Abstract
AIM
To investigate the effects of naringenin eye drops on N-methyl-N-nitrosourea (MNU)-induced photoreceptor cell death in rats.
METHODS
Photoreceptor cell death was induced by single intraperitoneal injection of MNU (60 mg/kg) in rats. Both eyes of all animals were instilled with one drop of vehicle, 0.5% or 1.0% naringenin eye drops three times per day from 7d before to 17d after MNU injection. Effects of naringenin on MNU-induced photoreceptor cell death were evaluated by electrophysiological and histological analysis.
RESULTS
Flash electroretinography (FERG) and oscillatory potentials (OPs) recordings showed that the vehicle control group had remarkable reduction of amplitudes and prolongation of latency times. FERG and OPs responses were significantly reversed in MNU-induced rats treated with 0.5% or 1.0% naringenin eye drops compared with the vehicle control. The retinal morphological results showed that naringenin dose-dependently preserved the outer nuclear layer, outer retina and total retina.
CONCLUSION
These results indicate that topical treatment with naringenin eye drops prevented retinal neurons from MNU-induced structural and functional damages.
Keywords: naringenin, N-methyl-N-nitrosourea, photoreceptor cell death, retinitis pigmentosa
INTRODUCTION
Retinitis pigmentosa (RP) is a group of inherited neurodegenerative diseases in human characterized by loss of photoreceptor cells leading to visual disturbance and eventually to blindness. The worldwide prevalence of RP in human is approximately 1/4000, making it one of the major causes of blindness. Although the genetic heterogeneity of RP is quite varied, both in vivo and in vitro evidences suggest that photoreceptor cell apoptosis is the final common pathway of degeneration in RP[1]. Thus, the key to defend RP diseases is to reduce retinal photoreceptor cell apoptosis. A number of therapeutic avenues, including gene therapy, retina transplantation, pharmacological treatment, are being explored on animal models and even in clinical trials on humans[2]. However, to date, no approved therapies are able to stop the evolution of RP or restore vision[3]. Therefore, the development of complementary and alternative treatment strategies to combat photoreceptor cell death is desperately needed.
Naringenin (4′,5,7-trihydroxy flavanone) is richly found in citrus and grape fruits. It has various ophthalmological activities. Naringenin markedly increases retinal function recovery after ischemic insult which may attribute to strong increase of ocular blood flow[4]–[7]. Naringenin has antioxidant activity on various oxidants induced injuries in ARPE-19 cells and human umbilical vein endothelial cells, and suppresses the development of endotoxin-induced uveitis in rats[8],[9]. So it is hypothesized that naringenin might prevent photoreceptor cell from apoptosis.
The photoreceptors are highly sensitive to toxic substances, and various chemicals are known to cause retinal toxicity[10]. Among the chemicals, N-methyl-N-nitrosourea (MNU), an alkylating agent, targets on photoreceptor cells and rapidly induces retinal photoreceptor cell death in rats by an apoptosis mechanism which mimics photoreceptor degeneration in humans[11]. This model has widely been used to study RP[12]. In this study, we evaluated the protective effects of naringenin against MNU-induced photoreceptor cell death in rats using flash electroretinography (FERG), oscillatory potentials (OPs) and retinal morphology.
MATERIALS AND METHODS
Materials
MNU was purchased from Sigma-Aldrich Co., Ltd (St Louis, MO, USA). Naringenin was bought from Xi'an Huike Plant Development Co., Ltd. Hydroxypropyl-β-cyclodextrin (HP-β-CD) and polycarbophil were from Zibo Qianhui Biotechnology Co., Ltd and Lubrizol Corporation (USA), respectively. Poloxamer 407 and disodium edetate were kindly provided by Germany's BASF Corporation and the Hangzhou Toshihito Pharmaceutical Co., Ltd., respectively. Benzalkonium chloride and sodium chloride were obtained from Shanghai Jingwei Chemical Co., Ltd. and Zigong Honghe Pharmaceutial Co., Ltd., respectively.
