Abstract
AIM
To investigate the mechanism underlying the anti-inflammatory effects of epigallocatechin-3-gallate (EGCG) in lipopolysaccharide (LPS)-stimulated human retinal endothelial cells (HRECs).
METHODS
HRECs pre-treated with EGCG (0-100 µmol/L) were stimulated with LPS (250 ng/mL). Levels of tumor necrosis factor alpha (TNF-α), vascular endothelial growth factor (VEGF), monocyte chemotactic protein-1 (MCP-1) and nitric oxide (NO) in the supernatants were determined by enzyme-linked immunosorbent assay (ELISA) and Griess assay. The protein expression of phosphorylated extracellular signal-regulated kinase (ERK) 1/2 and p38 mitogen-activated protein kinases (p38) were determined by Western blot analysis.
RESULTS
EGCG pre-treatment significantly inhibited the secretion of TNF-α, VEGF, MCP-1 and NO in LPS-stimulated HRECs. Moreover, EGCG effectively attenuated LPS-induced activation and phosphorylation of ERK1/2 and p38 in HRECs in a dose-dependent manner.
CONCLUSION
EGCG exhibited inhibitory effects on LPS-induced pro-inflammatory cytokines production by modulating ERK1/2 and p38 pathways in HRECs, suggesting EGCG as a potential candidate for anti-inflammatory intervention.
Keywords: epigallocatechin-3-gallate, human retinal endothelial cells, inflammatory factors
INTRODUCTION
According to the International Diabetes Federation, diabetes currently affects nearly 285 million people worldwide and this number is expected to reach 438 million by 2030[1]. Diabetic retinopathy (DR) is one of the most common complications of diabetes and a significant cause of severe vision loss and blindness in working-age adults. DR is a microvascular and neurodegenerative disorder that induces structural and functional abnormalities in all retinal cells[2]. In addition, increasing evidence suggests that the pathogenesis of DR is mediated by inflammatory processes, and therefore is regarded as an inflammatory disease.
A number of metabolic and molecular abnormalities typical of inflammatory state have been described in the retinas of diabetic animals or patients. These include increased various important cytokines [tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-1β], chemokines [monocyte chemotactic protein (MCP)-1, macrophage-derived inflammatory mediator (MIP)-α], oxygen free radicals and reactive nitrogen species [nitric oxide (NO), inducible nitric oxide synthase (iNOS) and reactive oxygen species (ROS)], and other angiogenesis factors [vascular endothelial growth factor (VEGF) and matrix metalloproteinase 2 (MMP-2)][3],[4].
In light of the significance of DR involving angiogenesis and inflammation, research on the development of new pharmacological interventions or prevention would be valuable[5]. Recent investigations have sought to identify natural products with antiangiogenic effects[6]. Epigallocatechin gallate (EGCG) is the major catechins found in green tea, which is produced from the Carmellia sinensis plant. Accumulating evidence has shown that EGCG have strong anti-oxidant activity and affect several signal transduction pathways including the suppression of cell proliferation, induction of apoptosis, and inhibition of tumor metastasis and angiogenesis[7]. Lipopolysaccharide (LPS) is the important pathogenic substances which induce inflammatory responses and angiogenesis and a potent activator for the mitogen-activated protein kinases (MAPKs) which are expressed in many inflammatory cells by stimulating the release of intermediary growth factors/cytokines[8],[9]. The effect of EGCG on LPS-induced inflammatory response of Human retinal endothelial cells (HRECs) has not been fully elucidated. In this study, we investigated the effect of EGCG on the cytotoxicity and expression of various inflammatory factors in LPS-stimulated HRECs. A better understanding of the underlying mechanisms may provide new basis for the prevention or treatment of DR.
MATERIALS AND METHODS
Materials
HRECs were purchased from the United States ScienCell (San Diego, USA), and LPS was purchased from American Sigma Company. EGCG (98% purity) was obtained from the Tea Research Institute of Zhejiang University. All other chemicals were of reagent grade. The CellTiter 96 AQueous cell viability assay kit (MTS) was purchased from Promega (Madison, USA). Enzyme-linked immunosorbent assay (ELISA) kits for Cytokine total VEGF (DVE00), TNF-α (DTA00C), MCP-1(DCP00) and NO (KGE001) assay kits were from R&D Systems (Minneapolis, USA). The cell lysis buffer and anti-total/anti-phosphorylated extracellular signal-regulated kinase (ERK) p38, ERK 1/2 antibodies were obtained from Cell Signaling (Beverly, USA). Rabbit anti-β-actin and all horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, USA). Dulbecco's modified eagle medium (DMEM) cell culture medium and fetal bovine serum were purchased from Invitrogen Company (Invitrogen, USA).
