Abstract
Opioids play an important role for the management of acute pain and in palliative care. The role of long-term opioid therapy in chronic non-malignant pain remains unclear and is the focus of much clinical research. There are concerns regarding analgesic tolerance, paradoxical pain and issues with dependence that can occur with chronic opioid use in the susceptible patient. In this review, we discuss how far human neuroimaging research has come in providing a mechanistic understanding of pain relief provided by opioids, and suggest avenues for further studies that are relevant to the management of chronic pain with opioids.
This article is part of the Special Issue Section entitled ‘Neuroimaging in Neuropharmacology’.
Keywords: Opioids, Analgesia, Pain, Neuroimaging
Highlights
-
•
Brain mechanisms are crucial to opioid analgesia in humans.
-
•
Opioids can have a direct effect on brain mechanisms for pain perception.
-
•
Opioids can also engage descending inhibition of spinal nociception.
-
•
Drug-induced tolerance, dependence and paradoxical pain may limit chronic opioid analgesic therapy.
1. Introduction
Basic science has advanced our understanding of nociception and suggests numerous receptors that can be targeted by drugs for pain relief in humans. Unfortunately, few novel compounds have emerged as clinically useful analgesics (Mogil, 2009). Opioids are the mainstay for the management of acute pain (Wu and Raja, 2011) and in palliative care (Portenoy, 2011), where the duration of treatment is limited.
The role of opioids for the treatment of chronic non-malignant pain remains debatable (Chou et al., 2009a). Chronic pain, by definition, persists beyond 3 months. In many patients, the fluctuations in the severity of such pain are not clearly correlated with demonstrable changes in a peripheral or central disease process. There are concerns that opioid-based medications may fail to maintain their efficacy when used indefinitely to relieve such pain. Robust data on opioid treatment efficacy in these patients are lacking (Noble et al., 2010), and concerns have grown over the escalating death rates from prescription-opioid overdose reported in the United States (Okie, 2010). Nonetheless, there is still general consensus amongst clinicians, that long-term management of pain with opiates can be beneficial, or at least safe with appropriate patient selection and dose titration (British Pain Society, 2010; Chou et al., 2009b; Kahan et al., 2011a, 2011b).
Opioid receptors are distributed throughout the nervous system. Experimental studies have demonstrated analgesic effects via stimulation of opioid receptors that are peripheral or centrally located (Dickenson and Kieffer, 2013). There are key spinal and supraspinal actions of opioids and the latter involves descending pathways. Furthermore, recent clinical trials with methyl-naltrexone, a peripherally restricted mu-opioid-receptor antagonist, suggest that the effects within the central nervous system are important to pain relief afforded by systemic delivery of opioids in palliative care and chronic non-malignant pain (Anissian et al., 2012; Michna et al., 2011; Thomas et al., 2008). Thus, human brain neuroimaging studies may further our understanding of the central processes through which opiates operate to provide pain relief.
Most human neuroimaging research in humans is focussed on the acute effects of opioids on experimentally induced pain in humans. Far less is known about the effects of chronic opioid analgesic therapy on the central nervous system in humans. Analgesic tolerance is a long-held limitation of chronic opioid therapy. More recent data now suggests that chronic opioid administration or withdrawal cause a hyperalgesic state (Bannister and Dickenson, 2010) where opioids can paradoxically worsen pain. Furthermore, there may be risks of cognitive decline (Kendall et al., 2010) and medication misuse, particularly in the vulnerable (Pergolizzi et al., 2012).
Here, we discuss how far human neuroimaging research has come in translating mechanistic data from other species, suggest avenues for further studies that are relevant to the long-term management of pain with opioids.
