Abstract
Background
Chronic hepatitis C (HCV) is a significant risk factor for cirrhosis and subsequently hepatocellular carcinoma (HCC). HCV patients with cirrhosis are screened for HCC every 6 months. Surveillance for progression to cirrhosis and consequently access to HCC screening is not standardized. Liver biopsy, the usual test to determine cirrhosis, carries a significant risk of morbidity and associated mortality. Transient ultrasound elastography (fibroscan) is a non-invasive test for cirrhosis.
Purpose
This study assesses the cost effectiveness of annual surveillance for cirrhosis in patients with chronic HCV and the effect of replacing biopsy with fibroscan to diagnose cirrhosis.
Method
A Markov decision analytic model simulated a hypothetical cohort of 10,000 patients with chronic HCV initially without fibrosis over their lifetime. The cirrhosis surveillance strategies assessed were: no surveillance; current practice; fibroscan in current practice with biopsy to confirm cirrhosis; fibroscan completely replacing biopsy in current practice (definitive); annual biopsy; annual fibroscan with biopsy to confirm cirrhosis; annual definitive fibroscan.
Results
Our results demonstrate that annual definitive fibroscan is the optimal strategy to diagnose cirrhosis. In our study, it diagnosed 20 % more cirrhosis cases than the current strategy, with 549 extra patients per 10,000 accessing screening over a lifetime and, consequently, 76 additional HCC cases diagnosed. The lifetime cost is £98.78 extra per patient compared to the current strategy for 1.72 additional unadjusted life years. Annual fibroscan surveillance of 132 patients results in the diagnosis one additional HCC case over a lifetime. The incremental cost-effectiveness ratio for an annual definitive fibroscan is £6,557.06/quality-adjusted life years gained.
Conclusion
Annual definitive fibroscan may be a cost-effective surveillance strategy to identify cirrhosis in patients with chronic HCV, thereby allowing access of these patients to HCC screening.
Keywords: Hepatitis C, Liver fibrosis, Hepatocellular carcinoma, Screening, Health economics, Outcomes research, Radiology/imaging
Introduction
The annual incidence of chronic hepatitis C virus (HCV) in the UK is approximately 10,000, with an estimated 216,000 prevalent cases in 2012 [1]. It is estimated that 20–30 % of these patients will develop cirrhosis after 30 years. Cirrhosis due to chronic HCV is associated with an increased risk of hepatocellular carcinoma (HCC) which is as high as 40-fold that of the general population [2, 3]. Cirrhosis develops through the progressive stages of fibrosis. These stages are described and defined by a variety of staging criteria, with the set of criteria most widely used at the present time being the Metavir system [4]. The results from various studies suggest that progression through these stages occurs in a linear fashion [5, 6]. Alcohol consumption, increased age at time of infection, and male gender increase progression to cirrhosis, while genotype and viral load seem to have no effect [6–10].
Reported rates of HCC in hepatitis C patients with cirrhosis vary from 1.2 to 8.0 % per year [11–14]. It was originally thought that HCV only leads to HCC in the presence of established cirrhosis. However, it has recently been found that patients with chronic HCV may develop HCC earlier, i.e., prior to the manifestation of cirrhosis. In two studies, the incidence of HCC in patients with Metavir stage 3 fibrosis was 0.8 % per year [3, 15]. This compares to 0.2–0.5 % per year in patients with hepatitis B without cirrhosis who are currently offered HCC screening [16, 17].
Once patients with HCV develop cirrhosis, international good practice guidelines recommend screening for HCC with ultrasound scanning (USS) and serum alpha-fetoprotein (AFP) every 6 months [18–20]. Current UK practice does not, however, recommend a specific interval for assessing fibrosis progression in order to diagnose cirrhosis and commence screening. Estimates range from 50 % of patients receiving biopsy in any 3-year period [21] to 87 % over 7.6 years [22].
Progression to cirrhosis and development of HCC are often clinically silent [5]. HCC has a typically poor prognosis, but if diagnosed early while still operable, survival improves considerably.
Liver biopsy is the gold standard diagnostic test for cirrhosis. With liver biopsy, a false–positive diagnosis is unlikely, but fibrosis is rarely uniform throughout the whole liver and downstaging is not infrequent. The sensitivity of liver biopsy has been stated to be as low as 0.65–0.75 [4, 23]. In addition, estimates of the proportion of patients not suitable for or refusing liver biopsy range from 33 to 60 % [8, 22]. Liver biopsy also has associated mortality risk [24].
Transient ultrasound elastography (fibroscan; Fibro-Scan®; Echosens, Paris, France) [25] assesses the stiffness of the liver as a marker of the fibrosis stage. Diagnostic accuracy requires the system to record ten valid readings with an interquartile range (IQR) that does not exceed 30 % of the median. Although there are no contraindications, the standard probe is unreliable for patients with a body mass index of >30 [26, 27]. A new XL probe has been shown to have excellent accuracy in obese patients [28], and the failure rate of this procedure, including that due to high BMI, has been reported to range from 3.1 to 41.7 % [27]. Meta-analyses have estimated the sensitivity and specificity of the fibroscan for diagnosing cirrhosis in chronic HCV to be 0.83 and 0.91, respectively. The area under the receiver operator curve is 0.94 [29–32]. It has consequently been recommended as a definitive investigation modality for diagnosing cirrhosis [27, 33].
The primary aim of this study was to assess the cost effectiveness of implementing annual surveillance of chronic HCV patients to diagnose cirrhosis and allow these patients access to HCC screening programs. The second aim was to calculate the impact and cost effectiveness of replacing liver biopsy with fibroscan for the diagnosis of cirrhosis in these patients, both in current practice and if annual surveillance were to be implemented.
Methods
Seven mutually exclusive strategies were adopted and compared to answer the questions posed in the study:
Natural history: investigations only conducted after patients have become symptomatic; no biopsy surveillance of fibrosis stage or HCC screening.
Current UK surveillance and screening: intermittent biopsy of patients with chronic HCV without cirrhosis to monitor fibrosis stage, followed by USS and AFP screening for HCC at 6-month intervals once cirrhosis has been diagnosed.
Annual biopsy of patients with chronic HCV without cirrhosis as surveillance of fibrosis stage, followed by HCC screening at 6-month intervals once cirrhosis has been diagnosed.
Management pattern following current UK surveillance and screening, replacing intermittent biopsy with fibroscan surveillance of fibrosis stage. Biopsy is used to confirm any diagnosis of cirrhosis, followed by HCC screening at 6-month intervals once cirrhosis is confirmed.
Management pattern following current UK surveillance and screening, replacing intermittent biopsy with fibroscan. Fibroscan is considered to be the definitive investigation with HCC screening at 6-month intervals once cirrhosis is diagnosed.
Annual fibroscan of patients with chronic HCV without cirrhosis as surveillance of fibrosis stage, with confirmation biopsy for a cirrhosis diagnosis, followed by HCC screening at 6-month intervals once cirrhosis is confirmed.
Annual fibroscan as a definitive investigation for surveillance of patients with chronic HCV for development of cirrhosis, followed by HCC screening at 6-month intervals once cirrhosis is diagnosed.
