Abstract
AIM
To evaluate histopathological retinal and renal response after one single dose of intravitreous injection of antiangiogenic drugs ranibizumab and bevacizumab in rats.
METHODS
Experimental study in 60d of life adults Wistar rats. Ten animals were included. Group 1 included 5 animals that were injected with 1 µL ranibizumab 1.25 mg in the right eye and with 1 µL of balanced salt solution (BSS) in the left eye, as control; Group 2 included 5 animals that were injected with 1 µL of bevacizumab in the right eye and with 1 µL of BSS in the fellow eye. All injections were performed with Hamilton syringes. After 15d of the interventions, all animals were sacrificed in CO2 chamber. Both eyes were enucleated and one kidney was removed, fixed and embedded in paraffin for histopathological analysis by optic microscopy. For statistical purposes the initial expected abnormal histopathological responses were defined as 0%.
RESULTS
Atypical histopathological retinal response was detected in 2 eyes injected with ranibizumab (40%) as well as in 2 control eyes in group 1. Same was detected in 1 eye injected with bevacizumab (20%) as well as in 1 control eye, in group 2. The noted atypical findings were lymphocytes and eosinophils in the vitreous posterior cavity and mild retinal inflammatory reaction with ganglion cell layer edema but without clinical significance. No atypical histopathological renal response was detected.
CONCLUSION
Unexpected atypical histopathological retinal response without clinical significance was observed in 3 eyes injected with antiangiogenic drugs (2 in group 1 and 1 in group 2) as well as in 3 control eyes (2 in group 1 and 1 in group 2). No atypical renal response was detected suggesting no extra ocular involvement of the intravitreous injected antiangiogenic drugs.
Keywords: antiangiogenic drugs, ranibizumab, bevacizumab, therapy, oxygen induced retinopathy, retinopathy of prematurity
INTRODUCTION
Many ocular diseases occur as a result of pathological angiogenesis. Among them are age related macular degeneration (ARMD), diabetic retinopathy (DR), diabetic macular edema (DME), retinal vein occlusion (RVO), and retinopathy of prematurity (ROP). Modern therapeutic approach to these diseases is the use of intravitreous anti-vascular endothelial growth factor (VEGF) drugs, especially bevacizumab and ranibizumab[1],[2]. Bevacizumab is full length human monoclonal antibody directed against all isoforms of VEGF approved by the Unites States Food and Drug Administration (FDA) as a first-line treatment for human metastatic colorectal cancer in intravenous use[3]. Many complications are related to the intravenous use of this drug such as hypertension, congestive heart failure, bleeding, neutropenia, proteinuria, thromboembolism, and neuropathy[4]–[7]. In these recent years, intravitreal injection of bevacizumab is being used in an off-label way for the treatment of those mentioned ophthalmological diseases related to angiogenesis[8]–[10]. However, bevacizumab escapes from the vitreous into the general circulation and reduces systemic VEGF concentrations for weeks to months. There are concerns regarding systemic safety of this drug for the use among preterm infants with very low birth weight for the treatment of severe cases of ROP[11],[12]. By the other hand, ranibizumab is a fraction of the bevacizumab molecule and it is an alternative anti-VEGF antibody with similar efficacy as bevacizumab but with the supposed advantage that it does not alter systemic VEGF concentrations in adults because this fragment does not contain the FcRn portion of the complete antibody[12],[13]. Ranibizumab is approved by FDA for use in intravitreous injections for the treatment of ARMD, DR, DME, and RVO. This drug is used in an off-label way for the treatment of premature babies in risk of blinding due to severe ROP[14],[15].
Intravitreal injections are a very common procedure and are the most effective route of drug delivery to the retina. Currently, there are several drugs available to treat retinal diseases related to angiogenesis and, even, more are in development, therefore, safety of these drugs, as well as, safety of the procedure in it, are very important issues when intravitreal injections are applied and the possibility of unexpected ocular or retinal inflammatory response should always be kept in mind. This study aims to evaluate histopathological retinal and renal responses after one single dose of intravitreous anti-VEGF drugs ranibizumab (Lucentis®) or bevacizumab (Avastin®), controlled by an intravitreous injection of balanced salt solution (BSS) in laboratory rats.