Preparation of naringenin eye drops
The detailed formulation of naringenin eye drops was listed in Table 1. The preparations of 0.5% or 1.0% naringenin eye drops were described as follows. Firstly, 10 g of polycarbophil were added to 400 mL water for injection with stirring and then swelled for 2h. The pH value of polycarbophil solution was adjusted to 6.0-6.5 with 2 mol/L NaOH and then autoclaved (Solution I). Secondly, 150 g of HP-β-CD were dissolved in 400 mL water for injection with stirring, and then 5 or 10 g of naringenin were added and dissolved by sonication for 2h. Two gram of poloxamer 407 was added and dissolved by sonication for 0.5h. Afterwards 1 g of disodium edentate, 2 g of sodium chloride and 0.1 g of benzalkonium chloride were added and dissolved (Solution II). Lastly, Solution I and Solution II were mixed evenly with stirring and diluted to 1000 mL with water for injection, and then the pH value of the mixture was adjusted to 6.0-7.0 with 2 mol/L sterile NaOH and filled into 100 bottles. Vehicle eye drops was prepared in similar way but without adding naringenin.
Table 1. Composition of naringenin eye drops.
| Ingredients | Percentage of composition (w/v) (%) |
| Naringenin | 0.5 or 1.0 |
| HP-β-CD | 15 |
| Polycarbophil | 1 |
| Poloxamer 407 | 0.2 |
| Disodium edentate | 1 |
| Benzalkonium chloride | 0.01 |
| Sodium chloride | 0.2 |
Animal model
Forty Sprague-Dawley (SD) rats, male, body weight approximately 130 g, were obtained from Experimental Animal Center, Guangzhou University of Chinese Medicine. All rats had free access to a standard diet and drinking water and were housed in a room maintained at 24.0±0.5°C and with a 12:12h cyclic lighting schedule. The experiment was performed in accordance with the Animal Ethics Committee of Guangzhou University of Chinese Medicine and conformed to the ARVO Resolution for the use of animals in ophthalmic and vision research.
Rats were randomly divided into five groups (eight rats per group), normal, MNU, MNU+V, MNU+LN and MNU+HN groups. V, LN and HN were vehicle, 0.5% and 1.0% naringenin eye drops, respectively. Normal group was injected with normal saline intraperitoneally, while other rats were intraperitoneally injected with a single dose of 60 mg/kg MNU (10 mg/mL) dissolved in normal saline after 8h of fasting. Both eyes of all rats were instilled with one drop of vehicle or naringenin eye drops 3 times per day from 7d before to 17d after MNU injection.
Methods
Retinal function assessment by flash electroretinography and oscillatory potentials recording
FERG and OPs were performed on 1, 7 and 17d after MNU injection for evaluating functional status of retina, respectively. Briefly, rats were dark adapted overnight, and then anesthetized with pentobarbital sodium (3%, 0.6 mL/kg) by intraperitoneal injection and Xylazine Hydrochloride Injection at 0.19 mL/kg by intramuscular injection. Pupils of all rats were dilated with Compound Tropicamide Eye Drops. Before recording, Oxybuprocaine Hydrochloride Eye Drops was given for surface anesthesia. Hypromellose Eye Drops was added to each eye to protect the eye and maintain a good electrical connection.
FERG and OPs were recorded from both eyes simultaneously using two silver ring corneal electrodes, two forehead reference electrodes, and one ground electrode in the tail. The ERG machine (APS-2000AER) was purchased from Kanghua Rui Ming Technology Co., Ltd. FERG was recorded from 5 responses to flashes of unattenuated white light (20ms, 0.05 Hz) from a photic stimulator (light-emitting diodes) set at brightness of 6.325e−4cd·s/m2 and filtered by a digital band-pass filter from 0.1-300 Hz. OPs was evaluated from 10 responses with an inter-stimulus interval of 20ms by filtering the original responses elicited by a stimulus luminance of 1.125e−3cd·s/m2 by a frequency band-pass filter (50-200 Hz).The amplitude of FERG a-wave was measured from the baseline to a-wave trough, and that of FERG b-wave was from a-wave trough to b-wave peak. The latency times of a-wave and b-wave were recorded the flash onset to the trough and peak of the wave. OPs 1 through 4 amplitudes and their latency times were derived from OPs waveforms. Amplitudes were measured from the trough immediately preceding the peak of each OP from OPs 1-4. Latency time for each OP was recorded from the flash onset to the peak of each OP.
