2.6; P = .01). When we analyzed each pubertal group separately, we found no gender difference in the prepubertal participants (P > .05), but a significant gender difference in the postpubertal participants (z = 1.98; P = .049; Fig 1A).
FIGURE 1.
Mean ± SEM changes in average heart rate (HR) (A) diastolic blood pressure (DBP) (B), and systolic blood pressure (SBP) (C) between 0- and 2-mg/kg doses of caffeine in prepubertal and postpubertal boys and girls. Because there were no effects of time and no differences between the 1- and 2-mg/kg doses, the data shown are the average changes from baseline and are from the 2-mg/kg condition. There were no gender differences in any of the measures in the prepubertal participants, but there were gender differences in all of the postpubertal participants. *Significantly different from postpubertal boys, P < .05.
Effects of Caffeine on Diastolic Blood Pressure
We found a main effect of time on diastolic blood pressure (z = 2.5; P = .014), but no interactions between time and any other factors. We also found significant main effects of gender (z = 2.2; P = .03) and pubertal group (z = 2.6; P = .008) and a significant interaction between gender and pubertal group (z = −2.0; P = 0.048). When we examined each pubertal group separately, we found a significant interaction between gender and caffeine dose in the postpubertal participants (P = .005) but not in the prepubertal participants (P = .71; Fig 1B).
Effects of Caffeine on Systolic Blood Pressure
There was a significant main effect of caffeine dose on systolic blood pressure (z = 2.1; P = .035), with both the 1- and 2-mg/kg doses increasing blood pressure compared with placebo (P = .009 and P < .0001, respectively). There was a significant interaction between pubertal group and gender, with no gender difference in systolic blood pressure in response to caffeine in the prepubertal participants (P = .49), but a significant gender difference in response to caffeine administration emerging after puberty (z = 2.2; P = .03; Fig 1C).
Changes in Response to Caffeine in Postpubertal Girls
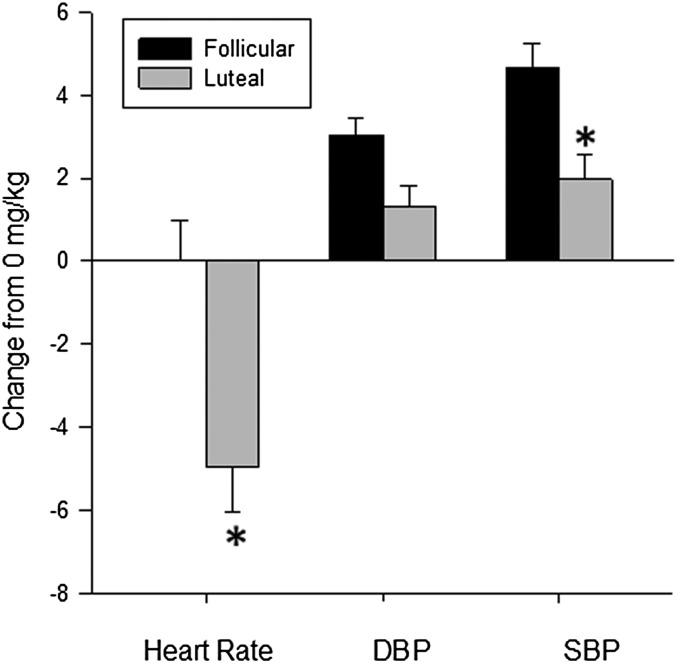
When data from postpubertal girls were analyzed, we found significant interactions between caffeine dose and menstrual cycle phase on heart rate (P = .02), diastolic blood pressure (P = .05), and systolic blood pressure (P = .002). When these effects were probed, we found that caffeine administration lowered heart rate significantly more in the midluteal phase and increased systolic blood pressure significantly more in the follicular phase. There was a trend toward a significantly greater increase in diastolic blood pressure in the follicular phase compared with the luteal phase (P = .05), but this trend did not reach significance (Fig 2).
FIGURE 2.
