Abstract
AIM
To assess the effects of hypoxia on human orbital fibroblasts (OF) on adipogenesis and adipocytokine production.
METHODS
Human OF were derived from tissues obtained from patients with Graves' ophthalmopathy (GO) and from patients without known thyroid diseases undergoing blepharoplasty. The OF were cultured separately under normoxic and hypoxic conditions. Comparisons of adipocytokine concentrations using multiplex ELISA and lipid accumulation in the cells using Oil Red O staining were subsequently performed.
RESULTS
There was increased adipogenesis in OF from GO subject when exposed to hypoxic culture conditions. This was not observed in OF from normal controls. Hypoxia led to an increase in leptin and a decrease in MCP-1 secretion in OF cultures.
CONCLUSION
Hypoxia induces adipogenesis in OF and may represent a mechanism by which smoking contributes to deterioration of GO. We also found novel changes to leptin and MCP-1 production in OF cultures exposed to hypoxia suggesting important roles of these cytokines in the disease process.
Keywords: hypoxia, smoking, Graves' ophthalmopathy
INTRODUCTION
Graves' ophthalmopathy (GO) is the most common extra-thyroidal manifestation of Graves' disease (GD). GO is clinically relevant in approximately 50% of patients with GD, with the severe forms affecting 3%-5% of patients[1]. Several factors have been implicated in the development of GO such as genetic attributes and environmental factors like smoking. Smoking has been a risk factor that has been most consistently linked to either the development or deterioration of GO. Recent studies have confirmed that smoking can influence the occurrence and the course of GO, and also impairs responsiveness to treatment, such as orbital radiotherapy and steroids[2]. In particular, current smokers face an up to 20- fold increased risk of GO compared to non- or never- smokers[3]. However, the biological mechanisms by which smoking influence the development of GO remains unknown[4].
The hallmark of GO are increased size of extraocular muscles and retrobulbar fat which have been associated with inflammatory cytokines and excessive glycosaminoglycan (GAG) secretion[5]. Current evidence suggest orbital fibroblasts (OF) play a key role in the pathogenesis of GO[6]. Previous studies have demonstrated that hypoxic culture conditions not only enhance glycosaminoglycan production but also protein and DNA synthesis in extraocular muscle fibroblasts from healthy subjects[7]. These extraocular muscle fibroblasts are capable of secreting inflammatory cytokines which are believed to play important roles in the pathogenesis of GO[5],[8]. These inflammatory cytokines have also been shown to exert differential effects on fibroblast cultures depending on the anatomical localisation of the cell types[7].
Although much is known about the effects of hypoxia on fibroblasts obtained from normal subjects, no reports to date have been published on the study of hypoxia on human OF obtained from patients with GO when compared to those obtained from healthy subjects. This study aims to understand 1) the influence of hypoxia in the secretion of inflammatory cytokines by OF; 2) the effects of hypoxia on adipogenesis by human OF.
SUBJECTS AND METHODS
Subjects
Orbital fat tissue was obtained from 2 patients with GO and from 2 control patients without known thyroid disease undergoing blepharoplasty. GO was diagnosed by an experienced ophthalmologist based on the diagnostic criteria defined by Bartley and Gorman[9] in the context of GD. All patients were euthyroid at the time of surgery. All tissues were obtained, after informed consent, in the Singapore National Eye Centre (Singapore) in accordance with the principles of the Declaration of Helsinki and after approval by the institutional ethics committee.
Methods
Human orbital fibroblast cultures
OF were grown from the fat tissue obtained. The fat tissue were minced and placed in plastic culture flasks in M199 medium (Gibco, Life Technologies) supplemented with 10% fetal bovine serum (FBS), gentamycin (20 µg/mL) and penicillin/streptomycin (100 U/mL) allowing the OF to emerge from the tissue as described in previously [10]. When confluent the fibroblasts were passaged into 150 cm2 culture flasks in M199 medium supplemented with 10% FBS and maintained at 37°C with 5% CO2. Low passages (1-5) were used in our experiments.
