Abstract
AIM
To evaluate the accuracy of axial length (AL) measurements obtained from immersion B-scan ultrasonography (immersion B-scan) for intraocular lens (IOL) power calculation in patients with high myopia and cataracts.
METHODS
Immersion B-scan, contact A-scan ultrasonography (contact A-scan), and the IOLMaster were used to preoperatively measure the AL in 102 eyes from 102 patients who underwent phacoemulsification and IOL implantation. Patients were divided into two groups according to the AL: one containing patients with 22 mm≤AL<26 mm(group A) and the other containing patients with AL≥26 mm (group B). The mean error (ME) was calculated from the difference between the AL measurement methods predicted refractive error and the actual postoperative refractive error.
RESULTS
In group A, ALs measured by immersion B-scan (23.48±1.15) didn't differ significantly from those measured by the IOLMaster (23.52±1.17) or from those by contact A-scan (23.38±1.20). In the same group, the standard deviation (SD) of the mean error (ME) of immersion B-scan (-0.090±0.397 D) didn't differ significantly from those of IOLMaster (-0.095±0.411 D) and contact A-scan (-0.099±0.425 D). In group B, ALs measured by immersion B-scan (27.97±2.21 mm) didn't differ significantly from those of the IOLMaster (27.86±2.18 mm), but longer than those measured by Contact A-scan (27.75±2.23 mm, P=0.009). In the same group, the standard deviation (SD) of the mean error (ME) of immersion B-scan (-0.635±0.157 D) didn't differ significantly from those of the IOLMaster (-0.679±0.359 D), but differed significantly from those of contact A-scan (-0.953±1.713 D, P=0.028).
CONCLUSION
Immersion B-scan exhibits measurement accuracy comparable to that of the IOLMaster, and is thus a good alternative in measuring AL in eyes with high myopia when the IOLMaster can't be used, and it is more accurate than the contact A-scan.
Keywords: axial length, measurement, immersion, ultrasonography, high myopia
INTRODUCTION
Achieving the desired postoperative refractive state requires the accurate measurement of corneal curvature and the axial length (AL), as well as the appropriate application of the formula used to determine intraocular lens (IOL) power, and the error in pre-operative AL measurement is the most significant error in IOL power calculation and equates to almost 2.5 D/mm in IOL power in a normal AL eye but decreases to 1.75 D/mm in a 30 mm eye and increases to 3.75 D/mm in a 20 mm eye[1]–[5]. Traditionally, ALs has been measured using ultrasound biometry-including contact A-scan ultrasonography and immersion A-scan ultrasonography[6]. Contact A-scan ultrasonography is a well established method for measuring AL, and immersion A-scan technique is potentially more accurate and is commonly accepted to be the gold standard in ocular echography, since it does not require indentation of the cornea, but it is less accurate in the presence of non-symmetrical posterior scleral staphyloma, macular degeneration, severe vitreal diseases, or retinal detachment[7]–[11]. Non-contact optical coherence interferometry(PCI) can improve the accuracy of AL measurement in patients with cataracts or the conditions listed above, however, in some cases (5%-8%), performance of the IOLMaster is impossible, because of limitations of the machine, such as dense media opacity (corneal or lens opacity), poor fixation by the patients, what's more, in most of the developing and underdeveloped countries, PCI device just can be used in large general hospital or professional eye hospital[11]–[14]. The immersion B-scan-a conventional method, which employs immersion A-scan, because it allows patients can be guided to adjust the eye positions to an ideal position at any time on the basis of the visual content of the images to make the measurement more accurate, thus it can be a good alternative and/or supplement in measuring AL in eyes with long AL when the IOLMster can't be used. However, the accuracy of the immersion B-scan hasn't been compared with conventional contact A-scan and IOLMaster synchronously. Therefore, we conducted a prospective, comparative trial to compare the accuracy of the three methods to measure the AL in eyes with high myopia.
