Abstract
AIM
To report the cytology results of 25-gauge transconjunctival (25G-TSV) diagnostic vitrectomy in cases suspicious for intraocular lymphoma (IOL), and compare the results to those reported in the literature.
METHODS
Clinical and cytopathological records of 18 vitreous biopsy specimens obtained via 25G-TSV diagnostic vitrectomy in 12 patients suspicious for IOL were reviewed retrospectively. A review of the literature in regards to the diagnostic yields of vitreous specimens obtained via 25-gauge and 20-gauge diagnostic vitrectomy in suspected cases of IOL was performed.
RESULTS
Eighteen eyes from 12 patients with clinical suspicion of IOL underwent diagnostic 25G-TSV. The cytopathological investigations demonstrated IOL in 15 eyes (83.3%). Vitreous analysis was non-diagnostic in 3 eyes (16.7%).
CONCLUSION
Twenty-five-gauge diagnostic vitrectomy yields adequate sample for cytological evaluation of the vitreous in cases suspicious for IOL. The diagnostic results of the 25G-TSV in the current study are superior to those reported for 20-gauge vitrectomy but equivalent to those reported for 25G-TSV in the published literature.
Keywords: 25-gauge vitrectomy, 20-gauge vitrectomy, intraocular lymphoma
INTRODUCTION
Intraocular lymphomas (IOLs) are a heterogeneous group of intraocular malignancies that are subdivided into primary vitreoretinal lymphomas, primary uveal lymphomas, and secondary uveal lymphomas. Primary vitreoretinal lymphoma as a subset of primary central nervous system lymphoma is the most common form of IOL. It is usually characterized by proliferation of large B-cells, but in rare instances it may be secondary to proliferation of T cells[1]–[3]. IOLs occur rarely and have poor prognosis, therefore, early diagnosis and prompt treatment can significantly influence the survival of the afflicted patients[4],[5]. These tumors often present with clinical features of vitritis and/or chronic granulomatous panuveitis, requiring a vitreous biopsy for definitive diagnosis and subsequent management[6]–[8]. Prior to implementation of vitrectomy techniques for obtaining vitreous samples, fine needle aspiration biopsy was the method of choice in uveitic eyes suspicious for having IOL. This technique, however, was associated with a high rate of false negative results[9],[10]. Diagnostic vitrectomy is a powerful tool for early detection of IOL and appears to be superior to fine needle aspiration biopsy. Diagnostic vitrectomy may be performed with either 20-gauge or 25-gauge techniques, both of which are known to produce sufficient amounts of vitreous with good preservation of cellular integrity necessary for cytopathological analysis[8],[11]–[15]. However, 25-gauge transconjunctival vitrectomy (25G-TSV) has some advantages over the standard 20-gauge vitrectomy. The 25G-TSV is performed transconjunctivally, eliminating the need for peritomy and due to the small size of the scleral incisions, no sutures are necessary. These in turn decrease the operating time, hasten patient recovery, and reduce the post-operative inflammation[16]–[20]. Although the safely and efficacy of 25G-TSV is well established, many surgeons remain uncertain about the utility of the 25G-TSV as a diagnostic tool[8]. This study demonstrates that 25-G TSV is adequate for obtaining vitreous specimens necessary for cytological analysis in cases suspicious for IOL. In addition, we reviewed the current literature with regards to the diagnostic results of 20- and 25-gauge vitrectomies in IOL.
SUBJECTS AND METHODS
Subjects
After obtaining approval from the ethics committee of Ophthalmic Research Center, a retrospective non-comparative study was performed on the medical and cytopathological records of 18 vitreous biopsy specimens received at the ocular pathology laboratory of Shahid Beheshti University of Medical Sciences in Tehran, Iran, between June 2006 and April 2011. The specimens were obtained via 25-gauge vitrectomy from 12 patients with chronic posterior or panuveitis, which were recalcitrant to corticosteroid therapy, and were suspicious for IOL.