Hypoxia treatment and adipocytokine concentration measurements
Prior to hypoxia/normoxia treatment, fibroblasts were seeded into 6-well culture plates at a cell density of 0.1×106 cell/well. When 80% confluent, the culture plates were placed under hypoxic conditions (1% O2, 94% N, 5% CO2) in a modular incubator chamber (Billups-Rothenburg, Del Mar, CA, USA) or atmospheric conditions (atmospheric O2, 5% CO2). Culture media (1 mL) was removed at specified times (0, 24h, 3, 4, 7, 8, 10, 11, 14, 15, 17, 18, 21 and 22d) from a single well and adipokine concentration determined using multiplex ELISA (Merck Millipore Cat#HADCYT-61K) in duplicate. Altogether 14 time point cytokine measurements were carried out for each fibroblast sample. The adipokine panel studied comprised IL-1β, IL-6, IL-8, leptin, TNF-α, MCP-1, Hepatocyte growth factor (HGF), adiponectin, resistin, Plasminogen activator inhibitor-1 (PAI-1, total) and Nerve growth factor (NGF).
Oil Red O staining
On completion of hypoxia/normoxia exposure, lipid accumulation in the cells was assessed using Oil Red O (ORO) staining. Cells were rinsed three times with cold phosphate buffered saline (PBS) and fixed in 10% formalin-calcium for 15min at room temperature and lipid stained using 0.21% ORO (Sigma Aldrich, Germany). The dye was extracted using 1 mL isopropanol and absorbance reading at 510 nm of the extracted dye taken as a measure of lipid accumulation. Absorbance readings of ORO stain in six well culture plates for each patient in both groups of patients were used for statistical analysis of lipid accumulation. Non readable wells were excluded from the dataset.
Statistical Analysis
Non-parametric test (Wilcoxon signed rank test) was used to compare statistical significance of ORO/cytokine measurements between hypoxia and normoxia treatment separately in GO and normal OF. P<0.0005 was taken as significant in order to adjust for the three cytokine comparisons carried out. Averages of the duplicate cytokine measurements were used for analysis. All statistical analyses were performed using Prism GraphPad software (Version 5.01). Median and interquartile range of the cytokine values is reported (25th percentile, 75th percentile). Only comparisons within paired samples (i.e. GO hypoxia vs GO normoxia, normal hypoxia vs normal normoxia) was carried out as the small sample size did not have enough power for unpaired samples (i.e. GO hypoxia vs normal hypoxia, GO normoxia vs normal normoxia was not carried out).
RESULTS
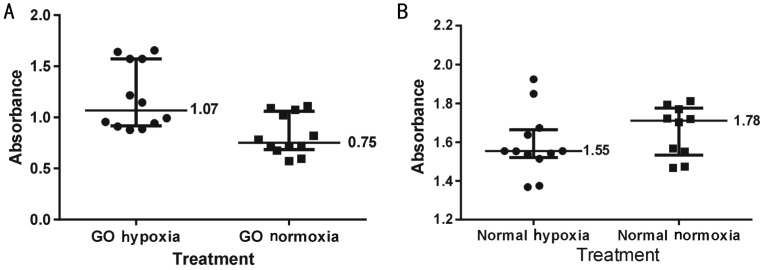
Hypoxia increased adipogenesis in OF from GO subjects measured by ORO staining (P=0.0003) but this was not observed in OF from normal controls (P=0.0037, Figures 1, 2).
Figure 1. ORO absorbance readings for OF cultures.
A: GO hypoxia: Graves' ophthalmopathy fibroblasts incubated in hypoxic conditions (5% CO2, 95% N2), GO normoxia: Graves' ophthalmopathy fibroblasts incubated under normoxia (atmospheric O2, 5% CO2); B: Normal hypoxia: Normal fibroblasts incubated under hypoxic conditions; Normal normoxia: Normal fibroblasts incubated under normoxia. The horizontal bars within each group represent 25th percentile, median and 75th percentile of ORO absorbance.
