Abstract
Twenty-six patients with recurrent CD20+ B-cell lymphoid malignancies received fludarabine, cyclophosphamide, and rituximab–based nonablative conditioning followed by either matched related (n = 18) or unrelated (n = 8) donor allogeneic stem cell transplantation (allo-SCT) between March 2008 and May 2011. Median age of patients at transplantation was 59 years (range, 41–64 years). At diagnosis, 20 (77%) had stage IV disease; 23 (88%) received ≥3 regimens, 14 (54%) received ≥4 regimens, and 4 (15%) had earlier autologous-SCT. All patients had either chemosensitive or stable disease and nine (35%) were in complete remission before transplantation. At the time of analysis, 17 patients were alive with an estimated 2-year overall survival and progression-free survival rate of 63% and nonrelapse mortality of 25%. Grade II to IV acute graft-vs-host-disease occurred in 8 (31%) and chronic graft-vs-host-disease in 6 (23%) patients (extensive, n = 3). Causes of death include progressive disease in four, acute graft-vs-host-disease in two (both after receiving donor lymphocyte infusion for mixed chimerism with residual disease), infection in one, and other (e.g., substance abuse, leukoencephalopathy) in two. Six patients required rehospitalization within 100 days of SCT (mean = 10 days; range, 3–18 days). Our data support fludarabine, cyclophosphamide, and rituximab–based nonablative conditioning allo-SCT in CD20+ B-cell lymphoid malignancies and it is time to compare this regimen with an alternative reduced-intensity conditioning regimen in B-cell malignancies.
Recurrent B-cell lymphoid malignancies have poor prog-noses with conventional chemotherapy. Autologous stem cell transplantation (auto-SCT) is widely accepted as an effective therapy with low transplant-related mortality (TRM) for patients with recurrent B-cell lymphoma. Although 40% to 60% of younger patients are expected to achieve long-term disease control after auto-SCT, a large proportion of patients will continues to relapse.
Allogeneic stem cell transplantation (allo-SCT) is limited by TRM, primarily mediated by graft-vs-host disease (GVHD). Unfortunately, because of this, allo-SCT is reserved mostly for heavily pretreated patients, which further escalates TRM, especially after myeloablative conditioning [1,2]. To decrease TRM and provide potential curative therapy to an extended spectrum of patients (e.g., older age, patients with comorbidities), reduced-intensity conditioning (RIC) allo-SCT is being performed increasingly. With an improvement in supportive care, outcomes after allo-SCT have improved significantly. Successful results have been reported in selected patient populations that have achieved long-term disease control after allo-SCT.
Disease-specific RIC regimens that offer acceptable GVHD-associated morbidity and TRM are preferred [3–5]. MD Anderson Cancer Center has shown the effectiveness of a fludarabine, cyclophosphamide, and rituximab (FCR) nonablative conditioning regimen (NST) allo-SCT in CD20+ B-cell lymphoid malignancies [6–8]. The regimen is also reported to be safe, with a low incidence of acute GVHD and nonrelapse mortality (NRM). However, most reported data using FCR NST allo-SCT are from a single institution. The only other study outside the single-center experience using FCR NST allo-SCT in recurrent follicular lymphoma (FL) closed early after enrolling only eight patients in FCR NST allo-SCT arm, due to slow accrual [9].
In this study, we report our experience using FCR NST allo-SCT in a variety of CD20+ B-cell lymphoid malignancies.
Materials and methods
FCR NST allo-SCT protocol was approved at Vanderbilt University Medical Center and Veterans Affairs Medical Center adult transplantation program for recurrent CD20+ B-cell lymphoid malignances as a standard of procedure clinical protocol in early 2008. Since then, 26 patients older than age 18 years who received FCR NST allo-SCT from March 2008 and May 2011 were included in this study. All patients received planned rituximab-based chemotherapy pre-SCT. Patients were required to have chemotherapy-sensitive or stable nonbulky (<5 cm) nodal disease documented pre-SCT after salvage chemotherapy. This study was approved by the Institutional Review Board of Vanderbilt University Medical Center and Veterans Affairs Medical Center. All patients provided informed consent in accordance with the Declaration of Helsinki.