Methods
The neat and diluted vitreous samples were fixed in 10% formalin and rapidly transferred to the pathology laboratory. Cytospins and cytoblocks were prepared and thin sections were taken from the cytoblocks. The specimens were stained with hematoxylin & eosin, May-Grünewald-Giemsa, Gram, and Periodic acid-Schiff stains. Furthermore, immunohistochemical stainings for CD3, CD20, and CD79a were performed. The cytopathological diagnoses were categorized into two groups: 1) intraocular lymphoma and 2) nonspecific and/or non-diagnostic vitreous fluid analysis. Specimens that showed a dominance of atypical B cells based on morphology and immune reactivity for CD20 and CD79a, or a dominance of atypical T cells based on cytomorphology and immune-phenotyping for CD3 were considered intraocular lymphoma. Nonspecific and/or non-diagnostic vitreous specimens were defined as the presence of either nonspecific chronic inflammation, when there was no atypia or lymphoid monoclonality, or the vitreous specimen was hypocellular. Microbiological cultures were obtained in all specimens.
We also performed a comprehensive review of the literature by searching the PubMed and ISI web of knowledge to identify the published articles on 20- and 25-gauge diagnostic vitrectomy in cases of uveitis suspicious for malignancy. We used the following keywords for our search: “intraocular lymphoma”, “diagnostic vitrectomy”, “vitrectomy and intraocular lymphoma”, “25-gauge vitrectomy”, “20-gauge vitrectomy”, and “sutureless vitrectomy”. The titles, abstracts, and the texts were reviewed to determine potentially eligible studies. The variables in our study included patient age, gender, the rate of IOL established by positive vitreous fluid analysis, and the rate of nonspecific and/or non-diagnostic vitreous specimens.
RESULTS
We reviewed the post-vitrectomy cytological diagnosis of 18 eyes from 12 patients with a mean age of 54.8±13.3y (age range 30-78y, F:M ratio 2:1) suspicious for IOL, who underwent 25G-TSV. The clinical and cytological data, as well as patient demographics, are summarized in Table 1.
Table 1. Summary of demographic data and clinical and cytological diagnoses for 18 eyes from 12 patients who underwent transconjunctival sutureless 25-gauge diagnostic vitrectomy.
| Case | Sex | Age(a) | Eye | Clinical diagnosis | Cytological diagnosis |
| 1 | F | 67 | OU | Bilateral granulomatous uveitis, suspicious for IOL | Bilateral Intraocular DLBCL |
| 2 | F | 60 | OU | Suspicious for IOL | Bilateral Intraocular DLBCL |
| 3 | F | 45 | OU | Suspicious for Intraocular lymphomaHistory of CNS lymphoma | Bilateral Intraocular DLBCL |
| 4 | F | 53 | OD | Suspicious for Intraocular lymphoma | Non-specific chronic inflammation |
| 5 | M | 57 | OS | Suspicious for IOL | Intraocular DLBCL |
| 6 | M | 30 | OD | Bilateral chronic uveitis, suspicious for IOL | Intraocular DLBCL |
| 7 | F | 78 | OU | Suspicious for IOLHistory of Mycosis Fungoides | Bilateral Intraocular T-cell lymphoma |
| 8 | M | 60 | OS | Suspicious for IOL | Hypocellular specimen |
| 9 | F | 64 | OS | Suspicious for IOL | Non-specific chronic inflammation |
| 10 | M | 35 | OU | Suspicious for IOL | Bilateral Intraocular DLBCL |
| 11 | F | 57 | OD | Chronic bilateral panuveitis, suspicious for IOL | Intraocular DLBCL |
| 12 | F | 51 | OU | Suspicious for IOL | Bilateral Intraocular DLBCL |
F: Female; M: Male; IOL: Intraocular lymphoma; DLBCL: Diffuse large B cell lymphoma; OD: Right eye; OS: Left eye; OU: Both eyes.
No post-operative complications, such as hypotony, endophthalmitis, cataract, glaucoma, and retinal tear or detachment developed in any of the cases in our series. Microbiological cultures were negative in all vitreous samples.
