Abstract
AIM
To detect whether Toxoplasma gondii (T. gondii) infection of mice can induce retinal DNA damage.
METHODS
A total of 20 laboratory-bred male Swiss albino mice were used and divided into four groups: control group (non-infected animals); T. gondii infected group; immunosuppressed infected group; and infected group treated with sulfadiazine and pyrimethamine. Mice eyes were collected 6wk post infection and retinas were obtained. Each retina was immediately processed for comet assay and the frequency of tailed nuclei (DNA damage) was calculated. In addition, retinal DNA damage was revealed by various comet assay parameters that were provided by the image analysis software including tail length, percentage of DNA in the tail, percentage of tailed cells and tail moment.
RESULTS
The obtained results showed that T. gondii infection induced a statistically significant increase in the frequency of tailed nuclei, tail length, percentage of DNA in the tail, and tail moment in mice retinal cells compared to the control group (which showed some degree of DNA damage). In immunosuppressed infected group, retinal DNA damage was severing and there was significant increase in various comet assay parameters compared to both control and infected groups. After treatment with sulfadiazine and pyrimethamine, retinal DNA damage decreased and all comet assay parameters showed a statistical significant decrease compared to infected groups.
CONCLUSION
T. gondii infection can induce DNA damage in mice retinal cells.
Keywords: Toxoplasma gondii, retinochoroiditis, DNA damage, comet assay
INTRODUCTION
Toxoplasma gondii (T. gondii), an intracellular protozoan parasite, affects the retina and the underlying choroid, causing retinochoroiditis which is the most common cause of infectious posterior uveitis in immunocompetent individuals[1]. In immunocompromised patients, Toxoplasma retinochoroiditis may have an atypical presentation and be difficult to diagnose[2]. Toxoplasmic retinochoroiditis may be either congenital or acquired and may recur months or years after the primary infection[3]. Active inflammation and infection in the eye typically lasts about 6wk, at which time the lesion will begin to regress, leaving behind a characteristic pigmented scar on the retina[4]. Upon funduscopic examination of the eye, a gray-white area of retinal necrosis with or without exudates with adjacent swelling of the optic disc, vitreitis, vasculitis, and retinal hemorrhage or detachment can be found. All of these conditions can lead to vision loss[5].
Ocular toxoplasmosis typically manifests itself through exacerbations of retinochoroiditis resulting from the rupture of quiescent Toxoplasma cysts in the retina releasing the bradyzoites, a form of the parasite with low levels of metabolic activity, which transform into tachyzoites, the form of the parasite with high levels of metabolic activity, and reactivate infection locally[6]. The intensity of damage to retina and choroid depends on the severity of the infection and the associated inflammatory reaction. Within the retina, lysosomal and other autolytic enzymes released by inflammatory cells predominantly macrophages and lymphocytes are thought to contribute to the pathogenic mechanisms of retinal tissue damage[7].
Single-cell gel electrophoresis assay (comet assay), a rapid, simple, visual and reliable biochemical technique was used to detect various genotoxic damages in mammalian cells[8]. This technique is able to detect single and double strand breaks and incomplete repair sites in eukaryotic cells. Quantitative analysis for DNA damage has yielded several parameters, including tailed nuclei, tail length, percentage of DNA in the tail and tail moment in the comet assay[9],[10].
In a previous study that assessed DNA damage in peripheral blood, liver and brain cells of mice by T. gondii infection using the comet assay, it was found that DNA damage occurred exclusively in leukocytic cells and not in liver and brain cells[11]. As retinal pigment epithelium, an integral part of the neuroretina in the posterior pole of the eye, acts as a barrier between the highly vascularized choroid and the retina with a complex architecture of neuronal cells. It is constantly subjected to contact with various infectious agents including T. gondii. So, this study aimed to detect whether T. gondii infection of mice can induce retinal DNA damage.
MATERIALS AND METHODS
Materials
Brain cysts of the Me49 non-virulent strain of T. gondii (kindly obtained from National Research Center, Giza, Egypt), regularly maintained by passage through Swiss albino mice every 8wk. The mice brains were ground with sterile pestle and mortars and diluted to a concentration of 1×102 cysts/mL obtaining brain cysts suspension.
