Abstract
Why do some individuals become dissatisfied with their marriages when levels of negative emotion are high and levels of positive emotions are low, whereas others remain unaffected? Using data from a 13-year longitudinal study of middle-aged and older adults in long-term marriages, we examined whether the 5-HTTLPR polymorphism in the serotonin transporter gene moderates the association between negative and positive emotional behavior (objectively measured during marital conflict) and changes in marital satisfaction over time. For individuals with two short alleles of 5-HTTLPR, higher negative and lower positive emotional behavior at Time 1 predicted declines in marital satisfaction over time (even after controlling for depression and other covariates). For individuals with one or two long alleles, emotional behavior did not predict changes in marital satisfaction. We also found evidence for a crossover interaction (individuals with two short alleles of 5-HTTLPR and low levels of negative or high levels of positive emotion had the highest levels of marital satisfaction). These findings provide the first evidence of a specific genetic polymorphism that moderates the association between emotional behavior and changes in marital satisfaction over time and are consistent with increasing evidence that the short allele of this polymorphism serves as a susceptibility factor that amplifies sensitivity to both negative and positive emotional influences.
Keywords: Emotional behavior, genetic polymorphisms, 5-HTTLPR, relationships
Marriage plays an important role in the social fabric of our lives. According to the 2009 census, 96% of Americans over the age of 65 had been married at least once in their life. The quality of marriage is an important contributor to health and well-being (Proulx, Helms, & Buehler, 2007). Spouses who are unhappy in their marriages are at a heightened risk for mental health problems (Whisman, 2007), physical health problems (Kiecolt-Glaser & Newton, 2001) as well as divorce (Gottman & Levenson, 2000). Thus, marital dissatisfaction has negative consequences for individuals, family members, and society alike.
In considering the foundations and functioning of marriage, emotions play an enormously important role (e.g., Gottman, 1994; Greenberg & Johnson, 1988). Early research on marriage was largely the province of sociologists, who developed excellent questionnaires to quantify spouses’ level of satisfaction with their marriages (e.g., Locke & Wallace, 1959). These questionnaires were used in survey studies that explored the association between marital satisfaction and select demographic, personality, and other variables (e.g., Bentler & Newcomb, 1978). As marriage became of increasing interest to psychologists, researchers began directly observing marital interaction and discovered the powerful role that emotion plays in marital satisfaction and marital stability (Birchler, Weiss, & Vincent, 1975; Levenson & Gottman, 1983). A large body of research, conducted over the ensuing decades, documented the general finding that high levels of negative emotion and low levels of positive emotion during marital interactions are associated with low levels of concurrent and future marital satisfaction (e.g., Carstensen, Gottman, & Levenson, 1995; Gottman & Levenson, 1992; Karney & Bradbury, 1995; Levenson & Gottman, 1983; Levenson & Gottman, 1985). Given this, it is not surprising that emotions have become a primary target for contemporary therapies that attempt to improve couples’ relationships (e.g., Gottman & Gottman, 2008; Greenberg & Johnson, 1988).
To paraphrase Longfellow, into each marriage some negative emotion must fall. Nonetheless, the effects of these negative emotions may not be as bad for some spouses as for others. Most of us know couples who seem to thrive in a negative emotional climate that is characterized by arguments and bickering. For other couples, even a droplet of negative emotion is toxic. Similarly, some couples may become much more satisfied with their marriages in the face of positive emotions, while others may remain relatively unaffected. Thus, the link between emotional behavior and marital satisfaction may differ greatly among individual couples (Bradbury, Fincham, & Beach, 2000; Karney & Bradbury, 1995; see also Sullivan, Pasch, Johnson, & Bradbury, 2010).
Previous research has pointed towards important moderating factors that could influence the association between emotion and marital satisfaction. These include attachment styles (Mikulincer & Shaver, 2003), coping processes (Karney & Bradbury, 1995; Randall & Bodenmann, 2009), and capacity for forgiveness (Fincham, Stanley, & Beach, 2007). Thus, for example, a person with an insecure attachment style, low coping skills, and an inability to forgive would be expected to experience particularly sharp declines in marital satisfaction in the face of high levels of negative and low levels of positive emotions.
Surprisingly, there has been almost no research on genetic moderators of the association between emotion and marital satisfaction. This is even more puzzling because marital satisfaction is known to be partly heritable (with heritabilities for aspects of marital quality ranging from 13% to 28%; Kendler & Baker, 2007; e.g., Spotts et al., 2004). Moreover, there is good evidence for genetic influences on attachment (Caspers, Paradiso, Yucuis, Troutman, Arndt, & Philibert, 2009), pair bonding (Walum et al., 2008) and sensitivity to spousal affect (Schoebi, Way, Karney, & Bradbury, 2012). Moreover, even though these kinds of studies confirm heritability, they do not establish the role that specific genes play in the association between emotion and marital satisfaction.
In the present study, we examined the 5-HTTLPR polymorphism in the promoter region of the serotonin transporter gene (SLC6A4)(e.g., Caspi et al., 2003; Lesch, Bengel, Heils, Sabol, & et al., 1996) as a potential moderator of the association between negative and positive emotional behavior and marital satisfaction. The 5-HTTLPR polymorphism has two common alleles (short and long), with the short allele leading to lower levels of serotonin uptake and lower transcriptional efficiency of the serotonin transporter protein (Lesch et al., 1996).
Early studies on 5-HTTLPR typically used gene x environment designs (often focusing on gene x stress interactions) to study its influence on distal outcomes such as the development of psychopathology. In this early work, the short allele was found to be associated with a heightened risk for depression and suicide in the face of stress and adversity (e.g., Caspi et al., 2003; Kendler, Kuhn, Vittum, Prescott, & Riley, 2005; Roy, Hu, Janal, & Goldman, 2007; Taylor, Way, Welch, Hilmert, Lehman, & Eisenberger, 2006). Initial enthusiasm for these findings was dampened by replication issues and the appearance of two meta-analyses that concluded that the interaction effect of 5-HTTLPR x stress did not predict depression (Munafò, Durrant, Lewis, & Flint, 2009; Risch et al., 2009). However, a more recent meta-analysis (Karg, Burmeister, Shedden, & Sen, 2011) that included all available studies (the earlier meta -analyses were more selective) found strong support for the effect. Similar affirmative meta-analytic findings have been obtained for effects of 5-HTTLPR on anxiety (Sen, Burmeister, & Ghosh, 2004).
The studies reviewed thus far focused on distal effects that may take decades to develop (e.g., depression). Another group of studies focused on more proximal effects of 5-HTTLPR, examining responses to well-controlled laboratory stimuli. These studies have consistently shown that individuals with the short allele exhibit heightened reactivity to negative stimuli including: (a) greater amygdala reactivity to negative faces (Hariri et al., 2002), (b) greater cortisol reactivity to social stress (Way & Taylor, 2010), (c) greater startle responses to loud noise (Brocke et al., 2006), and, of direct relevance to the present study, (d) greater reactivity to a partner’s negative affect (Schoebi et al., 2012). In two large laboratory studies using independent subject samples, we (Gyurak et al., 2013) recently found that the short allele of 5-HTTLPR was associated with two different kinds of heightened emotional reactivity. In the first study, individuals with two short alleles showed greater personal distress and greater physiological responding when watching films of others in distress. In the second study, individuals with two short alleles reported more anger and amusement and showed greater emotional behavior in response to an embarrassing situation (viewing a video of oneself singing in a Karaoke-like task).
As research on the distal and proximal effects of 5-HTTLPR has matured, a model has emerged that moves away from early views of the short allele of 5-HTTLPR as a risk factor and instead views it as a plasticity or susceptibility factor (for a review see Belsky & Pluess, 2009). In this view, the short allele is not directly linked to good or bad outcomes, but rather is associated with amplified reactions. Thus, the short allele is not only associated with greater reactivity to the kinds of negative stimuli reviewed above, but also with greater reactivity to more positive stimuli such as positive images (Beevers, Marti, Lee, Stote, Ferrell, Hariri, & Telch, 2011) and positive partner affect (Schoebi et al., 2012). Developing this model a bit further, individuals with the short allele would be likely to have the worst outcomes under unfavorable conditions (e.g., those high in negative and low in positive features) and the best outcomes under favorable conditions (e.g., those low in negative and high in positive features). Statistically, this differential susceptibility takes the form of “crossover interactions” 1, which have now been demonstrated between 5-HTTLPR and various factors including early family environment and current adversity (Taylor et al., 2006), parenting (Hankin et al., 2011), socioeconomic status (Mitchell et al., 2011), and current life events (Pluess, Belsky, Way, & Taylor, 2010) when predicting outcomes such as well -being, neuroticism, and depression. In contrast to earlier studies that almost always focused on gene x stress interaction effects, these studies explicitly consider gene x positive factor (or lack of stress) interaction effects (Belsky & Pluess, 2009).