These seven strategies were assessed by constructing and analyzing a state transition Markov model created using TreeAge Pro R2.0 (TreeAge Software, Williams-town, MA). Aggregated published data were used to inform the structure of this model and populate the parameters. Data were collated from systematic searches carried out in the UK National Health Service Economic Evaluation Database, Health Economic Evaluation Database, Harvard School of Public Heath Cost-Effectiveness Registry, Medline, EMBASE, and PubMed and with cross referencing of citations for all English language papers published from 1990 to 2012.
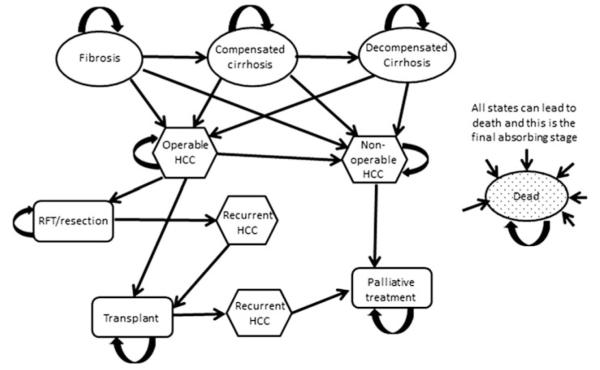
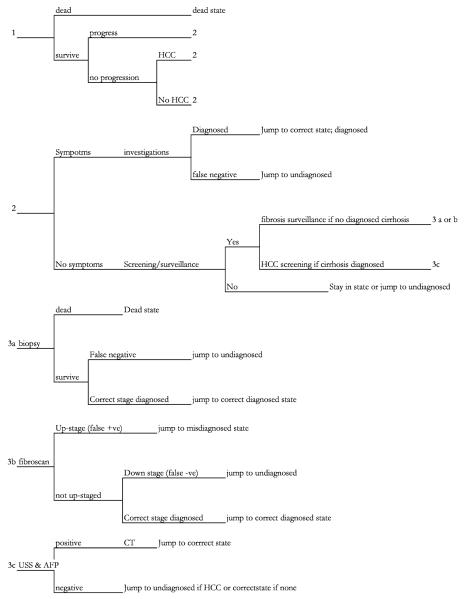
A hypothetical cohort of 10,000 patients in the UK aged 34 years [1] with chronic HCV and no fibrosis were simulated over their lifetime; cycle length was 3 months. Progression occurs through Metavir fibrosis stages. Patients with cirrhosis may decompensate. Patients with Metavir stage 3 fibrosis or cirrhosis can develop HCC. All HCC cases are initially operable and may progress to being inoperable. Figure 1 is a diagram of disease progression used for the transition states, and Fig. 2 is a representation of how the model informs transition between states in each cycle.
Fig. 1.
A simplified diagram of the health states through which the cohort can transition. All patients started without fibrosis (Metavir stage 0). In the model, fibrosis was broken down into Metavir stages 1–3. Separate stages were constructed for diagnosed, undiagnosed, and misdiagnosed states. HCC Hepatocellular carcinoma, RFT radiofrequency thermal ablation
Fig. 2.
Simplification of model structure informing cycle transition in each health state. CT Computed tomography, USS Ultrasound scanning, AFP serum alpha-fetoprotein
The model assumes that: (1) all states are progressive with no regression; (2) the probability of transition to HCC is the same from compensated or decompensated cirrhosis; (3) the clinical nature of decompensated cirrhosis means all decompensated cirrhosis is diagnosed; (4) probability of having a biopsy is the same in all stages of fibrosis.
Outcomes of interest were quality-adjusted life years (QALYs) expected from each strategy, unadjusted life years, number of patients with cirrhosis correctly diagnosed, and number of HCC cases detected. The QALY values used for each health state within the model are shown in Table 1. They are derived from published studies using EQ-5D, a standardized instrument for measuring health outcome (www.euroqol.org) that has been validated in chronic HCV and in the UK population [2, 9, 34–37].
Table 1.
Quality-adjusted life year values attached to health states within the model, including references and upper and lower limits explored in the sensitivity analysis
| Health state | QALY valuesa |
Definition information/source reference | Sensitivity analysis |
|
|---|---|---|---|---|
| Low | High | |||
| Biopsy | −0.05 | Liu et al. [34], Canadian Agency for Drugs and Technologies in Health [56] | 0.00 | −0.10 |
| Chronic HCV, no fibrosis | 0.92 | ADVANCE and REALIZE, Poordad et al. [35], Bacon et al. [36] | 0.89 | 0.98 |
| Metavir 1 | 0.92 | ADVANCE and REALIZE, Bacon et al. [36], Poordad et al. [35] | 0.89 | 0.96 |
| Metavir 2 | 0.89 | ADVANCE and REALIZE, Bacon et al. [36], Poordad et al. [35] | 0.86 | 0.93 |
| Metavir 3 | 0.89 | Bacon et al. [36], Poordad et al. [35] | 0.86 | 0.93 |
| Compensated cirrhosis | 0.85 | Wells et al. [37] | 0.80 | 0.90 |
| Decompensated cirrhosis | 0.74 | Wells et al. [37] | 0.70 | 0.78 |
| HCC with compensated cirrhosis |
0.28 | Wells et al. [37] | 0.25 | 0.35 |
| HCC with decompensated cirrhosis |
0.3 | Wells et al. [37] | 0.25 | 0.35 |
| HCC without cirrhosis | 0.44 | Grishchenko et al. [9] | 0.43 | 0.45 |
| Palliative care | 0.28 | Assumption: Wells et al. [37], Grishchenko et al. [9] compared with the health technology assessment of Sorafanib—Connock et al. [65]—SHARP trial showed no quality of life change with Sorafanib |
0.25 | 0.35 |
| Following successful ablation or resection |
0.7 | Argeudas et al. [2] | 0.62 | 0.84 |
| Recurrent HCC | 0.3 | Assumption from considering Wells et al. [37] and Grishchenko et al. [9] | 0.25 | 0.35 |
| Transplant recipient | 0.73 | Thompson Coon et al. [39] | 0.67 | 0.78 |
HCV Hepatitis virus C, HCC hepatocellular carcinoma, Metavir system (numbers refer to stages) to quantify the degree of inflammation and fibrosis of a liver biopsy
QALY value is the quality-adjusted life year value (utility value for undergoing procedures or linked to a given health state)
This analysis is from a healthcare service provider (UK National Health Service, NHS) perspective. Costs for many elements of chronic HCV care have previously been derived through micro-costing [4, 38–41]. For procedures or health states where no costing studies exist, NHS reference costs were used [42], as summarized in Table 2.
Table 2.