SUBJECTS AND METHODS
Subjects
This prospective study was conducted in the Chinese PLA General Hospital between January and May 2012. All participants provided written informed consent in accordance with the Declaration of Helsinki via protocols approved by the appropriate institutional Review Board. One hundred and two eyes from 102 consecutive patients scheduled to undergo phacoemulsification and IOL implantation were consecutively selected and divided into two groups according to the AL: group A contained 43 eyes, the AL range from 22.05 mm to 25.91 mm; group B contained 59 eyes, the AL range from 26.50 mm to 31.76 mm. The exclusion criteria were: 1) myopic traction maculopathy including macular retinoschisis and myopic macular holes; 2) history of surgery such as vitreoretinal surgery, cataract surgery, or refractive surgery; 3) retinal pathologies myopic chorioretinal atrophy or amblyopia that would prevent accurate fixation during the measurement of the AL; 4) dense cataract that would prevent an accurate measurement of the AL by the IOLMaster (≥Grade 4, Emery and Little classification[15]); 5) eyes with active choroidal neovascularization because of exudative changes such as serous retinal detachment which can affect the AL, and AL was measured using three methods: contact A-scan(SW-2100, Souer, Tianjin, China), immersion B-scan (SW-2100, A/B-mode ultrasound: A super probe frequency, 10 MHz; axial resolution, 0.12 mm; Souer, Tianjin, China), and the IOLMaster (version.5.4.3.0002, Carl Zeiss Mediate, Jena, Germany).
Each eye was evaluated on the same day using the IOLMaster, contact A-scan and an immersion B-scan. All the eyes were measured the AL and corneal curvature first using IOLMaster, then half the eyes in each category were measured the AL first using the contact A-scan and then using the immersion B-scan, and the other half, vice versa. One same examiner performed all measurements.
Methods
Contact A-scan ultrasonography
