Abstract
AIM
To investigate the long-term results of penetrating keratoplasty (PK) in patients with keratoconus (KC) and to evaluate factors that might influence the final visual outcome.
METHODS
We retrospectively reviewed the data of all patients with clinical KC who had undergone PK by a single corneal surgeon in a single center from May 1980 to December 2005. The age of the patients, preoperative best-corrected visual acuity (BCVA), corneal thickness, death to preservation time, and preservation to transplantation time were recorded. Additionally, postoperative complications such as graft rejection, development of glaucoma and specular microscopy were checked during the follow-up.
RESULTS
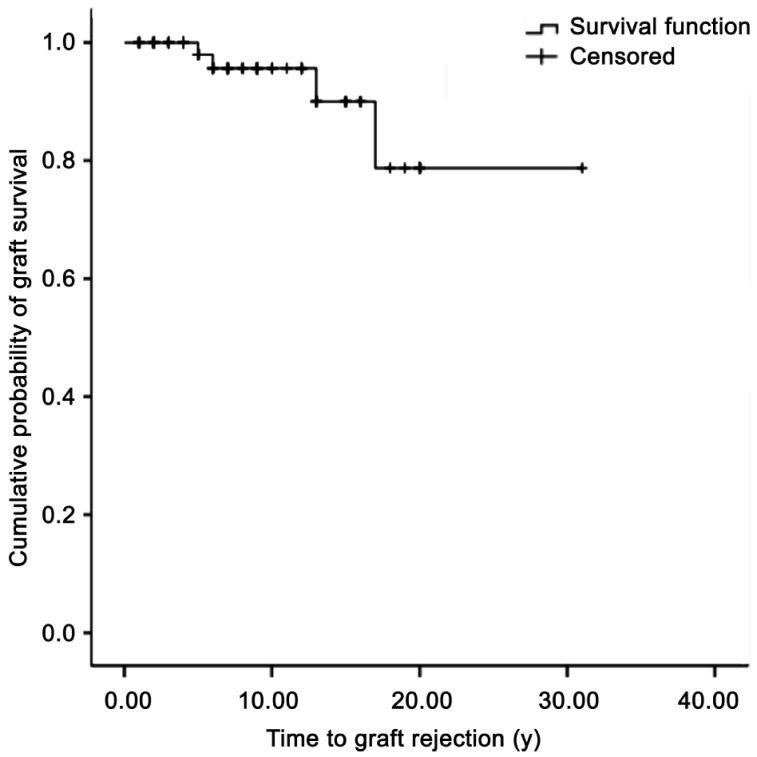
Sixty-nine eyes from 69 patients were finally included. The follow-up period was 8.64±6.13y. Graft rejection occurred in 4 eyes of 69 cases (5.8%), and the time to graft rejection was 2.1±1.3y. A Kaplan–Meier survival analysis showed that the estimated cumulative probability of graft rejection at 6, 13, and 17y after PK were 95.6%, 90.0%, and 78.8%, respectively. When we evaluated factors that might influence final BCVA in eyes, no disparity donor-host trephine size (same graft size) as well as higher spherical equivalent, and average K-value were associated with higher final BCVA. (P=0.006, 0.051, 0.092, and 0.021 in eyes with follow-up <8y; P=0.068, 0.065, and 0.030 in eyes with follow-up ≥8y, respectively).
CONCLUSION
The long-term results of PK in patients with KC were favorable with a high percentage of good BCVA. Less myopic change and low average K-reading, as well as a surgical technique using the same size donor-recipient button may provide better visual outcomes particularly in patients with KC.