Mean ± SEM changes in heart rate, diastolic blood pressure (DBP), and systolic blood pressure (SBP) in postpubertal girls in the follicular and luteal phases of the menstrual cycle. Caffeine administration lowered heart rate significantly more in the luteal phase compared with the follicular phase and increased SBP and DBP significantly more in the follicular phase compared with the luteal phase (all P < .05).
Responses on Behavioral Checklist
When data from the entire sample were analyzed, we found an interaction between caffeine dose and pre/post for “falling asleep” (F[2, 184] = 3.6; P = .03). We found interactions between caffeine dose, gender, and pre/post for “ringing in ears” (F [2, 184] = 5.4; P = .005), “energetic” (F[2, 184] = 5.8; P = .004), “stomach ache” (F[2, 184] = 17.7; P < .0001), “sleepy” (F[2, 184] = 13.1; P < .0001), and “tired” (F[2, 184] = 3.4; P = .034). We found interactions between caffeine dose, pubertal group, gender, and pre/post for “motivated” (F[2, 184] = 3.2; P = .04), “life is good” (F[2, 184] = 3.5; P = .03), “queasy” (F[2, 184] = 3.0; P = .046), “strong” (F[2, 184] = 4.4; P = .014), and “sweaty” (F[2, 184] = 7.9; P = .01). These data are shown in Table 2. When the analysis was restricted to postpubertal girls, we found menstrual cycle phase differences in response to caffeine for “body tired” (F[1, 19] = 4.75; P = .04) and “hungry” (F[1, 19] = 4.5; P = .047), but no difference by menstrual cycle phase for any other terms on this questionnaire.
TABLE 2.
Results From Behavioral Checklist by Gender and Pubertal Group
| Prepubertal | Postpubertal | |||||||
|---|---|---|---|---|---|---|---|---|
| Boys | Girls | Boys | Girls | |||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Nervous | 1.10 ± 0.07 | 1.02 ± 0.02 | 1.12 ± 0.08 | 1.04 ± 0.03 | 1.1 ± 0.05 | 1.02 ± 0.02 | 1.10 ± 0.06 | 1.00 ± 0.00 |
| Lively | 2.50 ± 0.2 | 2.33 ± 0.23 | 2.79 ± 0.26 | 2.69 ± 0.28 | 2.2 ± 0.21 | 2.12 ± 0.19 | 2.48 ± 0.18 | 2.00 ± 0.17 |
| Happy | 3.30 ± 0.3 | 3.27 ± 0.28 | 4.31 ± 0.20 | 4.19 ± 0.24 | 3.4 ± 0.19 | 3.37 ± 0.19 | 3.03 ± 0.20 | 3.13 ± 0.23 |
| Sad | 1.10 ± 0.06 | 1.02 ± 0.02 | 1.08 ± 0.07 | 1.00 ± 0.00 | 1.10 ± 0.06 | 1.06 ± 0.04 | 1.48 ± 0.16 | 1.35 ± 0.15 |
| Dizzy | 1.10 ± 0.05 | 1.1 ± 0.05 | 1.25 ± 0.12 | 1.15 ± 0.08 | 1.06 ± 0.03 | 1.00 ± 0.00 | 1.05 ± 0.05 | 1.13 ± 0.05 |
| Falling asleepa | 1.40 ± 0.12 | 1.48 ± 0.12 | 1.50 ± 0.19 | 1.52 ± 0.20 | 1.29 ± 0.08 | 1.39 ± 0.11 | 1.50 ± 0.15 | 1.65 ± 0.17 |
| Body tired | 1.25 ± 0.08 | 1.56 ± 0.15 | 1.42 ± 0.17 | 1.46 ± 0.17 | 1.40 ± 0.92 | 1.60 ± 0.12 | 1.88 ± 0.18 | 1.78 ± 0.17 |