The physician performed A-mode ultrasound using the handheld contact method, with a gain of about 80 dB in the lens measurement and automatic measurement storage modes. After the cornea was anesthetized using 0.4% oxybuprocaine hydrochloride, and patients were placed in a supine position, an A super probe (6 mm diameter) was gently placed in the center of the cornea and directed across the pupil to the macula. Careful attention was paid to avoid corneal indentation. Ten data points were obtained automatically, and the average value with a difference <0.05 mm was recorded for use in the analysis. The anterior chamber depth and axial mean were also recorded.
Immersion B-scan ultrasonography
Immersion B-scan was performed with a gain of 85 dB and the vector a sampling line located in the center of the screen. After the cornea surface anesthesia, a Hansen immersion shell was placed on the ocular surface and slowly injected with saline, the B-mode ultrasound probe was inserted into the immersion shell 5-10 mm from the cornea. Patients were asked to watch the center of the probe and to adjust their eye positions according to B-mode ultrasound images. When the ideal eye position was obtained, the image was frozen.
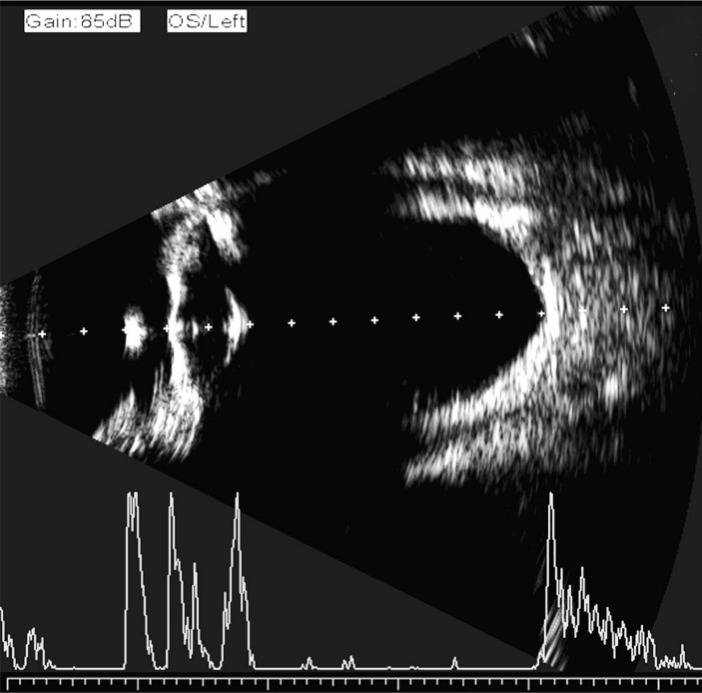
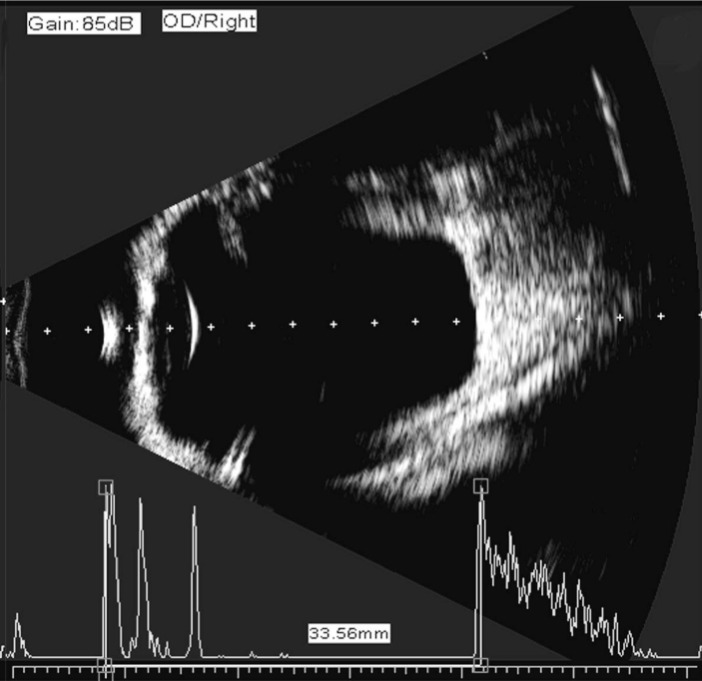
The AL obtained with immersion B-scan was determined by estimating an optical line through the eye (perpendicular to the cornea and lens) thus ensuring precise centration of the corneal peak. Therefore, in the presence of an eccentric staphyloma, the AL chosen for biometry calculations did not necessarily represent the maximum geometric AL of the eye (Figure 1).
Figure 1. First quality control measure for immersion B-scan ultrasound biometry.
The vector an ultrasound sampling line was determined to be exactly perpendicular to the double bands of the cornea and the center of the lens capsule, and directed at the center of the macula.
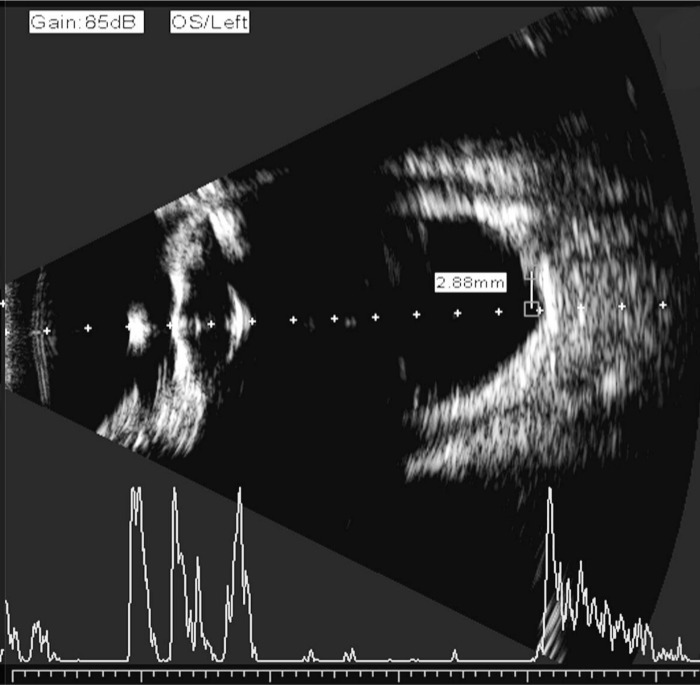
The point where the vector A super-sample line crossed the retina was approximately 4.5 mm from the disc center or approximately 3 mm from the optic disc edge (Figure 2). Final AL was obtained by the average value of three consecutive measurements of the distance from the corneal vertex to the macula, or from the crest of the anterior corneal surface to the crest of the macular retinal surface with an electronic measuring scale in the B ultrasound mode (Figure 3)[16],[17].