Clinical information was reviewed, and baseline characteristics, including common pretransplantation and transplant variable information, were recorded. Overall survival, progression-free survival, and NRM were defined by standard criteria.
Transplantation procedures
Donor and stem cell source
All patients received 10/10 HLA-matched (high-resolution typing) related or unrelated donor granulocyte colony-stimulating factor–mobilized peripheral blood stem cell transplantation. Requested stem cell dose for all donors was at least 4 × 106 CD34+ cells/kg and maximum cell dose of 8 × 106 CD34+ cells/kg for unrelated and 10 × 106 CD34+ cells/kg for related donor allo-SCT.
Conditioning regimen and GVHD prophylaxis
The conditioning regimen consisted of fludarabine intravenously over 1-hour infusion (30 mg/m2 daily for 4 days, on day –6, –5, –4, and –3), cyclophosphamide intravenous infusion 4 hours after fludarabine (750 mg/m2 daily for 3 days, on day –4, –3, and –2) and rituximab (375 mg/m2, on days –13, –6, +1, and +8). All patients received premedication with antihistamines and acetaminophen 30 minutes before infusion of rituximab. Stem cell infusion was performed on day 0. All patients received GVHD prophylaxis with tacrolimus and intravenous mini-methotrexate (5 mg/m2) with or without leucoverin on days +1 (24 hours after stem cell infusion), +3, and +6. Tacrolimus was administered daily orally from day –3 at a dosage of 0.09 mg/kg in a twice daily divided dosage. Dosages were adjusted to maintain whole blood trough blood levels at 5 to 15 mg/mL. Tacrolimus dosage was tapered by day +60 to +90, if there were signs of residual disease, or decreased or persistent mixed donor chimerism. Otherwise, it was maintained for 6 months and then tapered at 10% per week.
Patients receiving unrelated donor allo-SCT also received intravenous rabbit antithymocyte globulin (thymoglobulin; Genzyme Inc., Cambridge, MA, USA), total dosage of 5 to 7.5 mg/kg on days –3/–2, and –1 with standard premedications. The diagnosis and grading of acute and chronic GVHD were based on standard criteria [10,11].
Supportive care
All patients received supportive care per institutional standard of procedure protocols regarding antimicrobial prophylaxis, surveillance cultures, monitoring for viral infections, and treatment.
Chimerism and disease monitoring
Sorted (CD33, CD3) and unsorted (restriction fragment length polymorphism [RFLP]) chimerism and fluorescence in situ hybridization (FISH) for sex chromosomes (for gender-mismatched transplantation) were assessed as per institutional protocols. Briefly, chimerism analysis and disease evaluation with positron emission tomography/computed tomography or computed tomography scans were performed monthly post-transplantation until day +90. Bone marrow biopsies were also performed as a part of day +30 and day +90 post-SCT evaluation. Patients were transferred to long-term transplantation clinic beyond day +100 post-SCT once clinically stable and not requiring frequent clinic/laboratory visits. Frequency of chimerism and disease monitoring were modified in long-term transplantation clinic depending on chimerism and disease data on day +100.
Response criteria
Response criteria were based on guidelines from the International Workshop on Non-Hodgkin Lymphoma. Complete remission (CR) was defined as complete radiological regression of all previous measurable disease or bone marrow involvement. Partial response (PR) was defined as a reduction of ≥50% in the sum of the products of the longest and perpendicular diameter of measurable lesions within a 30-day period before transplantation and at days +30 and +100.
Statistical analysis
Overall survival, progression-free survival, and NRM were calculated from day of transplantation using Kaplan–Meier method. Dependent variables were adjusted for appropriate confounding factors. Statistical analyses were performed using SPSS software (version 19; IBM-SPSS, Chicago, IL, USA).
