Abstract
An autopsy case report of Trousseau's syndrome caused by intrahepatic cholangiocarcinoma is presented, and seven previously reported cases are reviewed. A 73-year-old woman experiencing light-headedness and dementia of unknown cause for 6 months developed severe hypotonia. A hypointense lesion compatible with acute cerebral infarction was detected by magnetic resonance imaging. Abdominal computed tomography revealed an ill-defined large liver mass in the right lobe. The mass was not further investigated because of the patient's poor condition. She died of multiple organ failure, and an autopsy was conducted. Postmortem examination revealed intrahepatic cholangiocarcinoma, fibrous vegetations on the mitral valves and multiple thromboemboli in the cerebrum, spleen and rectum. Trousseau's syndrome is defined as an idiopathic thromboembolism in patients with undiagnosed or concomitantly diagnosed malignancy. This syndrome is encountered frequently in patients with mucin-producing carcinomas, while the incidence in patients with intrahepatic cholangiocarcinoma is uncommon. We found that tissue factor and mucin tumor marker (CA19-9, CA15-3 and CA-125) expression in cancer cells may be involved in the pathogenesis of thromboembolism. A patient with unexplained thromboembolism may have occult visceral malignancy; thus, mucin tumor markers may indicate the origin of a mucin-producing carcinoma, and postmortem examination may play an important role in revealing the hidden malignancy.
Key words: Cholangiocarcinoma, Trousseau's syndrome, Tissue factor, CA19-9, CA15-3, CA-125
Introduction
Abnormal blood coagulation has been recognized in a subset of patients with malignancy. In 1865, Armand Trousseau first described the association of thromboembolic disorder and occult malignancies [1]. The incidence of thromboembolism in cancer patients has been reported to be 13% [2]. Different cancer types are associated with different rates of thromboembolism. This fatal complication is frequently encountered in patients with lung, pancreatic or ovarian cancer, but it is uncommon in patients with cholangiocarcinoma [3].
Intrahepatic cholangiocarcinomas mainly consist of adenocarcinomas arising from the intrahepatic biliary ductal epithelium, which is composed of columnar to cuboidal epithelium that forms glands and tubules. Intrahepatic cholangiocarcinoma is nearly 10 times more common in Japan than in European countries, and it is associated with a high rate of fatality because of the early occurrence of invasion and metastasis [4]. The major symptoms of this neoplasm are right upper quadrant abdominal pain, ascites, portal hypertension and jaundice, whereas patients who experience thrombosis are uncommon [5].
We herein report an autopsy case of intrahepatic cholangiocarcinoma that was detected following evidence of cerebral infarction due to thromboembolism. In addition, previously reported cases of patients with cholangiocarcinoma who developed thromboembolism in multiple organs are reviewed.
Case Report
A 73-year-old Japanese woman experienced light-headedness and dementia of unknown cause for 6 months. She had had diabetes for 20 years and had a history of surgical excision of breast cancer and laparoscopic cholecystectomy 10 and 20 years before, respectively. Her neurological disturbance had advanced, and she sought treatment at Kansai Medical University Kori Hospital.
At the first medical examination, no abnormalities were detected by brain computed tomography (CT) scan. However, 1 month after the consultation, the patient experienced general hypotonia with progressive dementia and was hospitalized. At the time of admission, her blood coagulation tests were as follows (normal range in parentheses): platelets 6.8 × 104/μl (14–34 × 104/μl), fibrinogen 74.0 mg/dl (180–355 mg/dl), D-dimer 101.6 μg/ml (0–0.9 μg/ml), prothrombin time 81.2% (75–130%) and activated partial thromboplastin time 27.9 s (23–35 s). Her serum albumin had decreased to 1.8 g/dl (3.8–5.0 mg/dl), and γ-GTP had increased to 172 U/l (8–45 U/l). The serum CA19-9 level was 376,100 U/ml (<37 U/ml), and the CA15-3 level was 124 U/ml (<27 U/ml), both of which were significantly increased. Upper abdominal CT scan revealed a large ill-defined mass in the periphery of the right liver lobe and multiple ischemic lesions in the spleen. Because of her poor condition, further examination of the liver mass was impossible. Cerebral magnetic resonance imaging showed multi-regional hypointense areas in the hemisphere of her right cortex, which were compatible with acute cerebral infarction. Cardiac ultrasound showed abnormal movements in the mitral and aortic valves.