Experimental Animals
A total of 20 laboratory-bred male Swiss albino mice, 10-week old, weighing approximately 40 g, were selected from the animal house of the Research Institute of Ophthalmology, Giza, Egypt. All mice had normal retinas on indirect ophthalmoscopic examination prior to the start of this study. They were housed in plastic cages (5 mice/cage) with white wood chips for bedding, fed by commercial complete food mixture and tap water for drinking, and maintained under controlled conditions of lighting (12h light/12h dark cycle) and temperature 25±2°C. The animal experiment was carried out according to the internationally valid guidelines and the research protocol was approved by the local ethical committee that applies the ARVO (the association for research in vision and ophthalmology) statements for using animals in ophthalmic and vision research.
Animals were divided into four groups of 5 mice each: Group I (GI): control non-infected mice; Group II (GII): T. gondii infected mice. Mice were inoculated intraperitoneally by 0.1 mL of the brain cysts suspension[12]. The efficiency of the experimental Toxoplasma infection in mice was confirmed by the complement fixation test for detection of specific anti-Toxoplasma antibodies[13]; Group III (GIII): Immunosuppressed mice and submitted to infection by T. gondii as GII. Each mouse was immunosuppressed by subcutaneous injection of methylprednisolone acetate (Depo-medrol®, Pfizer Inc.) 40 mg/d/mouse for five successive days before infection[14]; Group IV (GIV): T. gondii infected mice and treated with combination of sulfadiazine (Dohms Laboratories) at dose of 200 mg/kg/d and pyrimethamine (Sigma Chemical Co., St. Louis, Mo., USA), at dose of 12.5 mg/kg/d[15]. The drugs were provided in powder form and prepared daily as liquid suspensions; after brief sonication, the homogenized suspensions were administered orally to mice via tube feeding. Four weeks post-infection; treatments were administered daily at a fixed hour for 10d.
Six weeks post-infection, all mice were anesthetized and perfused through the heart with phosphate buffer serum (Mediatech, Manassas, VA, USA) to remove blood from the eyes before their collection. The eyes were collected and opened by corneal section through the ora serrata where the anterior segment constituents were removed therefore the retina was exposed and obtained. Each retina was immediately processed for comet assay.
Methods
Comet assay
(All chemicals were purchased from Sigma Chemical Co, USA). Crushed retinal samples of each group were transferred to 1 mL ice-cold phosphate buffer saline (PBS, pH7.9), stirred for 5min and filtered and 100 µL of cell suspension from each group were mixed with 600 µL of lowmelting agarose (0.8% in PBS), then 100 µL of the obtained mixture were pipetted onto the slides. The coated slides were immersed in lysis buffer [0.045 mol/L tris borate ethylenediaminetetraacetic acid (TBE) pH8.4, containing 2.5% sodium dodecyl sulfate (SDS)] for 15min. Then, the slides were placed in electrophoresis chamber containing the same TBE buffer, but devoid of SDS. The electrophoresis was conducted at 2 V/cm for 2min and 100 mA. Finally, the slides were stained with ethidium bromide 20 µg/mL at 4°C and the presence of comets was examined at ×40 magnification using fluorescence microscope [with excitation filter 420-490 nm (issue 510 nm)]. Using a Komet 5 image analysis software developed by Kinetic Imaging, Ltd. (Liverpool, UK) linked to a charge-coupled device (CCD) camera was assessed the quantitative and qualitative extent of DNA damage in the retinal cells by measuring the length of DNA migration, the percentage of migrated DNA and tail moment by observing 50 to 100 randomly selected cells per sample[16],[17]. The tail length was measured from the middle of the nucleus to the end of the tail. The percentage of DNA in the tail was calculated from the fraction of DNA in the tail divided by the amount of DNA in the nucleus multiplied by 100. The tail moment is defined as the product of the tail length and the fraction of total DNA in the tail (tail moment=tail length × % of DNA in the tail)[8].
Statistical Analysis
Collected data were coded and introduced to a PC using the Statistical Package for Social Science (SPSS) for windows version 11.0. Data were represented as the mean±standard deviation (SD) (n=5). The analysis of variance (ANOVA) procedure was used to clarify statistically significant differences between the studied groups. Values were considered statistically significant when P<0.05.