Keywords: keratoconus, penetrating keratoplasty, visual outcome
INTRODUCTION
Keratoconus (KC) is an asymmetric, bilateral, progressive disorder in which the cornea assumes a conical shape as a result of non-inflammatory thinning and protrusion. This corneal thinning induces irregular astigmatism, myopia, and protrusion, which leads to mild to marked impaired vision quality KC becomes apparent during the second decade of life and during puberty, and it progresses over 10-20y until the progression gradually stops[1]–[4]. The severity of the disorder at the time when the progression stops can range from very mild irregular astigmatism to severe thinning, protrusion, and scarring that requires penetrating keratoplasty (PK)[4]. Many therapeutic modalities including spectacles, contact lenses, intracorneal rings, and recently collagen cross linking has been developed. With recent improvement in the surgical technique of lamellar keratoplasty, deep anterior lamellar keratoplasty has been considered as an acceptable alternative by many surgeons. However, PK still remains the treatment of choice in many advanced cases. The prognosis of PK in patients with KC is excellent compared to that of other diseases[5],[6]. The graft survival rate after 5-12y of PK is >90%[7].
For the better visual outcome, the main challenge of PK in patients with KC is the reduction of postoperative refractive errors including astigmatism. It is known that the amount of astigmatism after PK is affected by factors such as severity of the disorder, trephination and suturing technique, graft size, and amount of donor–recipient disparity[8]. In the present study, we investigated the long-term results of PK in patients with KC by a single corneal surgeon in a single center and evaluated factors that might influence the final visual outcome.
SUBJECTS AND METHODS
Subjects
The subjects of this retrospective study included patients who had undergone PK for KC between May 1980 and December 2005 at Seoul St. Mary's Hospital, Seoul, Korea. Cases with ocular diseases other than KC or repeated transplantation were excluded from chart review. The following data were recorded: patient age, gender, laterality, preoperative best-corrected visual acuity (BCVA), axial lengths, white-to-white diameter, anterior chamber depth, keratometric astigmatism, average K-value, death to preservation time, preservation to transplant time, surgical technique, and donor/host graft sizes. Donor age was not available. Postoperative information such as BCVA, keratometric astigmatism, refractive spherical equivalent (SE), corneal thickness, endothelial cell count (ECD), coefficient of variation (CV), pleomorphism (measured as a percentage of 6 or 6A), and postoperative complications including graft rejection and presence of glaucoma were recorded. This study was approved by the institutional review board of Seoul St. Mary's Hospital, and we adhered to the tenets of the Declaration of Helsinki.
Methods
Surgeries were performed by one surgeon (MS Kim) under local or general anesthesia. Recipient corneal button diameter ranged from 7.00 mm to 8.00 mm, and the same size or 0.25/0.50 mm oversized donor buttons were used according to the white-to-white diameter and preoperative refraction. For the recipient trephination, we used a hand-held trephine (Moria Inc. France) or the Hessburg-Barron trephine (Katena Products, Inc, Benvilles, NJ). With both types of trephine, the cutting of recipient cornea was achieved by rotation of the trephine blade until it partially entered the anterior chamber, and the incision was completed with right-and-left cutting scissors. The same size or 0.25/0.50 mm oversized donor button were selected according to the preoperative refraction and white-to-white diameter, and punched on the endothelial side. The PK technique was interrupted 10-0 nylon sutures (20-24 separate sutures) or a combined four separate sutures and a 16-bite running suture. Intraoperative keratoscopy was performed with the Maloney keratoscope after completion of suturing. Postoperative treatment included a combination of topical antibiotics and steroids for 1y postoperatively.
Statistical Analysis
Statistical analyses were performed with SPSS for Windows version 14.0 (SPPS, Inc., Chicago, IL, USA). All data are mean±standard deviations. A P-value <0.05 was considered statistically significant. The Kaplan-Meier method was used to evaluate the cumulative incidence rate of graft survival. In the analysis, an event (graft rejection) was defined as a corneal endothelial khodadust line, keratic precipitates, and inflammation in the anterior chamber. Graft failure was defined as persistent cloudy graft for more than 3mo. We divided the patients into two groups according to the follow-up period (8y). Within each group, we evaluated factors that might affect postoperative BCVA and compared the preoperative and postoperative variables between the groups. The Student's t-test was conducted for quantitative traits, and the χ2 test was used to compare proportions of qualitative traits between groups.