| Need to pee | 1.17 ± 0.07 | 1.69 ± 0.18 | 1.15 ± 0.07 | 1.69 ± 0.19 | 1.12 ± 0.06 | 1.44 ± 0.16 | 1.58 ± 0.16 | 2.38 ± 0.28 |
| Headache | 1.29 ± 0.14 | 1.17 ± 0.07 | 1.21 ± 0.15 | 1.13 ± 0.08 | 1.17 ± 0.07 | 1.14 ± 0.05 | 1.40 ± 0.13 | 1.23 ± 0.09 |
| Can’t sleep | 1.27 ± 0.13 | 1.23 ± 0.09 | 1.40 ± 1.19 | 1.40 ± 0.20 | 1.08 ± 0.05 | 1.1 ± 0.06 | 1.13 ± 0.07 | 1.08 ± 0.04 |
| Irregular heart beat | 1.23 ± 0.12 | 1.17 ± 0.1 | 1.17 ± 0.11 | 1.15 ± 0.08 | 1.02 ± 0.02 | 1.08 ± 0.05 | 1.05 ± 0.05 | 1.03 ± 0.03 |
| Diarrhea | 1.02 ± 0.02 | 1.06 ± 0.04 | 1.02 ± 0.02 | 1.08 ± 0.08 | 1.02 ± 0.02 | 1.02 ± 0.02 | 1.00 ± 0.00 | 1.00 ± 0.00 |
| Impatient | 1.40 ± 0.16 | 1.31 ± 0.11 | 1.15 ± 0.08 | 1.27 ± 0.16 | 1.23 ± 0.08 | 1.23 ± 0.08 | 1.28 ± 0.09 | 1.53 ± 0.17 |
| Hungry | 2.48 ± 0.25 | 2.73 ± 0.28 | 2.46 ± 0.25 | 2.67 ± 0.26 | 2.27 ± 0.17 | 2.50 ± 0.22 | 1.95 ± 0.19 | 2.15 ± 0.24 |
| Cranky | 1.00 ± 0.00 | 1.00 ± 0.00 | 1.02 ± 0.02 | 1.02 ± 0.02 | 1.12 ± 0.06 | 1.1 ± 0.05 | 1.33 ± 0.12 | 1.20 ± 0.11 |
| Motivatedb | 1.94 ± 0.24 | 1.89 ± 0.23 | 2.77 ± 0.25 | 2.75 ± 0.32 | 2.04 ± 0.19 | 1.90 ± 0.19 | 2.25 ± 0.19 | 1.06 ± 0.14 |
| Life is goodb | 3.30 ± 0.28 | 3.23 ± 0.28 | 4.10 ± 0.24 | 4.23 ± 0.24 | 3.62 ± 0.20 | 3.40 ± 0.21 | 3.35 ± 0.25 | 3.08 ± 0.23 |
| Mood swings | 1.09 ± 0.06 | 1.1 ± 0.06 | 1.58 ± 0.25 | 1.58 ± 0.25 | 1.12 ± 0.06 | 1.04 ± 0.03 | 1.25 ± 0.11 | 1.20 ± 0.11 |
| Muscle twitch | 1.08 ± 0.05 | 1.19 ± 0.08 | 1.06 ± 0.05 | 1.06 ± 0.05 | 1.04 ± 0.03 | 1.06 ± 0.04 | 1.03 ± 0.03 | 1.10 ± 0.06 |
| Talkative | 1.46 ± 0.11 | 1.40 ± 0.12 | 1.67 ± 0.16 | 1.71 ± 0.19 | 1.65 ± 0.13 | 1.60 ± 0.14 | 1.75 ± 0.18 | 1.55 ± 0.13 |
| Queasyb | 1.04 ± 0.04 | 1.04 ± 0.03 | 1.10 ± 0.07 | 1.04 ± 0.03 | 1.02 ± 0.02 | 1.04 ± 0.03 | 1.03 ± 0.03 | 1.03 ± 0.03 |
| Heart racing | 1.02 ± 0.02 | 1.1 ± 0.05 | 1.23 ± 0.09 | 1.23 ± 0.09 | 1.08 ± 0.05 | 1.17 ± 0.09 | 1.03 ± 0.03 | 1.15 ± 0.09 |
| Can’t sit still | 1.15 ± 0.05 | 1.48 ± 0.19 | 1.48 ± 0.21 | 1.60 ± 0.24 | 1.35 ± 0.11 | 1.46 ± 0.13 | 1.13 ± 0.07 | 1.48 ± 0.14 |
| Ear ringingc | 1.09 ± 0.05 | 1.04 ± 0.04 | 1.31 ± 0.15 | 1.31 ± 0.15 | 1.08 ± 0.05 | 1.10 ± 0.04 | 1.00 ± 0.00 | 1.05 ± 0.05 |
| Energeticb | 1.94 ± 0.2 | 1.90 ± 0.2 | 2.34 ± 0.28 | 2.60 ± 0.31 | 2.02 ± 0.19 | 1.96 ± 0.18 | 1.83 ± 0.19 | 1.68 ± 0.15 |
| Stomachacheb | 1.1 ± 0.06 | 1.02 ± 0.02 | 1.21 ± 0.12 | 1.08 ± 0.05 | 1.10 ± 0.06 | 1.1 ± 0.05 | 1.10 ± 0.06 | 1.08 ± 0.06 |
| Stronga,b,c | 2.56 ± 0.24 | 2.52 ± 0.25 | 2.96 ± 0.30 | 2.92 ± 0.28 | 2.42 ± 0.23 | 2.25 ± 0.22 | 1.76 ± 0.18 | 1.33 ± 0.10 |