An important developmental theme has also emerged in studies of genetic polymorphisms (e.g., Lenroot & Giedd, 2011). Life-span developmental theories propose that human aging magnifies the importance of biological factors (Baltes, 1997), and empirical studies suggest that genes in particular become increasingly influential later in life (Lindenberger, Nagel, Chicherio, Li, Heekeren, & Bäckman, 2008). For example, human aging appears to magnify the effects of genetic polymorphisms associated with the dopamine system as the efficiency of the dopamine system declines with age (e.g., Nagel et al., 2008; Stürmer, Passow, Biesenack, & Li, 2012). The efficiency of the serotonin system has likewise been shown to decline with age in terms of both central serotonin transporter availability (van Dyck et al., 2000) and postsynaptic serotonin receptors (Meltzer et al., 1998). Thus, it is possible that the effects of serotonin-related genes such as 5-HTTLPR are also magnified with age. The longitudinal design utilized in the present study, which focuses on middle age and late life, may be particularly useful for examining the influence of aging on the effects of 5-HTTLPR(see also McArdle & Prescott, 2010).
The Present Study
We examined how 5-HTTLPR moderates the association between positive and negative emotional behavior (rated by trained coders) during marital conflict and changes in marital satisfaction in a 13-year longitudinal study of long-term marriages in middle age and late life. This study extends previous research on 5-HTTLPR by focusing on marital satisfaction, objectively coding emotional behavior that occurred during naturalistic marital interactions, including middle-aged and older participants, and using a longitudinal design. Building on prior work (Caspi, Hariri, Holmes, Uher, & Moffitt, 2010), our primary hypothesis was that for individuals with the short allele of 5-HTTLPR, higher negative and lower positive emotional behavior at Time 1 (T1) would predict greater declines in marital satisfaction over time. We designed our analyses to take into account: (a) associations between positive and negative emotional behavior; (b) different possibilities for the dominance structure of 5-HTTLPR (Caspi et al., 2010); (c) statistical nonindependence between husbands’ and wives’ data; (d) the alternative explanation that the effect of 5-HTTLPR x emotional behavior on marital satisfaction would be driven by depression (cf. Caspi et al., 2003) or other co variates; (e) the possibility of a crossover interaction of 5-HTTLPR x emotional behavior (Belsky & Pluess, 2009); and (f) whether findings generalized across gender (Caspi et al., 2010; Williams et al., 2003), cohort (Nagel et al., 2008), and ethnicity (Way & Lieberman, 2010; Williams et al., 2003).
Method
Participants
We analyzed data from a longitudinal study of long-term married couples consisting of a middle-aged (T1: age 40–50) and an older (T1: age 60–70) cohort. This sample of couples was recruited originally in 1989–1990 by a survey research company to be representative of a random sample of marriages in the San Francisco Bay Area in terms of socioeconomic status, religion, ethnicity, and marital satisfaction (for full details see Levenson, Carstensen, & Gottman, 1993). We examined a subsample (N= 125) who participated in genetic testing in 2009 (in 51 couples both spouses participated; in 23 couples one spouse participated). Spouses who participated in genetic testing did not differ from the other participants on the variables examined here except for showing higher positive emotional behavior at T1, p = .025. There were 74 middle-aged adults and 51 older adults (50% females; years of education: M= 15.97, SD = 2.74; 84.8% Caucasian, 4.8% African American, 4.0% Hispanic, 4.8% Latino/a, 1.6% Other). The genetic data from this sample have not been used in any prior publications.
Analyses draw from three waves of assessment [Time 1 (T1): 1989/90, n = 123 with complete data on all variables analyzed in the present study; Time 2 (T2): 1995/96, n = 117; Time 3 (T3): 2001/02, n = 107]. None of the variables examined here predicted drop-out over time, ps > .05.
Procedure
At each assessment, couples completed a set of questionnaires including measures of marital satisfaction (see below). They then came to our laboratory at Berkeley and participated in a well-established procedure for studying marital interaction (Levenson & Gottman, 1983). This procedure consisted of having couples engage in three 15-minute unrehearsed discussions of topics related to their marriage: (a) events of the day (at T1) or events since the last assessment (at subsequent waves); (b) a topic of continuing disagreement in their marriage (conflict topic); and (c) something they enjoyed doing together (pleasant topic). Conversations were recorded on videotape for subsequent analysis of emotional behavior and a number of physiological measures were recorded continuously from each spouse. For the present study, the emotional behavioral data obtained during the conflict conversation were used.
Measures
5-HTTLPR
DNA was collected and extracted from saliva using Oragene kits (DNA Genotek, Kanata, Ontario, Canada) according to manufacturer’s protocol. Anonymized DNA samples were extracted and purified by the Abbott-UCSF Viral Diagnostics and Discovery Center, San Francisco, CA. The extracted DNA was genotyped using the procedure described in Assal, Alarcon, Solomon, Masterman, Geschwind, and Cummings (2004) with slight modifications. A PCR product was amplified with primers (5′-GGCGTTGCCGCTCTGAATGC-3′ and 5′-GAGGGACTGAGCTGGACAACCA-3′) flanking the region containing the gene variation. The PCR conditions consisted of a 2-min denaturation step at 94°C, 35 cycles of 30-sec denaturation at 95°C, 30-sec annealing at 60°C, and 30-sec extension at 72°C, and a final 7-min extension step at 72°C. Genotype distribution [two short alleles: n = 21, 16.8%; one long allele: n = 70, 56%; two long alleles: n = 34, 27.2%] was consistent with previous studies (e.g., Caspi et al., 2003). Table 1 presents the distribution of the 5-HTTLPR genotypes in the total sample broken down by ethnicity as well as results for the Hardy-Weinberg equilibrium test. No deviations from Hardy -Weinberg equilibrium were detected, ps > .05, similar to other samples (e.g., Schoebi et al., 2012).
Table 1.
5-HTTLPR Genotype Frequencies by Ethnicity
| 5-HTTLPR Genotype |
HWE | |||
|---|---|---|---|---|
| s/s | s/l | l/l | χ2 (df = 1) | |
| Caucasians (n = 106) | 16 (15.1%) | 59 (55.7%) | 31 (29.2%) | 1.96, p = .162 |
| Other (n = 19) | 5 (26.3%) | 11 (57.9%) | 3 (15.8%) | .55, p = .458 |
| Total sample (N = 125) | 21 (16.8%) | 70 (56.0%) | 34 (27.2%) | 2.19, p = .139 |
Note. Absolute (relative) frequencies (s/s = short/short. s/l = short/long. l/l = long/long). HWE = Hardy-Weinberg equilibrium.
5-HTTLPR genotyping using the methods we used has generally shown high reliability. Specifically, when we conducted a blind re-analysis of 8 DNA samples from a larger genotyping project that included samples from all of the participants analyzed here, genotype reproducibility was 100%.
Emotional behavior
Emotional behavioral during the T1 conflict conversation was rated by trained coders (blind to participants ‘ genetic polymorphisms, marital satisfaction, and study hypotheses) using the Specific Affect Coding System (SPAFF; Gottman, 1996). SPAFF classifies emotional behaviors on a second-by-second basis based on verbal content, vocal tone, context, facial expression, gesture, and body movement. Inter-rater reliability for SPAFF was satisfactory in the present study (for detailed information see Carstensen et al., 1995). There were nine negative speaker codes, five positive speaker codes, and one neutral speaker code. For the present study, we focused on the negative and positive speaker codes and counted the relative frequency of each negative and positive speaker emotion coded during each second of the 15-minute conflict conversation (i.e., percentage of seconds in which each emotion code was present during the conversation). We obtained a composite score for negative emotional behavior by averaging across all negative speaker codes (i.e., anger, belligerence, contempt, defensiveness, disgust, domineering, fear/tension/worry, sadness, whining) and a composite score for positive emotional behavior by averaging across all positive emotion speaker codes (i.e., affection, humor, interest, joy, validation). As would be expected given the nature of the conflict conversation, the levels of negative emotional behavior (M = 4.86, SD= 3.34) were greater than the levels of positive emotional behavior (M = 2.23, SD= 1.86), t(122)= 6.38, p < .001.