Morphological evaluation by quantitative histology
Eyes were enucleated from rats after euthanasia at 17d of MNU- or normal saline-injection, and fixed for at least 24h with 4% paraformaldehyde in phosphate buffer saline. The eye tissues were embedded in paraffin after dehydration. The sections (3 µm) were then stained with hematoxylin and eosin (HE) and observed under an optical microscope (OLYMPUS BX50). The total retinal (TR) thickness (from the internal limiting membrane to the pigment epithelium cell layer), outer retinal (OR) thickness (the out plexiform layer to the pigment epithelium cell layer), and outer nuclear layer (ONL) were measured using Mingmei image processing system. The measurements were conducted at the central retina (approximately 400 µm from the optic nerve). The photoreceptor ratio was calculated as the following formula:
Photoreceptor ratio (%) =100%× (OR thickness/TR thickness)
Statistical Analysis
All data were presented as mean±SEM and analyzed by Statistical Package for the Social Sciences version 17.0 (SPSS 17.0). One-way analysis of variance (ANOVA) test was performed and post hoc multiple comparisons were conducted with LSD. P<0.05 was assumed to be significant.
RESULTS
Effects of Naringenin Eye Drops on Flash Electroretinography and Oscillatory Potentials
There were no differences for any parameters between MNU and MNU+V group (Figures 1–5), which indicates that vehicle eye drops had no effects on retinal morphology and retinal function. So the data of MNU+LN and MNU+HN group were compared with those of MNU+V group.
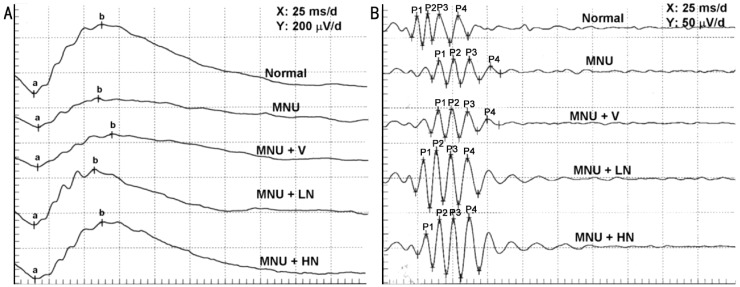
Figure 1. Representative examples of FERG (A) and OPs (B) recording in SD rats 17d after MNU injection, respectively.
a: a-wave; b: b-wave; P1: OP1; P2: OP2; P3: OP3; P4: OP4.
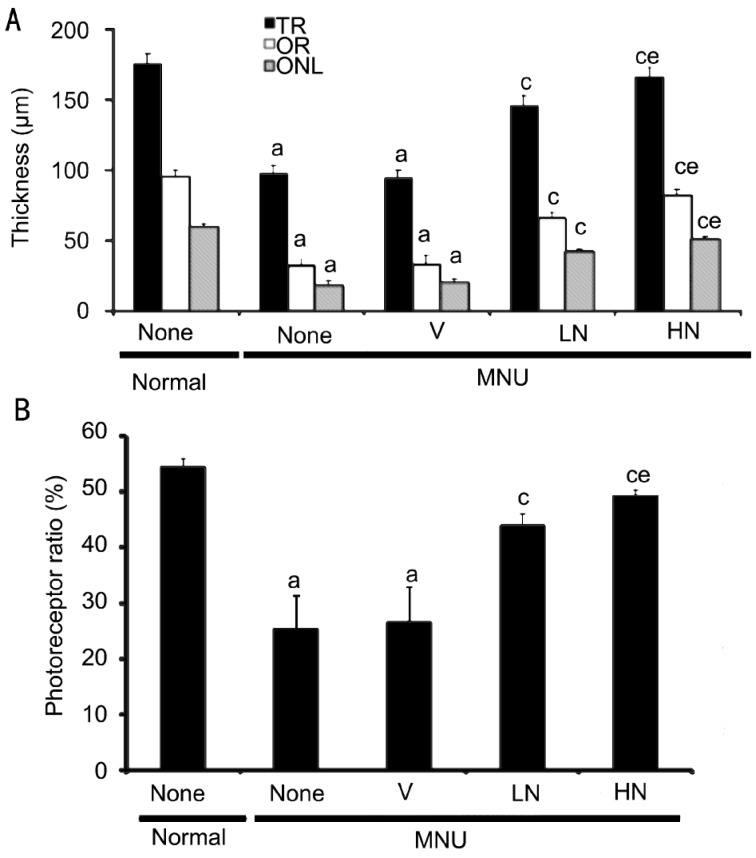
Figure 5. Effect of naringenin on retinal thickness and photoreceptor ratio of rats 17d after MNU injection.