2. Neuroimaging opioid-based analgesia
2.1. Acute administration of opioids
Neuroimaging research suggests that the experience of pain emerges from an extensive network of brain regions (Apkarian et al., 2005), which is unsurprising given how complex pain really is (Tracey, 2005). The International Association for the Study of Pain (IASP) defines pain as ‘an unpleasant sensory or emotional experience associated with actual or potential tissue damage, or described in terms of such damage’ (IASP, 1994). This definition is based on the concept of pain as a specific consciousness comprising of sensory, emotional and cognitive aspects. Brain imaging has provided evidence for neural mechanisms that contribute to pain perception and its modulation, but the neuro-signature of pain itself remains elusive (Tracey and Mantyh, 2007).
Nearly all neuroimaging studies of opioid analgesia have been performed in healthy volunteers, in whom the effects of opioids on pain from brief non-injurious noxious stimuli are examined (Adler et al., 1997; Oertel et al., 2008; Petrovic et al., 2002; Wagner et al., 2007; Wise et al., 2002). Experimentally induced pain cannot replicate the distress experienced by patients with long-term pain that is often difficult to treat. Nonetheless, studies in healthy volunteers allow for more precise stimulation of the nociceptive system and pharmacological modulation of the resultant experience of pain without illness, disease and treatment confounds. In these highly controlled experiments, short-acting intravenous opioids, for example remifentanil or alfentanil, are often employed to minimise variability from pharmacokinetics between individual subjects.
The early pharmacodynamic FMRI studies from our laboratory revealed that opioids decrease pain-related activations in a specific and dose dependent manner (Wise et al., 2002, 2004). Activations within the intra-calcarine cortex that are related to visual stimulation, a control task employed in these experiments, were not significantly affected by opioid administration. This finding suggests that the suppression of sensory-limbic activations was related to specific effects on neural processing, rather than global vascular effects from hypercarbia related to hypoventilation during opioid analgesia (Wise and Tracey, 2006). Furthermore, an investigation of the acute effects of remifentanil on FMRI activations after controlling for hypercarbia have not revealed direct effects of remifentanil on cerebral vascular reactivity itself (Pattinson et al., 2007). Interestingly, we found that activations evoked within the insular regions by noxious stimuli were particularly susceptible to remifentanil (Wise et al., 2002, 2004). The data are consisted with the known role of insular cortex in nociception and pain perception (Craig, 2003; Mazzola et al., 2009; Ostrowsky et al., 2002).
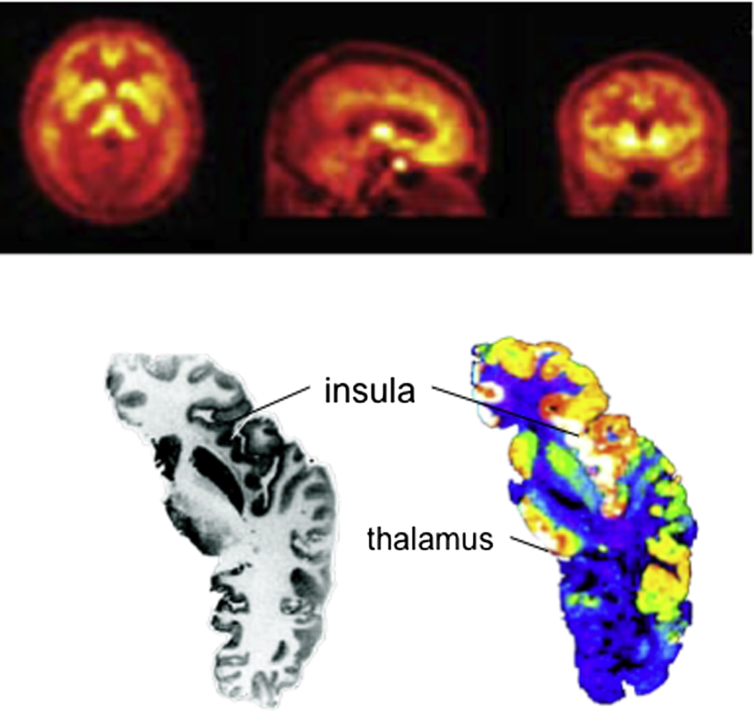
Oertel et al. (2008) have further demonstrated that opioid analgesic dose response functions differ between the posterior and anterior insula cortex. In their study, the posterior insula and other regions that encode the sensory aspects of pain, also demonstrate a linear reduction of activity with increasing intravenous opioids dose. However, they report that activations in the amygdala, anterior insula and cingulate cortices, which are regions implicated in the affective aspects of pain (Craig, 2009), are maximally suppressed at the lowest opioid dose. Their findings suggest that the limbic regions are exquisitely sensitive to opioid effects, consistent perhaps with their high opioid receptor densities (Fig. 1). The subjects only reported on the sensory aspects of pain in that study. Nonetheless, the FMRI data suggest that opioid analgesics can directly influence emotional responses at low doses that do not alter sensory aspects of pain (Fig. 2).