RESULTS
The results showed that T. gondii infection induced a statistically significant increase in the frequency of tailed nuclei (DNA damage) in mice retinal cells compared to the control group (which showed some degree of DNA damage). Tailed nuclei indicated by the ratio of the number of comet tails and the number of non-head shapes to the number of total nuclei in the comet assay. In immunosuppressed infected group (GIII); the frequency of tailed nuclei in retinal cells increased comparing with both control and infected groups. After treatment with sulfadiazine and pyrimethamine in GIV, DNA damage in retinal cells decreased comparing with infected group (Figure 1).
Figure 1. Representative photographs of comet showing DNA migration pattern in retinal cells of mice stained with ethidium bromide.
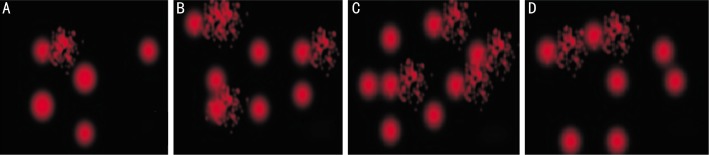
A: The control non-infected group (GI) in which most of the cells appeared with no comet. DNA was tightly compressed and maintained the circular disposition of the normal nucleus; B: T. gondii infected group (GII); the profile of the nuclear DNA in this group was altered with the appearance of a fluorescent streak extending from the nucleus; C: Immunosuppressed infected group (GIII) showed increase the number of damaged DNA in retinal cells; D: Infected treated group with sulfadiazine and pyrimethamine (GIV) showed less damage of retinal cells.
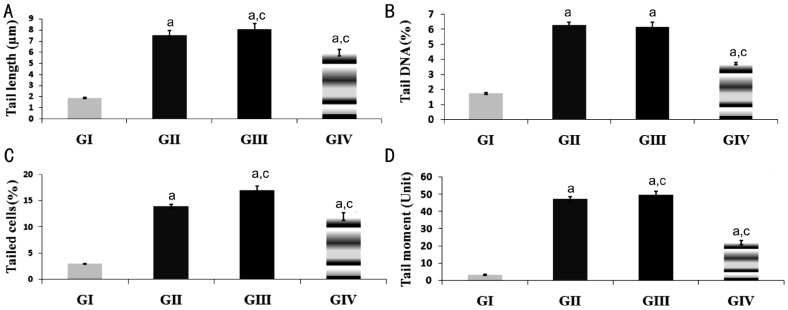
In this study, retinal DNA damage was revealed by various comet assay parameters that were provided by the image analysis software including tail length, percentage of DNA in the tail, percentage tailed cells and tail moment (Figure 2). Figure 2A illustrated that tail length statistically significant increased (P<0.05) after T. gondii infection (GII) by about 300% then reached the maximum percentage of increase in immunosuppressed infected group (GIII) by about 328% compared to control (GI) tail length value. After treatment with sulfadiazine and pyrimethamine (GIV) the percentage of tail length was about 216% that means some repair but did not reach the normal level. In addition, there was a significant difference increase (P<0.05) observed between GIII and GII. On the other hand, there was a significant difference decrease (P<0.05) between GIV and GII. Figure 2B illustrated the mean percentage of tail DNA reflecting the proportion of DNA that migrated from the head and was calculated as an average for the 50-100 cells selected for measurement. The mean percentage of tail DNA in control (GI) was 1.73%±0.06% then significantly increased (P<0.05) for infected (GII), immunosuppressed infected (GIII) and infected treated (GIV) groups to be 6.27±0.2 and 6.16±0.3 respectively. Comparing with infected group (GII), the percentage tail DNA for GIV, the group treated with sulfadiazine and pyrimethamine was significantly decreased (P<0.05) to be 3.72±0.09 while, there was no significant difference observed between GII and GIII. Figure 2C, 2D illustrated the percentage tailed cells and mean tail moment (whose magnitude reflects the frequency of DNA strand breaks per nucleus) for all groups compared to control. The obtained results of these parameters indicated the same phenomena for percentage of tail DNA that sulfadiazine and pyrimethamine reduced the degree of damage induced by T. gondii infection but did not reach the control limit.