Figure 2. Second quality control measure for immersion B-scan ultrasound biometry.
The intersection of the vector an ultrasound sampling line and the retina was determined to be 4.5 mm from the optic disc or 3 mm from the optic disc edge.
Figure 3. Axial length measurement by immersion B-scan ultrasound.
AL was defined as the distance from the corneal vertex to the macula or from the crest of the anterior corneal surface to the crest of the macular retinal surface with an electronic measuring scale in B-mode ultrasound.
IOLMaster
The phasic mode of the IOLMaster's AL menu was used to measure AL five times, from which an average value was recorded (signal-to-noise ratio >2) and used to calculate IOL power. The same senior physician performed all examinations with the IOLMaster.
IOL power calculation
IOL power was calculated using the SRK/T formula in the patients with the AL>24.5 mm and Hoffer Q formula in the patients with the AL≤24.5 mm. The same surgeon (ZL) performed all cataract surgeries using a 2.75 mm limbal incision. A foldable acrylic single-piece IOL (Rayner Superflex 620H; Rayner Intraocular Lenses Limited, Hove, East Sussex, UK) was implanted in the capsular bag in all cases. The final refractive error was obtained three months after cataract surgery.
Refraction was performed using an automated refractor at least 3 months postoperatively in order to stabilize the refraction.
Accuracy of the three techniques was evaluated in this study, and it was measured by mean actual postoperative refractive error using the following equation: Predictive error of each technique=Predicted postoperative error by that technique-postoperative spherical equivalent of the refractive error.
Statistical Analysis
All data were analyzed with the SPSS version 12.0 (SPSS Inc., Chicago, IL, USA). Values were recorded as mean ±SD (standard deviation). A paired t-test was used for parametric comparison of the means. The mean AL measurements and biometry errors were calculated. Deviation of the actual postoperative refraction from the assumed target preoperative refraction calculated and evaluated using a two-way analysis of variance (ANOVA). The statistical significance was set at P<0.05.
RESULTS
The patients' main details at the time of treatment were summarized in Table 1. All operations were performed successfully and all IOLs were implanted into the capsular bag. Comparisons of ALs measured by immersion B-scan, contact A-scan, and the IOLMaster were presented in Table 2.
Table 1. Characteristics of study population.
| Characteristics | Group A (22 mm≤AL<26 mm) | Group B (AL≥26 mm) |
| Number | 43 | 59 |
| Age | ||
| Range (a) | 27-87 | 28-84 |
| Mean | 60.4 | 62.8 |
| Standard deviation | ±9.8 | ±9.5 |
| Sex | ||
| M | 20 | 27 |
| F | 23 | 32 |
AL: Axial length; No differences were significant.
Table 2. Axial length of eyes with cataract(mean axial length±standard deviation).
| Method | Group A (43 eyes) (22 mm≤AL<26 mm) | Group B (59eyes) (AL≥26 mm) | |
| Immersion B-scan (mm) | 23.48+1.15 | 27.97±2.21 | |
| IOLMaster(mm) | 23.52+1.17 | 27.86±2.18 | |
| Contact A-scan (mm) | 23.38+1.20 | 27.75±2.23 | |
| P | Immersion B-scan/IOLMaster | 0.38 | 0.39 |
| Immersion B-scan/Contact A-scan | 0.46 | 0.01 | |
| IOLMaster/Contact A-scan | 0.89 | 0.03 | |
AL: Axial length.