RESULTS
Sixty-nine eyes from 69 patients (34 right eyes and 35 left eyes) were included in the study. Among the patients, 50 (72.5%) were male and 19 (27.5%) were female. The mean age at the time of surgery was 26.8±7.8y (range, 13-52y). The preoperative characteristic and postoperative characteristics at the last visit are shown in Table 1.
Table 1. Preoperative characteristics of the transplanted eyes.
| Variables | Mean±SD(Range) |
| Age (a) | 26.8±7.8(13.0-52.0) |
| BCVA (decimal) | 0.03±0.05(0.00-0.40) |
| Corneal thickness(µm) | 414.4±108.9(265.0-721.0) |
| Mean host size(mm) | 7.76±0.25(7.00-8.00) |
| Mean donor size(mm) | 7.87±0.26(7.25-8.50) |
| Death to preservation time (min) | 221.2±126.8(30.0-515.0) |
| Preservation to transplantation time (d) | 1.4±0.5(1.0-3.0) |
BCVA: Best-corrected visual acuity.
The mean host corneal button size was 7.76±0.25 mm (range, 7.00-8.00 mm), and the mean donor button size was 7.87±0.26 mm (range, 7.25-8.50 mm). The most commonly used trephine size was 8 mm for the host trephine (44.4%) and 8 mm for the donor trephine (32.4%). In 39 eyes (56.5%), donor and host sizes were the same; in 26 eyes (37.7%), a 0.25 mm oversized donor graft was used; and a 0.5 mm oversized donor graft was used in 4 eyes (5.8%). The surgical technique was interrupted sutures in 41 eyes (59.4%), and combined sutures in 28 eyes (40.6%).
The follow-up period was 8.64±6.13y (range, 5.0-31.0y). Graft rejection occurred in 4 eyes of 69 cases (5.8%). The time to graft rejection was 2.1±1.3y (range, 1-5y). The Kaplan–Meier survival analysis showed that the estimated cumulative probabilities of graft rejection at 6, 13, and 17y after PK were 95.6%, 90.0%, and 78.8%, respectively (Figure 1). All rejections were successfully treated, resulting in no cases of graft failure. When we compared ECD between patients with graft rejection and without graft rejection at the last examination, ECD was significantly lower in patients who experienced graft rejection (857.9±188.9 cells/mm2) compared to patients without graft rejection (1225.7±506.6 cells/mm2) (P=0.001). Other endothelial cell parameters such as CV and 6A were not statistically different between the two groups. (35.43±9.09 vs 36.35±8.19, P=0.778; 51.57±9.38 vs 54.05±12.63, P=0.392, respectively). Corneal thickness at the last visit was also not significantly different(521.89 84.22 µm vs 550.62±51.97 µm, P=0.344).
Figure 1. Kaplan–Meier analysis for the cumulative probability of graft survival.
The median time estimated from the survival analysis to graft rejection was 27.29y (95% confidence interval, 23.66–30.93).
Glaucoma developed in 4 eyes of 69 cases (5.8%). The time to development of glaucoma was 2.5±1.9y (range, 1-5y). Glaucoma was controlled with topical anti-glaucoma medication in all eyes. No additional glaucoma surgeries were needed.