Figure 2. Histograms showing the DNA damage in retinas of mice induced by T. gondii infection among the studied groups as revealed by various comet assay parameters.
A: Tail length; B: Percentage of tail DNA; C: Percentage of tailed cells; D: Tail moment. All values were expressed as mean±SD (n=5), aP<0.05 vs control (GI), cP<0.05 vs T. gondii infected group (GII).
DISCUSSION
The maintenance of DNA integrity is vital for genomic stability and cell viability. The genome is under constant attack from endogenous metabolic processes and exogenous environmental factors that can alter its chemical structure. DNA lesions consist of single strand breaks, double strand breaks, inter- and intra-strand crosslinks and base modifications, as well as oxidation and alkylation of bases, formation of bulky chemical adducts and crosslinking of adjacent nucleotides. DNA damage can lead to multiple lesions including mutations, deletions, insertions, translocations, and loss of chromosomes and essential genetic information. This genome instability can consequently induce apoptosis and fatal diseases[18]. Some studies have demonstrated that parasites including T. gondii can induce DNA damage of the cell critical components[19]–[21]. DNA damage could be endogenous due to attack by reactive oxygen species produced from normal metabolic products, especially the process of oxidative deamination[22]. Oxidative damage is thought to affect cell cycle control, gene expression, cytoplasm/mitochondria communication[23],[24].
By mean of the comet assay, the present study has elucidated some of the DNA changes in retinal cells of mice subsequent to T. gondii infection. In undamaged retinal cells, the DNA was tightly compressed and maintained the circular disposition of the normal nucleus. After T. gondii infection the profile of the nuclear DNA was altered with the appearance of a comet that had a bright head and tail, thus allowing the cells' differentiation from those that had normal DNA. Comet assay was used to detect DNA damage such as strand breaks and DNA-protein crosslinks. Cross-links may stabilize chromosomal DNA and inhibit DNA migration[25],[26]. In the current study, the observed increased DNA migration in T. gondii infected group may be attributed to the induction of DNA strand breaks. Reduced DNA migration in the control group may be attributed to the induction of crosslinks, which are relevant lesions with regard to mutagenesis.
In this study, mice retinal DNA damage induced by T. gondii infection was revealed by various comet assay parameters that were provided by the image analysis software including tail moment, percentage of DNA in the tail, tail length and percentage of tailed cells. These parameters showed a significant increase in T. gondii infected group compared to the control group (which showed some degree of DNA damage). DNA damage of retinal cells of T. gondii infected mice may be attributed to the host immune response against T. gondii infection including antibody production (humoral immune response) and activation of cellular mechanisms[27],[28]. In cellular immune response, macrophages are able to liberate nitric oxide, interferon-gamma (IFN-γ), tumor necrosis factor (TNF-α) and reactive oxygen species (ROS)[28]. While such mechanisms help to eradicate T. gondii, they inevitably expose target organs to certain endogenous genotoxic agents that react with DNA directly[29]. Degree of DNA damage observed in control group might be explained by the fact that about 10 000 oxidation hits to DNA per cell have been estimated to occur per day within the human body, and more than 35 different forms of oxidized bases are found in DNA in vitro[30],[31]. Most damage is repaired by effective DNA-repair enzymes, but some damage escapes repair, causing permanent damage[32].
Regarding immunosuppressed infected mice, DNA damage was more severe in mice retinal cells compared to T. gondii infected mice as change in the immune system increases the risk of Toxoplasma infection[33]. An interesting point to be considered is that the receiving immunosuppressive triggered the release of bradyzoites from tissue cysts which convert into tachyzoites, and proliferate in host tissue without restriction, leading to dissemination of Toxoplasma organisms to other cells. It was found that the use of prednisolone to immunosuppress mice prior to infection with T. gondii can help propagate a greater number of the parasite from both virulent (RH, type I) and avirulent (Me49, type II) strains in mice[34]. Clinically, the impact of DNA damage in retinal cells may be attributed to serious complications observed in immunocompromised patients especially in patients with AIDS and transplant patients[2].