| Sweatya,b,c | 1.1 ± 0.07 | 1.06 ± 0.03 | 1.08 ± 0.04 | 1.23 ± 0.05 | 1.23 ± 0.08 | 1.19 ± 0.08 | 1.08 ± 0.04 | 1.13 ± 0.09 |
| Shaky | 1.2 ± 0.07 | 1.23 ± 0.07 | 1.23 ± 0.1 | 1.52 ± 0.20 | 1.10 ± 0.05 | 1.19 ± 0.08 | 1.15 ± 0.07 | 1.33 ± 0.14 |
| Sleepyc | 1.6 ± 0.14 | 1.71 ± 0.15 | 1.48 ± 0.18 | 1.44 ± 0.18 | 1.35 ± 0.17 | 1.48 ± 0.18 | 1.93 ± 0.18 | 2.18 ± 0.20 |
| Tiredb | 1.62 ± 0.14 | 1.75 ± 0.15 | 1.71 ± 0.20 | 1.73 ± 0.20 | 1.56 ± 0.13 | 1.65 ± 0.11 | 2.10 ± 0.16 | 2.30 ± 0.17 |
Data are shown as pre and post caffeine administration. presented as means ± SEMs.
Significant interaction between caffeine treatment and pre/post (P < .05).
Significant interaction between caffeine treatment, pre/post, gender, and pubertal group (P < .05).
Significant interaction between caffeine treatment, pre/post, and gender (P < .05).
Discussion
This study showed that the cardiovascular effects of caffeine differ as a function of gender, with the gender differences emerging after puberty. We also found differences as a function of menstrual cycle phase in postpubertal girls, with caffeine administration resulting in more pronounced effects on heart rate during the luteal phase but more pronounced effects on blood pressure during the follicular. These data suggest that there are gender differences in cardiovascular responses to caffeine and that these gender differences are related to pubertal development. More work needs to be done to understand the mechanisms underlying this association.
It is well established that acute caffeine administration increases blood pressure and decreases heart rate. Our findings confirm this in both pre- and postpubertal boys and girls. There have been few studies conducted in children that examined correlations between dietary caffeine intake and blood pressure.14,15 Our previous study in 12- to 17-year-olds4 examined cardiovascular responses to acute caffeine administration in adolescents, but, to our knowledge, this is the first to study these effects in children as young as 8 years old. The caffeine doses used in adults are, on average, 2.5 to 5 mg/kg, in contrast to our doses of 1 to 2 mg/kg. Despite our doses being lower, we found cardiovascular effects at both the 1- and 2-mg/kg doses with no differences between these doses. The average body weight of the children in our study was ∼50 kg (33 kg in prepubertal and 69 kg in postpubertal participants), so the 1-mg/kg dose contained approximately the amount of caffeine in a 12-ounce can of soda.