Despite continuous anticoagulant therapy, her consciousness deteriorated and she died 3 months after the hospitalization due to multiple organ failure. The postmortem examination was performed 8 h after her death. The main object of the autopsy was to elucidate the pathological cause of the cerebral infarction.
Pathological Findings
Macroscopic Findings
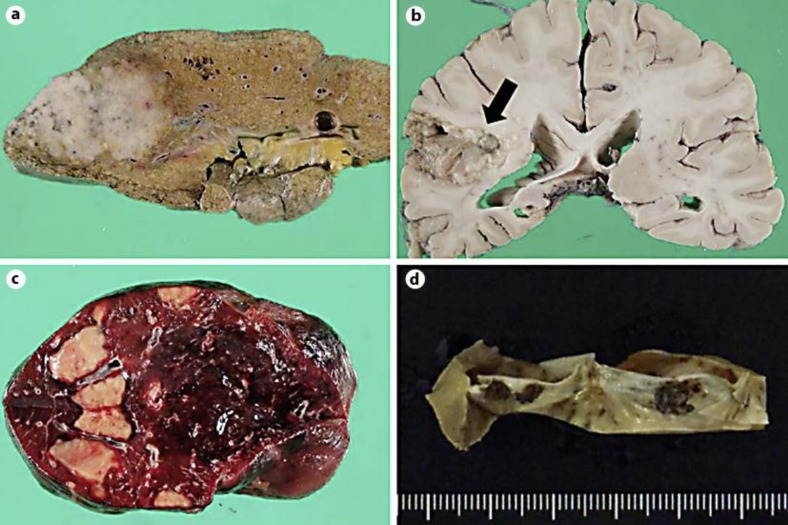
At autopsy, a small amount of serous ascites without evidence of hemorrhage was present. The liver wet weight was 830 g, and the right lobe was occupied by a single solid mass. The irregularly shaped whitish tumor measured 75 × 70 × 40 mm in diameter and infiltrated the surrounding parenchyma (fig. 1a). The hypointense lesion detected in the right hemisphere of the cerebrum was a liquefactive and necrotic infarction (fig. 1b). Small ulcers were found in the rectal mucosa, and geographical ischemic lesions were seen in the cut surface of the spleen (fig. 1c). In addition, fibrinous vegetations were detected on the mitral valves without evidence of infection (fig. 1d).
Fig. 1.
Macroscopic views. a Cut surface of formalin-fixed liver with an ill-defined single solid mass in the right lobe. b The arrow indicates acute infarction in the right hemisphere of the cerebral cortex. c Geographical ischemic lesions in the spleen. d Vegetations on mitral valves.