Methods
Cell culture
HRECs were cultured at 37°C in a 95% air/5% CO2 atmosphere in DMEM with 10% fetal bovine serum and antibiotics (penicillin and streptomycin, Gibco, Grand Island, USA). HRECs were pretreated with varying concentrations of EGCG (0, 6.25, 12.5, 25, 50, 100, 200 µmol/L) for 2h followed by further addition with or without LPS (250 ng/mL). After treatment for 24h, the cells and their supernatants were collected and processed as required for further studies.
Cell processing and viability assay
HRECs were seeded at 1×104 cells/well in 96-well plates and incubated for 2h to allow attachment, followed by treatment with different concentrations of EGCG and LPS for 24h. Cytotoxicity of EGCG to HRECs was determined by MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymeth oxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium salt] assay as described[10]. Briefly, 20 µL MTS/phenazine methosulfate mixtures were added to each well. After 2h incubation at 37°C, an absorbance at 490 nm was read on a microplate reader (model 680, Bio-Rad, USA). The background absorbance from the control well was subtracted from the actual absorbance value. Three duplicate studies were performed for each experimental condition.
Detection of vascular endothelial growth factor, tumor necrosis factor-α, monocyte chemotactic protein-1 and nitric oxide production
The supernatants of treated HRECs were collected into Eppendorf tubes. After centrifugation at 2000 g for 5min, the supernatants were transferred to new tubes, and the levels of VEGF, TNF-α and MCP-1 in those samples were determined by specific ELISA kits according to the manufacturer's instructions (R&D Systems, Minneapolis, USA). NO levels in culture supernatants were measured as its oxidized product nitrate. Reaction buffer and nitrate reductase were mixed with samples to convert nitrate to nitrite. After incubation for 30min at 37°C, Griess reagents were added into samples to promote oxidation and nucleophilic reaction. The absorbance of the product dye was determined at 540 nm using a flowthrough spectrophotometer (R&D Systems, Minneapolis, USA).
Western blot analysis
HRECs were treated as described in the section of ‘Cell Culture and Processing’ and the reaction was stopped on ice. Cells were washed twice with cold phosphate buffer solution and harvested with a cell scraper. For the preparation of whole cell lysates, cell pellets were extracted by lysis buffer. The extracts were centrifuged at 14 000 g for 5min and the supernatants were transferred to fresh tubes for further analysis. The extraction of nuclear protein was carried out using Nuclear and Cytoplasmic Extraction Reagents (Pierce, Rockford, USA) following the manufacturer's instructions. Protein concentrations were determined using the BCA method (Bio-Rad, Hercules, USA), and 40 µg of protein lysate per sample was mixed with 2×SDS loading buffer containing dithiothreitol, and heated at 100°C for 10min before resolving by SDS-PAGE. Proteins were transferred to polyvinylidene fluoride membrane and blocked with 7.5% non-fat dry milk in phosphate-buffered saline (PBS) with 0.1% (v/v) Tween20 (PBST). The membrane was incubated with the primary antibody in PBST for 24h at 4°C, washed 4 times and incubated for 1h at room temperature with an HRP-conjugated secondary antibody. After further washing, the immune complexes were visualized using the enhanced chemiluminescence method. Each protein band was analyzed with the VersaDoc 5000MP Image Analysis System (Bio-Rad, Hercules, USA).
Statistical Analysis
All experiments were performed in triplicate. The data were expressed as the mean±standard error of mean (SEM). One-way analysis of variance (ANOVA) and Dunnett's t-test were used to determine the statistical significance of differences between the experimental and control groups. A two-sided P<0.05 was considered to be statistically significant.