Marital satisfaction
Marital satisfaction was measured at T1, T2, and T3 by averaging two well-established self-report measures: (a) the Marital Adjustment Test (15 items; T1: α = .77; T2: α = .77; T3: α = .76; Locke & Wallace, 1959) and (b) the Marital Relationship Inventory (22 items;; T1: α = .87; T2: α = .85; T3: α = .87; Burgess, Locke, & Thomes, 1971). Across waves of data collection, these two self-report measures were highly correlated, T1: r= .89; T2: r= . 85; T3: r= .83. Across waves of measurement, there were no changes in mean levels of marital satisfaction, as indicated by nonsignificant slope means, ps > .05. However, there were sizeable interindividual differences in changes in marital satisfaction, as indicated by significant slope variances, ps < .05.
Covariates
Covariates were depression (using the respective subscale from the SCL-90; 13 items; T1: α = .88; T2: α = .90; T3: α = .92; Derogatis & Cleary, 1977), ethnicity (0 = else; 1 = Caucasian), gender (0 = male; 1 = female), cohort (0 = middle-aged; 1 = older), and education (in years). Analyses were also controlled for positive emotional behavior when analyzing negative emotional behavior (and vice versa).
Statistical Analyses
Statistical analyses involving the three 5-HTTLPR genotypes [i.e., short/short, short/long, long/long alleles] have been handled in different ways, reflecting different views as to the dominance or co-dominance of the alleles. A recent review (Caspi et al., 2010) found dominance of the short allele in 9 studies, dominance of the long allele in 10 studies, and co-dominance in 14 studies. Because this evidence is so mixed, we chose a statistical approach that enabled us to consider all possible dominance structures of 5-HTTLPR. Building on established “thermometer-coding” procedures (see Kendler et al., 2005) that we have used in previous studies (Gyurak et al., 2013), we coded 5-HTTLPR using two dummy variables, 5-HTTLPR (H1) (0 = long/long; 1 = short/long, short/short) and 5-HTTLPR (H2) (0 = short/long, long/long;1 = short/short). This coding is well-suited for studying the 5-HTTLPR genotype because it anchors the analyses at the most sensitive group, individuals with two short alleles (coded as 1, 1), extends all the way to individuals with two long alleles (coded as 0, 0), and assigns intermediate levels to individuals with one short and one long allele (coded as 1, 0). A significant effect for 5-HTTLPR (H1) indicates that individuals with two long alleles (l/l) differ from individuals with one or two short alleles (s/l or s/s), thus indicating dominance of the short allele. A significant effect for 5-HTTLPR (H2) indicates that individuals with two short alleles (s/s) differ from individuals with one or two long allele (s/l or l/l), thus indicating dominance of the long allele.
We conducted separate multiple linear regressions predicting marital satisfaction at T2 and T3 from (a) negative or positive emotional behavior at T1, (b) 5-HTTLPR thermometer-coded variables (H1 and H2), and (c) interaction terms between 5-HTTLPR (H1 and H2) x negative or positive emotional behavior. Covariates were depression, ethnicity, gender, cohort, education, the other kind of emotional behavior (positive when analyzing negative emotional behavior and vice versa), and marital satisfaction at T1. In follow-up analyses, to investigate whether findings generalized across gender and cohort, we examined 3-way interactions between gender or cohort, 5-HTTLPR (H2), and negative or positive emotional behavior. To evaluate effects of ethnicity, we also examined whether findings held when repeating analyses using only the Caucasian participants (n = 106). Finally, in a follow-up analysis, we investigated whether findings remained stable when controlling for changes in depression across the respective time interval instead of depression at T1. Unless noted otherwise, all findings remained stable. All continuous variables were mean-centered.
Analyses were conducted using STATA 10 (StataCorp., 2007) and focused on within -spouse associations. Because of the dyadic nature of our data, we used STATA’s cluster tool, which corrects all results for nonindependence between husbands and wives. Because we had complete data for only 51 couples (for the other 23 couples only one spouse per couple participated in genetic testing) we were underpowered to examine cross-spouse associations in greater depth. However, to obtain some idea of whether our primary hypothesis would also hold for cross-spouse associations, we employed a multi-group actor-partner modeling approach within a structural equation modeling framework (Olsen & Kenny, 2006) using AMOS (Arbuckle, 2008) to study how wives’ and husbands’ emotional behavior predicted changes in wives’ and husbands’ marital satisfaction depending on wives’ 5-HTTLPR genotype (the models studying the effects of husbands’ 5-HTTLPR genotype did not converge because of small cell sizes and thus were not tested.). For the actor-partner model, we used residualized change scores to model changes in marital satisfaction (by predicting marital satisfaction at T3 from marital satisfaction at T1 and saving the residuals for further analysis) to reduce the number of parameters to be estimated.
Results
Preliminary Analyses
Examination of intercorrelations between key study variables at T1 (see Table 2) showed that negative and positive emotional behavior were negatively correlated. As expected (Carstensen et al., 1995), low levels of marital satisfaction were associated with high levels of negative emotional behavior and low levels of positive emotional behavior at T1. The 5-HTTLPR variables (H1 and H2) were not correlated with negative or positive emotional behavior or with marital satisfaction.
Table 2.
Intercorrelations of Key Study Variables at T1
| 1. | 2. | 3. | 4. | 5. | |
|---|---|---|---|---|---|
| 1. Negative emotional behavior T1 | - | ||||
| 2. Positive emotional behavior T1 | −.51*** | - | |||
| 3. 5-HTTLPR (H1)a | −.01 | .01 | - | ||
| 4. 5-HTTLPR (H2)a | .07 | −.06 | .27** | - | |
| 5. Marital satisfaction T1 | −.27** | .21* | .05 | .00 | - |
Note.
5 -HTTLPR genotype was thermometer-coded (see Kendler et al., 2005; for a detailed description see text) using two dummy variables: 5-HTTLPR (H1) (0 = long/long; 1 = short/long, short/short) and 5-HTTLPR (H2) (0 = short/long, long/long; 1 = short/short).
p < .05.
p < .01.
p < .001.
5-HTTLPR Moderation of the Association between Emotional Behavior and Changes in Marital Satisfaction
Results from the regression analyses examing emotional behavior and 5-HTTLPR predicting changes in marital satisfaction are presented in Table 3 and 4. Consistent with our primary hypothesis, an interaction effect was found between negative emotional behavior at T1 and 5-HTTLPR (H2) when predicting changes in marital satisfaction over the longest (13 years) time interval, from T1 to T3, B = −2.65, SE(B) = 1.05, p = .015, Partial R2 = .08.2 Similarly, an interaction effect was found between positive emotional behavior at T1 and 5-HTTLPR (H2) when predicting changes in marital satisfaction from T1 to T3, B= 3.48, SE(B) = 1.27, p = .008, Partial R2 = .06. Statistical power for detecting these interaction effects (determined using GPower; Faul, Erdfelder, Lang, & Buchner, 2007) was .87 and .75, respectively. Interaction effects between negative or positive emotional behavior and 5-HTTLPR (H2) when predicting changes in marital satisfaction from T1 to T2 were not significant, ps > .05. No interaction effects emerged between negative or positive emotional behavior and the other dummy variable, 5-HTTLPR (H1), at any of the waves of data collection, ps > .05. Thus, in accordance with prior research (Kendler et al., 2005), we collapsed individuals with one or two long alleles into one group.
Table 3.