Data were expressed as mean±SEM, n=16. aP<0.05 vs normal group; cP<0.05 vs MNU+V group; eP<0.05 vs corresponding parameters of MNU+LN group.
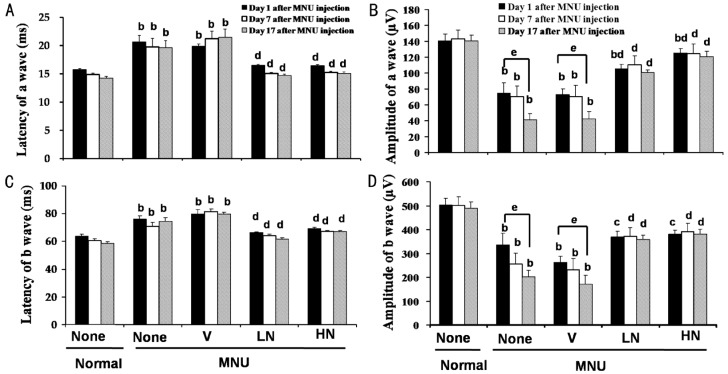
As shown in Figures 1, 2, the amplitudes of a- and b-wave markedly decreased in a time-dependent manner, while the latency times prolonged in the MNU+V group (P<0.05 vs normal group). These changes were reversed significantly by 0.5% and 1.0% naringenin eye drops (P<0.05 vs vehicle control group). One percent of naringenin eye drops increased the amplitudes of a- and b-wave by 182.3%±6.9% and 121.9%±23.0% 17d after MNU injection as compared with MNU+V group, respectively.
Figure 2. Effects of naringenin on the amplitude and latency of FERG 1, 7 and 17d after MNU injection in SD rats.
Data were expressed as mean±SEM, n=16. bP<0.01 vs normal group, cP<0.05 and dP<0.01 vs MNU+V group, and eP<0.05 vs recording 1d after MNU injection.
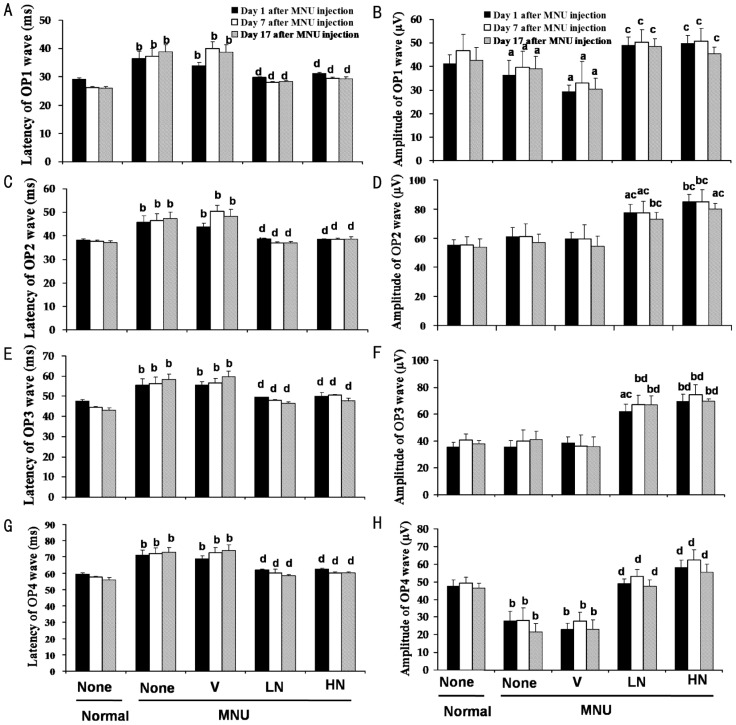
All latency times of four OP waves were significantly prolonged in MNU+V group as compared with normal group (P<0.05), and 0.5% and 1.0% naringenin eye drops reversed this parameter. Interestingly, the amplitudes of OP2 and OP3 in MNU+LN and MNU+HN group were larger than normal group (P<0.05, Figures 1, 3).