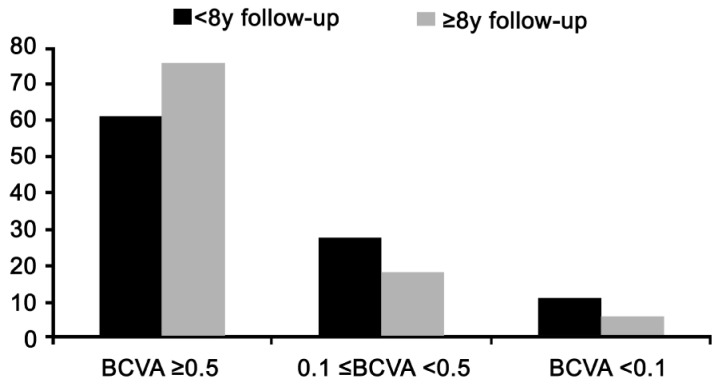
In eyes with follow-up <8y, 61.1% of eyes had BCVA at least 0.5. In eyes with follow-up ≥8y, 75.5% of eyes had BCVA at least 0.5 (Figure 2). In the evaluation of factors that might influence final BCVA, no disparity in donor-host trephine size (same graft size) was associated with higher BCVA at the last visit (P=0.006 in eyes with follow up <8y and P=0.068 in eyes with follow up ≥8y; Table 2). Additionally, postoperative refractive parameters such as less refractive SE and average K-value were associated with a postoperative BCVA ≥0.5 with marginal significance. (P=0.051 and 0.092 in eyes with follow up <8y; P=0.065 and 0.030 in eyes with follow up ≥8y; Table 2).
Figure 2. Best-corrected visual acuity at last follow-up examination.
Table 2. Comparison of pre and postoperative characteristics according to visual outcome at the last visit.
| Parameters | Eyes with follow up <8a |
Eyes with follow up ≥8a |
||||
| BCVA≥0.5 | BCVA<0.5 | P | BCVA≥0.5 | BCVA<0.5 | P | |
| n | 22 | 14 | 25 | 8 | ||
| Follow-up period | 4.3±1.8 | 4.7±1.5 | 0.450 | 13.2±3.7 | 15.3±7.8 | 0.497 |
| Preop. characteristics | ||||||
| Gender (% of M) | 81.8 | 64.2 | 0.267 | 78.2 | 70.0 | 0.673 |
| Laterality (% of OD) | 45.4 | 57.1 | 0.733 | 76.4 | 75.0 | 1.000 |
| Age (a) | 27.5±6.1 | 29.6±10.6 | 0.510 | 25.9±7.5 | 22.6±6.6 | 0.253 |
| BCVA (decimal) | 0.05±0.09 | 0.01±0.02 | 0.065 | 0.02±0.04 | 0.02±0.03 | 0.714 |
| Corneal thickness (µm) | 384.4±70.81 | 412.5±107.1 | 0.435 | 402.3±81.0 | 586.6±22.4 | 0.289 |
| Death to preservation time (min) | 273.7±42.05 | 223.3±157.3 | 0.483 | 150.2±94.3 | 360.0±107.6 | 0.054 |
| Preservation to transplantation time (d) | 1.2±0.5 | 1.5±0.6 | 0.537 | 1.6±0.9 | 2.0±1.0 | 0.581 |
| donor-recipient disparity (% of same size) | 72.7 | 23.0 | 0.006 | 73.9 | 28.5 | 0.068 |
| Suture technique [% of interrupted sutures (20-24 separate sutures)] | 63.6 | 71.4 | 0.727 | 45.4 | 87.5 | 0.092 |
| Postop. characteristics | ||||||
| Autorefractive SE (D) | -3.00±3.19 | -6.17±4.67 | 0.051 | -4.42±4.75 | -9.85±5.58 | 0.065 |
| Average K-value (D) | 43.03±1.66 | 46.25±4.55 | 0.092 | 43.89±2.09 | 48.40±4.70 | 0.030 |
| Keratometric astigmatism (D) | 4.67±2.62 | 5.05±2.97 | 0.700 | 2.80±1.70 | 4.76±1.98 | 0.029 |
| Corneal thickness (mm) | 547.0±70.4 | 529.0±42.5 | 0.347 | 548.7±45.6 | 585.8±48.2 | 0.080 |
| ECD (cells/mm2) | 1441.7±596.1 | 1069.6±478.0 | 0.057 | 1023.6±324.9 | 1224.2±384.5 | 0.261 |
| CV | 36.5±10.1 | 34.5±7.6 | 0.541 | 37.5±7.0 | 38.2±10.1 | 0.853 |
| 6A (%) | 56.5±12.3 | 60.6±13.0 | 0.376 | 51.0±10.3 | 51.1±13.7 | 0.991 |
BCVA: Best-corrected visual acuity; SE: Spherical equivalent; D: Diopter; ECD: Endothelial cell density; CV: Coefficient of variation; 6A: A percentage of 6.