Results
Median age of patients at transplantation was 59 years (range, 41–64 years). Ten patients had chronic lymphocytic leukemia, 7 had mantle cell lymphoma, 3 had diffuse large B-cell lymphoma, 3 had FL, and 3 had transformed lymphoma. At diagnosis, 20 (77%) patients had stage IV disease. Twenty-three patients (88%) received ≥3 regimens, 14 (54%) received ≥4 regimens, and 4 (15%) had prior auto-SCT. Nine (35%) patients were in CR pre-SCT after salvage therapy. Median stem cell dose was 2.81 × 106 CD34/kg (range, 2.3–9.8). Of 17 surviving patients at the time of analysis,14 (82%) patients are in CR, 2 are in PR (12%), and stable disease 1 (6%) at the time of analysis after a median follow-up of surviving patient after transplantation of 496 days (range, 130–1094 days).
Regimen tolerance
Conditioning regimen was tolerated very well, no patients developed grade III to IV mucositis and none required intravenous parenteral nutritional support. All patients tolerated rituximab and thymoglobulin infusion well and no infusion-related severe side effects were reported.
Engraftment and chimerism
All patients engrafted. Neutrophil counts recovered to >0.5 × 109/L a median of 14 days after transplantation (range, 6–19 days). Nineteen patients (73%) required no platelet transfusions; in the remaining patients, platelet counts recovered to >20 × 109/L at a median of 10 days after transplantation (range, 7–14 days).
Median values of donor RFLP by day 30 after transplantation was 70% (range, 60–100%). Median values of donor CD3+ and CD33+ cells by day 30 after transplantation were 80% and 83% (range, 9–100% and 50–100%), respectively. Median value of donor RFLP by day 100 after transplantation was 90% (range, 6–100%). Median values of donor CD3+ and CD33+ cells by day 100 after transplantation were 84% and 88% (range, 0–100% and 1–100%), respectively. Median level of donor RFLP, CD3+, and CD33+ had increased to ≥95% by day 100, in 11 (69%), 11 (42%), and 17 (65%) patients, respectively.
Outcomes
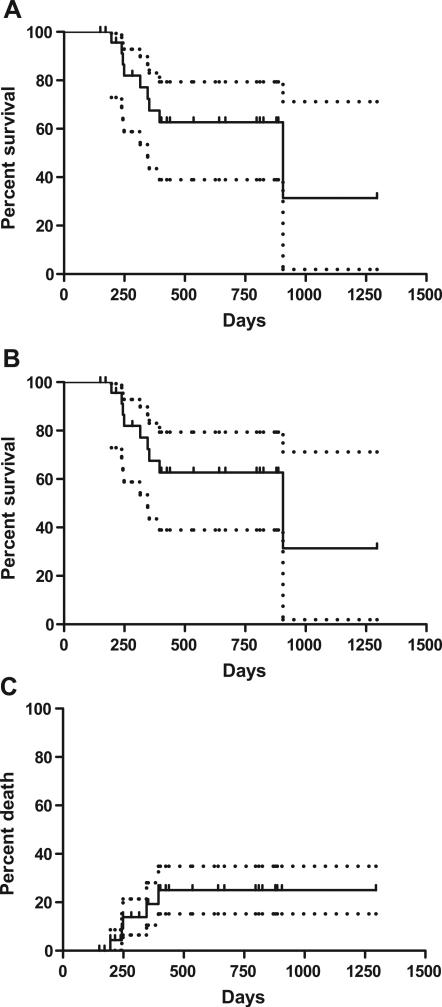
All patients survived 100 days post-transplantation and, at the time of analysis, 17 patients were alive with an estimated 2-year overall survival and progression-free survival rate of 63% and NRM rate of 25% (Fig. 1).
Figure 1.
Transplantation outcomes after FCR nonablative allo-SCT for CD20+ B-cell lymphoid malignancies: (A) overall survival; (B) progression-free survival, and (C) NRM.
Maximum acute GVHD grade II to IV occurred in 8 (31%) and chronic GVHD in 6 (23%) patients (extensive, n = 3). Of eight patients developing grade II to IV acute GVHD, two developed it only after receiving donor lymphocyte infusion (DLI; 107 CD3+ cells/kg one time dose) for residual disease with mixed donor chimerism. Both patients developed refractory grade IV gastrointestinal GVHD (one each related and unrelated donor allo-SCT recipients).