Six patients underwent bilateral vitrectomy while the rest underwent unilateral vitrectomy. Based on cytomorphology and immune phenotypic features, IOL was demonstrated in 15 eyes (83.3%) of nine patients (75%); 13 eyes from eight patients were diagnosed as having diffuse large B-cell lympohoma and 2 eyes from one patient was diagnosed with intraocular T-cell lymphoma (Figure 1). Cytopathologic examinations in cases with intraocular diffuse large B-cell lymphoma disclosed the presence of atypical lymphoid cells with large irregular nuclei and predominant immune reactivity for CD20 and CD79a. The lymphoid infiltrate in vitreous specimens with intraocular T-cell lymphoma was composed of large atypical lymphoid cells with diffuses immune reactivity for CD3 and lack of immune reactivity for CD20. Vitreous cytologic analysis was nonspecific and/or non-diagnostic in 3 eyes (16.7%) of 3 patients (25%); two eyes of two patients with nonspecific chronic inflammation and one with a hypocellular vitreous specimen. Cytopathologic examination of vitreous specimens in cases with nonspecific chronic inflammation disclosed the presence of small-sized bland-looking lymphoid cells with no evidence of monoclonality for T- or B-cells on immunocytochemical studies. Only one patient (both eyes in case 3) had a history of CNS involvement. Others did not have a history of CNS disease and did not show any evidence of CNS involvement at their one-year follow-up visit.
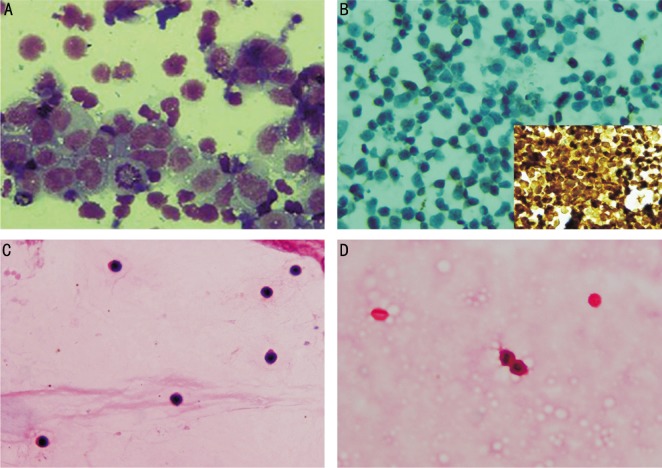
Figure 1. Photomicrographs of vitreous cytopathology in patients with clinical suspicion of IOL.
A: Diffuse large B-cell lymphoma in case 1 with aggregation of large pleomorphic mononuclear cells with irregular nuclei, prominent nucleoli and atypical mitosis in a vitreous cytospin preparation (Giemsa stain,×1000); B: Intraocular T cell lymphoma in case 7 with the presence of atypical lymphoid cells with mitosis, lack of immune-reactivity for CD20 (×400) and diffuse immune-reactivity (inset) for CD3 (×600); C: Nonspecific chronic inflammation in case 4 with the presence of small lymphocytes and no atypical features (hematoxylin & eosin,×400); D: Non-diagnostic and hypocellular vitreous specimen in case 8 with the presence of few red blood cells and few macrophage-like cells (hematoxylin & eosin, ×400).
DISCUSSION
Our study demonstrated a high diagnostic yield for the vitreous harvested via 25G-TSV (Table 2) in suspected cases of IOL (83.3%) compared to the rates reported for 20-gauge vitrectomy (a range of 9.9% to 50.0%) in the literature[21]–[27]. Additionally, our results were comparable with those of Yeh et al[14] that used the same technique for harvesting the vitreous. Our study had several limitations. These included limited number of cases, retrospective nature of the study, and probability of selection bias in our tertiary referral center. Despite the limitations of the current study, our results show that 25G-TSV is an effective method for harvesting adequate amounts of vitreous in eyes suspected for IOL and can be substituted for 20-gauge vitrectomy in such cases.