DISCUSSION
In the present study, the overall rate of graft rejection was 5.7%, which took an average of 2y. The Kaplan–Meier survival analysis showed that the estimated cumulative probability of graft rejection at 6, 13, and 17y after PK were 95.6%, 90.0%, and 78.8%, respectively. These results are consistent with previous studies that reported rejection rates of 4.3%-31.0%, and that most rejection episodes occur within 2y after PK[7],[9]–[15]. In this study, patients who experienced a rejection episode had a significantly lower ECD compared to that in patients who did not have a graft rejection. In one study that tried to predict endothelial cell loss and long-term corneal graft survival, the authors suggested that the rapid component of cell loss after PK lasts longer than that after cataract surgery, becoming negligible only after 4y, and reflecting more severe surgical trauma and postoperative complications, including cell-mediated immunological rejection[16]. In this regard, it seemed that the immunological rejection affected the ECD, although the effect was not reflected in other corneal endothelial cell parameters, such as 6A, CV, or pachymetry. The ECD at which corneal edema occurs has been estimated to be 300-700 cells/mm2[17],[18]. Although the ECD is an important marker, the ECD alone is not the most sensitive measure of endothelial cell health, as the endothelium functions even at low ECDs of <500 cells/mm2[19]. Several previous reports have suggested that polymegatism (CV) and pleomorphism (6A) are more sensitive measures of endothelium under stress[20]. In this study, the final CV was 40% and 6A was >50% even in patients who experienced immunological rejection, which can be considered as in a normal range. The reason for the relatively normal CV and 6A in patients who experienced rejection seemed to be related that the value was gained at the last visit when the acute stress had already recovered. Therefore, only ECD reflected the stress of immunological rejection.
We evaluated the risk factors that might influence the final BCVA. A BCVA ≥0.5 at the final visit occurred in 66.7% of the eyes in our study, which was similar with previous studies[7],[12]–[15]. Among the demographic, preoperative, and postoperative parameters, significantly less refractive error and lower average K-value were found in patients with a BCVA ≥0.5 at the final visit.
A refractive error that is reasonably corrected with spectacles as well as clear graft are needed for successful PK[12]. Thus, unexpectedly high average keratometry values and astigmatism after PK may affect the visual outcome unfavorably, and corneal surgeons should try to minimize postoperative astigmatism by applying an appropriate suturing technique.
In addition, no disparity between donor and recipient trephine size was associated with a better final BCVA (P=0.006 in eye with follow up <8y and P=0.068 in eyes with follow up ≥8y). The donor tissue trephine is routinely sized 0.25 mm larger than the host trephine for conventional PK. However, same-diameter trephines for both donor and host tissue have a benefit in patients with KC, because it helps to reduce postoperative myopia. Consistent with this, a larger disparity between cornea-recipient corneal sizes was shown to induce more refractive errors in patients with KC[12].