Cost data used in the model, including references and upper and lower bounds used in sensitivity analysis
| Procedures/health states | Costs (UK £) |
Definition information/source reference | Sensitivity analysis |
|
|---|---|---|---|---|
| Low | High | |||
| Procedures (total cost) | ||||
| Biopsy | 528 | NHS 2010–2011 Reference costs (DoH)—HRG GB04 (HRG codes to groups); sensitivity parameters informed by Lin et al. [38]; inflated to 2012 prices) |
188.30 | 882.26 |
| Fibroscan | 20.41 | Appendix two of the economic report from Stamuli et al. [40] (inflated to 2012 prices) |
13.00 | 35.48 |
| AFP and USS | 62.60 | AFP = £4; USS = £50, total = £54 [39] inflated to 2012 value [c × (1 + d)^t] |
33.43 | 128.65 |
| CT scan | 220.52 | HTA Thompson Coon et al. [39] inflated to 2012 £ plus £93 NHS tariff cost of Gastroenterology outpatient appointment |
152.70 | 266.27 |
| Ablation | 654.00 | NHS 2010/11 Reference cost: all OPSC codes = HRG group GB01 |
597.00 | 703.00 |
| Resection | 1,187.00 | NHS Reference costs 2010/11 code GA05 | 988.00 | 1,324.00 |
| Transplant | 16,377 | NHS 2010/11 Reference costs | 15,998.00 | 18,540.00 |
| Health state (attributable annual costs) | ||||
| Chronic HCV without cirrhosis | 0 | Assumption: no additional costs in this state; Cost of one medical physician (gastroenterology or infectious disease) out-patient appointment is £93 |
0 | 93.00 |
| Compensated cirrhosis | 1,357.51 | HTA Thompson Coon et al. [39], see also Hartwell et al. [41], Shepherd et al. [46] and Hospital and staff inflation index |
857.33 | 1,939.14 |
| First episode of decompensation (transition cost) |
2,512.00 | NHS 2011/12 tariff cost for episode of decompensated cirrhosis |
2,398.00 | 2,600.00 |
| Decompensated cirrhosis | 11,582.59 | HTA Thompson Coon et al. [39], Hartwell et al. [41], Shepherd et al. [46], Hospital community Staff inflation index |
7,650.12 | 14,761.42 |
| HCC without cirrhosis | 1,425.90 | Thompson Coon et al. [39]; inflated; assumed no extra cost as no cirrhosis |
734.31 | 2,461.19 |
| HCC in compensated cirrhosis | 2,587.51 | Thompson Coon et al. [39] (HTA) cost of HCC UK £1,425.90 (1,230/year INLATED) ? compensated cirrhosis 1,357.51/year |
2,091.82 | 3,818.70 |
| HCC in decompensated cirrhosis—not incl. decompensation costs |
13,008.50 | HTA Thompson Coon et al. [39], inflated. 11,582.59 ? 1,425.90 |
8,384.43 | 17,222.61 |
| Following successful resection or ablation | 4,094.56 | Thompson Coon et al. [39] inflated to 2012 UK £ | 2,782.22 | 5,669.39 |
| Transplant recipient | 1,759.60 | Thompson Coon et al. [39], Hartwell et al. [41], Shepherd et al. [46]; inflated |
976.99 | 2,754.55 |
| HCC recurrence | 5,520.46 | Cost of HCC plus cost of PRA state Thompson Coon et al. [39] HTA |
3,516.53 | 8,130.58 |
| Annual cost of palliative care | 35,765.64 | Cost of 1 year supply of Sorafanib from NICE technology appraisal 2010; additional palliative care/end of life costs not added |
35,200.00 | 36,000.00 |
AFP Serum alpha-fetoprotein, USS ultrasound scanning, CT computed tomography, NHS UK National Health Service
Costs and benefits were both discounted at 3.5 % [43]. Transition probabilities and test characteristics used within the model are summarized in Table 3, with a summary of the base case in Table 4.
Table 3.
State transition probabilities and test characteristics for biopsy, AFP, and USS combined and fibroscan that are used within the model
| HCV natural history/mortality rates | State transition rate and test characteristics |
Definition information/source reference | Sensitivity analysis | |
|---|---|---|---|---|
| Low | High | |||
| HCV natural history (TR) | ||||
| No cirrhosis to fibrosis stage Metavir 1 | Rate: 0.1098 Annual TP: 0.1040 |
Townsend et al. [10]; (Thein et al. [6]—0.1244) | 0.054 | 0.154 |
| Metavir 1 to Metavir 2 | Rate: 0.0954 Annual TP: 0.0910 |
Townsend et al. [10]; (Thein et al. [6]—0.0888) | 0.075 | 0.096 |
| Metavir 2 to Metavir 3 | Rate: 0.0954 Annual TP: 0.0910 |
Townsend et al. [10]; (Thein et al. [6]—0.1278) | 0.054 | 0.133 |
| Incidence of cirrhosis from Metavir 3 | Rate: 0.1233 Annual TP: 0.1160 |
Thein et al. [6]-transition from F3-F4 (Townsend et al. [10]—similar value calculable via probabilities and assumptions) |
0.104 | 0.129 |
| Decompensation of cirrhosis | Rate: 0.0429 Annual TP: 0.0420 |
The Global Burden of Hepatitis C Working Group [66], Chen et al. [67], Limits Townsend et al. [10] |
0.0161 | 0.0888 |
| HCC natural history | ||||
| Incidence of HCC in patients with Metavir 3 | Rate: 0.0080 Annual TP: 0.0020 |
Lok et al. [15]. (sensitivity from no risk without cirrhosis to same risk as in cirrhosis) |
0.0 | 0.0202 |
| Incidence of HCC in patients with cirrhosis | Rate: 0.0202 Annual TP: 0.0050 |
Salomon et al. [63] | 0.015 | 0.030 |
| Growth of HCC—from operable to inoperable | Rate: 0.145 Annual TP: 0.1349 |
Naugler and Sonnenberg [62], Lin et al. [38] | 0.1 | 0.7 |
| Mortality rates (probability) | ||||
| Biopsy | <40 years: 0.0005 40–59 years: 0.0011 60–79 years: 0.0048 >80 years: 0.0065 |
Population database study by West and Card [24] | <40: 0.0002 40–59: 0.0008 60–79: 0.0039 >80: 0.0028 Tornado: 0.00 |
0.0010 0.0016 0.0059 0.0127 Tornado: 0.0127 |
| Compensated cirrhosis | 0.084 | Fleming et al. [57] | 0.079 | 0.089 |
| Decompensated cirrhosis | 0.14 | The Global Burden of Hepatitis C Working Group [66], Chen et al. [67] |
0.10 | 0.30 |
| HCC with fibrosis stage Metavir 3 | 0.598 | Greten et al. [59] | 0.231 | 1.040 |
| HCC and compensated cirrhosis | 0.494 | Greten et al. [59] [–ln(0.49)] | 0.2466 | 1.188 |
| HCC with decompensated cirrhosis | 1.514 | Greten et al. [59] [–ln(0.8)] | 0.6397 | 2.0790 |
| Resection (perioperative) | 0.04 | Llovet and Ducreux [19], Lin et al. [38], Chang et al. [17] | 0.013 | 0.108 |
| Ablation (perioperative) | 0.03046 | Lin et al. [38], Livraghi et al. [60], Mondazzi et al. [61] | 0.0101 | 0.0618 |
| Patients who have received successful resection or ablation |
0.0693 | Llovet and Ducreux [19], Fong et al. [58], Bruix and Sherman [20] |
0.05 | 0.08 |
| Transplant (perioperative) | 0.0975 | Adam et al. [55]. Mortality is 9 % in first 6 months | 0.1450 | 0.2900 |
| Transplant recipients | 0.0301 | Mazzaferro et al. [68], Llovet et al. [69], Bismuth et al. [70], Bruix and Sherman [20] |
0.025 | 0.050 |
| HCC recurrence | 0.7713 | Ladabaum et al. [71] | 0.5108 | 0.9163 |