These findings replicate our earlier work showing gender differences in cardiovascular responses to caffeine3,4 and extend those findings by showing that these gender differences emerge after puberty. One potential explanation for this finding is that changes in steroid hormones that occur with pubertal development alter the metabolism of caffeine, which results in differential cardiovascular responses to caffeine in boys compared with girls. This possibility is supported by studies showing that estradiol inhibits cytochrome P450 activity,16,17 resulting in reduced caffeine clearance. An alternative explanation is that these gender differences are related to changes in caffeine consumption patterns as children age. When we examined caffeine sources in this study, we found that postpubertal participants were significantly more likely to consume caffeine from all major sources and with a greater frequency than were prepubertal children. These data suggest that caffeine use expands and increases as children get older. Gender differences in patterns of caffeine consumption have been shown in postpubertal participants in other studies as well.18
In addition to pubertal phase differences, we found that differences in cardiovascular responses varied as a function of menstrual cycle phase in postpubertal girls. Other studies have shown that the metabolism of caffeine varies as a function of menstrual cycle phase. For example, a study by Lane et al19 showed that caffeine clearance was slower during the luteal phase of the menstrual cycle compared with the follicular phase. In addition, previous studies have shown that there are differences in subjective responses to caffeine across the menstrual cycle as well, with decreased subjective responses during the follicular phase of the menstrual cycle.3,20 Taken together, these studies support our findings that responses to caffeine differ in girls as a function of menstrual cycle phase.
This study had several strengths. First, it was a double-blind, placebo-controlled, dose-response study. Second, this study was conducted in children and adolescents, who are an understudied population in this area. Third, we verified menstrual cycle phase by using salivary steroid hormone levels instead of relying strictly on self-report. This study was not without weaknesses. First, our sample, although large, was still primarily white, middle class, and well educated. It is not known whether these findings would generalize to other sociodemographic groups. Second, because of financial constraints, we were unable to verify caffeine abstinence empirically, so it is possible that some of the individuals were not compliant in the caffeine abstinence protocol. Third, although we did not explicitly reveal that we were administering caffeine, the caffeine use questionnaire and instructions about eliminating caffeine use might have increased expectancy about caffeine being studied. We attempted to minimize these concerns by using a placebo-controlled, within-subject design. Finally, this was a cross-sectional study, so we cannot determine how previous experience with caffeine influenced the response to caffeine in this study. Future studies will use longitudinal study designs.
Footnotes
Dr Temple was responsible for the study design, generation of randomization scheme, institutional review board approval, data analysis, and manuscript preparation and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Ms Ziegler was responsible for study design, participant randomization, institutional review board approval, data collection, training and supervising students and staff, and manuscript preparation and had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; Mr Graczyk was responsible for data collection, data quality control, and manuscript preparation; Ms Bendlin, Ms Sion, and Ms Vattana were responsible for data collection, data entry, and manuscript preparation; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was funded by a grant from the National Institute on Drug Abuse (RO1 DA030386) to Dr Temple. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Bender AM, Donnerstein RL, Samson RA, Zhu D, Goldberg SJ. Hemodynamic effects of acute caffeine ingestion in young adults. Am J Cardiol. 1997;79(5):696–699 [DOI] [PubMed] [Google Scholar]