Causes of death, rehospitalization, and Epstein–Barr virus reactivation
Causes of death include progressive disease in four, acute GVHD in two (both after receiving DLI for mixed chimerism with residual disease), infection in one, and others (e.g., substance abuse, leukoencephalopathy) in two. Only six patients required rehospitalization within 100 days of allo-SCT (mean = 10 days; range, 3–18 days). None of our patients in this series developed Epstein–Barr virus (EBV) reactivation post-transplantation.
Discussion
Our results indicate promising outcomes after FCR NST allo-SCT in heavily pretreated patients with B-cell lymphoid malignancies. Although there are very limited reported multi-institutional data using FCR NST allo-SCT for most CD20+ B-cell lymphoid malignancies, published data from MD Anderson Cancer Center have shown its effectiveness in FL, mantle cell lymphoma, and chronic lymphocytic leukemia [7,8,12]. This regimen is also reportedly safe, with a TRM of 10%, and 85% of patients were alive without disease at 8 years post-SCT in FL [7]. Similarly, recently published Blood and Marrow Transplant Clinical Trials Network results using FCR allo-SCT in FL (n = 8) showed a disease-free survival of 86% and no patient developed grade II to IV acute GVHD [9]; this study was closed early due to slow accrual.
There is no evidence that the more substantial RIC regimens (i.e., fludarabine and melphalan or busulfan) provide any advantage in disease control in patients with chemosensitive disease over FCR NST. Importantly, these alternative RIC regimens appear to be associated with more severe toxicities, GVHD, and NRM in lymphoma patients. The Grupo Español de Linfomas/Trasplante Autólogo de Médula Osea (GELTAMO) group, for example, has recently reported outcomes in 37 FL patients who underwent matched related donor using melphalan and fludarabine conditioning, a majority of patients had chemosensitive disease and 40% were in CR at the time of transplantation. After median follow-up of 52 months, 4-year disease-free survival rates for patients with progressive disease, PR, or CR at transplantation were 29%, 48%, and 64%, respectively, whereas the 4-year cumulative incidences of NRM were 71%, 33%, and 26%, respectively. The authors concluded that fludarabine and melphalan RIC allo-SCT was associated with significant NRM in heavily pretreated patients with FL [13]. However, this is not necessarily the case when using the fludarabine, intravenous busulfan, and thymoglobulin-based regimen.
In patients treated with rituximab close to the time of transplantation or given as a part of the conditioning regimen might attenuate disparate histocompatibility antigen presentation by B cells and the additional depletion of B cells may result in greater protection from GVHD [3,7,14–16]. We confirm this observation with only six (23%) patients developing acute grade II to IV (before DLI) and six (26%) developed chronic GVHD.
Risk of EBV reactivation is highest in older patients, T-cell–depleted SCT (in vivo or vitro), and in unrelated or mismatched SCT [17,18]. Cumulative numbers of patients with EBV reactivation and post-transplantation lymphoproliferative disorders are rising as more patients at high risk for EBV reactivation and post-transplantation lymphoproliferative disorders are receiving allo-SCT [19]. Elimination of host and donor memory B cells by low-dose rituximab in the conditioning regimen could reduce the incidence of EBV reactivation and post-transplantation lymphoproliferative disorders post-SCT. None of our patients developed EBV reactivation in this series similar to our earlier observation [20]. Excellent tolerance, decreased GVHD, minimal rehospitalization, and reduced risk of EBV reactivation might significantly reduce the expense, morbidity, and mortality associated with FCR NST allo-SCT. This is especially important when older heavily pretreated patients receiving unrelated donor T-cell–depleted (in vivo depletion with thymoglobulin) allo-SCT [18].
The optimal dosage of rituximab is unknown in the context of allo-SCT, and we decided to a standard dosing (375 mg/m2 for a total of four doses) instead of the higher dosage used in MD Anderson FCR regimens. A previous study has shown a correlation between the serum concentration of a therapeutic monoclonal antibody and the clinical response [21], and it is postulated that in the setting of allo-SCT, rituximab could augment the graft-vs-lymphoma effect through antibody-dependent cellular toxicity. The majority of lymphoma patients undergoing allo-SCT are heavily pre-treated, including multiple repeated rounds of rituximab therapy pre-SCT; in the treatment settings (FCR NST) where the burden of CD20 target cells is low, a low or standard dosing regimen could be as effective [22,23]. Our data support the effectiveness of standard rituximab dosing; however, further studies are needed to define the optimal dosage of rituximab with FCR NST allo-SCT.