MATERIALS AND METHODS
Study Design and Setting
This is an experimental study using adults Wistar laboratory rats conducted during 2012 at the Animal Experimental Unit of the Hospital de Clínicas de Porto Alegre (HCPA), Federal University of Rio Grande do Sul, Porto Alegre, Brazil.
Population and Methods
Ten adults Wistar rats with 60d of life were distributed in one of the 2 groups: group 1 included 5 animals that were injected with 1 µL ranibizumab 1.25 mg in the right eye and with 1 µL of BSS in the left eye, as control; group 2 included 5 animals that were injected with 1 µL of bevacizumab in the right eye and with 1 µL of BSS in the fellow control eye. All injections were performed with Hamilton syringes with 32-gauge needles under direct observation with a surgical microscope. All animals received sedation during the procedure and proparacaine hydrochloride 1% ophthalmic drops topically on the eyes. Povidone iodine 5% was placed on the conjunctiva of the eyes. Post-operatory therapy was performed with Maxitrol® (dexamethasone, neomycin, polymyxin B) eye drops, 3 times per day, during 7d in both eyes.
After 15d, all animals were sacrificed in CO2 chamber. The eyes were examined prior to enucleation. A dissecting technique under surgical microscope was used to remove both eyes and one of the kidneys. The enucleated eyes and kidneys were fixed in 4% paraformaldehyde for 45min at 4°C and embedded in paraffin and sectioned for histopathological analysis by light microscopy after staining by hematoxylin-eosyne (HE).
Statistical and Ethics
Study approval was obtained from the Institutional Committee of Animal Care at HCPA. The procedures adhered to the ARVO (Association for Research in Vision and Ophthalmology) Statement for the Use of Animals in Ophthalmic and Vision Research. To estimate the number of animals to be included in the study the initial expected atypical histopathological response for statistical purposes was defined as 0% according to the clinical observation of the use of these drugs in humans. The absolute margin of error was defined as 10% and the confidence level was defined as 95%. Each group should include at least 4 animals.
RESULTS
The post-operatory ophthalmological evaluation showed 1 case of cataract among ranibizumab injected eyes (group 1) and 1 case of cataract among controls in the group 2. There were no signs of endophthalmitis in all eyes.
Histological changes were evaluated by light microscopy. The inner retinal layers in all eyes were intact with no evidence of disorganization (Figure 1). In group 1 (comprised of 5 animals: 5 ranibizumab injected eyes, 5 control eyes) we reported 3 injected eyes without retinal abnormalities (60%), 3 control eyes without retinal abnormalities (60%), 2 injected eyes with abnormal histopathological response (40%) and 2 control eyes with abnormal histopathological response (40%). In group 2 (comprised of 5 animals: 5 bevacizumab injected eyes, 5 control eyes) we reported 4 injected eyes without retinal abnormalities (80%), 4 control eyes without retinal abnormalities (80%), 1 eye with abnormal response (20%) and 1 control eye with abnormal response (20%). In both groups there was mild vacuolization in the ganglion cell layer in both treated and control eyes. The histological retinal analysis showed inflammatory cellular response and fibroblasts were identified in both injected and control eyes in the same rate in both groups (Figures 2, 3). All histopathological evaluations on the sections of the kidneys were considered normal. We did not find renal glomerular sclerosis, renal interstitial fibrosis or renal tubular atrophy, classics markers of renal dysfunction (Figure 4).
Figure 1. Adult Wistar rat eye globe section showing normal vitreous cavity and normal retina after intravitreous injection of ranibizumab.
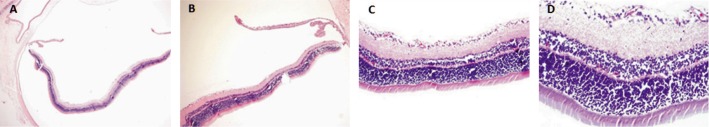
A: HE 40×; B: HE 100×; C: HE 200×; D: HE 400×.
Figure 2. Adult Wistar rat ocular globe after intravitreous injection of ranibizumab showing fibroblastic tissue and inflammation-like response.
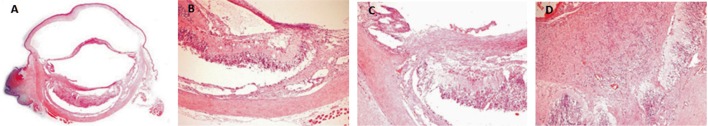
A: HE 20×; B: HE 100×; C: HE 200×; D: HE 400×.