Figure 3. Effects of naringenin on the amplitude and latency of OPs 1, 7 and 17 days after MNU injection in SD rats.
Data were expressed as mean±SEM, n=16. aP<0.05 and bP<0.01 vs normal group, cP<0.05 and dP<0.01 vs MNU+V group.
Effects of Naringenin on Retinal Histology
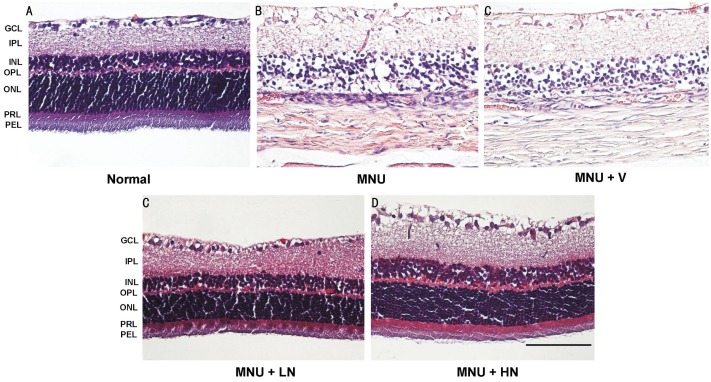
Normal rats had rows of well-arranged photoreceptor cells in OR. However, near-total loss of photoreceptor cells and disruption of OR were seen, and ONL contained no photoreceptor nuclei or only a few layers of nuclei at both the central and peripheral retina in MNU-injected rats. The remaining photoreceptor nuclei were lightly stained, and their chromatin was clumped. These morphological changes were not observed in retinas that were treatment with 0.5% and 1.0% naringenin eye drops (Figure 4). The thickness of TR, OR and ONL in MNU+V group were significantly decreased by 46.0%±4.0%, 65.5%±4.5% and 66.8%±4.9% as compared with normal group, respectively. Naringenin increased these parameters in a dose-dependent manner (Figure 5A). The photoreceptor ratio in MNU+V group was of 26.8%±6.1% as compared with normal group of 54.6%±1.4% (P<0.05). Naringenin dose-dependently prevented photoreceptor cell loss, and the photoreceptor ratio were 44.0%±2.0% and 49.4%±0.9% in MNU+LN and MNU+HN group, respectively (Figure 5B).
Figure 4. Representative pictures of HE-stained retinas of rats 17d after MNU injection.
GCL: Ganglion cell layer; IPL: Inner plexiform layer; INL: Inner nuclear layer; OPL: Outer plexiform layer; ONL: Outer nuclear layer; PRL: Photoreceptor cell layer; PEL: Pigment epithelial cell layer. Pictures were at the magnification of ×400. Scale bar: 50 μm.
Correlation Between Retinal Morphology and Retinal Function
As shown in Table 2, TR thickness had high correlations with FERG and the latency of OPs (P<0.05 or P<0.01). OR and ONL thickness had a high correlation with amplitudes of FERG a- and b- wave (P<0.05 or P<0.01). Furthermore, ONL thickness had a high correlation with the latency of OPs (P<0.05 or P<0.01).
Table 2. Pearson correlation coefficients between thickness of TR, OR and ONL, and retinal function.
| Parameters | Pearson correlation coefficient (r) |
||
| TR thickness | OR thickness | ONL thickness | |
| La | -0.953a | -0.867 | -0.907b |
| Lb | -0.896a | -0.828 | -0.867 |
| Aa | 0.997b | 0.953a | 0.970b |
| Ab | 0.974b | 0.997b | 0.997b |
| L1 | -0.970b | -0.936a | -0.960b |
| L2 | -0.943b | -0.876 | -0.911b |
| L3 | -0.966b | -0.926a | -0.952a |
| L4 | -0.964b | -0.916b | -0.945b |
| A1 | 0.741 | 0.569 | 0.637 |
| A2 | 0.453 | 0.310 | 0.354 |
| A3 | 0.480 | 0.327 | 0.379 |
| A4 | 0.937a | 0.877 | 0.903a |
La, Lb: Latency of a- and b-wave; Aa, Ab: Amplitude of a- and b-wave; L1, L2, L3 and L4: Latency of OP1, OP2, OP3 and OP4; A1, A2, A3 and A4: Amplitude of OP1, OP2, OP3 and OP4; TR: Total retina; OR: Outer retina; ONL: Outer nuclear layer. a,bCorrelation were significant at the 0.05 and 0.01 level (2-tailed).