In group A, ALs measured by immersion B-scan (23.48±1.15 mm) didn't differ significantly from those measured by the IOLMaster (23.52±1.17 mm) or from those by contact A-scan (23.38±1.20 mm). In the same group, the standard deviation (SD) of the mean error (ME) of immersion B-scan (-0.090±0.397 D, -0.97 to +1.25 D) didn't differ significantly from those of IOLMaster (-0.095±0.411 D, -0.95 to +1.23 D) and contact A-scan (-0.099±0.425 D, -1.11 to +1.35 D) at three months postoperative. The mean error of the three methods and the percentages of predictive error within ±0.50 D, ±1.00 D and ±1.50 D in group A were presented in Table 3.
Table 3. The mean error of three methods and the percentages of predictive error within ±0.50D, ±1.00D and ±1.50D in the two groups.
| Parameter | Group A (43 eyes) (22 mm≤AL<26 mm) |
Group B (59 eyes) (AL≥26 mm) |
||||
| Immersion B-scan | IOLMaster | Contact A-scan | Immersion B-scan | IOLMaster | Contact A-scan | |
| Mean error ±SD(D) | -0.090±0.397 | -0.095±0.411 | -0.099±0.425 | -0.635±0.157 | -0.679±0.359 | -0.953±1.713 |
| Range (D) | -0.97 to +1.25 | -0.95 to +1.23 | -1.11to + 1.35 | -1.80 to +1.17 | -1.41 to +1.27 | -2.48 to +1.63 |
| Prediction error | ||||||
| Within±0.50 D, n (%) | 22 (51.2) | 25 (58.1) | 23 (53.5) | 30 (50.8) | 31 (52.5) | 14 (23.7) |
| Within±1.00 D, n (%) | 36 (83.7) | 38 (88.4) | 36 (83.7) | 45 (76.3) | 48 (81.4) | 30 (50.8) |
| Within±1.50 D, n (%) | 43 (100.0) | 43 (100.0) | 43 (100.0) | 57 (96.6) | 57 (96.6) | 50 (84.7) |
ME: Mean predictive error; SD: Standard deviation; Predictive error=Predicted postoperative error—postoperative spherical equivalent of the refractive error; AL: Axial length.
In group B, ALs measured by immersion B-scan (27.97±2.21 mm) didn't differ significantly from those of the IOLMaster (27.86±2.18 mm), but significantly longer than those measured by Contact A-scan (27.75±2.23 mm, P=0.009). In the same group, the standard deviation (SD) of the mean error (ME) of immersion B-scan(-0.635±0.157 D, -1.80 to +1.17 D) didn't differ significantly from those of the IOLMaster (-0.679±0.359 D, -1.41 to +1.27 D), but differed significantly from those of Contact A-scan (-0.953±1.713 D, -2.48 to +1.63 D, P=0.028). The mean error of the three methods and the percentages of predictive error within ±0.50 D, ±1.00 D and ±1.50 D in group B were presented in Table 3.
DISCUSSION
With the increasing popularity of small incisions and phacoemulsification in cataract surgery, the accuracy of IOL power calculation has become the only factor affecting postoperative refractive error. This study was motivated by the need to minimize such AL measurement error. To improve the accuracy of IOL power measurement, a variety of high-quality IOLs and optimized formulae and measurement methods have been developed and improved.
In this study, we measured corneal curvature with the IOLMaster, calculated IOL power preoperatively using the SRK/T formula in the patients with the AL>24.5 mm and Hoffer Q formula in the patients with the AL≤24.5 mm[4],[18],[19]–[21]. We implanted the same type of IOL in all eyes to avoid any variation due to the use of different IOLs. AL was thus the only factor underlying differences among measurements obtained from the different methods.
The AL was defined as the distance from the corneal vertex to the foveal vitreoretinal interface. The error in pre-operative AL measurement was the most significant error in IOL power calculation and equates to almost 2.5D/mm in IOL power in a normal AL eye but decreases to 1.75D/mm in a 30mm eye and increases to 3.75D/mm in a 20mm eye[1]–[5]. Ultrasound biometry and optical biometry are two currently used ocular biometry methods that are based on different physical principles.