Figure 3. The histological retinal analysis showed inflammatory cellular response and fibroblasts were identified in ranibizumab injected eye. HE 400×.
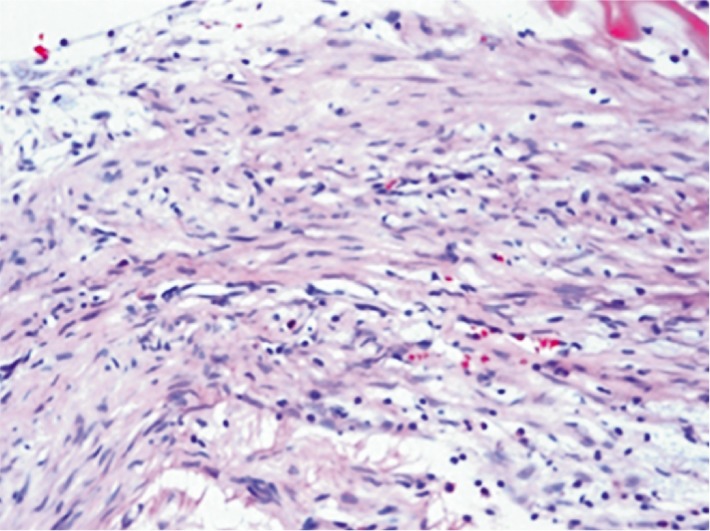
Figure 4. Adult Wistar rat normal kidney sections.
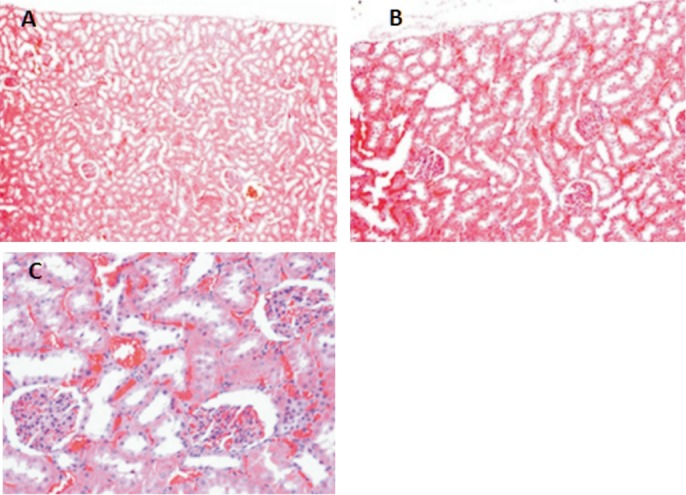
A: HE 100×; B: HE 200×; C: HE 400×.
DISCUSSION
The vacuolization in the ganglion cell layer in both treated and control eyes in our study were consistent with autolysis and tissue processing artifacts. These findings were previous related in rabbit model by Bakri et al[16]. The histopathological retinal analysis showed inflammatory cellular response. These findings were consistent with inflammation and fibrosis. These results were identified in both injected and control eyes in the same rate in both groups. That might have occurred due to retinal preparation or ocular trauma secondary to the injection itself and, possibly it was not dependent to the use of the anti-VEGF drugs. Another explanation to these findings could be the possibility of short period of time of treatment of the postoperative inflammation with inconsistent use of steroid-antibiotic drugs. We used topical Maxitrol® 3 times per day during only 7 of the 15d, in all animals. The ideal dose for adults 60d of life Wistar rats was not established previously. It could have been insufficient antinflammatory therapy in our experiment which may succeed to endophthalmitis-like or to non-specificity inflammation reaction caused by intravitreal injection. Dexamethasone has protective effects on the retina by improving the inflammatory response that involves lymphocyte and phagocyte cells[17]. Since we used in our experimental study Maxitrol® for 7d, a bias of experimental data could be produced and this can be a potential limitation of our study. On the other hand, there was no histological evidence of toxicity of the retina in any of the histological sections in all of the eyes injected with anti-VEGF drugs or with BSS in our study. Similar histopathological inflammatory-like finding was found after bevacizumab injection in rabbit's eyes despite the authors related that the drug was nontoxic to the retina of rabbits[18]–[20].