Histological and Immunohistochemical Findings
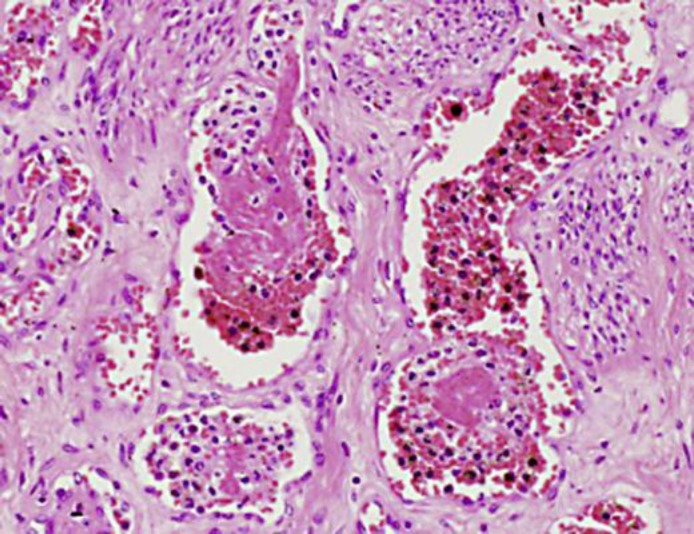
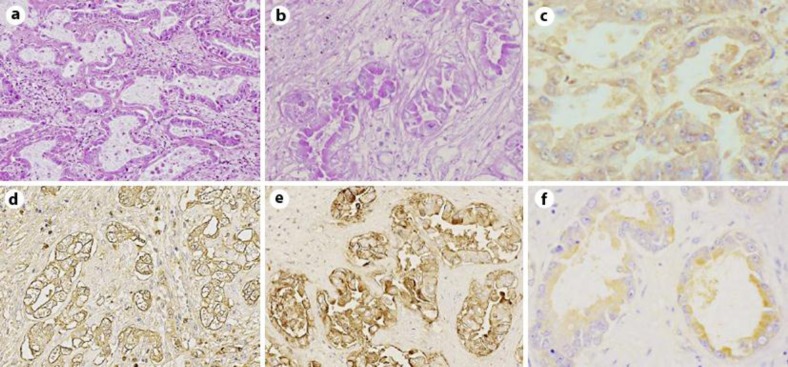
Brain sections showed acute cerebral infarction without evidence of tumor emboli. In other sites of the body, fibrinous thrombi were histologically confirmed, but again tumor embolus was not found (fig. 2). The liver tumor was a well-differentiated tubular adenocarcinoma (fig. 3a). Tumor cells formed irregularly shaped glands surrounded by fibrous stroma. The tumor cells had clear cytoplasm, and large amounts of mucin were present on the inside and outside of the cells (fig. 3b). The size and shape of the nuclei were relatively well arranged, and the nuclear polarities were fairly retained. The non-neoplastic part of the liver was not cirrhotic. Sections from portal hepatic and para-pancreatic nodes showed multiple metastasis of similar histology to the liver mass, a well-differentiated adenocarcinoma with mucin production. Although the patient had had breast cancer 10 years before, the liver mass was composed of a single mass, and tumor cells were cytokeratin 7- and cytokeratin 20-positive (data not shown), indicating intrahepatic biliary duct origin (intrahepatic cholangiocarcinoma), but no breast origin.
Fig. 2.
Thromboemboli detected in microvessels of uterine myometrium (HE).
Fig. 3.
Intrahepatic cholangiocarcinoma. a HE, ×100. b PAS, ×200. c–f Immunohistochemistry for TF (c), MUC-1/Y (d), MUC-1 (e) and MUC-16 (f), respectively, ×200. TF and MUC-16 were moderately expressed in the cytoplasm of cholangiocarcinoma cells. MUC-1/Y and MUC-1 were expressed strongly in the cytoplasm of cholangiocarcinoma cells.
To assess the correlation between intrahepatic cholangiocarcinoma and ubiquitous thromboemboli, immunohistochemistry in tumor sections was performed with the labeled streptavidin-biotin (LSAB) method (LSAB staining kit; Dako, Glostrup, Denmark). Tissue factor (TF) is an accelerator in hemocoagulation cascades, and TF is expressed in adenocarcinomas of several organs [6]. We performed immunohistochemistry for TF (CD142, polyclonal antibody; American Diagnostica Inc., Stamford, Conn., USA) and found moderate expression in the cytoplasm of cancer cells (fig. 3c). Mucin is a major component secreted from cancer cells, and the expression of MUC subtypes differs individually in mucin-producing carcinomas. In the present case, MUC-1/Y protein recognized by the CA19-9 epitope (clone C241; Nichirei Bioscience, Tokyo, Japan) (fig. 3d) and MUC-1 protein recognized by the CA15-3 epitope (clone, Ma695; Novocastra, Newcastle upon Tyne, UK) (fig. 3e) reacted strongly, and MUC-16 protein recognized by the CA-125 epitope (clone OV185; Nichirei Bioscience) reacted moderately in the cytoplasm of cancer cells (fig. 3f).