RESULTS
Effect of Epigallocatechin-3-gallate on Human Retinal Endothelial Cells Viability
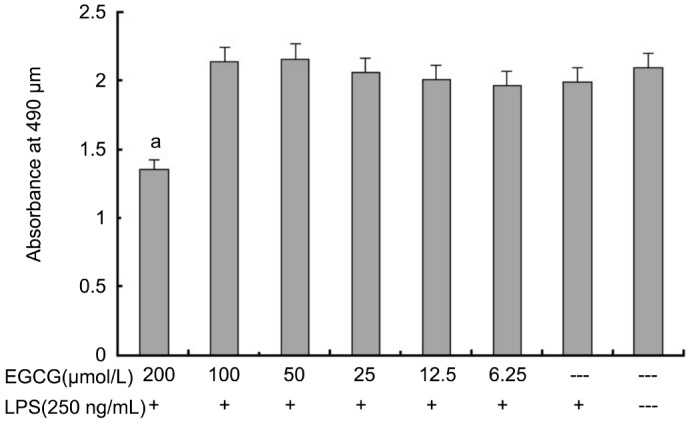
The effect of EGCG on the viability of HRECs was determined by MTS assay after 24h treatment. As shown in Figure 1, we found that an EGCG concentration of 200 µmol/L, absorbance is probably about 70% of that in normal control group, and the absorbance had no significant difference at concentrations of 0-100 µmol/L vs normal control group. EGCG did not show cytotoxicity on HRECs at concentrations up to 100 µmol/L, but significantly decreased viability in HRECs was observed when treated with EGCG at 200 µmol/L. Therefore, EGCG at concentrations of 100 µmol/L or below were used to evaluate its pharmacological activities in subsequent experiments.
Figure 1. Effects of EGCG on cell viability of HRECs.
Cells were incubated with indicated concentrations of EGCG for 24h. Cell viability was determined by MTS assay. a P<0.05 vs EGCG-/LPS+group
Inhibition of Lipopolysaccharide-induced Cytokine Secretion in Human Retinal Endothelial Cells by Epigallocatechin-3-gallate
To examine the effects of EGCG on LPS-induced pro-inflammatory cytokines release from HRECs, ELISA and Griess assay were performed. As shown in Table 1, the expression of VEGF, TNF-α, MCP-1 and NO in HRECs increased markedly upon 250 ng/mL LPS stimulation for 24h, and EGCG inhibited their production in a dose-dependent manner. These results indicated that EGCG suppressed LPS-mediated inflammatory responses in HRECs.
Table 1. Inhibitory effect of EGCG on LPS-induced TNF-α, VEGF, MCP-1 and NO production in HRECs.
| Groups | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| LPS (250 ng/mL) | + | + | + | + | + | + | -- |
| EGCG (µmol/L) | 100 | 50 | 25 | 12.5 | 6.25 | 0 | 0 |
| VEGF (pg/mL) | 75.7±14.6b | 99.3±15.7b | 145. 7±15.6b | 206.0±16.1 | 252.3±17.4 | 309.7±57.7d | 45.7±9.3 |
| NO (µmol/L) | 8.9±1.1b | 16.1±1.8b | 24.6±2.9b | 34.9±2.3 | 44.1±3.6 | 43.7±7.7d | 3.9±0.6 |
| MCP-1 (pg/mL) | 261.0±30.3b | 375.3±30.2b | 613.0±60.6b | 774.0±36.7 | 926.0±99.5 | 862.7±47.5d | 143.7±16.6 |
| TNF-α (pg/mL) | 49.7±12.0b | 52.7±17.6b | 81.0±12.7b | 110.7±13.5 | 125.7±11.7 | 133.0±12.5d | 29.7±6.0 |
HRECs were pre-treated with EGCG (0-100 µmol/L) for 2h followed by incubation with or without of LPS (250 ng/mL) for 24h. Levels of TNF-α, VEGF, MCP-1 and NO were determined by ELISA and Griess assay. Data represent Mean±SEM (n=3). bP<0.01 vs LPS alone (group 6); dP<0.01 vs control group (group 7).
Inhibition of p38 and Extracellular Signal-regulated Kinase Phosphorylation in Lipopolysaccharide -stimulated Human Retinal Endothelial Cells by Epigallocatechin-3-gallate
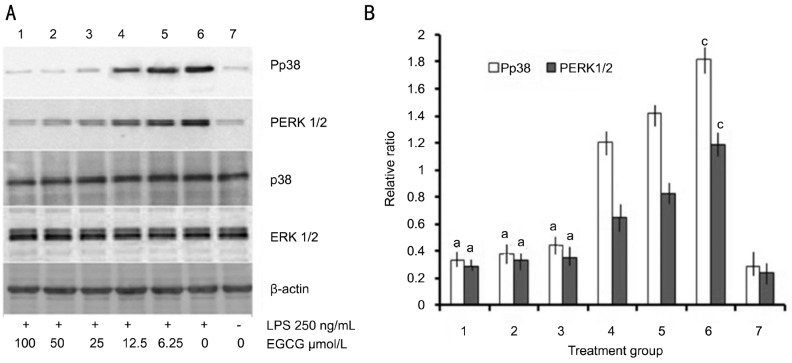
To determine the intracellular mechanisms involved in the anti-inflammatory effect of EGCG in LPS-stimulated HRECs, the levels of phospho-p38 and ERK1/2 were determined by Western blot. As shown in Figure 2, a remarkable increase in p38 and ERK1/2 phosphorylation was observed in HRECs after 250 ng/mL LPS treatment (Figure 2A). However, pretreatment with EGCG attenuated LPS-induced p38 and ERK1/2 phosphorylation in a dose-dependent manner (Figure 2B).