DISCUSSION
An acute systemic injection of MNU induces photoreceptor cell death in mice, rats and hamsters[12]. MNU causes DNA adduct formation in photoreceptor nuclei and then leads to retinal remodeling with morphological change of rapid loss of ONL and OR, whereas the other layers remaining structurally intact[13]–[16]. The present study demonstrated that the ONL in MNU+V group near-totally loss (Figures 4, 5), which was consistent with previous reports. Retinal function (Figures 1–3) were damaged, and the parameter had strong relationship with histopathology of retina (Table 2) in this RP model. These results indicate that RP model with MNU injection is a rapid and reproducible method and has great value for the study of RP.
FERG a-wave response reflects photoreceptor cell function, and b-wave reflects the functional status of bipolar and Müller cells which are the second-order neurons[17]. Naringenin reversed MNU-induced changes of latency times and amplitudes of FERG a- and b-wave, which suggests that naringenin remarkably prevents photoreceptor, bipolar and Müller cells from MNU-induced damage.
OPs provide noninvasive evaluation of inner retina function in vivo and is a useful tool in basic research as well as in the clinic[18]. Our results showed that naringenin reversed the prolongation of OP latency times induced by MNU. Moreover, naringenin not only reversed the decrease of OP1 and OP4 amplitudes, but also increased OP2 and OP3 amplitudes that were even larger than normal rats (P<0.05, Figure 3). The results suggest that naringenin improves MNU-induced inner retinal function.
Moreover, the protective effect of naringenin on retinal morphology was in a dose-dependent manner and rats' retinas in 1.0% naringenin eye drops group were almost completely preserved (Figures 4, 5), which indicate that naringenin has protective effect on retinal photoreceptor cells. The protective effect of naringenin may contribute to the preservation of retinal function (Table 2).
In summary, the results showed that naringenin eye drops successfully reduced retinal function damage, protected photoreceptor cells from death and improved behavior performance in MNU-induced photoreceptor cell death in rats. Therefore, naringenin may be a promising molecule for diseases caused by photoreceptor cell death such as RP which should be investigated further.
Acknowledgments
Foundation: Supported by Guangdong Provincial Department of Science and Technology Grant (No.2011B031700052)
Conflicts of Interest: Lin JL, None; Wang YD, None; Ma Y, None; Zhong CM, None; Zhu MR, None; Chen WP, None; Lin BQ, None.
REFERENCES
- 1.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11(4):273–284. doi: 10.1038/nrg2717. [DOI] [PubMed] [Google Scholar]
- 2.Musarella MA, MacDonald IM. Current concepts in the treatment of retinitis pigmentosa. J Ophthalmol. 2011;2011:753547. doi: 10.1155/2011/753547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sahni JN, Angi M, Irigoyen C, Semeraro F, Romano MR, Parmeggiani F. Therapeutic challenges to retinitis pigmentosa: from neuroprotection to gene therapy. Curr Genomics. 2011;12(4):276–284. doi: 10.2174/138920211795860062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiou GC, Xu XR. Effects of some natural flavonoids on retinal function recovery after ischemic insult in the rat. J Ocul Pharmacol Ther. 2004;209(2):107–113. doi: 10.1089/108076804773710777. [DOI] [PubMed] [Google Scholar]