The incidence of glaucoma in this study was 8.13%, which was consistent with a study that evaluated extended long-term outcomes after PK in patients with KC[7]. They reported that the post-PK glaucoma incidence was 5.3%. Factors associated with glaucoma after PK include preexisting glaucoma, aphakia, anterior segment inflammation, intraocular lens (IOL) removal vitrectomy, and host ocular diseases such as spontaneous perforation, mesodermal dysgenesis, trauma, iridocorneal endothelial syndrome, and aphakia[21],[22]. The incidence of glaucoma after PK in patients with KC is relatively low, as shown in this study (5.8%). An important mechanism for glaucoma development after PK is iridocorneal angle compression. Although an oversized donor graft may hypothetically alleviate an iridocorneal collapse, a statistically significant effect of oversized grafts on post-KP glaucoma had not been reported. In a study by Feizi et al[23], corneal hysteresis and corneal resistance factor which showed significant positive correlations with intraocular pressure, were affected by the donor-recipient disparity. In this study, no immediate IOP elevation after PK was noted, which may indicate that the disparity between donor and recipient trephine size was not associated with the development of postoperative glaucoma. In a study using pentacam, anterior chamber depth and volume was shown to be deeper and larger with the progression of keratoconus[24] The deeper anterior chamber in keratoconus compared to others may be beneficial in preventing glaucoma and other complications caused by same-size donor. Further studies regarding the effect of graft size difference on the postoperative outcomes are warranted.
In summary, the long-term results of PK in patients with KC were favorable with a high percentage of good BCVA. Less myopic shift and lower average K-reading, as well as clear graft are mandatory to achieve better visual rehabilitation. Additionally, using the same size donor-recipient button may provide better visual outcomes particularly in patients with KC.
Acknowledgments
Conflicts of Interest: Choi JA, None; Lee MA, None; Kim MS, None.
REFERENCES
- 1.Muftuoglu O, Ayar O, Ozulken K, Ozyol E, Akıncı A. Posterior corneal elevation and back difference corneal elevation in diagnosing forme fruste keratoconus in the fellow eyes of unilateral keratoconus patients. J Cataract Refract Surg. 2013;39(9):1348–1357. doi: 10.1016/j.jcrs.2013.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Piñero DP, Nieto JC, Lopez-Miguel A. Characterization of corneal structure in keratoconus. J Cataract Refract Surg. 2012;38(12):2167–2183. doi: 10.1016/j.jcrs.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 3.Choi JA, Kim MS. Progression of keratoconus by longitudinal assessment with corneal topography. Invest Ophthalmol Vis Sci. 2012;53(2):927–935. doi: 10.1167/iovs.11-8118. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Jiménez M, Santodomingo-Rubido J, Wolffsohn JS. Keratoconus: a review. Contact Lens & Anterior Eye. 2010;33(4):157–166. doi: 10.1016/j.clae.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Muraine M, Sanchez C, Watt L, Retout A, Brasseur G. Long-term results of penetrating keratoplasty. A 10-year-plus retrospective study. Graefes Arch Clin Exp Ophthalmol. 2003;241(7):571–576. doi: 10.1007/s00417-003-0691-z. [DOI] [PubMed] [Google Scholar]
- 6.Claesson M, Armitage WJ. Ten-Year Follow-up of Graft Survival and Visual Outcome After Penetrating Keratoplasty in Sweden. Cornea. 2009;28(10):1124–1129. doi: 10.1097/ICO.0b013e3181a2a7a6. [DOI] [PubMed] [Google Scholar]
- 7.Niziol LM, Musch DC, Gillespie BW, Marcotte LM, Sugar A. Long-term outcomes in patients who received a corneal graft for keratoconus between 1980 and 1986. Am J Ophthalmol. 2013;155(2):213–219. doi: 10.1016/j.ajo.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Woodford SV. Corneal Surgery: Theory, Technique and Tissue. 3rd ed. New York: Mosby; 1999. pp. 431–440. [Google Scholar]