Figure 2. Effect of EGCG on LPS-induced P38 and ERK1/2 activation in HRECs.
A: Cells were pre-treated with indicated concentrations of EGCG in the presence or absence of LPS (250 ng/mL). Whole cell lysates were prepared and probed for total and phospho-p38 and ERK1/2 by western blotting. β-actin was used as a loading control; B: The levels of P38 and ERK1/2 phosphorylation were quantified by densitometric analysis, and Pp38 or PERK1/2 data were expressed as the relative ratio of the corresponding value phospho-p38/p38 or phosphor-ERK1/2/ERK1/2 of each group with the correction of β-actin (mean±SEM, n=3/group). aP<0.05 vs group 6, cP<0.05 vs group 7.
DISCUSSION
DR is a leading cause of blindness among working-age adults. Despite tight glycemic control, blood pressure control and lipid-lowering therapy, the number of DR patients keeps growing and therapeutic approaches are limited. There are significant limitations and side effects associated with the current therapies. Thus, there is a great need for development of new strategies for prevention and treatment of DR. Many of the molecular and physiologic abnormalities in the retina of diabetic patients are related to inflammation. Moreover, a number of anti-inflammatory therapies have been found to significantly inhibit development of DR in animal models[11]. Therefore, it is important to study the inflammatory mediators and their relationship with development of early and late DR, this may help develop the anti-inflammatory approaches to inhibit development and progression of DR. The purpose of this study was to evaluate the anti-inflammatory activity of EGCG as representative of a new class of effective natural agent that has never been studied in this context of anti-inflammation to HRECs.
EGCG, a natural anti-oxidant flavonoid that is abundant in green tea, has been shown to suppress the migration and adhesion of many cell types. The compound has been studied widely due to its excellent chemo-preventive properties in the context of most cell types[12],[13]. EGCG has been demonstrated to have both anti-inflammatory and antioxidant properties in multiple cell types. EGCG is associated with a decrease in the activation of various types of tyrosine kinase receptors, such as the VEGF/VEGFR system, and directly inhibits phosphorylation of the MAPKs p38 and c-Jun N-terminal kinase (JNK), and NF-κB, resulting in significant inhibition of proinflammatory and proangiogenic mediators such as IL-1b, VEGF, and MMP-2 and MCP-1[14],[15]. Because of these properties, it is thought that EGCG may have therapeutic benefit in diabetes, and also has been proposed that the administration of EGCG to the ocular surface represents a new chemopreventive alternative to suppress the corneal neovascularization induced by inflammation[16],[17]. In the present study, we sought to evaluate the efficacy of EGCG in HRECs cell culture models. LPS is the important pathogenic substances, and can induce TNF-a, IL-6, NO, VEGF and prostaglandin. The purpose of our study was to extend the observations to include EGCG as representative of a new class of effective natural agent, which has never been studied in this context of anti-inflammation to HRECs. As shown in Table 1, the expression levels of proinflammatory cytokines in HRECs were remarkably increased when the cells were cultured in the presence of LPS (250 ng/mL). However, the pretreatment with EGCG markedly blocked the expression of all cytokines in a dose-dependent manner, respectively. These results indicated that EGCG affected LPS-mediated inflammatory responses in HRECs.
These proinflammatory cytokines and mediators produced and released depend on the inducible gene expression, and were usually affected by different transcription factor regulation. A variety of extracellular signals were acted on through a series of signal transduction, such as the ERK and p38 phosphorylation. Phosphorylation of ERK and p38 have a wide range of kinase activity, can cause inflammatory molecular activation and related gene expression[18]. Therefore, ERK and p38 may be potential anti-inflammatory drug development targets. As shown in Figure 2, the phosphorylated levels of the two signal transducers in HRECs were remarkably increased by LPS (250 ng/mL), but down-regulated by pre-treatment of EGCG in a dose-dependent manner.