Discussion
Trousseau's syndrome is an idiopathic thromboembolism that occurs before or at the same time as a diagnosis of malignancy [1]. This syndrome is also termed migratory thrombophlebitis due to non-bacterial endocarditis occurring in patients with undiagnosed cancer [1]. In our case, cardiac ultrasound showed abnormal movements in the valves, and vegetations composed of non-infectious fibrin clots were histologically detected on the surface of mitral valves. These findings suggested the presence of a hemocoagulation disorder.
The pathogenesis of thromboembolism in cancer patients is complex, and multiple factors are involved. One of the main factors is TF, which activates the coagulation cascade via factor VII [7]. Wang et al. [8] demonstrated the activation of blood coagulation systems by tumor-producing TF by using a mouse pancreatic cancer xenograft model. According to previous immunohistochemical reports, TF is found more frequently in epithelial malignancies than in benign or non-epithelial tumors, and tumors with high TF expression such as pancreatic cancer tend to increase the incidence of thromboembolism [6, 9]. In pancreatic cancer, patients with high TF expression had about a five times higher rate of thromboembolism as compared to patients with low expression [10]. In the present case, a moderate level of TF expression was immunohistochemically detected in the cytoplasm of cholangiocarcinoma cells. However, other mechanisms may also be involved in the genesis of thromboembolism in cases of malignancy.
Intravascular coagulation has been frequently associated with mucin-producing carcinoma. Mucin is a term for glycoproteins of the major structural component of mucus produced by epithelial cells, and each subtype of mucins has individual functions. Purified mucin extracts from adenocarcinoma cells can activate coagulation mechanisms both in vitro and in vivo [11], and the activation of factor X by cancer-producing mucin plays an important role in hemocoagulation cascades. In the present case, serum tumor marker CA19-9 (also known as MUC-1/Y) and CA15-3 (also known as MUC-1) showed significant elevation (376,100 and 124 U/ml, respectively), and intrahepatic cholangiocarcinoma cells strongly expressed MUC-1/Y, MUC-1, and also MUC-16 (CA-125) immunohistochemically. MUC-1 and MUC-16 (CA-125) expression is correlated with the incidence of thromboembolism in cancer patients [12, 13]. In our case, in addition to CA15-3 and CA-125, elevated CA19-9 may also be involved in the formation of thromboembolism.
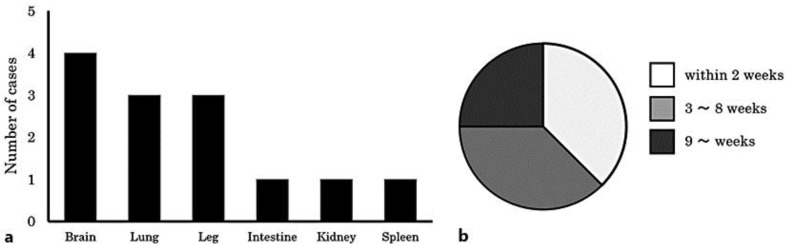
The tumors associated with Trousseau's syndrome are usually mucin-producing adenocarcinomas derived from visceral organs. However, there are few reports of an association between cholangiocarcinoma and thromboembolism. Trousseau's syndrome associated with primary cholangiocarcinoma was first reported by Ching in 1991 [14], and as far as we know, a total of 7 cases in 6 reports have been published in the English language literature [7, 14, 15, 16, 17, 18]. Including our case, the average age at onset was 58 years (range 39–76 years), and the patients consisted of 3 men and 5 women. All of the cases were histologically confirmed to be adenocarcinoma. Cerebral vessels were the most frequent site of thromboembolism, and lung and femoral vessels were secondary common sites (fig. 4a). Anticoagulant therapy with low molecular weight heparin was successful in only 1 patient [18]. In the remaining 7 patients, deep vessel thrombosis was fatal. Three of the 8 patients (38%) died within 2 weeks of diagnosis, and 6 of the 8 patients (75%) died within 8 weeks of the diagnosis (fig. 4b). Postmortem examination was performed in only 3 of the 8 cases.