Table 2. Diagnostic results of vitreous analysis harvested via 25G-TSV versus 20-gauge diagnostic vitrectomy in cases suspected for IOL.
| Vitrectomy technique used for vitreous sampling | Clinically suspected IOL eyes | IOL established by vitreous fluid analysis |
| 25G-TSV (current study) | 18 (100) | 15 (83.3) |
| 25G-TSV (Yeh et al) | 12 (100) | 8 (66.7) |
| 20-gauge vitrectomy (Davis et al) | 28 (33) | 14 (50.0) |
| 20-gauge vitrectomy (Akpek et al study) | 26 (100) | 10 (38.5) |
| 20-gauge vitrectomy (Priem et al) | 21 (53.8) | 5 (23.8) |
| 20-gauge vitrectomy (Verbraeken) | 28 (100) | 6 (21.4) |
| 20-gauge vitrectomy (Wittenberg et al) | 71 (25.5) | 14 (19.7) |
| 20-gauge vitrectomy (Liu et al) | 74 (100) | 8 (10.8) |
| 20-gauge vitrectomy (Mruthyunjaya et al) | 71 (78.9) | 7 (9.9) |
25G-TSV: 25-gauge transconjunctival vitrectomy; IOL: intraocular lymphoma.
n(%)
Although 25G-TSV was accepted amongst vitreoretinal surgeons as both safe and effective for obtaining vitreous specimens and establishing a diagnosis in cases suspected for IOL, only a few authors have published their experiences with this method[12],[14]. Yeh et al[14] used 25G-TSV in 12 eyes with suspected IOL and confirmed the diagnosis in 8 (66.7%) eyes (Table 2); in his case series cytopathological changes consistent with IOL were found in only 3 eyes (25%), however, the diagnostic yield for IOL was increased to 66.7% when gene arrangement investigations, cytokine studies, and/or flow cytometry were used in addition. In our series, 25G diagnostic-TSV preserved the integrity of the harvested cells and revealed the diagnosis of intraocular lymphoma in the majority of the specimens using cytopathology alone, without the implementation of complementary studies (i.e. genetic studies).
Compared to the 20-gauge vitrectomy that necessitates conjunctival incisions, sclerotomies, and scleral sutures, small-gauge transconjunctival vitrectomy such as the 25G-TSV offers significant reduction of post-operative pain, rapid wound healing and speedy visual recovery[17],[19],[20],[28]–[32]. It can be the preferable method in cases that require diagnostic vitrectomy with less intraocular manipulation[20].
Although rare events such as bleb formation at the sclerotomy site, transient ocular hypotony during the early post-operative period may be higher in 25G-TSV; no significant increase in rate of severe complications such as endophthalmitis was noted following 25G-TSV when compared to the conventional 20G vitrectomy[31],[35]–[40]. The risk of other post-operative complications, such as retinal detachment and vitreous hemorrhage, was lower in 25G-TSV compared to the 20-gauge vitrectomy[20],[32],[41]. In the series by Yeh et al[14], 4 eyes developed transient postoperative hypotony. However, in our series no such complications were seen.
In conclusion, diagnostic vitrectomy using the 25G-TSV is superior to the tradition 20G vitrectomy for obtaining adequate vitreous samples for cytological analysis in suspected cases of IOL, where minimal intraocular manipulation is preferred.
Acknowledgments
The authors thank Mrs Kefayat Mohammadi Roshanagh for her assistance in this study.
Conflicts of Interest: Kanavi MR, None; Soheilian M, None; Hosseini SB, None; Azari AA, None.