It was reported that serum chemokines and cell adhesion in patients with severe nonproliferative DR were significantly elevated when compared to those without severe DR[19]. These findings provide evidence to support the role of inflammation in the pathogenesis of DR. EGCG which reduced circulating levels of chemokines, and inhibited secretions of chemokines and adhesion molecules, has a direct protective effect against vascular inflammation in diabetes[20]. Significant decline of adenosine monophosphate activated protein kinase (AMPK) activity in the diabetic retina has been reported when compared with the normal retina[21]. Our study results showed that EGCG inhibited the LPS-induced phosphorylation of ERK1/2 and p38 activity as evidenced by the reduction of NO, VEGF, MCP-1 and TNF-α in HRECs, thus providing a possible mechanistic explanation for the anti-inflammatory effects of EGCG. This is consistent with recent data on diabetic nephropathy and glucose-stimulated skeletal muscle, both of which were associated with AMPK deactivation[22]. These findings are explained by and compatible with recent in vitro data showing that AMPK activation led to NF-κB suppression in vascular endothelial cells and inflammatory leukocytes, both of which are the key cellular components responsible for the pathogenesis of DR. DR is usually affected by different transcription factor regulation[23]. Our results demonstrated the EGCG-mediated reduction in proinflammatory cytokines levels, suggesting that EGCG may has potential use as an anti-inflammatory agent for the treatment DR.
In summary, our results showed that EGCG blocked LPS-induced production of a variety of inflammatory factors in HRECs in a dose-dependent manner, probably via p38 and ERK1/2 pathways. Exploring the direct targets of EGCG will greatly contribute to a better understanding of its pharmacological actions. Future studies will further explore the underlying molecular mechanisms, determine the optimal EGCG concentration used and understand the limited bioavailability of EGCG in vivo. Our study findings demonstrate EGCG may a promising agent that has potential value for prevention and treatment of DR.
Acknowledgments
Foundations: Supported by Public Technology Application Research Grant of Zhejiang Province (No.2011C33029); Natural Science Foundation of Zhejiang Province, China (No.LY13B020002)
Conflicts of Interest: Zhang HY, None; Wang JY, None; Yao HP, None.
REFERENCES
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Barber AJ. A new view of diabetic retinopathy: a neurodegenerative disease of the eye. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(2):283–290. doi: 10.1016/S0278-5846(03)00023-X. [DOI] [PubMed] [Google Scholar]
- 3.Izuora KE, Chase HP, Jackson WE, Coll JR, Osberg IM, Gottlieb PA, Rewers MJ, Garg SK. Inflammatory markers and diabetic retinopathy in type 1 diabetes. Diabetes Care. 2005;28(3):714–718. doi: 10.2337/diacare.28.3.714. [DOI] [PubMed] [Google Scholar]
- 4.Koleva-Georgieva DN, Sivkova NP, Terzieva D. Serum inflammatory cytokines IL-1beta, IL-6, TNF-alpha and VEGF have influence on the development of diabetic retinopathy. Folia Med (Plovdiv) 2011;53(2):44–50. doi: 10.2478/v10153-010-0036-8. [DOI] [PubMed] [Google Scholar]
- 5.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95–103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta SK, Kumar B, Nag TC, Agrawal SS, Agrawal R, Agrawal P, Saxena R, Srivastava S. Curcumin prevents experimental diabetic retinopathy in rats through its hypoglycemic, antioxidant, and anti-inflammatory mechanisms. J Ocul Pharmacol Ther. 2011;27(2):123–130. doi: 10.1089/jop.2010.0123. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Deng Y, Lu BM, Liu YX, Li J, Bao JK. Green tea catechins: a fresh flavor to anticancer therapy. Apoptosis. 2014;19(1):1–18. doi: 10.1007/s10495-013-0908-5. [DOI] [PubMed] [Google Scholar]
- 8.Kim HJ, Park GM, Kim JK. Anti-inflammatory effect of pristimerin on lipopolysaccharide-induced inflammatory responses in murine macrophages. Arch Pharm Res. 2013;36(4):495–500. doi: 10.1007/s12272-013-0054-1. [DOI] [PubMed] [Google Scholar]