| HCC given palliative care in line with NICE recommendations (including Sorafanib) |
1.1215 | Carr et al. [72] | 0.895 | 1.245 |
| Test characteristics: biopsy | ||||
| Sensitivity of biopsy | 0.669 | Regev et al. [23]: 33.1 % false negatives; Bedossa et al. [4] | 0.60 | 1.00 |
| Likelihood of being biopsied through routine surveillance when asymptomatic |
0.2309 | Foster et al. [21]: 50 % of chronic HCV patients without already diagnosed cirrhosis will receive a biopsy in 3 years; Sweeting et al. [22] from two cohorts: 54 % at 16 years and 87 % in 7.6 years (rate = 0.2310) |
0.0354 | 0.5358 |
| Probability of biopsy when symptoms develop (includes probability that patient is suitable for biopsy) |
0.4 | Freeman et al. [8]: 59 % in community studies, none from UK; Sweeting et al. [22]: 0.33 in UK population (Trent group) |
0.3 | 0.6 |
| Probability of incidental HCC being diagnosed on routine biopsy | 0.0379 | Based on data of incidental diagnosis from Trevisani et al. [64] |
0.00 | 0.0488 |
| Test characteristics: AFP and USS | ||||
| Sensitivity of combined AFP and USS screening | 0.85 | Lin et al. [38] | 0.55 | 0.95 |
| Specificity of combined AFP and USS screening for HCC in HCV | 0.8 | Lin et al. [38] | 0.70 | 0.90 |
| Test characteristics: fibroscan | ||||
| Probability of patient not being suitable for fibroscan: e.g.>BMI |
0.031 | Castera et al. [27] [BMI[30]; High parameter limit informed from UK obesity statistics |
0.00 | 0.461 |
| Probability of scan failure (10 valid shots not achieved) | 0.2 | Degos et al. [32], Stamuli et al. [40] (sensitivity analysis high limit informed by Castera et al. [27]) |
0.01 | 0.417 |
| Probability of incidental diagnosis of HCC during fibroscan | 0 | Fibroscan measures only liver stiffness and does not produce images; no evidence of assessment or calibration of any changes in output in HCC |
0 | 0 |
| Sensitivity of fibroscan in diagnosing Metavir 3 | 0.82 | Tzochatzis et al. [31] | 0.77 | 0.87 |
| Sensitivity of fibroscan for diagnosing cirrhosis | 0.83 | Tzochatzis et al. [31] | 0.79 | 0.86 |
| Specificity of fibroscan for diagnosing Metavir 3 | 0.86 | Tzochatzis et al. [31] | 0.80 | 0.91 |
| Specificity of fibroscan for diagnosing cirrhosis | 0.91 | Tzochatzis et al. [31] | 0.84 | 0.95 |
TR Transition rate, BMI body mass index, NICE UK National Institute of Clinical Evidence
Probability of false positive results is 1 – specificity and the probability of a false negative is 1 – sensitivity. References for values used and the upper and lower limits used in the sensitivity analysis
Table 4.
Base case summary
| Decision problem (scope) |
|
| Comparators | Liver biopsy and fibroscan |
| Model structure | State transition Markov model with cohort simulation analysis |
| Population | Hypothetical cohort of 10,000 34–year–old patients newly diagnosed with chronic HCV and no fibrosis |
| Setting | UK NHS |
| Time horizon | Lifetime |
| Cycle length | 3 months |
| Benefits | QALYs; unadjusted life years; number with cirrhosis correctly diagnosed; number with HCC diagnosed |
| Costs perspective | NHS: costs from micro–costing studies and reference tables valued in 2012 £ sterling |
| Discounting | Costs and benefits discounted at 3.5 % |
| Equity weighting | All additional QALYs have equal weight regardless of the beneficiary |
Model Calibration and Validation
Calibration ensures a clinically relevant and meaningful model [44, 45]. To calibrate the baseline model we identified two targets. The first was the proportion of cohort diagnosed with cirrhosis. The UK National Institute of Clinical Evidence (NICE) and the program of Health Technology Assessment (HTA) both use a benchmark expectation of 30 % of patients with chronic HCV developing cirrhosis 20–30 years following diagnosis [41, 46]. The secondary target was the proportion of patients the model predicts will be diagnosed with HCC. Estimates from empirical studies in Europe and the USA vary from 4.8 [13] to 28 % [15] with between 5 and 25 years follow-up [13–15, 47, 48].
The adequacy of calibration was assessed by visual fit to the target values. The model outputs were additionally verified by assessing life expectancy prediction compared to published reports. The expected cost effectiveness results this model generated for current HCC screening compared to natural history were also assessed and compared to the literature for cross-validity.
Sensitivity Analysis
Univariate deterministic sensitivity analyses were conducted on all independent parameters to assess the robustness of the optimal strategy choice.
Results
Calibration and Validation
The base case model of current UK practice predicts 28.9 % of the cohort will be diagnosed with cirrhosis 30 years after the diagnosis of chronic HCV. This correlates well with the first target and represents patients with HCV who are either untreated or for whom the virus is treatment resistant. The second calibration target was also adequately met, as can be seen in Fig. 3.
Fig. 3.
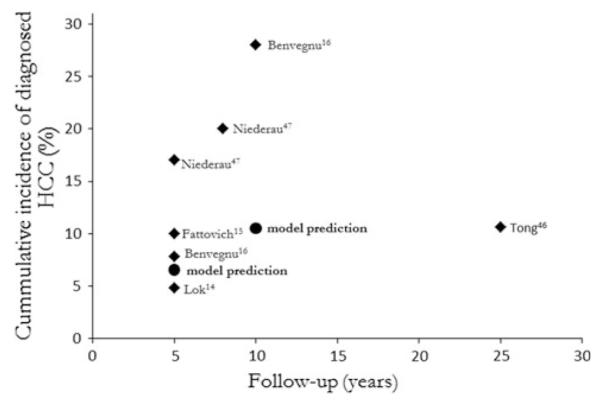
Visual fit of model prediction of cumulative incidence of HCC diagnosis following the current practice strategy compared to Europe and USA data
Life expectancy in the natural history strategy is 71.27 years (37.27 years from diagnosis). This increases to 71.62 years with current surveillance and screening (an additional 128 days). The additional survival changes with age at diagnosis: From 0.39 years (142 days) if diagnosed at aged 20 years to 0.13 years (47 days) when diagnosed at aged 50 years. This is similar to previous findings [39].
The portion of the model reflecting current HCC screening practice has been compared to disease natural history in previous studies, and the results of these studies were used to cross-validate the present model. None of the previous models take into account the reliability of diagnosing cirrhosis, and all commence their cohorts with diagnosed cirrhosis. To compare our model with those of previous studies, we conducted a cohort analysis with a hypothetical population of 10,000 patients starting in the diagnosed cirrhosis state. The current surveillance strategy then produces a QALY gain of 0.24 over the natural history strategy at an additional cost of £4,323.17. The incremental cost-effectiveness ratio (ICER) was £18,013.21/QALY, which is comparable to that reported in three previous studies [2, 38, 49].