Fig. 1.
Opioid receptor distribution in the human brain. [Above] The distribution of opiate receptors in the medial and lateral aspects of brain is revealed by an in-vivo study of opiate binding potential using radiolabeled carfentanil, a mu-opiate receptor ligand. There is increased binding in the frontal-limbic relative to sensory regions (Rabiner et al., 2011). [Below] Axial sections showing the gross anatomy (left) and radio-ligand binding to mu opiate receptors. Note the high opioid receptor densities in the insular region (Martin et al., 2007). Courtesy of Professor Bruce Morton.
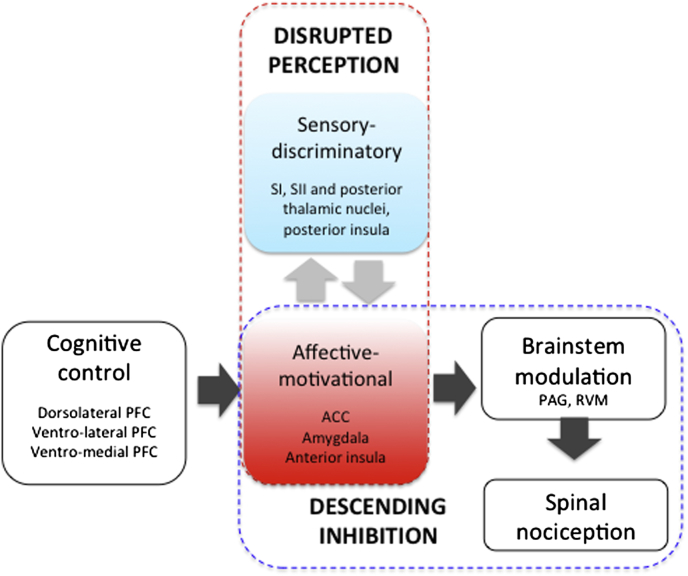
Fig. 2.
Brain mechanisms of opioid analgesia. Pain perception is generated by neural activation in the inter-connected (light-grey arrows) brain regions that have sensory-discriminatory (blue) and affective-motivational (red) functions. Opioids may alter the consciousness of pain directly by preferential targeting of limbic regions (in red box). Additionally, opioids may engage limbic-brainstem inhibition of spinal nociception (dark grey arrows), which also occurs during cognitive control of pain that involves the prefrontal cortex (PFC).
Opioid analgesia is not exclusively associated with suppression of brain activity. Wagner and colleagues found increased activation in the perigenual anterior cingulate cortex (ACC) and the periaqueductal grey (PAG) activations during opioid analgesia (Wagner et al., 2007). Converging evidence suggests that these regions comprise the neural circuit for the descending modulation of nociception, which include frontal-limbic and brainstem regions (Tracey, 2010). Baseline fluctuations of activity within regions in this circuit can predict moment-to-moment variation in the threshold of pain sensation within individuals, and relates to differences in trait anxiety and pain vigilance between individuals (Ploner et al., 2010). Endogenous opioids are known to contribute to the function of this descending neural circuit (Bencherif et al., 2002; Sprenger et al., 2006; Zubieta et al., 2001). Opioids can activate this same neural circuit for analgesic effects (Petrovic et al., 2002), which is shown to be active across FMRI studies during noxious stimulation itself (Fig. 2).