- 9.Lin CM, Chang H, Chen YH, Li SY, Wu IH, Chiu JH. Protective role of wogonin against lipopolysaccharide-induced angiogenesis via VEGFR-2, not VEGFR-1. Int Immunopharmacol. 2006;6(11):1690–1698. doi: 10.1016/j.intimp.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Tang X, Yao K, Zhang L, Yang Y, Yao H. Honokiol inhibits H(2)O(2)-induced apoptosis in human lens epithelial cells via inhibition of the mitogen-activated protein kinase and Akt pathways. Eur J Pharmacol. 2011;650(1):72–78. doi: 10.1016/j.ejphar.2010.09.076. [DOI] [PubMed] [Google Scholar]
- 11.Tang J, Kern TS. Inflammation in diabetic retinopathy. Prog Retin Eye Res. 2011;30(5):343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, Ho W. (-)-Epigallocatechin gallate inhibits endotoxin-induced expression of inflammatory cytokines in human cerebral microvascular endothelial cells. J Neuroinflam. 2012;9:161–166. doi: 10.1186/1742-2094-9-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peairs A, Dai R, Gan L, Shimp S, Rylander MN, Li L, Reilly CM. Epigallocatechin-3-gallate (EGCG) attenuates inflammation in MRL/lpr mouse mesangial cells. Cell Mol Immunol. 2010;7(2):123–132. doi: 10.1038/cmi.2010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang J, Han Y, Chen C, Sun H, He D, Guo J, Jiang B, Zhou L, Zeng C. EGCG attenuates high glucose-induced endothelial cell inflammation by suppression of PKC and NF-kappaB signaling in human umbilical vein endothelial cells. Life Sci. 2013;92(10):589–597. doi: 10.1016/j.lfs.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 15.Trompezinski S, Denis A, Schmitt D, Viac J. Comparative effects of polyphenols from green tea (EGCG) and soybean (genistein) on VEGF and IL-8 release from normal human keratinocytes stimulated with the proinflammatory cytokine TNFalpha. Arch Dermatol Res. 2003;295(3):112–116. doi: 10.1007/s00403-003-0402-y. [DOI] [PubMed] [Google Scholar]
- 16.Huang SM, Chang YH, Chao YC, Lin JA, Wu CH, Lai CY, Chan KC, Tseng ST, Yen GC. EGCG-rich green tea extract stimulates sRAGE secretion to inhibit S100A12-RAGE axis through ADAM10-mediated ectodomain shedding of extracellular RAGE in type 2 diabetes. Mol Nutr Food Res. 2013;57(12):2264–2268. doi: 10.1002/mnfr.201300275. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Huerta V, Gutierrez-Sanchez L, Flores-Estrada J. (-)-Epigallocatechin 3-gallate (EGCG) at the ocular surface inhibits corneal neovascularization. Med Hypoth. 2011;76(3):311–313. doi: 10.1016/j.mehy.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 18.Li XY, He JL, Liu HT, Li WM, Yu C. Tetramethylpyrazine suppresses interleukin-8 expression in LPS-stimulated human umbilical vein endothelial cell by blocking ERK, p38 and nulear factor-kappaB signaling pathways. J Ethnopharmacol. 2009;125(1):83–88. doi: 10.1016/j.jep.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 19.Meleth AD, Agron E, Chan CC, Reed GF, Arora K, Byrnes G, Csaky KG, Ferris FL, 3rd, Chew EY. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46(11):4295–4301. doi: 10.1167/iovs.04-1057. [DOI] [PubMed] [Google Scholar]
- 20.Silva KC, Rosales MA, Hamassaki DE, Saito KC, Faria AM, Ribeiro PA, Lopes de Faria JB, Lopes de Faria JM. Green tea is neuroprotective in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2013;54(2):1325–1336. doi: 10.1167/iovs.12-10647. [DOI] [PubMed] [Google Scholar]
- 21.Kubota S, Ozawa Y, Kurihara T, Sasaki M, Yuki K, Miyake S, Noda K, Ishida S, Tsubota K. Roles of AMP-activated protein kinase in diabetes-induced retinal inflammation. Invest Ophthalmol Vis Sci. 2011;52(12):9142–9148. doi: 10.1167/iovs.11-8041. [DOI] [PubMed] [Google Scholar]
- 22.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol. 2011;2(69):1–15. doi: 10.3389/fphar.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muramatsu D, Wakabayashi Y, Usui Y, Okunuki Y, Kezuka T, Goto H. Correlation of complement fragment C5a with inflammatory cytokines in the vitreous of patients with proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251(1):15–17. doi: 10.1007/s00417-012-2024-6. [DOI] [PubMed] [Google Scholar]