- 2.Lane JD, Williams RB, Jr. Cardiovascular effects of caffeine and stress in regular coffee drinkers. Psychophysiology. 1987;24(2):157–164 [DOI] [PubMed] [Google Scholar]
- 3.Temple JL, Ziegler A.M. Gender differences in subjective and physiological responses to caffeine and the role of steroid hormones. J Caffeine Res. 2011;1:41–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Temple JL, Dewey AM, Briatico LN. Effects of acute caffeine administration on adolescents. Exp Clin Psychopharmacol. 2010;18(6):510–520 [DOI] [PubMed] [Google Scholar]
- 5.Waring WS, Goudsmit J, Marwick J, Webb DJ, Maxwell SR. Acute caffeine intake influences central more than peripheral blood pressure in young adults. Am J Hypertens. 2003;16(11 pt 1):919–924 [DOI] [PubMed] [Google Scholar]
- 6.Farag NH, Vincent AS, Sung BH, Whitsett TL, Wilson MF, Lovallo WR. Caffeine tolerance is incomplete: persistent blood pressure responses in the ambulatory setting. Am J Hypertens. 2005;18(5 pt 1):714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lovallo WR, Wilson MF, Vincent AS, Sung BH, McKey BS, Whitsett TL. Blood pressure response to caffeine shows incomplete tolerance after short-term regular consumption. Hypertension. 2004;43(4):760–765 [DOI] [PubMed] [Google Scholar]
- 8.Frary CD, Johnson RK, Wang MQ. Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc. 2005;105(1):110–113 [DOI] [PubMed] [Google Scholar]
- 9.Miller KE. Wired: energy drinks, jock identity, masculine norms, and risk taking. J Am Coll Health. 2008;56(5):481–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richardson NJ, Rogers PJ, Elliman NA, O’Dell RJ. Mood and performance effects of caffeine in relation to acute and chronic caffeine deprivation. Pharmacol Biochem Behav. 1995;52(2):313–320 [DOI] [PubMed] [Google Scholar]
- 11.Hughes JR, Higgins ST, Bickel WK, et al. Caffeine self-administration, withdrawal, and adverse effects among coffee drinkers. Arch Gen Psychiatry. 1991;48(7):611–617 [DOI] [PubMed] [Google Scholar]
- 12.Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110(4):743–747 [DOI] [PubMed] [Google Scholar]
- 13.Schlossberger NM, Turner RA, Irwin CE Jr. Validity of self-report of pubertal maturation in early adolescents. J Adolesc Health. 1992;13(2):109–113 [DOI] [PubMed]
- 14.Savoca MR, Evans CD, Wilson ME, Harshfield GA, Ludwig DA. The association of caffeinated beverages with blood pressure in adolescents. Arch Pediatr Adolesc Med. 2004;158(5):473–477 [DOI] [PubMed] [Google Scholar]
- 15.Savoca MR, MacKey ML, Evans CD, Wilson M, Ludwig DA, Harshfield GA. Association of ambulatory blood pressure and dietary caffeine in adolescents. Am J Hypertens. 2005;18(1):116–120 [DOI] [PubMed] [Google Scholar]
- 16.Pollock BG, Wylie M, Stack JA, et al. Inhibition of caffeine metabolism by estrogen replacement therapy in postmenopausal women. J Clin Pharmacol. 1999;39(9):936–940 [DOI] [PubMed] [Google Scholar]
- 17.Zaigler M, Rietbrock S, Szymanski J, Dericks-Tan JS, Staib AH, Fuhr U. Variation of CYP1A2-dependent caffeine metabolism during menstrual cycle in healthy women. Int J Clin Pharmacol Ther. 2000;38(5):235–244 [DOI] [PubMed] [Google Scholar]
- 18.Penolazzi B, Natale V, Leone L, Russo PM. Individual differences affecting caffeine intake: analysis of consumption behaviours for different times of day and caffeine sources. Appetite. 2012;58(3):971–977 [DOI] [PubMed] [Google Scholar]
- 19.Lane JD, Steege JF, Rupp SL, Kuhn CM. Menstrual cycle effects on caffeine elimination in the human female. Eur J Clin Pharmacol. 1992;43(5):543–546 [DOI] [PubMed] [Google Scholar]
- 20.Terner JM, de Wit H. Menstrual cycle phase and responses to drugs of abuse in humans. Drug Alcohol Depend. 2006;84(1):1–13 [DOI] [PubMed] [Google Scholar]