In the present study, sulfadiazine and pyrimethamine were given in combination because the considerable individual inhibitory effect of each drug. These drugs have a synergistic action by inhibiting T. gondii folic acid synthesis which is essential for parasite survival and replication[35]. Pyrimethamine interferes with replication of the parasite as it inhibits the enzyme dihydrofolate reductase in the folate production pathway while, sulfadiazine acts as a competitive antagonist for para-aminobenzoic acid (PABA), one of the precursors of folate production[36]. Their synergistic activity in ocular toxoplasmosis has been documented in animal models, rendering this combination most effective and recommended for the treatment of vision-threatening toxoplasmosis[37]. Additionally, mice infected and treated with sulfadiazine and pyrimethamine in this study displayed a significant decrease in DNA damage compared to T. gondii infected group. The observed decrease in DNA damage might be due to the repair of the lesions induced by treatment that reduced the number of damage cells and inhibit the accumulation of DNA damage[38]. In fact, prompt treatment may achieve rapid resolution, minimize induced T. gondii genotoxic effects in retinal cells, minimize inflammatory damage, prevent widespread tissue destruction and decrease the chances of dissemination. This in agreement with Soheilian et al[39] who observed that classic therapy (pyrimethamine, sulfadiazine) for Toxoplasma retinochoroiditis has the ability to reduce lesion size and vitreal inflammation as well as improve visual acuity.
In conclusion, the findings of the present study suggested that T. gondii infection can induce DNA damage in retinal cells.
Acknowledgments
Conflicts of Interest: El-Sayed NM, None; Aly EM, None.
REFERENCES
- 1.Delair E, Creuzet C, Dupouy-Camet J, Roisin M-P. In vitro effect of TNF-α and IFN-γ in retinal cell infection with Toxoplasma gondii. Invest Ophthalmol Vis Sci. 2009;50(4):1754–1760. doi: 10.1167/iovs.07-1376. [DOI] [PubMed] [Google Scholar]
- 2.Hazan A, Patel RM, Levinson D, Mian U, Gritz DC. A typical bilateral Toxoplasma retinochoroiditis in a bone marrow transplant patient with negative serum titers. J Ophthalmic Inflamm Infect. 2013;3(1):23. doi: 10.1186/1869-5760-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silveira C, Vallochi AL, da Silva RU, Muccioli C, Holland GN, Nussenblatt RB, Belfort R, Rizzo LV. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95(3):396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- 4.Smith JR, Cunningham ET. Atypical presentations of ocular toxoplasmosis. Curr Opin Ophthalmol. 2002;13(6):387–92. doi: 10.1097/00055735-200212000-00008. [DOI] [PubMed] [Google Scholar]
- 5.London NJ, Hovakimyan A, Cubillan LD, Siverio CD, Jr, Cunningham ET., Jr Prevalence, clinical characteristics, and causes of vision loss in patients with ocular toxoplasmosis. Eur J Ophthalmol. 2011;21(6):811–819. doi: 10.5301/EJO.2011.6403. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson JP, Hutchison WM, Pettersen E. Tissue cyst rupture in mice chronically infected with Toxoplasma gondii. Parasitol Res. 1989;75:599–603. doi: 10.1007/BF00930955. [DOI] [PubMed] [Google Scholar]
- 7.Jabs DA. Ocular toxoplasmosis. Int Ophthalmol Clin. 1990;30(4):264–270. doi: 10.1097/00004397-199030040-00009. [DOI] [PubMed] [Google Scholar]
- 8.Olive PL, Banath JP, Durand RE. Heterogeneity in radiation-induced DNA damage in individuals' mammalian cells. Biochem Biophys Res Commun. 1990;23:291–293. [Google Scholar]