Proportion of Cirrhosis Diagnosed by Each Strategy
Implementing annual surveillance of patients, instead of current practice, increases the proportion of patients correctly diagnosed as having cirrhosis by 30 % using fibroscan and 40 % using biopsy. An annual biopsy increases the number of chronic HCV patients receiving HCC screening 10 years after diagnosis by 80 patients per 10,000 receiving surveillance, or an extra 100 patients using definitive fibroscan. After 20 years, this increases to 492 and 549 additional patients, respectively. Twenty percent more cases of HCC in patients with cirrhosis is consequently diagnosed, which is an extra 76 HCC cases over 30 years.
Proportion Diagnosed with Metavir 3 Fibrosis
Current HCC screening does not commence until cirrhosis has been diagnosed. The results of studies suggest, however, that patients with chronic HCV are at risk of HCC in the absence of cirrhosis [3, 13]. To enable assessment of the total burden of undiagnosed HCC, we have analyzed the diagnosis of Metavir stage 3 fibrosis along with HCC in patients with Metavir 3 fibrosis. The model predicts that 20 years following diagnosis of chronic HCV, 15.9 % of patients will have Metavir stage 3 fibrosis. Almost 3 % of these will have HCC. With current surveillance, 50 % of Metavir stage 3 fibrosis is diagnosed. Since none of these patients enter screening, only 17 % of patients with HCC in Metavir stage 3 are diagnosed. Annual screening with biopsy does not increase the number of HCC cases detected with Metavir stage 3 fibrosis as biopsy is considered to have a perfect specificity so there are no false–positive diagnoses of cirrhosis and no additional screening.
Used as a definitive investigation, fibroscan misdiagnoses 1.2 % cases of Metavir stage 3 fibrosis as cirrhosis when it replaces biopsy in current surveillance. This increases to 6.9 % if used for annual surveillance. Consequently, with definitive annual fibroscan, 137 extra patients enter HCC screening per 10,000 under surveillance. This enables an additional 30 HCC cases to be detected before the patient develops cirrhosis. These results suggest that commencing HCC screening earlier in fibrosis progression may be worthwhile.
Proportion of HCC Cases Diagnosed
According to the model, 65 % of all HCC in patients with chronic HCV and cirrhosis is detected by the current strategy in the UK and 17 % of HCC that develops in Metavir stage 3 fibrosis patients. Implementing annual definitive fibroscan surveillance and commencing HCC screening once cirrhosis is diagnosed potentially increases the HCC cases diagnosed in cirrhosis to 75 %. As fibroscan is not 100 % specific and we found that definitive fibroscan misdiagnosed 7 % of Metavir stage 3 cases as cirrhosis, this would result in an additional 1.4 % of the patient cohort entering screening (139 extra patients per 10,000 in surveillance). Extending screening to these patients allows 40 % more of the HCC developing before cirrhosis to be diagnosed.
The underlying prevalence of cirrhosis increases from 0.16 % at 5 years from diagnosis to 42.00 % after 30 years with chronic HCV. Annual definitive fibroscan diagnoses 23.21 % more cirrhosis than the current strategy. In practice, 2,000 patients need to be under surveillance to diagnose one additional case of cirrhosis 5 years after diagnosis; this drops to ten patients at 30 years. Thus, 132 patients need to be under annual surveillance to diagnose one additional HCC through the increased numbers entering HCC screening.
Cost Effectiveness of Strategies
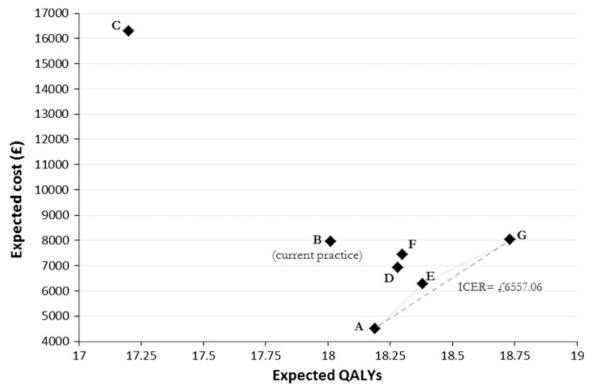
With an acceptability threshold of £30,000/QALY gained, annual definitive fibroscan is the optimal strategy. It generates an ICER of £6,557.06/QALY over the next best strategy: symptomatic investigation and treatment with no fibrosis surveillance or HCC screening. Definitive fibroscan replacing biopsy in current surveillance is an extendedly dominated strategy; all other strategies are strictly dominated (Fig. 4).
Fig. 4.
Cost-effectiveness plane measuring benefit in terms of quality-adjusted life years (QALYs) for all strategies (A–G; see Methods) comparing fibroscan and biopsy for monitoring fibrosis progression in patients with chronic HCV. Dominated strategies are B, C, D, F; E is an extendedly dominated strategy; strategies A and G are on the cost-effective frontier. ICER Incremental cost-effectiveness ratio
The model was modified to allow hypothetical patients to be analyzed in a Monte Carlo simulation. In total, 10,000 patients were tracked through the model over their lifetime and the number of biopsies counted. This model predicts that within the context of the program of current surveillance and screening strategy in the UK the expectation is that one biopsy would be conducted in every two patients diagnosed with chronic HCV over a lifetime. This is reduced to one biopsy in every four patients if biopsy is only used to confirm cirrhosis following a fibroscan. Using fibroscan without confirmation biopsy would save over 2,000 liver biopsies per year in the UK using current prevalence data [1].
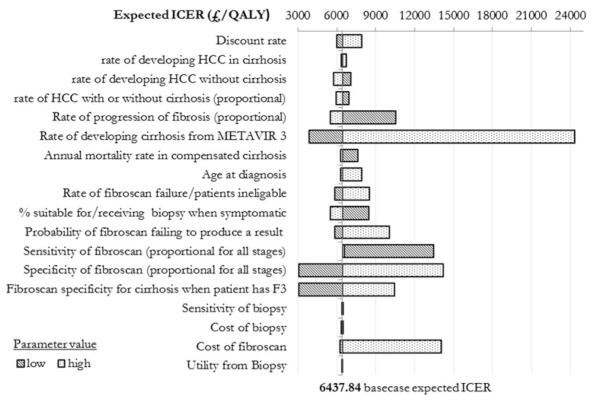
Sensitivity Analysis
The key results of the univariate sensitivity analysis are shown in Fig. 5. The expected ICER is most sensitive to change in the underlying rate of cirrhosis. More patients with diagnosed cirrhosis require more screening and treatment with higher total cost, but as all strategies face higher costs, incremental cost is not much changed. At a high incidence of cirrhosis, total and incremental QALYs fall by 25 % as more patients are in worse health states and an increasing incidence of cirrhosis causes an increased incidence of HCC. Even at the highest feasible rate for developing cirrhosis, which might reflect a high-risk population such as HCV–human immunodeficiency virus co-infected patients, an annual fibroscan is still cost effective using a threshold of £30,000/QALY.
Fig. 5.