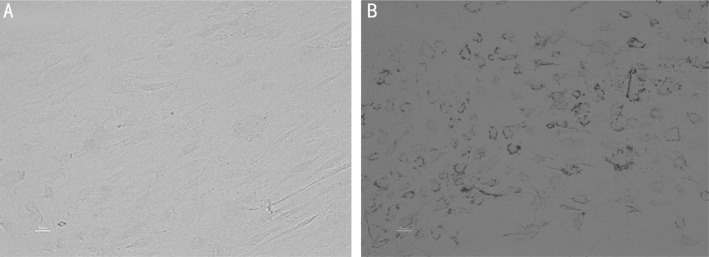
Figure 2. Digital photographs (at ×20 magnification) of OF from GO subjects showing adipogenic effects of hypoxia.
The dark spots are intracellular lipid droplets stained with ORO. A: Normoxia; B: Hypoxia.
Adipocytokine Measurement
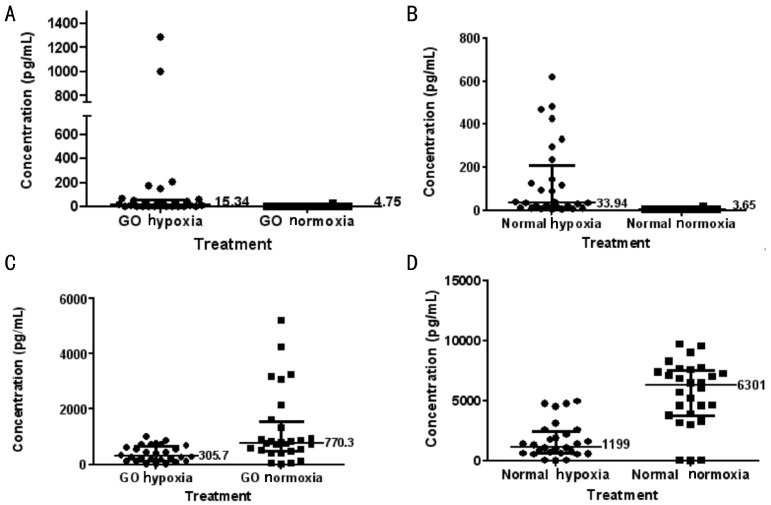
A panel of 11 cytokines in the multiplex ELISA were measured but only significant results for adipocytokine measurements for paired samples are reported here (Figure 3). Leptin levels increased significantly in OF cultures from both GO and normal controls when exposed to hypoxia (P=0.0003 and P=0.0001 respectively). Monocyte chemotactic protein-1 (MCP-1) secretion was decreased significantly in both groups under hypoxic conditions (both P<0.0001).
Figure 3. Adipocytokine concentrations of leptin (A and B) and MCP-1 (C and D) for OF cultures in different culture conditions.
GO hypoxia: Graves' ophthalmopathy fibroblasts incubated in hypoxic conditions (5% CO2, 95% N2); GO normoxia: Graves' ophthalmopathy fibroblasts incubated under normoxia (atmospheric O2, 5% CO2); Normal hypoxia: Normal fibroblasts incubated under hypoxic conditions; Normal normoxia: Normal fibroblasts incubated under normoxia. The horizontal bars within each group represent 25th percentile, median and 75th percentile.