Negative Emotional Behavior, 5-HTTLPR, and their Interaction as Predictors of Changes in Marital Satisfaction Over Time
| T2 Marital Satisfaction | T3 Marital Satisfaction | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B | SE(B) | 95% CI | B | SE (B) | 95% CI | |||
| Negative emotional behavior T1 | −.30 | .78 | −1.86 | 1.25 | −.29 | .62 | −1.53 | .94 |
| 5-HTTLPR (H1)a | 3.93 | 2.15 | −.37 | 8.23 | −.14 | 1.95 | −4.04 | 3.77 |
| 5-HTTLPR (H2)b | −3.96 | 2.35 | −8.66 | .73 | −3.97 | 2.39 | −8.75 | .81 |
|
| ||||||||
| Negative emotional behavior T1 x 5-HTTLPR (H1)a | .65 | .81 | −.97 | 2.27 | .47 | .71 | −.96 | 1.90 |
| Negative emotional behavior T1 x 5-HTTLPR (H2)b | −.66 | 1.21 | −3.08 | 1.77 | −2.65* | 1.05 | −4.76 | −.54 |
|
| ||||||||
| F(12,66) = 18.51; R2 = .77 | F(12,58) = 14.27; R2 = .78 | |||||||
Note.
5 -HTTLPR (H1) (0 = long/long; 1 = short/long, short/short).
5 -HTTLPR (H2) (0 = short/long, long/long; 1 = short/short). Results from regression analyses controlling for depression, ethnicity, gender, cohort, education, positive emotional behavior, and marital satisfaction at T1. Standard errors corrected for clustering within couples.
p < .05.
Table 4.
Positive Emotional Behavior, 5-HTTLPR, and their Interaction as Predictors of Changes in Marital Satisfaction Over Time
| T2 Marital Satisfaction | T3 Marital Satisfaction | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| B | SE(B) | 95% CI | B | SE (B) | 95% CI | |||
| Positive emotional behavior T1 | 2.51* | 1.05 | .42 | 4.61 | −.86 | 1.37 | −3.61 | 1.89 |
| 5-HTTLPR (H1)a | 3.94 | 2.06 | −.17 | 8.06 | −.18 | 1.95 | −4.07 | 3.72 |
| 5-HTTLPR (H2)b | −3.85 | 2.19 | −8.21 | .52 | −3.11 | 2.33 | −7.77 | 1.55 |
|
| ||||||||
| Positive emotional behavior T1 x 5-HTTLPR (H1)a | −2.36 | 1.22 | −4.79 | .07 | −.50 | 1.43 | −3.37 | 2.37 |
| Positive emotional behavior T1 x 5-HTTLPR (H2)b | 2.23 | 1.41 | −.60 | 5.04 | 3.48** | 1.27 | .95 | 6.02 |
|
| ||||||||
| F(12,66) = 16.75; R2 = .78 | F(12,58) = 14.27; R2 = .78 | |||||||
Note.
5 -HTTLPR (H1) (0 = long/long; 1 = short/long, short/short).
5 -HTTLPR (H2) (0 = short/long, long/long; 1 = short/short). Results from regression analyses controlling for depression, ethnicity, gender, cohort, education, negative emotional behavior, and marital satisfaction at T1. Standard errors corrected for clustering within couples.
p < .05.
p < .01.
To control for possible spurious effects due to multiple testing (8 tests total, consisting of 4 interaction tests predicting changes in marital satisfaction at T2 and T3) using procedures developed by Storey and colleagues (e.g., Storey & Tibshirani, 2003). Results showed that 5-HTTLPR still interacted with both negative and positive emotional behavior to predict changes in marital satisfaction (all q’s < .05 at a false discovery rate of 5%).
Follow-up analyses were then conducted to decompose the significant interaction effects, contrasing individuals with two short alleles with the other 5-HTTLPR variants. These analyses provided strong support for our primary hypothesis. For individuals with two short alleles of 5-HTTLPR, higher negative emotional behavior at T1 predicted declines in marital satisfaction from T1 to T3, B= −2.41, SE(B) = 1.00, p = .019. For individuals with one or two long alleles of 5-HTTLPR, negative emotional behavior at T1 did not predict changes in marital satisfaction from T1 to T3, B= .02, SE(B) = .29, p = .947. In a similar vein, for individuals with two short alleles of 5-HTTLPR, lower positive emotional behavior at T1 predicted declines in marital satisfaction from T1 to T3, B= 2.10, SE(B) = 1.04, p = .048. Somewhat unexpectedly, for individuals with one or two long alleles, higher positive emotional behavior at T1 predicted declines in marital satisfaction, B= −1.23, SE(B) = .58, p = .037. However, this association was no longer statistically significant at the .05 level when controlling for changes in depression from T1 to T3 (instead of depression at T1), B= −1.08, SE(B) = .58, p = .067.
Crossover Interactions
As shown in Figure 1 and 2, interaction effects between 5-HTTLPR and emotional behavior were in fact crossover interactions. That is, individuals with two short alleles of 5-HTTLPR had: (a) the lowest marital satisfaction at high levels of negative and low levels of positive emotional behavior and (b) the highest marital satisfaction at low levels of negative and high levels of positive emotional behavior. To illustrate the nature of the crossover interaction further, Figure 3 plots the changes in marital satisfaction from T1 to T3 at low vs. high levels of negative emotional behavior at T1 depending on the 5-HTTLPR genotype.
Figure 1.
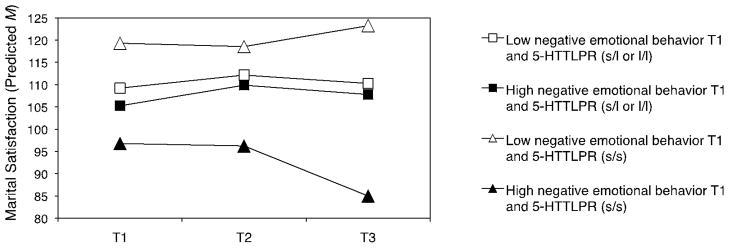
Marital Satisfaction over 13 Years by Negative Emotional Behavior and 5-HTTLPR
Note. Predicted uncentered means of marital satisfaction at T1, T2, and T3 for individuals with high (M+ 1 SD) or low (M − 1 SD) levels of negative emotional behavior at T1 and two short alleles (s/s) or one or two long alleles (s/l or l/l) of 5-HTTLPR. Analyses controlled for depression, ethnicity, gender, cohort, education, and positive emotional behavior at T1. T1: 1989/90. T2: 1995/96. T3: 2001/02.
Figure 2.
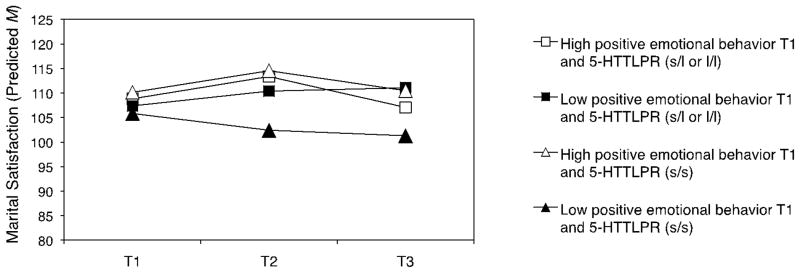
Marital Satisfaction over 13 Years by Positive Emotional Behavior and 5-HTTLPR
Note. Predicted uncentered means of marital satisfaction at T1, T2, and T3 for individuals with high (M+ 1 SD) or low (M − 1 SD) levels of positive emotional behavior at T1 and two short alleles (s/s) or one or two long alleles (s/l or l/l) of 5-HTTLPR. Analyses controlled for depression, ethnicity, gender, cohort, education, and negative emotional behavior at T1. T1: 1989/90. T2: 1995/96. T3: 2001/02.
Figure 3. Crossover Interaction between Negative Emotional Behavior and 5-HTTLPR Predicting Changes in Marital Satisfaction over 13 Years.
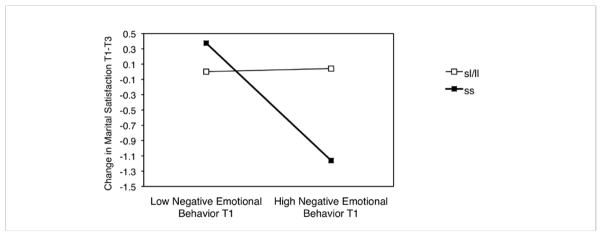
Predicting Changes in Marital Satisfaction over 13 Years.