Fig. 4.
Clinicopathological features of reported cases of Trousseau's syndrome caused by cholangiocarcinoma. a Distribution of thromboemboli. b Survival time from diagnosis.
In a previous report, about 10% of patients with cholangiocarcinoma developed thromboemboli, and the incidence of thromboembolism was considered to be a prognostic factor in patients with cholangiocarcinoma [19]. Screening for coagulation time and TF are regarded as important factors for hypercoagulability in cancer patients, and mucin production by cancer cells should be speculated by elevation of mucin tumor markers such as CA19-9, CA-125 and CA15-3. Clinically, general screening with abdominal/pelvic CT may be useful [20]. To treat these thromboemboli, subcutaneous injections of low molecular weight heparin are effective [21]. However, the prognosis of cholangiocarcinoma is relatively poor compared with other visceral malignancies. Approximately 13% of undiagnosed cancer patients who developed thromboemboli had an occult malignancy [2]. Mucin tumor markers may indicate the origin of mucin-producing carcinoma. Our case emphasizes the importance of postmortem examination to identify the cause of multiple thromboembolism and possible pathological mechanisms of this lethal syndrome.
Disclosure Statement
The authors have no conflict of interest to declare.
Acknowledgement
The authors thank Ms. Akane Shudo for preparing the manuscript.
References
- 1.Varki A. Trousseau's syndrome: multiple definitions and multiple mechanisms. Blood. 2006;110:1723–1729. doi: 10.1182/blood-2006-10-053736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Shami K, Griffiths E, Stereiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist. 2007;12:518–523. doi: 10.1634/theoncologist.12-5-518. [DOI] [PubMed] [Google Scholar]
- 3.Rickles FR, Edwards RL. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983;62:14–31. [PubMed] [Google Scholar]
- 4.Ishak KG, Goodman ZD, Stocker JT. Atlas of Tumor Pathology Third Series, Fascicle 31. Washington D.C.: Armed Forces Institute of Pathology; 1999. Tumors of the liver and intrahepatic bile ducts. [Google Scholar]
- 5.Nakanuma Y, Curado MP, Franceschi S, Gores G, Paradis V, Sripa B. Intrahepatic cholangiocarcinoma. In: Bosmann FT, Carneiro F, Hruban RH, Theise ND, editors. WHO Classification of Tumours of the Digestive System. ed 4. Lyon: IARC; 2010. pp. 217–224. [Google Scholar]
- 6.Callandar NS, Varki N, Rao LVM. Immunohistochemical identification of tissue factor in solid tumors. Cancer. 1992;70:1194–1201. doi: 10.1002/1097-0142(19920901)70:5<1194::aid-cncr2820700528>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Ortego J, Blanco Lopez L, Carbonell Abello J, Monfort Faure J. Multiple thromboemboli associated to two occult tumors: a case mimicking catastrophic antiphospholipid syndrome. Joint Bone Spine. 2011;78:405–408. doi: 10.1016/j.jbspin.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 8.Wang JG, Geddings JE, Aleman MM, Cardenas JC, Chantrathammachart P, Williams JC, Kirchhofer D, Bogdanov VY, Bach RR, Rak J, Church FC, Wolberg AS, Pawlinski R, Key NS, Yeh JJ, Mackman N. Tumor-derived tissue factor activates coagulation and enhances thrombosis in a mouse xenograft model of human pancreatic cancer. Blood. 