DISCUSSION
Extensive research has been done to examine the effects of hypoxia on human adipose tissues. Tissue hypoxia represents the common denominator of many pathological processes that occurs in cardiovascular, haematological, pulmonary, inflammatory processes and fibrosis[11]. Tissue hypoxia has also been proposed as the mechanism by which smoking affects GO. Limited data is available on the effects of hypoxia on orbital adipogenesis and adipocytokine production in GO. The OF have recently emerged as a key player in the pathogenesis of GO, which participates through differentiation to adipocytes or myofibroblasts, enhancement of orbital inflammation and excessive production of extra-cellular matrix[12]. Sorisky and colleagues have demonstrated that cultures derived from human orbital tissue contain adipocyte precursor cells (5%-10% in total) that are capable of differentiating into lipid filled adipocytes when cultured under conditions known to stimulate adipogenesis[7]. The resultant expansion of fat within the orbital confines results in proptosis characteristics of GO. Cawood et al[13] have shown cigarette smoke extract increased adipogenesis in an in vitro model of GO using primary cultures of OF from patients with GO. Similar to their study, we used the human model of GO, which involves culturing OF taken from patients undergoing surgery for GO and our results suggest hypoxia could represent a mechanism by which smoking contributes to pathogenesis of GO by promoting adipogenesis. However, our results were at variance to their study, who found no difference in adipogenesis between OF from GO subjects and normal controls. In our experiments, the GO fibroblasts exposed to hypoxic conditions responded with increase lipid accumulation but this was not observed in fibroblasts obtained from normal controls. Tsai et al[14] has recently reported that cultured OF from patients with GO displayed increased intracellular superoxide anions and H2O2 compared to normal fibroblasts. This elevated reactive oxygen species can attack polyunsaturated fatty acids in cell membranes and result in formation of lipid peroxidation products. The results of this study and that obtained by our investigations suggest OF from GO subjects are inherently different from normal controls and their response to hypoxia could have been modulated by environmental factors as a result of the systemic and orbital disease processes. These novel observations may also shed some light into the possible mechanisms of action of antioxidants in modulating the course of GO[15].
Apart from enhancing adipogenesis, hypoxia also induces cellular adaptation via coordinated expression numerous genes, which may represent another mechanism by which hypoxia contributes to GO pathogenesis. An important and well characterised key regulator of this adaptive response to alterations in oxygen tension is hypoxia inducible factor-1 (HIF-1). In particular, HIF-1 α has been identified in human adipose tissue. This transcription factor activates transcription by binding to a specific cis-acting regulatory sequence referred to as hypoxia response element (HRE), a hallmark of hypoxia-sensitive target genes[16]–[18]. Leptin has been identified as one of the many specific hypoxia- sensitive target genes, and increased expression of this gene and its protein has been found in the subset of OF that undergo adipogenesis[19]. Leptin, the product of the obese (ob) gene, is expressed uniquely in cells committed to the adipocyte lineage and is expressed exclusively by mature adipocytes, hence serving as a marker of adipocyte differentiation[20]. It is also thought to possess pro-inflammatory and pro-angiogenic activities [21]–[25]. Hence, the increased leptin levels observed in OF cultures exposed to hypoxia in our study not only represent a marker of increased adipogenesis, but may have pathogenic roles in stimulating angiogenesis and contributes to the inflammation characteristic of GO.
Several cytokines are produced by the OF that form the inflammatory mileu in GO. In particular, MCP-1 is a powerful chemoattractant that targets mononuclear and T lymphocyte infiltration and promotes inflammation[26],[27]. Our study results interestingly show a reduction in MCP-1 secretion on OF exposed to hypoxic conditions. Hypoxia has been previously shown to reduce TNF-α induced MCP-1 mRNA expression and protein secretion in conditioned media of human adipocytes due to downregulation of nuclear factor-κB (NF-κB) signalling [28]. This results in inhibition of migration of monocytes and macrophages. This unexpected response by adipocytes to low oxygen concentrations could reflect a mechanism to limit inflammation and immune cell infiltration in areas of adipose tissue, where the adipocytes are exposed to high levels of inflammatory cytokines such as TNF-α and represent an attempt to maintain tissue function[28]. We propose a similar mechanism could explain the low MCP-1 secretion in response to hypoxia in OF in our study. Hence, although evidence so far suggest the role of leptin and MCP-1 in the pathogenesis of GO, this is the only study that investigates the direct influence of hypoxia on OF in patients with GO.
In our study, OF from subjects with GO and normal controls responded similarly in term of adipocytokine production, but only those from GO subjects undergo increased adipogenesis. It is possible that the adipocytokine production represents a common cellular response to exposure to hypoxia regardless of the underlying disease process, or hypoxia may have some additive or synergistic effect in combination with other pathogenic influences in the orbit in patients with underlying autoimmune thyroid disease since orbital tissue expansion do not occur in smokers without these diseases. However, these reasons remain speculative at this point until further investigations.