- 9.Olive PL, Banath JP. The comet assay: a method to measure DNA damage in individual cells. Nat Protoc. 2006;1(1):23–29. doi: 10.1038/nprot.2006.5. [DOI] [PubMed] [Google Scholar]
- 10.Hovhannisyan GG. Fluorescence in situ hybridization in combination with the comet assay and micronucleus test in genetic toxicology. Mol Cytogenet. 2010;3:17. doi: 10.1186/1755-8166-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ribeiro DA, Pereira PC, Machado JM, Silva SB, Pessoa AW, Salvadori DM. Does toxoplasmosis cause DNA damage? An evaluation in isogenic mice under normal diet or dietary restriction. Mutat Res. 2004;559(1-2):169–76. doi: 10.1016/j.mrgentox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 12.Aghwan SS, Al-Taee AF, Suliman EG. Detection of Toxoplasma gondii infection in domestic rabbits by using multiple techniques. Iraqi J Vet Sci. 2010;24(2):65–69. [Google Scholar]
- 13.Ondriska F, Čatár G, Vozarová G. The significance of complement fixation test in clinical diagnosis of toxoplasmosis. Bratisl Lek Listy. 2003;104(6):189–196. [PubMed] [Google Scholar]
- 14.El-Sobky MM, El-Mazar HM. The efficacy of nitazoxanid as a new promising antiparasitic drug on experimental Hymenolepis nana infection. Egypt J Med Sci. 2004;25(1):87–99. [Google Scholar]
- 15.Romand S, Pudney M, Derouin F. In vitro and in vivo activities of the hydroxynaphthoquinone atovaquone alone or combined with pyrimethamine, sulfadiazine, clarithromycin, or minocycline against Toxoplasma gondii. Antimicrob Agents Chemother. 1993;37(11):2371–2378. doi: 10.1128/aac.37.11.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nandhakumar S, Parasuraman S, Shanmugam MM, Ramachandra RK, Parkash C, Vishnu B. Evaluation of DNA damage using single-cell gel electrophoresis (Comet Assay) J Pharmacol Pharmacother. 2011;2(2):107–111. doi: 10.4103/0976-500X.81903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang Y, Rachid Z, Jean-Claude B J. MGMT is a molecular determinant for potency of the DNA-EGFR-combi-molecule ZRS1. Mol Cancer Res. 2011;9(3):320–331. doi: 10.1158/1541-7786.MCR-10-0407. [DOI] [PubMed] [Google Scholar]
- 18.López-Camarillo C, Lopez-Casamichana M, Weber C, Guillen N, Orozco E, Marchat LA. DNA repair mechanisms in eukaryotes: special focus in Entamoeba histolytica and related protozoan parasites. Infect Genet Evol. 2009;9(6):1051–1056. doi: 10.1016/j.meegid.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Harba NM, Afifi AF. Evaluation of DNA damage by DNA fragmentation and comet assays in experimental toxoplasmosis with virulent strain. PUJ. 2012;5(2):189–198. [Google Scholar]
- 20.Ribeiro DA, Calvi SA, Picka MM, Persi E, de Carvalho TB, Caetano PK, Nagoshi LR, Lima CR, Machado JM, Salvadori DM. DNA damage and nitric oxide synthesis in experimentally infected balb/c mice with Trypanosoma cruzi. Exp Parasitol. 2007;116(3):296–301. doi: 10.1016/j.exppara.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya N, Kumar N. Identification and molecular characterization of DNA damaging agent induced expression of Plasmodium falciparum recombination protein PfRad51. Int J Parasitol. 2003;33(12):1385–92. doi: 10.1016/s0020-7519(03)00212-1. [DOI] [PubMed] [Google Scholar]
- 22.Watson JD, Baker TA, Bell SP, Gann A, Levine M, Losick R. Molecular Biology of the Gene, 5th ed, ch.9 and 10. Peason Benjamin Cummings; CSHL Press; 2004. [Google Scholar]
- 23.Singh NP. Microgel electrophoresis of DNA from individual cells: principles and methodology. In: Pfeifer G.P., editor. Technologies for Detection of DNA Damage and Mutations. New York and London: Plenum Press; 1996. pp. 3–23. [Google Scholar]