Kim et al[8], in 2008, and Thaler et al[20], in 2010, related absence of intravitreal bevacizumab-induced neuronal toxicity in the mouse retina. Our study did not detect specific retinal toxicity of intravitreous anti-VEGF drugs ranibizumab and bevacizumab, as expected according to the clinical experience in humans, but unexpected histopathological retinal response related to inflammation was observed. Some possibilities for this finding were established in order to improve quality for new experiments, as well as to demonstrate the potential inflammatory risk of intravitreal injections as a first line option for treatment of the ocular diseases. All histopathological evaluation on the sections of the kidneys was considered normal suggesting no extra ocular toxicity of the intravitreous injected anti-VEGF drugs. Pellé et al[21], previously, in a case report, related renal dysfunction after four times repeated ranibizumab intravitreal injections. The authors described findings of tubular atrophy and interstitial fibrosis. In our experimental work, we did not find renal glomerular sclerosis, renal interstitial fibrosis or renal tubular atrophy, maybe because only one intravitreal injection was performed in each eye.
Acknowledgments
Conflicts of Interest: Fortes Filho JB, None; Maia M, None; Tartarella MB, None; Meyer FS, None; Fortes BGB, None; Kliemann LM, None
REFERENCES
- 1.Penha FM, Rodrigues EB, Furlani BA, Dib E, Melo GB, Farah ME. Toxicological considerations for intravitreal drugs. Expert Opin Drug Metab Toxicol. 2011;7(8):1021–1034. doi: 10.1517/17425255.2011.585970. [DOI] [PubMed] [Google Scholar]
- 2.Penha FM, Rodrigues EB, Maia M, Furlani BA, Regatieri C, Melo GB, Magalhães O, Jr, Manzano R, Farah ME. Retinal and ocular toxicity in ocular application of drugs and chemicals--part II: retinal toxicity of current and new drugs. Ophthalmic Res. 2010;44(4):205–224. doi: 10.1159/000316695. [DOI] [PubMed] [Google Scholar]
- 3.Hurwitz H, Kabbinavar F. Bevacizumab combined with standard fluoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology. 2005;69(3):17–24. doi: 10.1159/000088480. [DOI] [PubMed] [Google Scholar]
- 4.Hurwitz H, Saini S. Bevacizumab in the treatment of metastatic colorectal cancer: safety profile and management of adverse events. Semin Oncol. 2006;33(5 Suppl 10):26–34. doi: 10.1053/j.seminoncol.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Borzomati D, Nappo G, Valeri S, Vincenzi B, Ripetti V, Coppola R. Infusion of Bevacizumab increases the risk of intestinal perforation: results on a series of 143 patients consecutively treated. Updates Surg. 2013;65(2):121–124. doi: 10.1007/s13304-013-0207-2. [DOI] [PubMed] [Google Scholar]
- 6.Ababneh OH, Yousef YA, Gharaibeh AM, bu Ameerh MA, bu-Yaghi NE, Al B. Intravitreal bevacizumab in the treatment of diabetic ocular neovascularization. Retina. 2013;33(4):748–755. doi: 10.1097/IAE.0b013e3182721153. [DOI] [PubMed] [Google Scholar]
- 7.Lubezky N, Winograd E, Papoulas M, Lahat G, Shacham-Shmueli E, Geva R, Nakache R, Klausner J, Ben-Haim M. Perioperative complications after neoadjuvant chemotherapy with and without bevacizumab for colorectal liver metastases. J Gastrointest Surg. 2013;17(3):527–532. doi: 10.1007/s11605-012-2108-y. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Kim C, Kim JH, Lee BJ, Yu YS, Park KH, Kim KW. Absence of intravitreal bevacizumab-induced neuronal toxicity in the retina. Neurotoxicology. 2008;29(6):1131–1135. doi: 10.1016/j.neuro.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Akkoyun I, Karabay G, Haberal N, Dagdeviren A, Yilmaz G, Oto S, Erkanil L, Akova YA. Structural consequences after intravitreal bevacizumab injection without increasing apoptotic cell death in a retinopathy of prematurity mouse model. Acta Ophthalmol. 2012;90(6):564–570. doi: 10.1111/j.1755-3768.2010.01963.x. [DOI] [PubMed] [Google Scholar]