- 5.Park YH, Chiou GC. Structure-activity relationship (SAR) between some natural flavonoids and ocular blood flow in the rabbit. J Ocul Pharmacol Ther. 2004;20(1):35–42. doi: 10.1089/108076804772745446. [DOI] [PubMed] [Google Scholar]
- 6.Park XH, Xu XR, Chiou GC. Structure requirements of flavonoids for increment of ocular blood in the rabbit and retinal function recovery in the rat. J Ocul Pharmacol Ther. 2004;20(3):189–200. doi: 10.1089/1080768041223666. [DOI] [PubMed] [Google Scholar]
- 7.Xu XR, Park XH, Chiou GC. Effects of dehydrogenation of flavones and number of hydroxy groups in the molecules on ocular blood flow in rabbits and retinal function recovery in rats. J Ocul Pharmacol Ther. 2004;20(4):311–320. doi: 10.1089/1080768041725290. [DOI] [PubMed] [Google Scholar]
- 8.Lin BQ, Chiou GC. Antioxidant activity of naringenin on various oxidants induced damages in ARPE-19 cells and HUVEC. Guoji Yanke Zazhi. 2008;8(10):1963–1967. [Google Scholar]
- 9.Shiratori K, Ohgami K, Ilieva I, Jin XH, Yoshida K, Kase S, Ohno S. The effects of naringin and naringenin on endotoxin-induced uveitis in rats. J Ocul Pharmacol Ther. 2005;21(4):298–304. doi: 10.1089/jop.2005.21.298. [DOI] [PubMed] [Google Scholar]
- 10.Stone J, Maslim J, Valter-Kocsi K, Mervin K, Bowers F, Chu Y, Barnett N, Provis J, Lewis G, Fisher SK, Bisti S, Gargini C, Cervetto L, Merin S, Pe'er J. Mechanisms of photoreceptor death and survival in mammalian retina. Prog Retin Eye Res. 1999;18(6):689–735. doi: 10.1016/s1350-9462(98)00032-9. [DOI] [PubMed] [Google Scholar]
- 11.Nagar S, Krishnamoorthy V, Cherukuri P, Jain V, Dhingra NK. Early remodeling in an inducible animal model of retinal degeneration. Neuroscience. 2009;160(2):517, 529. doi: 10.1016/j.neuroscience.2009.02.056. [DOI] [PubMed] [Google Scholar]
- 12.Tsubura A, Lai YC, Miki H, Sasaki T, Uehara N, Yuri T, Yoshizawa K. Animal models of N-methyl-N-nitrosourea-induced mammary cancer and retinal degeneration with special emphasis on therapeutic trials. In Vivo. 2011;25(1):11–22. [PubMed] [Google Scholar]
- 13.Ogino H, Ito M, Matsumoto K, Yagyu S, Tsuda H, Hirono I, Wild CP, Montesano R. Retinal degeneration induced by N-methyl-N-nitrosourea and detection of 7-methyldeoxyguanosine in the rat retina. Toxicol Pathol. 1993;21(1):21–25. doi: 10.1177/019262339302100103. [DOI] [PubMed] [Google Scholar]
- 14.Tsubura A, Yoshizawa K, Kuwata M, Uehara N. Animal models for retinitis pigmentosa induced by MNU; disease progression, mechanisms and therapeutic trials. Histol Histopathol. 2010;25(7):933–944. doi: 10.14670/HH-25.933. [DOI] [PubMed] [Google Scholar]
- 15.Nakajima M, Yuge K, Senzaki H, Shikata N, Miki H, Uyama M, Tsubura A. Photoreceptor apoptosis induced by a single systemic administration of N-methyl-N-nitrosourea in the rat retina. Am J Pathol. 1996;148(2):631–641. [PMC free article] [PubMed] [Google Scholar]
- 16.Boudard DL, Mendoza J, Hicks D. Loss of photic entrainment at low illuminances in rats with acute photoreceptor degeneration. Eur J Neurosci. 2009;30(8):1527–1536. doi: 10.1111/j.1460-9568.2009.06935.x. [DOI] [PubMed] [Google Scholar]
- 17.Homma K, Osakada F, Hirami Y, Jin ZB, Mandai M, Takahashi M. Detection of localized retinal malfunction in retinal degeneration model using a multielectrode array system. J Neurosci Res. 2009;87(9):2175–2182. doi: 10.1002/jnr.22024. [DOI] [PubMed] [Google Scholar]
- 18.Dong CJ, Agey P, Hare WA. Origins of the electroretinogram oscillatory potentials in the rabbit retina. Vis Neurosci. 2004;21(4):533–543. doi: 10.1017/S0952523804214043. [DOI] [PubMed] [Google Scholar]