Two types of A-scan ultrasound biometry are currently in use. They are immersion A-scan and contact A-scan. Immersion A-scan measurement had been widely recognized as the standard method of AL measurement in clinic, which was more accurate than contact A-scan[7],[8]. However, because of the relatively cumbersome of the immersion A-scan, contact A-scan was used more widely. The immersion B-scan(also known as B-mode-guided vector-A-mode) was an immersion A-scan method with B-mode-guided image, which integrated the advantages of immersion A-scan and the B-scan and overcame the weaknesses of the two methods.
The AL measurement from the immersion A-scan or contact A-scan was difficult in the patients with high myopia and cataract, especially in patients with asymmetric scleral staphyloma. It was because that in patients with asymmetric scleral staphyloma and cataract, the ideal waveform of the A-scan caught not been obtained easily due to their poor fixation, then, the off-axis measurement error would have occurred, which would lead to poor postoperative visual[9]–[11]. In our study, the average AL in the group A (patients without high myopia) measured from immersion B-scan was 0.10 mm longer than measured from contact A-scan. Some others had shown that the average AL measured by immersion A-scan was 0.03-0.27 mm longer than measured by contact A-scan[6]–[8],[22],[23]. The difference might be due to on the one hand, the different degrees of corneal depression, on the other hand, the ultrasonic instrument we used were not exactly the same as it used in other studies, and the results might be different when the ultrasonic instrument different. In the group B (patients with high myopia), the average AL measured from immersion A-scan was 0.22 mm longer than measured from contact A-scan, and the comparison of the mean error of the two methods showed that the immersion B-scan was more accurate. As the examiner and the ultrasonic device were extremely the same in the two groups, the difference might be due to the off-axis measurement error occurred when the AL measured by contact A-scan. The accuracy of the immersion B-scan was better than A-scan may be due to: It is a two-dimensional modality with brightness modulation, and allows the intuitive visualization of various biometric reference interfaces, especially the morphology of the macula and the posterior scleral staphyloma. In addition, patients can be guided in the adjustment of eye positions at any time on the basis of the visual content of the images. The sampling line of vector an ultrasound can also provide accurate information to guide the placement of the electronic measuring scale, which can prevent A-mode ultrasound–induced errors in alignment. Wang et al[17] recorded that the average AL measured by immersion A-scan was 0.44 mm shorter than measured by contact A-scan, and the difference might be due to the patients with longer AL in their study. In eyes with axial lengths ≥30 mm, a posterior pole staphyloma temporal to the fovea was common, and the corneal vertex-fovea distance was approximately 0.5-1.5 mm, shorter than the distance from the corneal vertex to the bottom of the staphyloma, which was where the A-scan usually found the perpendicular axis and recorded the AL[6].
The IOLMaster was an optical biometry that had shown good repeatability and reliability in the measurement of AL. Our findings were similar to the findings reported by Fontes et al[24], who concluded that the accuracy of AL measured with the IOLMaster had been shown to be similar to that of AL measured by immersion A-scan ultrasound in patients without high myopia, and the IOLMaster had been recognized as a new standard AL measurement. Some others concluded that the AL measurement by the IOLMaster was more accurate than that by contact or immersion A-Scan in eyes with or without high myopia[11],[12],[25],[26]. In our study, the results showed that in the group B (patients with high myopia), the AL measurements from the IOLMaster were more accurate than from the contact A-scan. However, the application of the IOLMaster was limited in some patients (5%-8%) with corneal diseases or dense cataracts[13],[14]. What's more, in most of the developing and underdeveloped countries, it just can be used in large general hospital or professional eye hospital. In our study, the results showed that the immersion B-scan exhibits measurement accuracy comparable to that of the IOLMaster in patients with cataract with or without high myopia. We concluded that this result due to the strict control of image quality as following: First, we obtained clear images of the bands of the cornea, anterior and posterior lens, and retina with complete optic nerve visualization in B-mode echo ultrasonography by adjusting the patient's eye position and B-scan ultrasound immersion depth. Second, by adjusting the patient's eye position, we ensured that the sampling line of vector an ultrasound was perpendicular to the corneal–retinal interface and passed through the center of the pupil. Third, the fovea is approximately 4.5 mm (3 disc diameter or 15°) from the center of the optic nerve [16],[17].