Interestingly, pain from noxious stimulation is also associated with activations in the several brain regions that are better known for their roles in processing rewards, such as monetary gain, palatable food, drugs and pleasurable social interactions (Becerra et al., 2001). These regions include the orbitofrontal cortex (OFC), nucleus accumbens (NA) and the ventral tegmental area (VTA), all of which are densely populated by opioid receptors. FMRI activations of these ‘reward’ regions during noxious stimulation may reflect increased endogenous opioidergic transmission for the regulation of pain. This increased opioidergic tone has been considered an opponent-process (pain-opposing) that persists briefly after cessation of the noxious stimulation to explain the experience of relief (from pain) (Leknes and Tracey, 2008; Seymour et al., 2005). Supporting the opponent-theory of pain are studies from our group and others studies demonstrating increased activations in similar reward regions when pain relief is experienced from the cessation or diminution of noxious stimulation (Baliki et al., 2010; Leknes et al., 2011). More recently, we demonstrated that pain relief related to opioids could be predicted from the baseline reactivity of ‘reward-regions’ within the brain in an FMRI study (Wanigasekera et al., 2012). In that study, activations within the OFC, NA and VTA evoked by painful noxious stimulation prior to opioid administration were positively correlated to reductions in subjective reports of pain intensity from identical noxious stimulation during opioid administration in healthy individuals. Opioid analgesia was also positively correlated with a psychometric measure of reward responsiveness, though neuroimaging data better accounted for the variance in opioid effects on pain ratings. Together these data suggest that opioid analgesia also involves neural mechanisms for reward processing. Interestingly, activity in these ‘reward regions’ evoked by noxious stimulation during opioid administration did not correlate with analgesic effect, suggesting that exogenous opioids do not directly modulate these reward regions for analgesic effect but may operate on shared mechanisms that are further downstream. For example, the baseline reactivity of the VTA also significantly predicted the opioid-induced changes in neuronal activity in the right amygdala and the left hippocampus, which may in turn influence the expression of behavioural analgesia in the study.
As with any treatment for pain, opioid analgesia can be influenced by the belief (expectancy) of efficacy held by the individual. Expectancy is often used to explain the placebo effect, which is now substantiated by neuroimaging research as a genuine psychobiological event. Endogenous opioids are known to contribute to placebo effect (Scott et al., 2008; Wager et al., 2007). Opioidergic mechanisms for placebo analgesia operate within the neural circuit for the descending control of pain, including the ACC, the PAG and the spinal dorsal horn (Eippert et al., 2009). More recently, we investigated whether effects from positive and negative expectancies of opioid analgesia engaged similar brain regions in healthy subjects (Bingel et al., 2011). The experiment involved a fixed and constant infusion of remifentanil. Subjects reported slightly lower pain compared to baseline when the infusion commenced but knowledge of that was hidden. Pain relief became significantly superior when subjects were told that the infusion had commenced. When informed that the infusion had ceased (though in reality the infusion was continued), their ratings of pain returned to baseline. The FMRI data revealed that effects of positive and negative expectancies on opioid analgesia involve distinct neuroanatomical substrates. Expectation of poor analgesic effect was accompanied by activation of the entorhinal cortex, a hippocampal region that mediates the exacerbation of pain through anxiety, through inhibition of perigenual ACC activity (Ploghaus et al., 2001). Conversely, positive expectation of analgesic effect was associated with increased perigenual ACC activation, suggesting the descending mechanisms of pain inhibition were engaged.