Note. Residualized change in marital satisfaction from T1 to T3 for individuals with low (M− 1 SD) or high (M + 1 SD) levels of negative emotional behavior at T1 and two short alleles (s/s) or one or two long alleles (s/l or l/l) of 5-HTTLPR. Analyses controlled for depression, ethnicity, gender, cohort, education, and positive emotional behavior at T1. T1: 1989/90. T3:2001/02.
Effects of Gender, Cohort, and Ethnicity
We explored the generalizability of these findings across gender, cohort, and ethnicity. Findings for positive emotional behavior generalized across gender and cohort, as indicated by nonsignificant 3-way interactions involving gender or cohort, 5-HTTLPR (H2), and positive emotional behavior when predicting changes in marital satisfaction from T1 to T3, ps > .05.
In contrast, findings for negative emotional behavior were qualified by gender and cohort, as indicated by significant 3-way interactions, ps < .05. In terms of gender, the prediction of declines in marital satisfaction by having two short alleles appeared stronger for wives, B = −3.47, SE(B) = 1.21, p = .006, than for husbands, B= −2.13, SE(B) = 1.03, p = .043. Among individuals with one or two long alleles, negative emotional behavior did not predict declines in marital satisfaction for wives, B= −.24, SE(B) = .31, p = .439, or for husbands, B= −.37, SE(B) = .41, p = .379. In terms of cohort, higher negative emotional behavior predicted a decline in marital satisfaction for older adults with two short alleles, B= −3.07, SE(B) = .69, p < .001, but not for middle-aged adults with two short alleles, B= 1.22, SE(B) = 1.39, p = .385. Among individuals with one or two long alleles, negative emotional behavior did not predict declines in marital satisfaction for older adults, B= −.11, SE(B) = .47, p = .821, or for middle-aged adults, B = .20, SE(B) = .32, p = .527. To summarize, the moderating effect of 5-HTTLPR on the association between negative emotional behavior and changes in marital satisfaction appeared stronger for wives than for husbands and was present for older spouses but not for middle-aged spouses.
Finally, when repeating all analyses including only Caucasian participants (n = 106), the results remained essentially unchanged. One association (negative emotional behavior predicting decline in marital satisfaction from T1 to T3 among husbands with two short alleles) was reduced to trend level, p = .070.
Cross-Spouse Associations
Exploratory actor-partner analyses provided some evidence for cross-spouse associations in the expected direction.3 For wives with two short alleles, both wives’ higher negative emotional behavior, B= −.26, SE(B) = .08, p = .001 as well as husbands’ higher negative emotional behavior, B= −.19, SE(B) = .08, p = .017, predicted declines in wives’ marital satisfaction from T1 to T3 (controlling for husbands’ and wives’ positive emotional behavior). For wives with one or two long alleles, neither wives’ own negative emotional behavior, B = −.03, SE(B) = .06, p = .641, nor husbands’ negative emotional behavior, B = .03, SE(B) = .09, p = .715, predicted changes in wives’ marital satisfaction from T1 to T3. These results remained stable when controlling for covariates. The multi-group model exploring the effects of husbands’ 5-HTTLPR genotype were not tested because of small cell sizes.
Discussion
To our knowledge, the present findings are the first to implicate a specific genetic polymorphism as playing an important role in moderating the association between emotional behavior and changes in marital satisfaction. Consistent with our primary hypothesis, for individuals who had two short alleles of 5-HTTLPR, higher negative and lower positive emotional behavior at T1 predicted declines in marital satisfaction over time. In contrast, for individuals with one or two long alleles, levels of negative and positive emotional behavior did not predict changes in marital satisfaction. Although one effect could already be seen at T1, the findings were most pronounced when predicting changes in marital satisfaction across the full 13-year range of data collection. This is consistent with a view that genetic influences may increase over time and/or age (Lindenberger et al., 2008; McArdle & Prescott, 2010). Given earlier concerns about the replicability of these kinds of single-gene effects (e.g., Risch et al., 2009), we designed our analyses to be maximally conservative by: (a) controlling for variance in marital satisfaction shared by positive and negative emotional behavior; (b) considering different possibilities for the dominance structure of 5-HTTLPR; (c) correcting for nonindependence between husbands and wives; and (d) controlling for the possible effects of depression as well as ethnicity, gender, cohort, and education.
Although our primary hypothesis involved the association between an unfavorable emotional climate (i.e., high levels of negative emotion and low levels of positive emotion) and declines in marital satisfaction, our findings also provided evidence for the kind of crossover interaction involving 5-HTTLPR that has been reported by others (e.g., Belsky & Pluess, 2009; Mitchell et al., 2011; Taylor et al., 2006). In our study, individuals with two short alleles of 5 -HTTLPR also had the highest marital satisfaction in a favorable emotional climiate (i.e., low levels of negative emotion and high levels of positive emotion). This finding is consistent with a growing body of empirical work showing that the short allele amplifies reactivity to positive as well as negative emotional stimuli (e.g., Beevers et al., 2011; Gyurak et al., 2013; Hariri et al., 2002; Homberg & Lesch, 2011; Schoebi et al., 2012; Way & Taylor, 2010). These findings suggest that viewing a particular 5 -HTTLPR variant as inherently “bad” or “good” is unwise. Rather, whether the short allele results in bad or good outcomes in the long term will depend on the presence or absence of positive and negative factors.
For negative emotional behavior, our analyses also revealed several interesting differences related to gender and age. In terms of gender, the 5-HTTLPR effects on the relationship between negative emotional behavior and marital satisfaction were stronger for females than for males. This is consistent with prior findings that 5-HTTLPR effects can be more pronounced for females; however, this pattern has not always been found (for an overview see, for example, Caspi et al., 2010). In terms of age, the 5-HTTLPR effects on the association between negative emotional behavior and marital satisfaction were present for older spouses but not for middle-aged spouses. These findings are consistent with both theory (Lindenberger et al., 2008) and empirical findings (e.g., Nagel et al., 2008; Stürmer et al., 2012) that suggest that genes become increasingly influential in late life. Interestingly, these gender and age effects were only found for negative emotions and not for positive emotions. This is consistent with findings that negative behaviors have a greater overall effect on marital satisfaction than positive behaviors (see Karney & Bradbury, 1995) and with the more generalized notion that “bad is stronger than good” (Baumeister, Bratslavsky, Finkenauer, & Vohs, 2001). However, it is important to realize that the present study was based on emotional behaviors that occurred when couples discussed a relationship problem. Thus, it is possible that moderation effects for positive emotions might have emerged in a context more conducive to the production of positive emotional behaviors.
Understanding the role that specific genes play in moderating the association between emotional behaviors and marital satisfaction may help explain the variability in this association that is found in popular lore (e.g., some loving couples love to bicker) and in the marital research literature (Bradbury et al., 2000; Karney & Bradbury, 1995). Our study examined broad classes of emotional behaviors (negative and positive) during a particular kind of marital interaction (discussing a marital problem). We expect that this association could be profitably explored in terms of more specific emotions and other contexts. For example, some negative emotions, such as sadness, may promote marital satisfaction when they involve revealing vulnerabilities and attempting to elicit support (Clark, Oullette, Powell, & Milberg, 1987; Graham, Huang, Clark, & Helgeson, 2008). Some positive emotions, for example amusement at the expense of the partner, could undermine marital satisfaction (Levenson, Haase, Bloch, Holley, & Seider, in press). Cultural variations likely also come into play, with different traditions of emotional expression (Soto, Levenson, & Ebling, 2005) and different views as to which emotions and levels of emotional intensity are most desirable (Tsai, Knutson, & Fung, 2006). One implication of the present study is that short-allele carriers of 5-HTTLPR would be expected to show stronger associations between emotional behavior and marital satisfaction regardless of the direction of that association, as determined by specific emotions, contexts, and cultures.