In conclusion, immersion B-scan exhibits measurement accuracy comparable to that of the IOLMaster, and is thus a good alternative in measuring AL in eyes with high myopia when the Ironmaster can't be used, and it is more accurate than the contact A-scan.
Acknowledgments
Foundations: Supported by National Key Basic Research Program of China (973 Program, No.2013CB967000); National Natural Science Foundation of China (No. 81271052)
Conflicts of Interest: Yang QH, None; Chen B, None; Peng GH, None; Li ZH, None; Huang YF, None.
REFERENCES
- 1.Olsen T. Calculation of intraocular lens power: a review. Acta Ophthalmol Scand. 2007;85(5):472–485. doi: 10.1111/j.1600-0420.2007.00879.x. [DOI] [PubMed] [Google Scholar]
- 2.Lee AC, Qazi MA, Pepose JS. Biometry and intraocular lens power calculation. Curr Opin Ophthalmol. 2008;19(1):13–17. doi: 10.1097/ICU.0b013e3282f1c5ad. [DOI] [PubMed] [Google Scholar]
- 3.Jin H, Rabsilber T, Ehmer A, Borkenstein AF, Limberger IJ, Guo H, Auffarth GU. Comparison of ray-tracing method and thin-lens formula in intraocular lens power calculations. J Cataract Refract Surg. 2009;35(4):650–662. doi: 10.1016/j.jcrs.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 4.Aristodemou P, Knox Cartwright NE, Sparrow JM, Johnston RL. Formula choice Hoffer Q, Holladay 1, or SRK/T and refractive outcomes in 8108 eyes after cataract surgery with biometry by partial coherence interferometry. J Cataract Refract Surg. 2011;37(1):63–71. doi: 10.1016/j.jcrs.2010.07.032. [DOI] [PubMed] [Google Scholar]
- 5.Norrby S. Sources of error in intraocular lens power calculation. J Cataract Refract Surg. 2008;34(3):368–376. doi: 10.1016/j.jcrs.2007.10.031. [DOI] [PubMed] [Google Scholar]
- 6.Trivedi RH, Wilson ME. Prediction error after pediatric cataract surgery with intraocular lens implantation: Contact versus immersion A-scan biometry. J Cataract Refract Surg. 2011;37(3):501–505. doi: 10.1016/j.jcrs.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 7.Hennessy MP, Chan DG. Contact versus immersion biometry for axial length before cataract surgery. J Cataract Refract Surg. 2003;29(11):2195–2198. doi: 10.1016/s0886-3350(03)00224-4. [DOI] [PubMed] [Google Scholar]
- 8.Schelenz J, Kammann J. Comparison of contact and immersion techniques for axial length measurement and implant power calculation. J Cataract Refract Surg. 1989;15(4):425–428. doi: 10.1016/s0886-3350(89)80062-8. [DOI] [PubMed] [Google Scholar]
- 9.Zaldivar R, Shultz MC, Davidorf JM, Holladay JT. Intraocular lens power calculation in patients with extreme myopia. J Cataract Refract Surg. 2000;26(5):668–674. doi: 10.1016/s0886-3350(00)00367-9. [DOI] [PubMed] [Google Scholar]
- 10.Ghanem AA, El-Sayed HM. Accuracy of intraocular lens power calculation in high myopia. Oman J Ophthalmol. 2010;3(3):126–130. doi: 10.4103/0974-620X.71888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lege BA, Haigis W. Laser interference biometry versus ultrasound biometry in certain clinical conditions. Graefes Arch Clin Exp Ophthalmol. 2004;242(1):8–12. doi: 10.1007/s00417-003-0672-2. [DOI] [PubMed] [Google Scholar]