In addition to the areas described above that were expectancy ‘specific’, activations within the primary somatosensory, insular and mid-anterior cingulate cortices regions followed subjective reports of pain that was modulated by expectancy under a fixed remifentanil dose. It should be noted, though, that activations in those regions did not return to baseline levels during the nocebo arm unlike the subjectively reporting pain ratings. Hence, they reflect the true pharmacodynamic action that occurred and illustrate nicely the value of imaging that is not a simple surrogate marker of pain rating slavishly following the subjective report but are something additional. However, activations in many of these same somatosensory, insular and cingulate regions also correlate with pain modulated by varying doses of remifentanil without psychological manipulation (Wise et al., 2002, 2004). This raises the question about whether placebo and intrinsic opioid effects might interact significantly to impact estimates of drug effectiveness in placebo-controlled clinical trials, where a brain ‘on drug’ might not be able to mount the same placebo effect and mechanism as a brain ‘off drug’.
Atlas et al. (2012) employed a full factorial experimental design in their recent study to investigate potential interaction effects between remifentanil and expectation on pain ratings as well as brain activity. They did not find significant interaction effects on pain ratings, or brain activity. Pain relief from remifentanil and expectation were indistinguishable based on subjective pain ratings. FMRI did not reveal regions where interaction effects were significant. Instead, expectancy and remifentanil effects were distinguishable because they influenced different brain regions. Additionally, expectancy and remifentanil effects had different onsets. Expectancy effects occurred soon after the subjects became aware the infusion had started, whereas remifentanil effects become prominent only after peak blood concentrations were achieved from the infusion. These studies on drug and expectancy effects suggest that expectancy effects possess distinct functional neuro-anatomies. Further research is needed to explore the possibility of isolating the contribution of placebo or nocebo effects on pain based on neuroimaging data during clinical drug trials.
2.2. Opioid receptor availability – PET studies
Neuroimaging studies on the effects of opioids are scant in patients with chronic non-malignant pain. However, central effects appear critical to opioid analgesia in humans. Hence data regarding the distribution of opioid-receptors in the brain in patient populations can be instructive (Fig. 1).
Patients with chronic non-malignant pain are a heterogeneous group. Clinical trials have not revealed any particular clinical pain syndrome for which opioids are particularly efficacious. The earliest PET study suggests that opioid-receptor binding is decreased during a period of increased pain related to inflammation in a small group of patients with rheumatoid arthritis (Jones et al., 1994). An interpretation for the decreased opioid-receptor binding observed in the longitudinal study is increased occupancy by release of brain endorphins; possibly centrally regulated pain caused by the joint inflammation.
Other investigators have employed case–control designs in their PET studies of individuals with well-characterized neuropathic pain. In general, they demonstrate decreased opioid-receptor binding in patients compared to age-matched healthy controls (Jones et al., 2004; Maarrawi et al., 2007; Willoch et al., 2004). Complex regional pain syndrome (CRPS) and fibromyalgia are currently diagnoses of exclusion: the contributing pathologies remain unclear in symptomatic individuals. In these pain syndromes, opioid-receptor binding is again altered primarily in the frontal and limbic regions, and further accounts in part for the variance observed in the self-reports of affect and mood related to pain in patients (Harris et al., 2007; Klega et al., 2010).
A reduced opioid-receptor density has been proposed as an explanation for the efficacy of exogenous opioids or the lack thereof in patients (Harris et al., 2007; Maarrawi et al., 2007). However, binding potential is an estimate of available receptors, which depends on the affinity and selectivity of ligand (Henriksen and Willoch, 2008). A change in binding potentials may be related to receptor occupancy by endogenous opioid peptides or change in absolute quantity of receptors. Additionally, the action of different opioids varies depending on the selectivity of their binding to opioid-receptor subtypes (Melichar et al., 2005).