REFERENCES
- 1.Coupland SE, Heimann H, Bechrakis NE. Primary intraocular lymphoma: a review of the clinical, histopathological and molecular biological features. Graefes Arch Clin Exp Ophthalmol. 2004;242(11):901–913. doi: 10.1007/s00417-004-0973-0. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC, Rubenstein JL, Coupland SE, Davis JL, Harbour JW, Johnston PB, Cassoux N, Touitou V, Smith JR, Batchelor TT, Pulido JS. Primary Vitreoretinal Lymphoma: A Report from an International Primary Central Nervous System Lymphoma Collaborative Group Symposium. Oncologist. 2011;16(11):1589–1599. doi: 10.1634/theoncologist.2011-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coupland SE, Damato B. Understanding intraocular lymphomas. Clin and Exp Ophthalmol. 2008;36(6):564–578. doi: 10.1111/j.1442-9071.2008.01843.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis JL. Intraocular lymphoma: a clinical perspective. Eye (Lond) 2013;27(2):153–162. doi: 10.1038/eye.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura K, Usui Y, Goto H, Japanese Intraocular Lymphoma Study Group Clinical features and diagnostic significance of the intraocular fluid of 217 patients with intraocular lymphoma. Jpn J Ophthalmol. 2012;56(4):383–389. doi: 10.1007/s10384-012-0150-7. [DOI] [PubMed] [Google Scholar]
- 6.Smit RLMJ, Baarsma GS, de Vries J. Classification of 750 consecutive uveitis patients in the Rotterdam Eye Hospital. Int Opthalmol. 1993;17(2):71–76. doi: 10.1007/BF00942778. [DOI] [PubMed] [Google Scholar]
- 7.Zamiri P, Boyd S, Lightman S. Uveitis in the elderly-is it easy to identify the masquerade? Br J Ophthalmol. 1997;81(10):827–831. doi: 10.1136/bjo.81.10.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Margolis R. Diagnostic vitrectomy for the diagnosis and management of posterior uveitis of unknown etiology. Curr Opin Ophthalmol. 2008;19(3):218–224. doi: 10.1097/ICU.0b013e3282fc261d. [DOI] [PubMed] [Google Scholar]
- 9.Wakefield D, Zierhut M. Intaocular Lymphoma: More questions than answers. Ocul Immunol Inflamm. 2009;17(1):6–10. doi: 10.1080/09273940902834413. [DOI] [PubMed] [Google Scholar]
- 10.Sen HN, Bodaghi B, Hoang PL, Nussenblatt R. Primary Intraocular Lymphoma: Diagnosis and Differential Diagnosis. Ocul Immunol Inflamm. 2009;17(3):133–141. doi: 10.1080/09273940903108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uy HS, Foster CS. Diagnostic vitrectomy and uveitis. Int Ophthalmol Clin. 1999;39(1):223–235. doi: 10.1097/00004397-199903910-00020. [DOI] [PubMed] [Google Scholar]
- 12.Kanavi MR, Soheilian M, Bijanzadeh B, Peyman GA. Diagnostic vitrectomy (25-gauge) in a case with intraocular lymphoma masquerading as bilateral granulomatous panuveitis. Eur J Ophthalmol. 2010;20(4):795–798. doi: 10.1177/112067211002000426. [DOI] [PubMed] [Google Scholar]
- 13.Trikha R, Yeung C, Modjtahedi S, Martin S, Dwyre DM, Telander DG. B-cell lymphoma line (Raji) viability and surface marker expression minimally affected by 20- and 25-gauge vitrectomy systems analyzed by flow cytometry. Retina. 2010;30(9):1505–1510. doi: 10.1097/IAE.0b013e3181d87e1f. [DOI] [PubMed] [Google Scholar]