Viewed in a broader framework, the present study extends previous research that has demonstrated genetic influences on relationship outcomes in adulthood (e.g., Schoebi et al., 2012; Walum et al., 2008) into the realm of marital satisfaction. Similarly, it ex tends research that has demonstrated that 5-HTTLPR moderates the association between risk factors and outcomes in the domain of individual psychopathology (e.g., Caspi et al., 2003; Karg et al., 2011) to show similar effects in the social, interpersonal domain of marriage. Our study can be seen as falling within the gene x stress framework that characterized early studies of 5-HTTLPR (Caspi et al., 2003), although the early studies focused on more “external” factors (e.g., stressful life events) and ours focused more on “internal” factors (e.g., emotional behaviors that emerged during spousal interactions). The present findings thus contribute to a biopsychosocial view of marriage and family (e.g., Horwitz & Neiderhiser, 2011; Kiecolt-Glaser & Newton, 2001).
Strengths and Limitations
To our knowledge, this is the first study that shows that a specific genetic polymorphism (5-HTTLPR) moderates the association between objectively coded emotional behaviors and marital satisfaction in a longitudinal design. The study had several additional strengths: (a) the duration of the longitudinal follow-up was substantial (13 years), (b) participants were in well-established long-term marriages in middle age and late life, and (c) emotional behaviors were sampled during a naturalistic discussion of a marital problem. Our finding that having two short alleles of 5-HTTLPR was associated with a stronger association between the emotional quality of marital interaction and spousal marital satisfaction is consistent with newer views of the role this allele variant plays as a plasticity factor (Belsky & Pluess, 2009) that increases susceptibility to negative as well as positive conditions. The findings also extend our prior work investigating the influence of this polymorphism in the realm of emotional reactivity (Gyurak et al., 2013). In one of these studies, we found that having two short alleles was associated with greater emotional reactivity (self-report and physiology) to viewing the distress of others. In the other study, we found that having two short alleles was associated with greater emotional reactivity (self-report and objectively coded behavior) in an embarrassing situation. The present study extended this work into the realm of gene x stress associations, finding that having two short alleles strengthened the association between emotional behavior and a more distal outcome (marital satisfaction). Given that these three studies were performed on three independent samples of participants and utilized three different experimental paradigms underscores the robustness of the effects of 5-HTTLPR in the realm of emotion (or at least when using these kinds of well-controlled laboratory procedures and multi-method assessments of emotion).
This study also had several limitations worthy of note. Our sample was recruited to be representative of the San Francisco Bay Area and thus was primarily Caucasian and well-educated. Thus, the generalizability of these findings to other populations needs to be established. Our sample size (N= 125 individuals) was small by the standards of population-based genetics studies (although it is quite respectable by the standards of observational studies of marital interaction) and thus we might have been relatively underpowered to explore the full range of influences of factors such as gender and age. A larger sample size would have enabled us to explore cross-spouse associations in greater depth (our exploratory analyses suggested that these might exist and that they parallel those found in within-spouse analyses). A larger sample size would have also given us greater power to detect possible co-dominant effects of the 5-HTTLPR alleles (i.e., additional differences between individuals with one and two long alleles) and possible 3-way interaction effects involving positive emotional behavior (we found that some associations only held for negative emotional behavior). Finally, there are additional subdivisions of 5-HTTLPR (e.g., SNP rs25531; Wendland, Martin, Kruse, Lesch, & Murphy, 2006) that merit additional study using these kinds of longitudinal experimental designs and behavioral data.
Conclusions
The search for the recipe that produces a successful marriage has long occupied scientists, therapists, and laypeople alike. Among the key ingredients, an important role for emotion has been endorsed in many empirical studies (e.g., Carstensen et al., 1995; Gottman & Levenson, 1992; Karney & Bradbury, 1995; Levenson & Gottman, 1983), in a number of marital therapies (Gottman & Gottman, 2008; Lebow, Chambers, Christensen, & Johnson, 2012), and in folk wisdom. Here we present the first evidence that a common genetic polymorphism in the serotonin transporter gene plays an important role in determining how powerfully negative and positive emotions will influence marital satisfaction over time. For those with two short alleles of 5-HTTLPR, we would expect that marital satisfaction will be most likely: (a) to decline in a climate characterized by high levels of negative emotional behavior and low levels of positive emotional behavior; and (b) to increase in a climate characterized by low levels of negative emotional behavior and high levels of positive emotional behavior. For those with one or more long alleles, this association between emotional behavior and marital satisfaction should be much weaker.
What are we to do with information about how sensitive a marriage might be to the emotions expressed by spouses? We are probably a long way from being able to use it to make important life decisions such as whom to marry and how much attention to pay to our partner’s emotion (e.g., “marry someone with long alleles of 5-HTTLPR and you won’t have to worry about how they feel”). Clearly, these kinds of genetic effects do not fully determine the fate of marriage, but rather represent small biases in a particular direction. Our findings (and those of others) that suggest that genetic influences on behavior become stronger with age suggest that such biases do aggregate and become more substantial over the decades of adult development. Nonetheless, many, many other factors affect the fate of an individual marriage and can override or counteract the biases associated with particular genetic influences. Still, these findings do raise the intriguing question of whether knowing your and your partner’s 5-HTTLPR genotype would be useful information for relationship partners, much in the same way as knowing their temperament, attachment style, or level of neuroticism might be.
As conceptual models and experimental paradigms for studying common functional genetic polymorphisms continue to improve, we expect that the importance of this approach in emotion research will increase. Clearly, this research has great potential to help us understand individual differences in emotional functioning and the implications these differences have for the lives people live. The present study shows the important role that one particular genetic polymorphism plays in influencing the association between emotion and the quality of marriage, arguably one of the most important intimate relationships of adulthood. We expect that these kinds of influences will be found to extend to other kinds of intimate adult relationships as well. Given the critical role that social relationships play in physical health (e.g., Cacioppo & Patrick, 2008; House, Landis, & Umberson, 1988; Robles & Kiecolt-Glaser, 2003), mental health (e.g., Whisman, 2007), and general well-being (e.g., Coan, 2008; Proulx et al., 2007), there will be many opportunities to explore the way that our genes influence the complex relationships between emotions, intimate relationships, and health.
Acknowledgments
This research was supported by a National Institute on Aging (American Recovery and Reinvestment Act) grant 3R37-AG017766-09S3 to Robert W. Levenson and a grant from the German Research Foundation HA 4475/2-1 to Claudia M. Haase.
Footnotes
However, not all crossover interactions indicate differential susceptibility (for details see Belsky & Pluess, 2009).
Our hypotheses focused on predicting changes in marital satisfaction over time. Note that an interaction effect between negative emotional behavior at T1 and 5-HTTLPR (H2) was already found when predicting marital satisfaction at T1, B= −2.44, SE(B) = 1.18, p = .042. When decomposing the interaction, a similar pattern of results was obtained. For individuals with two short alleles of 5-HTTLPR, higher negative emotional behavior predicted lower marital satisfaction, B= −3.37, SE(B) = 1.12, p = .004. For individuals with one or two long alleles of 5-HTTLPR, negative emotional behavior did not predict marital satisfaction, B= −.59, SE(B) = .48, p = .220.
In addition, this actor-partner model approach revealed that changes in wives’ and husbands’ marital satisfaction from T1 to T3 were not correlated (r = .03, p = .854), consistent with the notion that there may be two marriages, his and hers (Barnard, 1982). Moreover, both change residuals for wives’ and husbands’ marital satisfaction showed significant variability, σ2 = 1.02, p < .001 and σ2 = .85, p < .001, indicating that different individuals changed in different directions over time.