- 12.MacLaren RE, Sagoo MS, Restore M, Allan BD. Biometry accuracy using zero – and negative-powered intraocular lenses. J Cataract Refract Surg. 2005;31(2):280–290. doi: 10.1016/j.jcrs.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 13.Burnham WP. In: Optical coherence biometry. Wallace RB III, editor. Refractive Cataract Surgery and Multifocal IOLs Thorofare: Slack; 2001. pp. 21–35. [Google Scholar]
- 14.Tehrani M, Krummenauer F, Blom E, Dick HB. Evaluation of the practicality of optical biometry and applanation ultrasound in 253 eyes. J Cataract Refract Surg. 2003;29(4):741–746. doi: 10.1016/s0886-3350(02)01740-6. [DOI] [PubMed] [Google Scholar]
- 15.Emery JM. Kelman phacoemulsification,patient selection. In: Emery JM, McIntyre DJ, editors. Extracapsular cataract surgery. London St Louis: CV Mosby; 1983. pp. 95–100. [Google Scholar]
- 16.Bergès O, Puech M, Assouline M, Letenneur L, Gastellu-Etchegorry M. B-mode-guided vector-A-mode versus A-mode biometry to determine axial length and intraocular lens power. J Cataract Refract Surg. 1998;24(4):529–535. doi: 10.1016/s0886-3350(98)80297-6. [DOI] [PubMed] [Google Scholar]
- 17.Wang YH, Xiao Y, Hong H, Fu ZY. Accuracy of Immersion B-scan Ultrasound Biometry in High Myopic Patients. Zhongguo Chaosheng Yixue Zazhi. 2005;21(8):578–581. [Google Scholar]
- 18.Raouf EN, Ashraf M, Hanem K, Walid G. Intraocular lens power calculation in patients with high axial myopia before cataract surgery. Saudi J Ophthalmol. 2010;24(3):77–80. doi: 10.1016/j.sjopt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stopyra W. The accuracy of IOL power calculation formulas for eyes of axial length exceeding 24.5 mm. Klin Oczna. 2013;115(2):93–95. [PubMed] [Google Scholar]
- 20.Retzlaff JA, Sanders DR, Kraff MC. Development of the SRK/T intraocular lens implant power calculation formula. J Cataract Refract Surg. 1990;16(3):333–340. doi: 10.1016/s0886-3350(13)80705-5. [DOI] [PubMed] [Google Scholar]
- 21.Hoffer KJ. The HofferQ formula: a comparison of theoretic and regression formulas. J Cataract Refract Surg. 1993;19(6):700–712. doi: 10.1016/s0886-3350(13)80338-0. [DOI] [PubMed] [Google Scholar]
- 22.Watson A, Armstrong R. Contact or immersion technique for axial length measurement? Aust N Z J Ophthalmol. 1999;27(1):49–51. doi: 10.1046/j.1440-1606.1999.00162.x. [DOI] [PubMed] [Google Scholar]
- 23.Shammas HJ. A comparison of immersion and contact techniques for axial length measurements. J Cataract Refract Surg. 1984;10(4):444–447. doi: 10.1016/s0146-2776(84)80044-0. [DOI] [PubMed] [Google Scholar]
- 24.Fontes BM, Fontes BM, Castro E. Intraocular lens power calculation by measuring axial length with partial optical coherence and ultrasonicbiometry. Arq Bras Oftalmol. 2011;74(3):166–170. doi: 10.1590/s0004-27492011000300004. [DOI] [PubMed] [Google Scholar]
- 25.Roessler GF, Dietlein TS, Plange N, Roepke AK, Dinslage S, Walter P, Mazinani BA. Accuracy of intraocular lens power calculation using partial coherence interferometry in patients with high myopia. Ophthalmic Physiol Opt. 2012;32(3):228–233. doi: 10.1111/j.1475-1313.2012.00903.x. [DOI] [PubMed] [Google Scholar]
- 26.Shekharan SC, Pawar N, Maheshwari D, Ramakrishnan R. IOL master optical biometry vs conventional ultrasound Biometry in intraocular lens power calculations in high myopic eyes. AIOC 2009 PROCEEDINGS. 2009:136–139. [Google Scholar]