- 14.Yeh S, Weichel ED, Faia LJ, Albini TA, Wroblewski KK, Stetler-Stevenson M, Ruiz P, Sen HN, Chan CC, Nussenblatt RB. 25-Gauge transconjunctival sutureless vitrectomy for the diagnosis of intraocular lymphoma. Br J Ophthalmol. 2010;94(5):633–638. doi: 10.1136/bjo.2009.167940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolis R, Brasil OFM, Lowder CY, Singh RP, Kaiser PK, Smith SD, Perez VL, Sonnie C, Sears JE. Vitrectomy for diagnosis and management of uveitis of unknown cause. Ophthalmology. 2007;114(10):1893–1897. doi: 10.1016/j.ophtha.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 16.Fabian ID, Moisseiev J. Sutureless vitrectomy: evolution and current practices. Br J Ophthalmol. 2011;95(3):318–324. doi: 10.1136/bjo.2009.176495. [DOI] [PubMed] [Google Scholar]
- 17.Fujii GY, de Juan E, Humayun MS, Pieramici DJ, Chang TS, Awh C, Ng E, Barnes A, Wu SL, Sommerville DN. A new 25-gauge instrument system for transconjunctival sutureless vitrectomy surgery. Ophthalmology. 2002;109(10):1807–1812. doi: 10.1016/s0161-6420(02)01179-x. [DOI] [PubMed] [Google Scholar]
- 18.Eckardt CM. Transconjunctival sutureless 23-gauge vitrectomy. Retina. 2005;25(2):208–211. doi: 10.1097/00006982-200502000-00015. [DOI] [PubMed] [Google Scholar]
- 19.Williams GA. 25-, 23-, or 20-gauge instrumentation for vitreous surgery? Eye (Lond) 2008;22(10):1263–1266. doi: 10.1038/eye.2008.20. [DOI] [PubMed] [Google Scholar]
- 20.Kellner L, Wimpissinger B, Stolba U, Brannath W, Binder S. 25-gauge vs 20-gauge system for pars plana vitrectomy: a prospective randomised clinical trial. Br J Ophthalmol. 2007;91(7):945–948. doi: 10.1136/bjo.2006.106799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mruthyunjaya P, Jumper JM, McCallum R, Patel DJ, Cox TA, Jaffe GJ. Diagnostic yield of vitrectomy in eyes with suspected posterior segment infection or malignancy. Ophthalmology. 2002;109(6):1123–1129. doi: 10.1016/s0161-6420(02)01033-3. [DOI] [PubMed] [Google Scholar]
- 22.Liu K, Klintworth GK, Dodd LG. Cytologic findings in vitreous fluids. Analysis of 74 specimens. Acta Cytol. 1999;43(2):201–206. doi: 10.1159/000330977. [DOI] [PubMed] [Google Scholar]
- 23.Wittenberg LA, Maberley DA, Ma PE, Wade NK, Gill H, White VA. Contribution of vitreous cytology to final clinical diagnosis: Fifteen-year review of vitreous cytology specimens from one institution. Ophthalmology. 2008;115(11):1944–1950. doi: 10.1016/j.ophtha.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Verbraeken H. Diagnostic vitrectomy and chronic uveitis. Graefes Arch Clin Exp Ophthalmol. 1996;234(Suppl. 1):S2–7. doi: 10.1007/BF02343040. [DOI] [PubMed] [Google Scholar]
- 25.Priem H, Verbraeken H, de Laey JJ. Diagnostic problems in chronic vitreous inflammation. Graefes Arch Clin Exp Ophthalmol. 1993;231(8):453–456. doi: 10.1007/BF02044231. [DOI] [PubMed] [Google Scholar]
- 26.Akpek EK, Ahmed I, Hochberg FH, Soheilian M, Dryja TP, Jakobiec FA, Foster CS. Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999;106(9):1805–1810. doi: 10.1016/S0161-6420(99)90341-X. [DOI] [PubMed] [Google Scholar]
- 27.Davis JL, Miller DM, Ruiz P. Diagnostic testing of vitrectomy specimens. Am J Ophthalmol. 2005;140(5):822–829. doi: 10.1016/j.ajo.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 28.O'Malley C, Heintz RM., Sr Vitrectomy with an alternative instrument system. Ann Ophthalmol. 1975;7(4):585–588, 591–594. [PubMed] [Google Scholar]