References
- Arbuckle JL. Amos 17.0 user’s guide. Chicago: SPSS and Amos Development Corporation; 2008. [Google Scholar]
- Assal F, Alarcon M, Solomon EC, Masterman D, Geschwind DH, Cummings JL. Association of the serotonin transporter and receptor gene polymorphisms in neuropsychiatric symptoms in Alzheimer disease. Archives of Neurology. 2004;61:1249–1253. doi: 10.1001/archneur.61.8.1249. [DOI] [PubMed] [Google Scholar]
- Baltes PB. On the incomplete architecture of human ontogeny: Selection, optimization, and compensation as foundation of developmental theory. American Psychologist. 1997;52:366–380. doi: 10.1037/0003-066x.52.4.366. [DOI] [PubMed] [Google Scholar]
- Barnard J. The future of marriage. New Haven, CT: Yale University Press; 1982. [Google Scholar]
- Baumeister RF, Bratslavsky E, Finkenauer C, Vohs KD. Bad is stronger than good. Review of General Psychology. 2001;5:323–370. doi: 10.1037/1089-2680.5.4.323. [DOI] [Google Scholar]
- Beevers CG, Marti CN, Lee HJ, Stote DL, Ferrell RE, Hariri AR, Telch MJ. Associations between serotonin transporter gene promoter region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of Abnormal Psychology. 2011;120:187–197. doi: 10.1037/a0022125. [DOI] [PubMed] [Google Scholar]
- Belsky J, Pluess M. Beyond diathesis stress: Differential susceptibility to environmental influences. Psychological Bulletin. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Bentler PM, Newcomb MD. Longitudinal study of marital success and failure. Journal of Consulting and Clinical Psychology. 1978;46:1053–1070. [Google Scholar]
- Birchler G, Weiss R, Vincent J. Multimethod analysis of social reinforcement exchange between maritally distressed and nondistressed spouse and stranger dyads. Journal of Personality and Social Psychology. 1975;31:349–360. doi: 10.1037/h0076280. [DOI] [Google Scholar]
- Bradbury TN, Fincham FD, Beach SRH. Research on the nature and determinants of marital satisfaction: A decade in review. Journal of Marriage and the Family. 2000;62:964–980. doi: 10.1111/j.1741-3737.2000.00964.x. [DOI] [Google Scholar]
- Brocke B, Armbruster D, Müller J, Hensch T, Jacob CP, Lesch KP, Kirschbaum C, Strobel A. Serotonin transporter gene variation impacts innate fear processing: Acoustic startle response and emotional startle. Molecular Psychiatry. 2006;11:1106–1112. doi: 10.1038/sj.mp.4001908. [DOI] [PubMed] [Google Scholar]
- Burgess EW, Locke HJ, Thomes MM. The family. New York: Van Nostrand Reinhold; 1971. [Google Scholar]
- Cacioppo JT, Patrick W. Loneliness: Human nature and the need for social connection. New York, NY: W. W. Norton & Company; 2008. [Google Scholar]
- Carstensen LL, Gottman JM, Levenson RW. Emotional behavior in long-term marriage. Psychology and Aging. 1995;10:140–149. doi: 10.1037/0882-7974.10.1.140. [DOI] [PubMed] [Google Scholar]
- Caspers KM, Paradiso S, Yucuis R, Troutman B, Arndt S, Philibert R. Association between the serotonin transporter promoter polymorphism (5-HTTLPR) and adult unresolved attachment. Developmental Psychology. 2009;45:64–76. doi: 10.1037/a0014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Hariri AR, Holmes A, Uher R, Moffitt TE. Genetic sensitivity to the environment: The case of the serotonin transporter gene and its implications for studying complex diseases and traits. The American Journal of Psychiatry. 2010;167:509–527. doi: 10.1176/appi.ajp.2010.09101452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Clark MS, Oullette R, Powell MC, Milberg S. Recipient’s mood, relationship type, and helping. Journal of Personality and Social Psychology. 1987;53:94–103. doi: 10.1037/0022-3514.53.1.94. [DOI] [PubMed] [Google Scholar]
- Coan JA. Toward a neuroscience of attachment. In: Cassidy J, Shaver PR, editors. Handbook of attachment: Theory, research, and clinical applications. 2. New York, NY: Guilford Press; 2008. pp. 241–265. [Google Scholar]
- Derogatis LR, Cleary PA. Confirmation of the dimensional structure of the SCL-90: A study in construct validation. Journal of Clinical Psychology. 1977;33:981–989. doi: 10.1002/1097-4679(197710)33:4<981::aid-jclp2270330412>3.0.co;2-0. [DOI] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39:175–191. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- Fincham FD, Stanley SM, Beach SRH. Transformative processes in marriage: An analysis of emerging trends. Journal of Marriage and Family. 2007;69:275–292. doi: 10.1111/j.1741-3737.2007.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottman JM. What predicts divorce? The relationship between marital processes and marital outcomes. Hillsdale, NJ: Lawrence Erlbaum; 1994. [Google Scholar]
- Gottman JM. What predicts divorce: The measures. Hillsdale, NJ: Lawrence Erlbaum; 1996. [Google Scholar]
- Gottman JM, Gottman JS. Gottman method couple therapy. In: Gurman AS, editor. Clinical handbook of couple therapy. 4. New York, NY: Guilford Press; 2008. pp. 138–164. [Google Scholar]
- Gottman JM, Levenson RW. Marital processes predictive of later dissolution: Behavior, physiology, and health. Journal of Personality and Social Psychology. 1992;63:221–233. doi: 10.1037/0022-3514.63.2.221. [DOI] [PubMed] [Google Scholar]
- Gottman JM, Levenson RW. The timing of divorce: Predicting when a couple will divorce over a 14-year period. Journal of Marriage & the Family. 2000;62:737–745. doi: 10.1111/j.1741-3737.2000.00737.x. [DOI] [Google Scholar]
- Graham SM, Huang JY, Clark MS, Helgeson VS. The positives of negative emotions: Willingness to express negative emotions promotes relationships. Personality and Social Psychology Bulletin. 2008;34:394–406. doi: 10.1177/0146167207311281. [DOI] [PubMed] [Google Scholar]
- Greenberg LS, Johnson SM. Emotionally focused therapy for couples. New York, NY: Guilford Press; 1988. [Google Scholar]
- Gyurak A, Haase CM, Sze J, Goodkind MS, Coppola G, Lane J, Miller BL, Levenson RW. The effect of the serotonin transporter polymorphism (5-HTTLPR) on empathic and self-conscious emotional reactivity. Emotion. 2013;13:25–35. doi: 10.1037/a0029616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Nederhof E, Oppenheimer CW, Jenness J, Young JF, Abela JRZ, Smolen A, Ormel J, Oldehinkel AJ. Differential susceptibility in youth: Evidence that 5-HTTLPR x positive parenting is associated with positive affect ‘for better and worse’. Translational Psychiatry. 2011;1:e44. doi: 10.1038/tp.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Kolachana B, Fera F, Goldman D, Egan MF, Weinberger DR. Serotonin transporter genetic variation and the response of the human amygdala. Science. 2002;297:400–403. doi: 10.1126/science.1071829. [DOI] [PubMed] [Google Scholar]
- Homberg JR, Lesch KP. Looking on the bright side of serotonin transporter gene variation. Biological Psychiatry. 2011;69:513–519. doi: 10.1016/j.biopsych.2010.09.024. [DOI] [PubMed] [Google Scholar]
- Horwitz BN, Neiderhiser JM. Gene -environment interplay, family relationships, and child adjustment. Journal of Marriage and the Family. 2011;73(4):804–816. doi: 10.1111/j.1741-3737.2011.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: Evidence of genetic moderation. Archives of General Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karney BR, Bradbury TN. The longitudinal course of marital quality and stability: A review of theory, methods, and research. Psychological Bulletin. 1995;118:3–34. doi: 10.1037/0033-2909.118.1.3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Baker JH. Genetic influences on measures of the environment: A systematic review. Psychological Medicine. 2007;37:615–626. doi: 10.1017/S0033291706009524. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kuhn JW, Vittum J, Prescott CA, Riley B. The interaction of stressful life events and a serotonin transporter polymorphism in the prediction of episodes of major depression. Archives of General Psychiatry. 2005;62:529–535. doi: 10.1001/archpsyc.62.5.529. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Newton TL. Marriage and health: His and hers. Psychological Bulletin. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- Lebow JL, Chambers AL, Christensen A, Johnson SM. Research on the treatment of couple distress. Journal of Marital and Family Therapy. 2012;38:145–168. doi: 10.1111/j.1752-0606.2011.00249.x. [DOI] [PubMed] [Google Scholar]