- 24.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer. Biochem J. 1996;313(Pt1):17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yousef H, Afify A, Hasan H, Meguid A. DNA damage in hemocytes of Schistocerca gregaria (Orthoptera: Acrididae) exposed to contaminated food with cadmium and lead. Nat Sci. 2010;2(4):292–297. [Google Scholar]
- 26.Merk O, Speit G. Detection of crrosslinks with the comet assay in relationship to genotoxicity and cytotoxicity. Environ Mol Mutagen. 1999;33(2):167–172. doi: 10.1002/(sici)1098-2280(1999)33:2<167::aid-em9>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 27.Silva JC, Gennari SM, Ragozo AM, Amajones VR, Magnabosco C, Yai LE, Ferreira-Neto JS, Dubey JP. Prevalence of Toxoplasma gondii antibodies in sera of domestic cats from Guarulhos and São Paulo, Brazil. J Parasitol. 2002;88(2):419–420. doi: 10.1645/0022-3395(2002)088[0419:POTGAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Kawazoe U. Neves DP, de Melo AL, Genaro O, Linardi PM, editors. Toxoplasmose. Parasitologia Humana, Atheneu, São Paulo. 2000:147–156. [Google Scholar]
- 29.Fitzpatrick FA. Inflammation, carcinogenesis and cancer. Int Immunopharmacol. 2001;1(9-10):1651–1667. doi: 10.1016/s1567-5769(01)00102-3. [DOI] [PubMed] [Google Scholar]
- 30.Ames BN, Shigenage MK, Hagen TM. Oxidants, antioxidants and the degenerative diseases of aging. Proceed Nat Acad Sci USA. 1993;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Halliwell B. Why and how should we measure oxidative DNA damage in nutritional studies? How far have we come? Am J Clin Nutr. 2000;72(5):1082–1087. doi: 10.1093/ajcn/72.5.1082. [DOI] [PubMed] [Google Scholar]
- 32.Jackson AL, Loeb LA. The contribution of endogenous sources of DNA damage to the multiple mutations in cancer. Mutat Res. 2001;477(1-2):7–21. doi: 10.1016/s0027-5107(01)00091-4. [DOI] [PubMed] [Google Scholar]
- 33.Flegr J, Stříž L. Potential immunomodulatory effects of latent toxoplasmosis in humans. BMC Infect Dis. 2011;11:274. doi: 10.1186/1471-2334-11-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Puvanesuaran VR, Nowroji K, Sreenivasan S, Noordin R, Balakrishnan V. Use of prednisolone to aid propagation of Toxoplasma gondii in mice. Eur Rev Med Pharmacol Sci. 2012;16(8):1028–1032. [PubMed] [Google Scholar]
- 35.Doliwa C, Xia D, Escotte-Binet S, Newsham EL, Sanderson SJ, Aubert D, Randle N, Wastling JM, Villena I. Identification of differentially expressed proteins in sulfadiazine resistant and sensitive strains of Toxoplasma gondii using difference-gel electrophoresis (DIGE) Int J Parasitol Drugs Drug Resist. 2013;3:35–44. doi: 10.1016/j.ijpddr.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ng P, McCluskey PJ. Treatment of ocular toxoplasmosis. Aust Prescriber. 2002;25(4):88–90. [Google Scholar]
- 37.Nussenblatt RB, Whitcup SM, Palestine AG. Ocular Toxoplasmosis. In: Nussenblatt RB, Whitcup SM, Palestine AG, editors. Fundamentals and Clinical Practice. St. Louis: Mosby Year Book Inc; 1996. pp. 211–228. Uveitis. [Google Scholar]
- 38.Oshida K, Iwanaga E, Kuramitsu MK, Miyamoto Y. An in vivo comet assay of multiple organs (liver, kidney and bone marrow) in mice treated with methyl methanesulfonate and acetaminophen accompanied by hematology and/or blood chemistry. J Toxicol Sci. 2008;33(5):515–524. doi: 10.2131/jts.33.515. [DOI] [PubMed] [Google Scholar]
- 39.Soheilian M, Ramezani A, Azimzadeh A, Sadoughi MM, Dehghan MH, Shahghadami R, Yaseri M, Peyman GA. Randomized trial of intravitreal clindamycin and dexamethasone versus pyrimethamine, sulfadiazine, and prednisolone in treatment of ocular toxoplasmosis. Ophthalmology. 2011;118(1):134–141. doi: 10.1016/j.ophtha.2010.04.020. [DOI] [PubMed] [Google Scholar]