DLI is often used to augment disease control in patients with progressive or resistant lymphoma, but can also be given to patients with mixed chimerism in an effort to achieve full donor chimerism, even in the absence of measurable disease. Administration of DLIs is associated with a risk for severe GVHD, which can be life-threatening [24]. Two patients in our series died from severe GVHD after DLI for a mixed chimerism with residual disease. The precise criteria for DLI in patients undergoing FCR allo-SCT are not clear and our policy is to wait and watch, especially for those who are early post-SCT or in CR.
Patients with B-cell lymphoma are typically severely immunosuppressed because of earlier therapy with purine analogs and multiple chemotherapy combinations including rituximab. It is assumed that these patients are at further higher risk of infectious complications after FCR allo-SCT. Rehospitalization rate was minimal in our series and we did not observe excess infectious complications compared to alternative RIC regimens (unpublished data); however, this needs to be studied in prospective studies.
Summary
Our data confirm that a CD20+ B-cell lymphoma–specific NST FCR conditioning regimen is safe and effective in heavily pretreated B-cell lymphoid malignancies. Intensified therapies to improve disease control before SCT are needed to make safer allo-SCT an eligible option for a majority of patients. We recommend FCR allo-SCT be considered early, and novel strategies (e.g., radioimmuno-therapy, tandem auto-allo-SCT) be incorporated to prevent disease progression post-SCT, a major cause of failure.
Acknowledgments
Funding disclosure
N.R. was supported by National Center for Research Resources, National Institute of Health (grant no. 5K-12 CA090625-09).
Footnotes
Conflict of interest disclosure
No financial interest/relationships with financial interest relating to the topic of this article have been declared.
References
- 1.Freytes CO, Lazarus HM. Second hematopoietic SCT for lymphoma patients who relapse after autotransplantation: another autograft or switch to allograft? Bone Marrow Transplant. 2009;44:559–569. doi: 10.1038/bmt.2009.214. [DOI] [PubMed] [Google Scholar]
- 2.Hari P, Carreras J, Zhang MJ, et al. Allogeneic transplants in follicular lymphoma: higher risk of disease progression after reduced-intensity compared to myeloablative conditioning. Biol Blood Marrow Transplant. 2008;14:236–245. doi: 10.1016/j.bbmt.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barrett AJ, Savani BN. Stem cell transplantation with reduced-intensity conditioning regimens: a review of ten years experience with new transplant concepts and new therapeutic agents. Leukemia. 2006;20:1661–1672. doi: 10.1038/sj.leu.2404334. [DOI] [PubMed] [Google Scholar]
- 4.Blaise D, Farnault L, Faucher C, et al. Reduced-intensity conditioning with Fludarabin, oral Busulfan, and thymoglobulin allows long-term disease control and low transplant-related mortality in patients with hematological malignancies. Exp Hematol. 2010;38:1241–1250. doi: 10.1016/j.exphem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 5.Michallet M, Le QH, Mohty M, et al. Predictive factors for outcomes after reduced intensity conditioning hematopoietic stem cell transplantation for hematological malignancies: a 10-year retrospective analysis from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Exp Hematol. 2008;36:535–544. doi: 10.1016/j.exphem.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Khouri IF. Reduced-intensity regimens in allogeneic stem-cell transplantation for non-Hodgkin lymphoma and chronic lymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2006:390–397. doi: 10.1182/asheducation-2006.1.390. [DOI] [PubMed] [Google Scholar]