In conclusion, the key pathological features of GO are inflammation, excess production of glycosaminoglycan, and adipogenesis[29]. The results of our study and others suggest that hypoxia induced by smoking contributes to deterioration of GO via its influence on all these processes[7],[13]. Further studies are certainly required to confirm our findings as there may be limitations to generalizing the results from our small study.
Acknowledgments
Foundation: Supported by Endocrinology and Metabolic Society of Singapore.
Conflicts of Interest: Chng CL, None; Lai OF, None; Chew CS, None; Peh YP, None; Fook-Chong SM, None; Seah LL, None; Khoo DH, None.
REFERENCES
- 1.Wiersinga WM, Bartalena L. Epidemiology and prevention of Graves' ophthalmopathy. Thyroid. 2002;12(10):855–860. doi: 10.1089/105072502761016476. [DOI] [PubMed] [Google Scholar]
- 2.Stan MN, Bahn RS. Risk factors for development or deterioration of Graves' ophthalmopathy. Thyroid. 2010;20(7):777–783. doi: 10.1089/thy.2010.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornton J, Kelly SP, Harrison RA, Edwards R. Cigarette smoking and thyroid eye disease: a systematic review. Eye (Lond) 2007;21(9):1135–1145. doi: 10.1038/sj.eye.6702603. [DOI] [PubMed] [Google Scholar]
- 4.Wiersinga WM. Smoking and thyroid. Clin Endocrinol (Oxf) 2013;79(2):145–151. doi: 10.1111/cen.12222. [DOI] [PubMed] [Google Scholar]
- 5.Bahn RS. Graves' ophthalmopathy. N Engl J Med. 2010;362(8):726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Steensel L, Dik WA. The orbital fibroblast: a key player and target for therapy in Graves' ophthalmopathy. Orbit. 2010;29(4):202–206. doi: 10.3109/01676831003668443. [DOI] [PubMed] [Google Scholar]
- 7.Metcalfe RA, Weetman AP. Stimulation of extraocular muscle fibroblasts by cytokines and hypoxia: possible role in thyroid-associated ophthalmopathy. Clin Endocrinol (Oxf) 1994;40(1):67–72. doi: 10.1111/j.1365-2265.1994.tb02445.x. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34(+) fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013;98(7):2783–2790. doi: 10.1210/jc.2013-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartley GB, Gorman CA. Diagnostic criteria for Graves' ophthalmopathy. Am J Ophthalmol. 1995;119(6):792–795. doi: 10.1016/s0002-9394(14)72787-4. [DOI] [PubMed] [Google Scholar]
- 10.Valyasevi RW, Erickson DZ, Harteneck DA, Dutton CM, Heufelder AE, Jyonouchi SC, Bahn RS. Differentiation of human orbital preadipocyte fibroblasts induces expression of functional thyrotropin receptor. J Clin Endocrinol Metab. 1999;84(7):2557–2562. doi: 10.1210/jcem.84.7.5838. [DOI] [PubMed] [Google Scholar]
- 11.Semenza GL. Hypoxia and human disease-and the Journal of Molecular Medicine. J Mol Med (Berl) 2007;85(12):1293–1294. doi: 10.1007/s00109-007-0285-z. [DOI] [PubMed] [Google Scholar]
- 12.Hemmrich K, von Heimburg D, Cierpka K, Haydarlioglu S, Pallua N. Optimization of the differentiation of human preadipocytes in vitro. Differentiation. 2005;73(1):28–35. doi: 10.1111/j.1432-0436.2005.07301003.x. [DOI] [PubMed] [Google Scholar]
- 13.Cawood TJ, Moriarty P, O'Farrelly C, O'Shea D. Smoking and thyroid-associated ophthalmopathy: a novel explanation of the biological link. J Clin Endocrinol Metab. 2007;92(1):59–64. doi: 10.1210/jc.2006-1824. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CC, Wu SB, Cheng CY, Kao SC, Kau HC, Chiou SH, Hsu WM, Wei YH. Increased oxidative DNA damage, lipid peroxidation, and reactive oxygen species in cultured orbital fibroblasts from patients with Graves' ophthalmopathy: evidence that oxidative stress has a role in this disorder. Eye(Lond) 2010;24(9):1520–1525. doi: 10.1038/eye.2010.31. [DOI] [PubMed] [Google Scholar]