Tornado diagram showing change in overall expected ICER for the optimal strategy of an annual definitive fibroscan as individual parameters are varied. Metavir System to quantify the degree of inflammation and fibrosis of a liver biopsy
The sensitivity and specificity of fibroscan also have a significant effect on the expected ICER. Low sensitivity reduces diagnosed cirrhosis, significantly reducing the cost of the strategy as fewer people are screened or treated (half the total cost and a 25 % decrease in the incremental cost). Without screening more HCC cases are missed and therefore mortality is greater and QALYs fewer (incremental QALY decreases to 0.06). The dramatic fall in QALYs gained doubles the ICER, halving the expected cost effectiveness of implementing the policy. Lower specificity means more false–positive diagnoses of cirrhosis so more patients are screened. This is reflected in higher costs (30 % more than base case), but substantially more QALYs (incremental QALY 2.81 compared to 0.55 in the base case). These results indicate the potential magnitude of QALY benefit from screening all patients at Metavir stage 3 fibrosis.
The highest feasible cost for fibroscan suggested by a micro-analysis conducted for the NHS purchasing committee was £36 per scan [39]. Use of definitive fibroscan to replace biopsy in current practice becomes preferred if the cost per procedure is higher than £164. At a cost of £658 per scan no strategy using fibroscan would be cost effective with a threshold of £30,000/QALY.
As age increases, the QALYs expected become so small that the ICER crosses the cost-effectiveness threshold. At age 72 years current practice with definitive fibroscan becomes optimal; at age 84 years, symptomatic management becomes optimal.
Discussion
In the UK, annual surveillance strategies are effective for the surveillance of patients with chronic HCV to diagnose cirrhosis and allow access to HCC screening with the aim of improving HCC diagnosis and prognosis. The most cost-effective surveillance strategy is annual definitive fibroscan, which would allow 20 % more cirrhosis cases to be diagnosed than current practice. This means that from a cohort of 10,000 patients, an extra 549 patients would access screening over a lifetime, resulting in an expected 76 additional HCC cases diagnosed.
Compared to current UK practice, annual definitive fibroscan surveillance delivers an additional 1.72 unadjusted life years and costs an additional £98.78 per patient over a lifetime. Implementing annual screening with fibroscan is half the cost of using biopsy. Fibroscan is also non-invasive so more people are able to have the procedure without attributable mortality or increased morbidity. Annual fibroscan surveillance of 132 patients will diagnose one additional HCC case over a lifetime. The ICER for annual fibroscan is £6,557.06/QALY gained, which is well below the acceptability threshold used by NICE. There are no previous studies considering this question to compare this result with.
Annual definitive fibroscan is optimal for patients aged between 20 and 72. Above the age of 72 years, patients should only receive fibroscan if they would have had biopsy in current practice. After the age of 82 years, patients should no longer receive fibrosis surveillance or HCC screening and should only be investigated if they become symptomatic. Fibroscan is cost-effective in populations where the prevalence of obesity is <80 %, and it the remains optimal strategy as long as compliance is >20 %. Annual definitive fibroscan remains the optimal strategy and becomes even more cost effective as the risk of HCC increases. This means that this strategy would be cost effective in populations with a high HIV or HBV co-infection and concomitant alcohol use and in injecting drug users, all of which are factors that increase the risk of HCC in the UK.
Fibroscan is a new technology and as such the cost to the NHS of developing surveillance programs is unclear. Based on a recent study [40] that completed a micro-costing assessment (assuming that procedures took 5–15 min; machine lifespan was 5–10 years; number of procedures annually were 2,500–7,000), the authors concluded that the expected cost per procedure would be £13–35. Fibroscan should be rationed to replacing biopsy in current practice if it costs £164 to £658 per procedure to implement. If costs for any hospital are more than £658 per procedure the technology is not cost effective for this indication.
Within this model imperfect specificity of the fibroscan meant that patients with Metavir stage 3 fibrosis, who are at risk of HCC but not currently eligible for screening programs, could enter such screening programs. This would lead to the diagnosis of 25 additional HCCs in patients with Metavir stage 3 fibrosis. A future study is needed to comprehensively consider the effectiveness and cost effectiveness of screening patients with Metavir stage 3 fibrosis for HCC rather than waiting until these patients actually develop cirrhosis. This strategy might provide the opportunity to diagnose more HCC cases earlier when the HCC is at operable and potentially curable stages, which could significantly improve prognosis. Annual definitive fibroscan provides a potentially cost-effective mechanism to diagnose Metavir stage 3 fibrosis that is acceptable to patients. Studies by Castera [26, 27] suggest that to achieve a sufficiently accurate diagnosis of Metavir 3 fibrosis, the fibroscan should be combined with serum tests. This would need to be modeled along with all other mutually exclusive relevant comparisons that may arise in this scenario.
The model qualitatively met the calibration targets well. The life expectancy predictions and cost-effectiveness results for current HCC screening compared to natural history verified that the model outputs were credible [43].
Sensitivity analyses begin to assess the parameter uncertainty and their implications upon the choice of an optimal strategy. Increases in the ICER (i.e., greater cost per QALY gained) from this parameter uncertainty predicted results in the loss of health service efficiency. The sensitivity analysis suggests that definitive annual fibroscan is a robust optimal strategy; however, it reflects only single parameters changed at any time.
Standardizing the surveillance of fibrosis in patients with chronic HCV is a cost-effective strategy in terms of more patients being diagnosed with cirrhosis, entering screening programs, and subsequently having HCC diagnosed. Diagnosing and treating more HCC cases did provide a survival benefit. Some researchers may raise the argument that diagnosing asymptomatic people with a terminal condition with few effective treatments may have limited benefit. This model has not considered the possible benefit of better cirrhosis treatment to prevent decompensation or reduce mortality; we also did not consider other potential benefits to patients of being part of a regular screening program. These could include counseling to reduce alcohol consumption and earlier referral for treatment of comorbid conditions, which in turn could delay progression to cirrhosis. These additional benefits would make fibrosis surveillance with fibroscan even more cost effective. We also did not analyze different surveillance intervals dependent on the stage of fibrosis diagnosed. It is possible that patterns of surveillance that have longer intervals for mild or no fibrosis have a potential for even greater cost effectiveness. Patients with chronic HCV are typically young, in their most productive years. Extending the scope of this analysis to consider societal cost and benefit may reveal that keeping these patients well and functional for as long as possible further improves the benefits. Additionally, some aspect of productivity will be captured in the QALY attributed to the health states.
There have been a number of recent studies suggesting the possibility of personalized medicine in terms of in risk-stratification of patients with the potential prevention of HCC [50–52]. Further research may prove that fibroscan plays a fundamental role in the surveillance of fibrosis stage in patients with chronic HCV. Currently the potential to diagnose HCC earlier, both in terms of fibrosis stage and cancer stage, has only a small benefit. Recent studies into multi-targeted tyrosine kinase inhibitors suggest improved treatment and life expectancy for patients with HCC may be on the horizon, which would make annual surveillance even more beneficial [53, 54].
In summary, annual definitive fibroscan may be a cost-effective surveillance strategy to identify cirrhosis in patients with chronic HCV, allowing these patients access to HCC screening and resulting in reduced mortality and morbidity due to HCC.
Acknowledgments
CC is funded by MRC Population Health Scientist Fellowship RA0837.
Footnotes
Conflict of interest None.