3. Opioid tolerance and drug-induced hyperalgesia
3.1. Analgesic tolerance
Opioid-induced tolerance is simply described as a shift to the right in the dose–response curve: a higher dose is required over time to maintain analgesic effect. Progressive disease may lead to higher opioid requirements. Tolerance also results from pharmacokinetic change, for example, when the drug up-regulates the activity of a metabolic process that facilitates its elimination from the body. For this review, “opioid tolerance” refers to pharmacodynamic tolerance, which reflects drug-activated changes in the response of the neural systems.
Mechanisms for analgesic tolerance include altered molecular signalling pathways and down-regulation of opioid receptors (Nagi and Pineyro, 2011). These mechanisms are largely elucidated in healthy animal models. Data regarding opioid tolerance in animal models of inflammation, injury and disease are few and inconsistent. Some behavioural studies suggest that tolerance to the anti-nociceptive effects (withdrawal effects) of opioids develop more slowly in inflammatory (Vaccarino et al., 1993; Zollner et al., 2008), cancer (Urch et al., 2005) and neuropathic models (Iwai et al., 2012) when compared to sham controls. Other studies demonstrate enhanced development of analgesic tolerance, particularly in neuropathic models (Raghavendra et al., 2002). The inconsistencies appear related to the species, disease model and the opioid and dose administered (Mogil, 2009).
3.2. Opioid-induced hyperalgesia
Distinct from tolerance, the excitatory effects of opioids at the cellular level are thought to cause opioid-induced hyperalgesia (Muscoli et al., 2010; Simonnet and Rivat, 2003; van Dorp et al., 2009; Waxman et al., 2009). In clinical practice, opioid-induced hyperalgesia is diagnosed when generalised pain develops and worsens with escalating opioid dose (Tompkins and Campbell, 2011). Improvement following opioid dose reduction is recommended to help distinguish between opioid tolerance and excitatory effects (Baron and McDonald, 2006). However, discomfort related to drug withdrawal can negatively influence the overall pain experienced and cloud the diagnosis.
Opioid-induced hyperalgesia is mainly demonstrated after prolonged administration of opioids in healthy animal models (Bannister and Dickenson, 2010; Heinl et al., 2011). These data appear consistent with findings of decreased tolerance of noxious stimuli in human individuals maintained on long-term methadone to manage opioid dependence (Compton et al., 2000; Doverty et al., 2001; Pud et al., 2006). However, a recent study in which oxycodone was administered orally over days in healthy volunteers without a history of drug misuse revealed no analgesic tolerance or hyperalgesia (Cooper et al., 2012). Hence, the development of tolerance or hyperalgesia may vary with the duration of administration, dose and opioid chemistry (Angst and Clark, 2006).
Susceptibility to these hyperalgesic drug effects may also differ between individuals. In healthy volunteers, cessation of remifentanil (after about 40 min of infusion) can induce (Jensen et al., 2009; Luginbuhl et al., 2003; Wanigasekera et al., 2011) or enhance hyperalgesia (Angst et al., 2003; Petersen et al., 2001) but not consistently. The phenomenon of opioid post-infusion hyperalgesia has been investigated recently in a combined psychophysical-fMRI study (Wanigasekera et al., 2011). Only half of all subjects recruited developed post-infusion hyperalgesia to noxious thermal stimuli, implying considerable inter-individual variation for the phenomenon, possibly related to genetic susceptibility (Jensen et al., 2009). Midbrain reticular formation activation was significantly increased only in hyperalgesic subjects but correlated negatively with the degree of hyperalgesia, which suggest that brainstem activity regulates, rather than drives sensitisation effects during opioid withdrawal.
In that human FMRI study (Wanigasekera et al., 2011), subjects who developed opioid post-infusion hyperalgesia were indistinguishable from the rest of study cohort during the remifentanil infusion itself: decreases in subjective ratings of pain during opioid infusion were similar in both groups. In subjects who subsequently developed post-infusion hyperalgesia, FMRI revealed increased activation in the midbrain reticular formation during the opioid infusion, suggesting initiation of pro-nociceptive process in that brief period. This was an acute administration study with an hour of opioid infusion, it remains unclear whether opioid-induced hyperalgesia would have occurred eventually had the infusion been prolonged in the susceptible individuals.