- 7.Khouri IF, McLaughlin P, Saliba RM, et al. Eight-year experience with allogeneic stem cell transplantation for relapsed follicular lymphoma after nonmyeloablative conditioning with fludarabine, cyclophosphamide, and rituximab. Blood. 2008;111:5530–5536. doi: 10.1182/blood-2008-01-136242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khouri IF, Bassett R, Poindexter N, et al. Nonmyeloablative allogeneic stem cell transplantation in relapsed/refractory chronic lymphocytic leukemia: long-term follow-up, prognostic factors, and effect of human leukocyte histocompatibility antigen subtype on outcome. Cancer. 2011 Mar 31; doi: 10.1002/cncr.26091. doi:10.1002/cncr.26091. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomblyn MR, Ewell M, Bredeson C, et al. Autologous versus reduced-intensity allogeneic hematopoietic cell transplantation for patients with chemosensitive follicular non-Hodgkin lymphoma beyond first complete response or first partial response. Biol Blood Marrow Transplant. 2011;17:1051–1057. doi: 10.1016/j.bbmt.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 11.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 12.Tam CS, Bassett R, Ledesma C, et al. Mature results of the MD Anderson Cancer Center risk-adapted transplantation strategy in mantle cell lymphoma. Blood. 2009;113:4144–4152. doi: 10.1182/blood-2008-10-184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinana JL, Martino R, Gayoso J, et al. Reduced intensity conditioning HLA identical sibling donor allogeneic stem cell transplantation for patients with follicular lymphoma: long-term follow-up from two prospective multicenter trials. Haematologica. 2010;95:1176–1182. doi: 10.3324/haematol.2009.017608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reddy N, Savani BN. Treatment options for transformed lymphoma: incorporating allogeneic stem cell transplantation in a multimodality approach. Biol Blood Marrow Transplant. 2011;17:1265–1272. doi: 10.1016/j.bbmt.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inamoto Y, Flowers ME. Treatment of chronic graft-versus-host disease in 2011. Curr Opin Hematol. 2011;18:414–420. doi: 10.1097/MOH.0b013e32834ba87d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crocchiolo R, Castagna L, El-Cheikh J, et al. Prior rituximab administration is associated with reduced rate of acute GVHD after in vivo T-cell depleted transplantation in lymphoma patients. Exp Hematol. 2011;39:892–896. doi: 10.1016/j.exphem.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Landgren O, Gilbert ES, Rizzo JD, et al. Risk factors for lymphoproliferative disorders after allogeneic hematopoietic cell transplantation. Blood. 2009;113:4992–5001. doi: 10.1182/blood-2008-09-178046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peric Z, Cahu X, Chevallier P, et al. Features of Epstein-Barr virus (EBV) reactivation after reduced intensity conditioning allogeneic hematopoietic stem cell transplantation. Leukemia. 2011;25:932–938. doi: 10.1038/leu.2011.26. [DOI] [PubMed] [Google Scholar]
- 19.Reddy N, Rezvani K, Barrett AJ, Savani BN. Strategies to prevent EBV reactivation and posttransplant lymphoproliferative disorders (PTLD) after allogeneic stem cell transplantation in high-risk patients. Biol Blood Marrow Transplant. 2011;17:591–597. doi: 10.1016/j.bbmt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savani BN, Pohlmann PR, Jagasia M, et al. Does peritransplantation use of rituximab reduce the risk of EBV reactivation and PTLPD? Blood. 2009;113:6263–6264. doi: 10.1182/blood-2009-04-213892. [DOI] [PubMed] [Google Scholar]
- 21.Berinstein NL, Grillo-Lopez AJ, White CA, et al. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 22.Aue G, Lindorfer MA, Beum PV, et al. Fractionated subcutaneous rituximab is well-tolerated and preserves CD20 expression on tumor cells in patients with chronic lymphocytic leukemia. Haematologica. 2010;95:329–332. doi: 10.3324/haematol.2009.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vianna RM, Mangus RS, Fridell JA, et al. Induction immunosuppression with thymoglobulin and rituximab in intestinal and multivisceral transplantation. Transplantation. 2008;85:1290–1293. doi: 10.1097/TP.0b013e31816dd450. [DOI] [PubMed] [Google Scholar]
- 24.Tomblyn M, Lazarus HM. Donor lymphocyte infusions: the long and winding road: how should it be traveled? Bone Marrow Transplant. 2008;42:569–579. doi: 10.1038/bmt.2008.259. [DOI] [PubMed] [Google Scholar]