Contributor Information
C. Canavan, Division of Epidemiology and Public Health, Nottingham University, Clinical Sciences Building, City Hospital Campus, Hucknall Road, Nottingham NG5 1PB, UK
J. Eisenburg, Institute for Technology Assessment, Massachusetts General Hospital, Boston, MA, USA
L. Meng, Institute for Technology Assessment, Massachusetts General Hospital, Boston, MA, USA
K. Corey, Gastrointestinal Unit, Massachusetts General Hospital, Boston, MA, USA
C. Hur, Institute for Technology Assessment, Massachusetts General Hospital, Boston, MA, USA; Gastrointestinal Unit, Massachusetts General Hospital, Boston, MA, USA
References
- 1.London: Health Protection Agency . Hepatitis C in the UK. Health Protection Agency, Colindale; London: 2012. [Google Scholar]
- 2.Argeudas MR, Chen VK, Eloubeidi MA, Fallon MB. Screening for hepatocellular carcinoma in patients with hepatitis C cirrhosis: a cost-utility analysis. Am J Gastroenterol. 2003;98:679–690. doi: 10.1111/j.1572-0241.2003.07327.x. [DOI] [PubMed] [Google Scholar]
- 3.Lemon SM, McGiven DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–1278. doi: 10.1053/j.gastro.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bedossa P, Poynard T and the French METAVIR Cooperative Study Group An algorithm for grading activity in chronic hepatitis C. Hepatology. 1996;24:289–293. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 5.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Cost-effectiveness of treatment for chronic hepatitis C infection in an evolving patient population. J Am Med Assoc. 2003;290:228–237. doi: 10.1001/jama.290.2.228. [DOI] [PubMed] [Google Scholar]
- 6.Thein HH, Yi Q, Dore GJ, Krahn MD. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatology. 2008;48:418–431. doi: 10.1002/hep.22375. [DOI] [PubMed] [Google Scholar]
- 7.Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost effectiveness of a single course of inter-feron-alpha 2b in patients with histologically mild chronic hepatitis C. Ann Intern Med. 1997;127:855–865. doi: 10.7326/0003-4819-127-10-199711150-00001. [DOI] [PubMed] [Google Scholar]
- 8.Freeman AJ, Dore GJ, Law MG, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 9.Grishchenko M, Grieve RD, Sweeting MJ, et al. the Trent HCV Study group Cost-effectiveness ofpegylated interferon and ribivarin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care. 2009;25:171–180. doi: 10.1017/S0266462309090229. [DOI] [PubMed] [Google Scholar]
- 10.Townsend R, McEwan P, Kim R, Yuan Y. Structural frameworks and key model parameters in cost-effectiveness analyses for current and future treatments of chronic hepatitis C. Value Health. 2011;14:1068–1077. doi: 10.1016/j.jval.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 12.Benvegnu L, Alberti A. Patterns of hepatocellular carcinoma in HCV infection. Dig Dis Sci. 1996;14:49S–55S. doi: 10.1007/BF02087876. [DOI] [PubMed] [Google Scholar]
- 13.Poynard T, Ratziu V, Charlotte F, Goodman Z, McHutchison J, Albrecht J. Rates and risk factors of liver fibrosis progression in patients with chronic hepatitis C. J Hepatol. 2001;34:730–739. doi: 10.1016/s0168-8278(00)00097-0. [DOI] [PubMed] [Google Scholar]
- 14.Yano M, Kumada H, Kage M, et al. A long term pathological evolution of chronic hepatitis C. Hepatology. 1996;23:1334–1340. doi: 10.1002/hep.510230607. [DOI] [PubMed] [Google Scholar]
- 15.Lok AS, Seeff LB, Morgan TR, et al. the HALT-C Trial Group Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology. 2009;136:138–148. doi: 10.1053/j.gastro.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Di Bisceglie AM. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S–38S. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 17.Chang Y, Lairson DR, Chan W, Lu S-N, Aoki N. Cost effectiveness of screening for hepatocellular carcinoma among subjects at different levels of risk. J Eval Clin Pract. 2011;17:261–267. doi: 10.1111/j.1365-2753.2010.01432.x. [DOI] [PubMed] [Google Scholar]
- 18.Ryder SC. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut. 2003;52:iii1–iii8. doi: 10.1136/gut.52.suppl_3.iii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llovet M, Ducreux M. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 21.Foster GR, Goldin RD, Main J, Murray-Lyon I, Hargreaves S, Thomas HC. Management of chronic hepatitis C: clinical audit of biopsy based management algorithm. BMJ. 1997;315:453–458. doi: 10.1136/bmj.315.7106.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sweeting MJ, De Angelis D, Neal KR, Ramsay ME, Irving WL, Wright M. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. J Clin Epidemiol. 2006;59:144–152. doi: 10.1016/j.jclinepi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intra-observer variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614–2618. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 24.West J, Card T. Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology. 2010;139:1230–1237. doi: 10.1053/j.gastro.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 25.Echosens . Registered technology of EchoSens. EchoSens; Paris: Available at: www.echosens.com. [Google Scholar]
- 26.Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Clin Hepatol. 2008;28:835–847. doi: 10.1016/j.jhep.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 27.Castera L, Sebastiani G, Le Bail B, de Ledinghen V, Couzigou P, Alberti A. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J Hepatol. 2010;52:191–198. doi: 10.1016/j.jhep.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Feasibility and diagnostic performance of the FibroScan XL probe for liver stiffness measurement in overweight and obese patients. Hepatology. 2012;55:199–208. doi: 10.1002/hep.24624. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134:960–974. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 30.Stebbing J, Farouk L, Panos G, et al. A meta-analysis of transient elastography for the detection of hepatic fibrosis. J Clin Gastroebterol. 2010;44:214–219. doi: 10.1097/MCG.0b013e3181b4af1f. [DOI] [PubMed] [Google Scholar]
- 31.Tzochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas BR, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol. 2011;54:650–659. doi: 10.1016/j.jhep.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 32.Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicentre study (the FIBROSTIC study) J Hepatol. 2010;53:1013–1021. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 33.Castera L, Vergniol J, Foucher J, et al. Prospective comparison of transient elastography, fibrotest, APRI and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343–350. doi: 10.1053/j.gastro.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLoS ONE. 2011;6:e26783. doi: 10.1371/journal.pone.0026783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poordad F, McCone J, Bacon BR, et al. for the SPRINT-2 investigators Boceprevir for untreated chronic HCV Genotype 1 infection. N Engl J Med. 2011;364:1195–1206. doi: 10.1056/NEJMoa1010494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bacon BR, Gordon SC, Lawitz E, et al. for the HCV RESPOND-2 Investigators Boceprevir for previously treated chronic HCV Genotype 1 infection. N Engl J Med. 2011;364:1207–1217. doi: 10.1056/NEJMoa1009482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells CD, Murrill WB, Arguedas MR. Comparison of health-related quality of life preferences between physicians and cirrhotic patients: implications for cost-utility analysis in chronic liver disease. Dig Dis Sci. 2004;49:453–458. doi: 10.1023/b:ddas.0000020502.46886.c1. [DOI] [PubMed] [Google Scholar]
- 38.Lin OS, Keeffe EB, Sanders GD, Owens DK. Cost-effectiveness of screening for hepatocellular carcinoma in patients with cirrhosis due to chronic hepatitis C. Aliment Pharmacol Ther. 2004;19:1159–1172. doi: 10.1111/j.1365-2036.2004.01963.x. [DOI] [PubMed] [Google Scholar]
- 39.Thompson Coon J, Rogers G, Hewson P, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess. 2007;11:1–206. doi: 10.3310/hta11340. [DOI] [PubMed] [Google Scholar]
- 40.Stamuli E, Kruger J, Hutton J. Cost-effectiveness of ultrasound elastography in the assessment of liver fibrosis. Economic report for the NHS Centre for Evidence-based Purchasing. 2009 CEP08053. [Google Scholar]
- 41.Hartwell D, Jones J, Baxter L, Shepherd J. Peginterferon alfa and ribavirin for chronic hepatitis C in patients eligible for shortened treatment, re-treatment or in HCV/HIV co-infection: a systematic review and economic evaluation. Health Technol Assess. 2011;15 doi: 10.3310/hta15170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Department of Health [Accessed July 2012];2010-11 Reference costs publication. Available at: www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_131140.