3.3. Opioid-induced hyperalgesia in injury or disease
In contrast to opioid-induced hyperalgesia in healthy animals, relatively few animal studies demonstrate opioid-induced hyperalgesia in the presence of tissue injury (Celerier et al., 2006; Li et al., 2001; Liang et al., 2008), inflammation (Rivat et al., 2009) or cancer (King et al., 2007), suggesting that these pathological states are protective. Notably, the doses of opioids used to induce hyperalgesia appear higher than those employed to study opioid tolerance.
Several clinical investigators have reported increased post-surgical pain scores and analgesic requirements following intra-operative remifentanil administration (Guignard et al., 2000; Rauf et al., 2005). However, the effects are small and are not reproduced in other comparable studies (Cortinez et al., 2001; Lee et al., 2005), leading to questions about the relevance of opioid tolerance or withdrawal-induced hyperalgesia in peri-operative pain management (Fishbain et al., 2009). Quantitative sensory testing in patients whose pain is managed by chronic opioid therapy reveals decreased pain thresholds to noxious stimuli during opioid therapy (Chen et al., 2009; Chu et al., 2006) but not consistently (Reznikov et al., 2005). Again, the findings are difficult to interpret, as the patients were heterogeneous in terms of pain phenotypes. Also, the relevance of QST findings to the clinical pain state is yet to be fully established.
4. Pain and opioid addiction
4.1. Opioid self-administration
In laboratory settings, unrestrained and healthy rats exposed to morphine by chance will subsequently self-administer opiates regularly (Gardner, 2000). This behavioural model has long been employed as a tool for studying neurobiology of addiction (Weeks, 1962). There are less data on how inflammatory or neuropathic pain might modulate volitional opioid self-administration. Rats with experimental arthritis increase opioid self-administration with progression of inflammation. Opioid self-administration decreased with alleviation of inflammation by indomethacin or dexamethasone treatment (Colpaert et al., 1982, 2001; Lyness et al., 1989). Also, opiates do not seem to act as reinforcers in neuropathic models of pain (Ozaki et al., 2003, 2004). Sustained activation of the kappa-opioidergic system within the nucleus accumbens is observed in the inflammatory pain state, and is thought to suppress drug-rewarding effects (Narita et al., 2005).
More recently, Ewan and colleagues compared the effects of morphine and cocaine on intracranial electrical self-stimulation (ICSS) of the VTA in the nerve-ligated rat, a model of neuropathic pain (Ewan and Martin, 2011). ICSS is an operant paradigm in which rats will press a lever repeatedly in order to receive intracranial electrical stimulation, which is highly rewarding by itself. In healthy rats, cocaine and opioids facilitated ICSS. In contrast, cocaine facilitated ICSS in nerve-ligated rats but opioids, which produced analgesic effects, did not. A subsequent experiment by the same investigators revealed opposite effects of these drugs on ICSS of the paraventricular nucleus (PVN) of the hypothalamus: opioids facilitate ICSS to a greater extent than cocaine (Ewan and Martin, 2012). Their studies reveal the complex interaction between opioid analgesia and reward-seeking behaviour. The reinforcing properties of opioids are modulated by neuropathic pain and appear to be mediated differently from those of cocaine, a clearly non-analgesic but addictive drug.
Overall, the experimental data suggest that opioids do not act as primary reinforcers in the presence of pain that is clearly attributable to peripheral or central pathology. This is consistent with the bulk of clinical experience of opioid use for post-surgical (Wu and Raja, 2011), and cancer-related pain (Portenoy, 2011).