- 10.Aisenbrey S, Ziemssen F, Volker M, Gelisken F, Szurman P, Jaissle G, Grisanti S, Bartz-Schmidt KU. Intravitreal bevacizumab (Avastin) for occult choroidal neovascularization in age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2007;245(7):941–948. doi: 10.1007/s00417-006-0471-7. [DOI] [PubMed] [Google Scholar]
- 11.Sato T, Wada K, Arahori H, Kuno N, Imoto K, Iwahashi-Shima C, Kusaka S. Serum concentrations of bevacizumab (avastin) and vascular endothelial growth factor in infants with retinopathy of prematurity. Am J Ophthalmol. 2012;153(2):327–333. doi: 10.1016/j.ajo.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Hoerster R, Muether P, Dahlke C, Mehler K, Oberthur A, Kirchhof B, Fauser S. Serum concentrations of vascular endothelial growth factor in an infant treated with ranibizumab for retinopathy of prematurity. Acta Ophthalmol. 2013;91(1):74–75. doi: 10.1111/j.1755-3768.2012.02469.x. [DOI] [PubMed] [Google Scholar]
- 13.Carneiro AM, Costa R, Falcão MS, Barthelmes D, Mendonca LS, Fonseca SL, Gonçalves R, Gonçalves C, Falcão-Reis FM, Soares R. Vascular endothelial growth factor plasma levels before and after treatment of neovascular age-related macular degeneration with bevacizumab or ranibizumab. Acta Ophthalmol. 2012;90(1):25–30. doi: 10.1111/j.1755-3768.2011.02240.x. [DOI] [PubMed] [Google Scholar]
- 14.Orozco-Gomez LP, Hernandez-Salazar L, Moguel-Ancheita S, Ramirez-Moreno MA, Morales-Cruz MV. Laser-ranibizumab treatment for retinopathy of prematurity in umbral-preumbral disease. Three years of experience. Cir Cir. 2011;79(3):207–232. [PubMed] [Google Scholar]
- 15.Mota A, Carneiro A, Breda J, Rosas V, Magalhaes A, Silva R, Falcão-Reis F. Combination of intravitreal ranibizumab and laser photocoagulation for aggressive posterior retinopathy of prematurity. Case Report Ophthalmol. 2012;3(1):136–141. doi: 10.1159/000338623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006;142(1):162–164. doi: 10.1016/j.ajo.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 17.Manzano RP, Peyman GA, Khan P, Kivilcim M. Testing intravitreal toxicity of bevacizumab (Avastin) Retina. 2006;26(3):257–261. doi: 10.1097/00006982-200603000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Migliorati G, Nicoletti I, D'Adamio F, Spreca A, Pagliacci C, Riccardi C. Dexamethasone induces apoptosis in mouse natural killer cells and cytotoxic T lymphocytes. Immunology. 1994;81(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- 19.Kong L, Mintz-Hittner HA, Penland RL, Kretzer FL, Chevez-Barrios P. Intravitreous bevacizumab as anti-vascular endothelial growth factor therapy for retinopathy of prematurity: a morphologic study. Arch Ophthalmol. 2008;126(8):1161–1163. doi: 10.1001/archophthalmol.2008.1. [DOI] [PubMed] [Google Scholar]
- 20.Thaler S, Fiedorowicz M, Choragiewicz TJ, Bolz S, Tura A, Henke-Fahle S, Yoeruek E, Zrenner E, Bartz-Schmidt KU, Ziemssen F, Schuettauf F. Toxicity testing of the VEGF inhibitors bevacizumab, ranibizumab and pegaptanib in rats both with and without prior retinal ganglion cell damage. Acta Ophthalmol. 2010;88(5):170–176. doi: 10.1111/j.1755-3768.2010.01927.x. [DOI] [PubMed] [Google Scholar]
- 21.Pellé G, Shweke N, Duong Van Huyen JP, Tricot L, Hessaïne S, Frémeaux-Bacchi V, Hiesse C, Delahousse M. Systemic and kidney toxicity of intraocular administration of vascular endothelial growth factors inhibitors. Am J Kidney Dis. 2011;57(5):756–759. doi: 10.1053/j.ajkd.2010.11.030. [DOI] [PubMed] [Google Scholar]