- Lenroot RK, Giedd JN. Annual Research Review: Developmental considerations of gene by environment interactions. Journal of Child Psychology and Psychiatry. 2011;52:429–441. doi: 10.1111/j.1469-7610.2011.02381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, Sabol SZ, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Carstensen LL, Gottman JM. Long-term marriage: Age, gender, and satisfaction. Psychology and Aging. 1993;8:301–313. doi: 10.1037/0882-7974.8.2.301. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Marital interaction: Physiological linkage and affective exchange. Journal of Personality and Social Psychology. 1983;45:587–597. doi: 10.1037/0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Gottman JM. Physiological and affective predictors of change in relationship satisfaction. Journal of Personality and Social Psychology. 1985;49:85–94. doi: 10.1037/0022-3514.49.1.85. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Haase CM, Bloch L, Holley S, Seider BJ. Emotion regulation in couples. In: Gross JJ, editor. Handbook of emotion regulation. 2 in press. [Google Scholar]
- Lindenberger U, Nagel IE, Chicherio C, Li SC, Heekeren HR, Bäckman L. Age-related decline in brain resources modulates genetic effects on cognitive functioning. Frontiers in Neuroscience. 2008;2:234–244. doi: 10.3389/neuro.01.039.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke HJ, Wallace KM. Short marital-adjustment and prediction tests: Their reliability and validity. Marriage and Family Living. 1959;21:251–255. doi: 10.2307/348022. [DOI] [Google Scholar]
- McArdle JJ, Prescott CA. Contemporary modeling of gene x environment effects in randomized multivariate longitudinal studies. Perspectives on Psychological Science. 2010;5:606–621. doi: 10.1177/1745691610383510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer CC, Smith G, DeKosky ST, Pollock BG, Mathis CA, Moore RY, Kupfer DJ, Reynolds CF. Serotonin in aging, late-life depression, and Alzheimer’s disease: The emerging role of functional imaging. Neuropsychopharmacology. 1998;18:407–430. doi: 10.1016/S0893-133X(97)00194-2. [DOI] [PubMed] [Google Scholar]
- Mikulincer M, Shaver PR. The attachment behavioral system in adulthood: Activation, psychodynamics, and interpersonal processes. Advances in Experimental Social Psychology. 2003;35:53–152. doi: 10.1016/S0065-2601(03)01002-5. [DOI] [Google Scholar]
- Mitchell C, Notterman D, Brooks-Gunn J, Hobcraft J, Garfinkel I, Jaeger K, Kotenko I, McLanahan S. Role of mother’s genes and environment in postpartum depression. Proceedings of the National Academy of Sciences. 2011;108:8189–8193. doi: 10.1073/pnas.1014129108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Durrant C, Lewis G, Flint J. Gene X environment interactions at the serotonin transporter locus. Biological Psychiatry. 2009;65:211–219. doi: 10.1016/j.biopsych.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Nagel IE, Chicherio C, Li SC, von Oertzen T, Sander T, Villringer A, Heekeren HR, Backman L, Lindenberger U. Human aging magnifies genetic effects on executive functioning and working memory. Frontiers in Human Neuroscience. 2008;2:1–8. doi: 10.3389/neuro.09.001.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JA, Kenny DA. Structural equation modeling with interchangeable dyads. Psychological Methods. 2006;11:127–141. doi: 10.1037/1082-989x.11.2.127. [DOI] [PubMed] [Google Scholar]
- Pluess M, Belsky J, Way BM, Taylor SE. 5-HTTLPR moderates effects of current life events on neuroticism: Differential susceptibility to environmental influences. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1070–1074. doi: 10.1016/j.pnpbp.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx CM, Helms HM, Buehler C. Marital quality and personal well-being: A meta-analysis. Journal of Marriage and Family. 2007;69:576–593. doi: 10.1111/j.1741-3737.2007.00393.x. [DOI] [Google Scholar]
- Randall AK, Bodenmann G. The role of stress on close relationships and marital satisfaction. Clinical Psychology Review. 2009;29:105–115. doi: 10.1016/j.cpr.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Risch N, Herrell R, Lehner T, Liang KY, Eaves L, Hoh J, Griem A, Kovacs M, Ott J, Merikangas KR. Interaction between the serotonin transporter gene (5-HTTLPR), stressful life events, and risk of depression: A meta-analysis. JAMA. 2009;301:2462–2471. doi: 10.1001/jama.2009.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robles TF, Kiecolt-Glaser JK. The physiology of marriage: pathways to health. Physiology & Behavior. 2003;79:409–416. doi: 10.1016/s0031-9384(03)00160-4. [DOI] [PubMed] [Google Scholar]
- Roy A, Hu XZ, Janal MN, Goldman D. Interaction between childhood trauma and serotonin transporter gene variation in suicide. Neuropsychopharmacology. 2007;32:2046–2052. doi: 10.1038/sj.npp.1301331. [DOI] [PubMed] [Google Scholar]
- Schoebi D, Way BM, Karney BR, Bradbury TN. Genetic moderation of sensitivity to positive and negative affect in marriage. Emotion. 2012;12:208–212. doi: 10.1037/a0026067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2004;127B:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Soto JA, Levenson RW, Ebling R. Cultures of moderation and expression: emotional experience, behavior, and physiology in Chinese Americans and Mexican Americans. Emotion. 2005;5:154–165. doi: 10.1037/1528-3542.5.2.154. [DOI] [PubMed] [Google Scholar]
- Spotts EL, Neiderhiser JM, Towers H, Hansson K, Lichtenstein P, Cederblad M, Pederson NL, Reiss D. Genetic and environmental influences on marital relationships. Journal of Family Psychology. 2004;18:107–119. doi: 10.1037/0893-3200.18.1.107. [DOI] [PubMed] [Google Scholar]
- StataCorp. Stata statistical software: Release 10. College Station, TX: StataCorp LP; 2007. [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proceedings of the National Academy of Sciences. 2003;100:9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer VS, Passow S, Biesenack J, Li SC. Dopaminergic and cholinergic modulations of visual-spatial attention and working memory: Insights from molecular genetic research and implications for adult cognitive development. Developmental Psychology. 2012;48:875–889. doi: 10.1037/a0026198. [DOI] [PubMed] [Google Scholar]
- Sullivan KT, Pasch LA, Johnson MD, Bradbury TN. Social support, problem solving, and the longitudinal course of newlywed marriage. Journal of Personality and Social Psychology. 2010;98:631–644. doi: 10.1037/a0017578. [DOI] [PubMed] [Google Scholar]
- Taylor SE, Way BM, Welch WT, Hilmert CJ, Lehman BJ, Eisenberger NI. Early family environment, current adversity, the serotonin transporter promoter polymorphism, and depressive symptomatology. Biological Psychiatry. 2006;60:671–676. doi: 10.1016/j.biopsych.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Knutson B, Fung HH. Cultural variation in affect valuation. Journal of Personality and Social Psychology. 2006;90:288–307. doi: 10.1037/0022-3514.90.2.288. [DOI] [PubMed] [Google Scholar]
- van Dyck CH, Malison RT, Seibyl JP, Laruelle M, Klumpp H, Zoghbi SS, Baldwin RM, Innis RB. Age-related decline in central serotonin transporter availability with [(123)I] beta-CIT SPECT. Neurobiology of Aging. 2000;21:497–501. doi: 10.1016/S0197-4580(00)00152-4. [DOI] [PubMed] [Google Scholar]
- Walum H, Westberg L, Henningsson S, Neiderhiser JM, Reiss D, Igl W, Ganiban JM, Spotts EL, Pedersen NL, Eriksson E, Lichtenstein P. Genetic variation in the vasopressin receptor 1a gene (AVPR1A) associates with pair-bonding behavior in humans. Proceedings of the National Academy of Sciences. 2008;105:14153–11415. doi: 10.1073/pnas.0803081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Lieberman MD. Is there a genetic contribution to cultural differences? Collectivism, individualism and genetic markers of social sensitivity. Social Cognitive and Affective Neuroscience. 2010;5:203–211. doi: 10.1093/scan/nsq059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biological Psychiatry. 2010;67:487–492. doi: 10.1016/j.biopsych.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Molecular Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- Whisman MA. Marital distress and DSM-IV psychiatric disorders in a population-based national survey. Journal of Abnormal Psychology. 2007;116:638–643. doi: 10.1037/0021-843x.116.3.638. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28:533–541. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]