- 9.Lim L, Pesudov K, Coster DJ. Penetrating keratoplasty for keratoconus: Visual outcomes and success. Ophthalmology. 2000;107(6):1125–1131. doi: 10.1016/s0161-6420(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 10.Brierly SC, Izquierdo L, Mannis MJ. Penetrating keratoplasty for keratoconus. Cornea. 2000;19(3):329–332. doi: 10.1097/00003226-200005000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Duman F, Kosker M, Suri K, Reddy JC, Ma JF, Hammersmith KM, Nagra PK, Rapuano CJ. Indications and Outcomes of Corneal Transplantation in Geriatric Patients. Am J Ophthalmol. 2013;156(3):600–607.e2. doi: 10.1016/j.ajo.2013.04.034. [DOI] [PubMed] [Google Scholar]
- 12.Javadi MA, Motlagh BF, Jafarinasab MR, Rabbanikhah Z, Anissian A, Souri H, Yazdani S. Outcomes of penetrating keratoplasty in keratoconus. Cornea. 2005;24(8):941–946. doi: 10.1097/01.ico.0000159730.45177.cd. [DOI] [PubMed] [Google Scholar]
- 13.Basu S, Reddy JC, Vaddavalli PK, Vemuganti GK, Sangwan VS. Long-term outcomes of penetrating keratoplasty for keratoconus with resolved corneal hydrops. Cornea. 2012;31(6):615–620. doi: 10.1097/ICO.0b013e31823d03e3. [DOI] [PubMed] [Google Scholar]
- 14.Olson RJ, Pingree M, Ridges R, Lundergan ML, Alldredge C, Jr, Clinch TE. Penetrating keratoplasty for keratoconus: a long-term review of results and complications. J Cataract Refract Surg. 2000;26(7):987–991. doi: 10.1016/s0886-3350(00)00430-2. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka S, Honda N, Ono K, Mimura T, Usui T, Amano S. Extended long-term results of penetrating keratoplasty for keratoconus. Cornea. 2010;29(5):528–530. doi: 10.1097/ICO.0b013e3181c29705. [DOI] [PubMed] [Google Scholar]
- 16.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44(8):3326–3331. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 17.Joyce NC. Proliferative capacity of the corneal endothelium. Prog Retin Eye Res. 2003;22(3):359–389. doi: 10.1016/s1350-9462(02)00065-4. [DOI] [PubMed] [Google Scholar]
- 18.Holladay JT, Bishop JE, Prager TC. The resiliency of the corneal endothelium to refractive and intraocular surgery. Cornea. 2000;19(3):263–273. doi: 10.1097/00003226-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Lass JH, Sugar A, Benetz BA, Beck RW, Dontchev M, Gal RL, Kollman C, Gross R, Heck E, Holland EJ, Mannis MJ, Raber I, Stark W, Stulting RD, Cornea Donor Study Investigator Group Endothelial cell density to predict endothelial graft failure after penetrating keratoplasty. Arch Ophthalmol. 2010;128(1):63–69. doi: 10.1001/archophthalmol.2010.128.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krachmer JH, Mannis MJ, Holland EJ. Cornea. 3rd ed. 1. St Louis: Mosby; 2011. Specular microscopy; pp. 186–188. [Google Scholar]
- 21.Moisseiev E, Varssano D, Rosenfeld E, Rachmiel R. Intraocular pressure after penetrating keratoplasty and Descemet's stripping automated endothelial keratoplasty. Can J Ophthalmol. 2013;48(3):179–185. doi: 10.1016/j.jcjo.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Almousa R, Nanavaty MA, Daya SM, Lake DB. Intraocular pressure control and corneal graft survival after implantation of ahmed valve device in high-risk penetrating keratoplasty. Cornea. 2013;32(8):1099–1104. doi: 10.1097/ICO.0b013e31828d2a17. [DOI] [PubMed] [Google Scholar]
- 23.Feizi S, Einollahi B, Yazdani S, Hashemloo A. Graft biomechanical properties after penetrating keratoplasty in keratoconus. Cornea. 2012;31(8):855–858. doi: 10.1097/ICO.0b013e31823f8ce4. [DOI] [PubMed] [Google Scholar]
- 24.Emre S, Doganay S, Yologlu S. Evaluation of anterior parameters in keratoconic eyes measured with the pentacam system. J Cataract Refract Surg. 2007;33(10):1708–1712. doi: 10.1016/j.jcrs.2007.06.020. [DOI] [PubMed] [Google Scholar]