- 43.Treasury HM. Appraisal and Evaluation in Central Government. Treasury Guidance. TSO; London: 2003. The Green Book. (amended 2011) [Google Scholar]
- 44.Stout NK, Knudsen AB, Kong CY, McMahon P, Gazelle S. Calibration methods used in cancer simulation models and suggested guidelines. Pharmacoeconomics. 2009;27:533–545. doi: 10.2165/11314830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the IPSOR-SMDM modelling good practice task force 7. Med Decis Mak. 2012;32:733–743. doi: 10.1177/0272989X12454579. [DOI] [PubMed] [Google Scholar]
- 46.Shepherd J, Jones J, Hartwell D, Davidson P, Price A, Waugh N. Interferon alfa (pegylated and non-pegylated) and ribavirin for the treatment of mild chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess. 2007;11:11. doi: 10.3310/hta11110. [DOI] [PubMed] [Google Scholar]
- 47.Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusion associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 48.Niederau C, Lange S, Heintges T, et al. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687–1695. doi: 10.1002/hep.510280632. [DOI] [PubMed] [Google Scholar]
- 49.Andersson KL, Solomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2008;6:1418–1424. doi: 10.1016/j.cgh.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abu Dayyeh BK, Yang M, Fuchs BC, et al. for the HALT-C Trial Group A functional polymorphism in the Epydermal growth factor gene is associated with risk of hepatocellular carcinoma. Gastroenterology. 2011;141:141–149. doi: 10.1053/j.gastro.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donadon V, Balbi M, Mas MD, Casarin P, Zanette G. Metformin and reduced risk of hepatocellular carcinoma in diabetics with chronic liver disease. Liver Int. 2010;30:750–758. doi: 10.1111/j.1478-3231.2010.02223.x. [DOI] [PubMed] [Google Scholar]
- 52.Pacanowski M, Amur S, Zinek I. New genetic discoveries and treatment for Hepatitis C. JAMA. 2012;307:1921–1922. doi: 10.1001/jama.2012.3516. [DOI] [PubMed] [Google Scholar]
- 53.Han C, Michalopoulos GK, Wu T. Prostaglandin E2 receptor EP1 transactivates EGFR/MET receptor tyrosine kinases and enhances invasiveness in human hepatocellular carcinoma cells. J Cancer Physiol. 2006;207:261–270. doi: 10.1002/jcp.20560. [DOI] [PubMed] [Google Scholar]
- 54.Marquardt JU, Galle PR, Teufel A. Molecular diagnosis and therapy of hepatocellular carcinoma: an emerging field for advanced technologies. J Hepatol. 2012;56:267–275. doi: 10.1016/j.jhep.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 55.Adam R, Cailliez V, Majno P, et al. Normalised intrinsic mortality risk in liver transplantation: European liver transplant registry study. Lancet. 2000;356:621–627. doi: 10.1016/s0140-6736(00)02603-9. [DOI] [PubMed] [Google Scholar]
- 56.Canadian Agency for Drugs and Technologies in Health Transient elastography (FibroScan) for non-invasive assessment of liver fibrosis. 2012. [PubMed]
- 57.Fleming KM, Aithal GP, Card TR, West J. All-cause mortality in people with cirrhosis compared with the general population: a population-based cohort study. Liver Int. 2012;32:79–84. doi: 10.1111/j.1478-3231.2011.02517.x. [DOI] [PubMed] [Google Scholar]
- 58.Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for meta-static colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Greten TF, Papendorf F, Bleck JS, et al. Survival rate in patients with hepatocellular carcinoma: a retrospective analysis of 389 patients. Br J Cancer. 2005;92:1862–1868. doi: 10.1038/sj.bjc.6602590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Livraghi T, Giorgio A, Marin G, et al. Hepatocellular carcinoma and cirrhosis in 746 patients: long-term results of percutaneous ethanol injection. Radiology. 1995;197:101–108. doi: 10.1148/radiology.197.1.7568806. [DOI] [PubMed] [Google Scholar]
- 61.Mondazzi L, Bottelli R, Brambilla G, et al. Transarterial oily chemoembolization for the treatment of hepatocellular carcinoma: a multivariate analysis of prognostic factors. Hepatology. 1994;19:1115–1123. [PubMed] [Google Scholar]
- 62.Naugler WE, Sonnenberg A. Survival and cost-effectiveness analysis of competing strategies in the management of small hepatocellular carcinoma. Liver Transpl. 2010;16:1186–1194. doi: 10.1002/lt.22129. [DOI] [PubMed] [Google Scholar]
- 63.Salomon JA, Weinstein MC, Hammitt JK, Goldie SJ. Empirically calibrated model of hepatitis C virus infection in the United States. Am J Epidemiol. 2002;156:761–773. doi: 10.1093/aje/kwf100. [DOI] [PubMed] [Google Scholar]
- 64.Trevisani F, Santi V, Gramenzi A, et al. for Italian Liver Cancer Group Surveillance for early diagnosis of hepatocellular carcinoma: is it effective in intermediate/advanced cirrhosis? Am J Gastroenterology. 2007;102:2448–2457. doi: 10.1111/j.1572-0241.2007.01395.x. [DOI] [PubMed] [Google Scholar]
- 65.Connock M, Round J, Bayliss S, Tubeuf S, Greenheld W, Moore D. Sorafenib for the treatment of advanced hepatocellular carcinoma. Health Technol Assess. 2010;14:17–21. doi: 10.3310/hta14Suppl1/03. [DOI] [PubMed] [Google Scholar]
- 66.The Global Burden of Hepatitis C Working Group Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44:20–29. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 67.Chen MS, Li JQ, Zheng Y, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–700. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 69.Llovet JM, Mas X, Aponte JJ, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bismuth H, Majno PE, Adam R. Liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 1999;19:311–322. doi: 10.1055/s-2007-1007120. [DOI] [PubMed] [Google Scholar]
- 71.Ladabaum U, Cheng SL, Yao FY, Roberts JP. Cost effectiveness of screening for recurrent hepatocellular carcinoma after liver transplantation. Clin Transplant. 2011;25:283–291. doi: 10.1111/j.1399-0012.2010.01212.x. [DOI] [PubMed] [Google Scholar]
- 72.Carr B, Carroll S, Muszbek N, Gondek K. Economic evaluation of Sorafenib in unresectable hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1739–1746. doi: 10.1111/j.1440-1746.2010.06404.x. [DOI] [PubMed] [Google Scholar]