- 15.Duntas LH. The evolving role of selenium in the treatment of graves' disease and ophthalmopathy. J Thyroid Res. 2012;2012:736161. doi: 10.1155/2012/736161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Semenza GL. HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol (1985) 2000;88(4):1474–1480. doi: 10.1152/jappl.2000.88.4.1474. [DOI] [PubMed] [Google Scholar]
- 17.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92(12):5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang GL, Semenza GL. Purification and characterization of hypoxia-inducible factor 1. J Biol Chem. 1995;270(3):1230–1237. doi: 10.1074/jbc.270.3.1230. [DOI] [PubMed] [Google Scholar]
- 19.Erickson DZ, Harteneck DA, Erickson BJ, Dutton CM, Bahn RS. Induction of leptin expression in orbital preadipocyte fibroblasts. Thyroid. 2001;11(3):221–226. doi: 10.1089/105072501750159570. [DOI] [PubMed] [Google Scholar]
- 20.Mitchell SE, Rees WD, Hardie LJ, Hoggard N, Tadayyon M, Arch JR, Trayhurn P. ob gene expression and secretion of leptin following differentiation of rat preadipocytes to adipocytes in primary culture. Biochem Biophys Res Commun. 1997;230(2):360–364. doi: 10.1006/bbrc.1996.5964. [DOI] [PubMed] [Google Scholar]
- 21.Santos-Alvarez J, Goberna R, Sanchez-Margalet V. Human leptin stimulates proliferation and activation of human circulating monocytes. Cell Immunol. 1999;194(1):6–11. doi: 10.1006/cimm.1999.1490. [DOI] [PubMed] [Google Scholar]
- 22.Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97(9):2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiguchi N, Maeda T, Kobayashi Y, Fukazawa Y, Kishioka S. Leptin enhances CC-chemokine ligand expression in cultured murine macrophage. Biochem Biophys Res Commun. 2009;384(3):311–315. doi: 10.1016/j.bbrc.2009.04.121. [DOI] [PubMed] [Google Scholar]
- 24.Zarkesh-Esfahani H, Pockley AG, Wu Z, Hellewell PG, Weetman AP, Ross RJ. Leptin indirectly activates human neutrophils via induction of TNF-alpha. J Immunol. 2004;172(3):1809–1814. doi: 10.4049/jimmunol.172.3.1809. [DOI] [PubMed] [Google Scholar]
- 25.Sierra-Honigmann MR, Nath AK, Murakami C, Garcia-Cardena G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor. Science. 1998;281(5383):1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- 26.Chen MH, Chen MH, Liao SL, Chang TC, Chuang LM. Role of macrophage infiltration in the orbital fat of patients with Graves' ophthalmopathy. Clin Endocrinol (Oxf) 2008;69(2):332–337. doi: 10.1111/j.1365-2265.2008.03219.x. [DOI] [PubMed] [Google Scholar]
- 27.Gustafson B, Hammarstedt A, Andersson CX, Smith U. Inflamed adipose tissue: a culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27(11):2276–2283. doi: 10.1161/ATVBAHA.107.147835. [DOI] [PubMed] [Google Scholar]
- 28.Famulla S, Horrighs A, Cramer A, Sell H, Eckel J. Hypoxia reduces the response of human adipocytes towards TNFalpha resulting in reduced NF-kappaB signaling and MCP-1 secretion. Int J Obes (Lond) 2012;36(7):986–992. doi: 10.1038/ijo.2011.200. [DOI] [PubMed] [Google Scholar]
- 29.Prabhakar BS, Bahn RS, Smith TJ. Current perspective on the pathogenesis of Graves' disease and ophthalmopathy. Endocr Rev. 2003;24(6):802–835. doi: 10.1210/er.2002-0020. [DOI] [PubMed] [Google Scholar]