- 29.Fujii GY, De Juan E, Jr, Humayun MS, Chang TS, Pieramici DJ, Barnes A, Kent D. Initial experience using the transconjunctival sutureless vitrectomy system for vitreoretinal surgery. Ophthalmology. 2002;109(10):1814–1820. doi: 10.1016/s0161-6420(02)01119-3. [DOI] [PubMed] [Google Scholar]
- 30.Ibarra MS, Hermel M, Prenner JL, Hassan TS. Longer-term outcomes of transconjunctival sutureless 25-gauge vitrectomy. Am J Ophthalmol. 2005;139(5):831–836. doi: 10.1016/j.ajo.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Yanyali A, Celik E, Horozoglu F, Oner S, Nohutcu AF. 25-Gauge transconjunctival sutureless pars plana vitrectomy. Eur J Ophthalmol. 2006;16(1):141–147. doi: 10.1177/112067210601600123. [DOI] [PubMed] [Google Scholar]
- 32.Ghoraba HH, Elgouhary SM, Ellakwa AF. Different techniques of transconjunctival cannulated vitrectomy versus conventional non-cannulated vitrectomy in various vitreoretinal disorders. Clin Ophthalmol. 2013;7:1859–1865. doi: 10.2147/OPTH.S38997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizzo S, Genovesi-Ebert F, Murri S, Belting C, Vento A, Cresti F, Manca ML. 25-gauge, sutureless vitrectomy and standard 20-gauge pars plana vitrectomy in idiopathic epiretinal membrane surgery: a comparative pilot study. Graefes Arch Clin Exp Ophthalmol. 2006;244(4):472–479. doi: 10.1007/s00417-005-0173-6. [DOI] [PubMed] [Google Scholar]
- 34.Wickham L, Bunce C, Kwan AS, Bainbridge J, Aylward GW. A pilot randomised controlled trial comparing the post-operative pain experience following vitrectomy with a 20-gauge system and the 25-gauge transconjunctival system. Br J Ophthalmol. 2010;94(1):36–40. doi: 10.1136/bjo.2008.153411. [DOI] [PubMed] [Google Scholar]
- 35.Lakhanpal RR, Humayun MS, de Juan E, Jr, Lim JI, Chong LP, Chang TS, Javaheri M, Fujii GY, Barnes AC, Alexandrou TJ. Outcomes of 140 consecutive cases of 25-gauge transconjunctival surgery for posterior segment disease. Ophthalmology. 2005;112(5):817–824. doi: 10.1016/j.ophtha.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 36.Yoon YH, Kim DS, Kim JG, Hwang JU. Sutureless vitreoretinal surgery using a new 25-gauge transconjunctival system. Ophthalmic Surg Lasers Imaging. 2006;37(1):12–19. [PubMed] [Google Scholar]
- 37.Liu DT, Chan CK, Fan DS, Lam SW, Lam DS, Chan WM. Choroidal folds after 25 gauge transconjunctival sutureless vitrectomy. Eye (Lond) 2005;19(7):825–827. doi: 10.1038/sj.eye.6701662. [DOI] [PubMed] [Google Scholar]
- 38.Sommerville DN, Hainsworth DP. Bacterial endophthalmitis following 25-gauge transconjunctival sutureless vitrectomy. Clin Ophthalmol. 2008;2(4):935–936. doi: 10.2147/opth.s2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Acar N, Unver YB, Altan T, Kapran Z. Acute endophthalmitis after 25-gauge sutureless vitrectomy. Int Ophthalmol. 2007;27(6):361–363. doi: 10.1007/s10792-007-9081-6. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SR, Aylward GW. Endophthalmitis following 25-gauge vitrectomy. Eye (Lond) 2005;19(11):1228–1229. doi: 10.1038/sj.eye.6701737. [DOI] [PubMed] [Google Scholar]
- 41.Scott IU, Flynn HW, Jr, Acar N, Dev S, Shaikh S, Mittra RA, Arevalo JF, Kychenthal A, Kunselman A. Incidence of endophthalmitis after 20-gauge vs 23-gauge vs 25-gauge pars plana vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2011;249(3):377–380. doi: 10.1007/s00417-010-1505-8. [DOI] [PubMed] [Google Scholar]