2012;119:5543–5552. doi: 10.1182/blood-2012-01-402156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasthuri RS, Taubman MB, Mackman N. Role of tissue factor in cancer. J Clin Oncol. 2009;27:4834–4838. doi: 10.1200/JCO.2009.22.6324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khorana AA, Ahrendt SA, Ryan CK, Francis CW, Hruban RH, Hu YC, Hostetter G, Harvey J, Taubman MB. Tissue factor expression, angiogenesis, and thrombosis in pancreatic cancer. Clin Cancer Res. 2007;13:2870–2875. doi: 10.1158/1078-0432.CCR-06-2351. [DOI] [PubMed] [Google Scholar]
- 11.Pieno GF, Brain MC, Gallus AS, Hirsh J, Hatton MW. Tumors, mucus production, and hypercoagulability. Ann N Y Acad Sci. 1974;230:262–270. doi: 10.1111/j.1749-6632.1974.tb14458.x. [DOI] [PubMed] [Google Scholar]
- 12.Tesselaar ME, Romijn FP, Van Der Linden IK, Prins FA, Bertina RM, Osanto S. Microparticle-associated tissue factor activity: a link between cancer and thrombosis? J Thromb Haemost. 2007;5:520–527. doi: 10.1111/j.1538-7836.2007.02369.x. [DOI] [PubMed] [Google Scholar]
- 13.Jovin TG, Boosupalli V, Zivkovic SA, Wechsler LR, Gebel JM. High titers of CA-125 may be associated with recurrent ischemic strokes in patients with cancer. Neurology. 2005;64:1944–1945. doi: 10.1212/01.WNL.0000163850.07976.63. [DOI] [PubMed] [Google Scholar]
- 14.Ching CK. Trousseau syndrome in a patient with cholangiocarcinoma. Am J Gastroenterol. 1991;86:928–929. [PubMed] [Google Scholar]
- 15.Martins EG, Fleming KA, Garrido MC, Hine KR, Chapman RG. Superficial thrombophlebitis, dysplasia, and cholangiocarcinoma in primary sclerosing cholangitis. Gastroenterology. 1994;107:537–542. doi: 10.1016/0016-5085(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez JL, Riancho JA, Gonzalez-Macias J. Cholangiocarcinoma presenting as Trousseau's syndrome. Am J Gastroenterol. 1998;93:847–848. doi: 10.1111/j.1572-0241.1998.847a_a.x. [DOI] [PubMed] [Google Scholar]
- 17.Tasi SH, Juan CJ, Dai MS, Kao WY. Trousseau's syndrome related to adenocarcinoma of the colon and cholangiocarcinoma. Eur J Neurol. 2004;11:493–496. doi: 10.1111/j.1468-1331.2004.00814.x. [DOI] [PubMed] [Google Scholar]
- 18.Jang JW, Yeo CD, Kim JD, Bae SH, Choi JY, Jung ES, Rha SE, Byun JY, Yoon SK. Trousseau's syndrome in association with cholangiocarcinoma: positive tests for coagulation factors and anticardiolipin antibody. J Korean Med Sci. 2006;21:155–159. doi: 10.3346/jkms.2006.21.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeon HK, Kim DU, Baek DH, Ha DW, Lee BE, Ryu DY, Cheong JH, Kim GH, Song GA, Jang AE. Venous thromboembolism in patients with cholangiocarcinoma: focus on risk factors and impact on survival. Eur J Gastroenterol Hepatol. 2012;24:444–449. doi: 10.1097/MEG.0b013e328350f93c. [DOI] [PubMed] [Google Scholar]
- 20.Di Nisio M, Otten HM, Piccioli A, Lensing AWA, Prandoni P, Buller HR, Prins MH. Decision analysis for cancer screening in idiopathic venous thromboembolism. J Thromb Haemost. 2005;3:2391–2396. doi: 10.1111/j.1538-7836.2005.01606.x. [DOI] [PubMed] [Google Scholar]
- 21.Walsh-McMonagle D, Green D. Low-molecular-weight heparin in the management of Trousseau's syndrome. Cancer. 1997;80:649–665. [PubMed] [Google Scholar]