4.2. Subjective effects of opioids
The positive subjective effects induced by opioids are thought to be reinforcing in humans but there is evidence that pain modulates these effects. In healthy subjects without clinical pain or history of recreational drug dependence, positive subjective effects of opioids are decreased during pain caused by hand-immersion in icy-cold water (Conley et al., 1997; Zacny et al., 1996). The subjective effects of opioids may differ in the presence of chronic pain, or when there is continued exposure of opioids. An early study in the 1950s reported that in contrast to healthy controls and in the opioid dependent subjects, most chronically ill patients reported the drug as pleasant only because it reduced pain (Lasagna et al., 1955). A later study found that opioid-dependent subjects reported more positive effects (‘Liking’ and ‘Good drug effects’), and had lower ratings for negative effects (‘Coasting’ and “Tired or sluggish’) (Azolosa et al., 1994).
The rate of drug delivery to the central nervous system is widely held to be a key predictor of the reinforcing strength of a drug. Extensive data in non-human species suggest that rapid delivery is associated with increased addictive potential for several recreational drugs, including opioids (Samaha and Robinson, 2005). In humans, under well-controlled experimental settings, more rapid infusions of opioids produced increased positive effects of the drug in healthy volunteers (Marsch et al., 2001) as well as opioid-dependent subjects (Comer et al., 2009). It remains unclear whether the positive subjective reactions of rapid drug administration are modulated in the presence of pain. Also subjective reactions to opioids do not themselves explain differences in self-administration in the presence of a painful stimulus. A recent study found that opioid use was linked to the presence of painful stimulation in the control group but not in the drug-dependent group even though the positive subjective effects of opioids (oxycodone) were similar in opioid-dependent and control groups (Comer et al., 2010).
4.3. Neuroimaging the subjective effect of opioids
Neuroimaging research has largely focussed on the analgesic effect of opioids. There are relatively few data on the neural correlates of subjective or reinforcing effects of opioids per se in healthy drug-naïve volunteers. Several PET studies have revealed significant changes in regional cerebral blood flow particularly in the ACC and OFC following acute administration (single dose) of opioids in healthy volunteers (Firestone et al., 1996). Similar activity is observed in other MR-perfusion-based studies (Leppa et al., 2006; Zelaya et al., 2012).
These changes might be anticipated given the distribution of opioid receptors in the brain, and are likely neural correlates to the subjective effects of the drug in the absence of pain (Leppa et al., 2006; Zelaya et al., 2012).
4.4. Opioids and the brain in chronic pain
There are as yet no published data regarding how the brains of patients with pain respond to long-term opioid therapy. The decreased opioid receptor binding in PET studies suggest that brain responses to opioids should differ and account for clinically relevant effects (see previous section). Further support for the hypothesis comes from the numerous MR-based studies that suggest volumetric changes in the reward-circuitry in several clinical pain populations compared to healthy controls (Schmidt-Wilcke, 2008). However, it remains unclear whether these findings from neuroimaging (PET or MRI) studies relate to chronic pain or to effects from long-term medication use (including opioids).
The issue was partly addressed in a recent longitudinal MR study (Younger et al., 2011). Individuals with chronic low back pain were administered oral morphine daily for 1 month, and compared to those who used placebo. Significant volumetric differences were observed in several regions, including the amygdala, and anterior cingulate cortex between patients who were treated with opioids compared to those who received placebo. However, patients who were treated with morphine reported significant reduction of reported pain scores, compared to those who were on placebo. Thus, the MR findings may relate to pain relief in general, rather than to the effects of opioid specifically. Interestingly, the volumetric changes in the morphine group persisted for months after cessation of opioids but no data regarding pain at that stage were reported.
It is still unclear whether structural changes in the limbic system determine the subjective effects of exogenous opioids. A recent FMRI study explored activity in the NA in patients with chronic back pain (Baliki et al., 2010). This limbic structure forms part of the mesostriatal dopamine pathway of the reward-circuitry. It encodes information that helps determine motivation and hence the behavioural response to external stimuli (Leknes and Tracey, 2008). In that study, noxious heat stimulation engendered NA responses in patients with chronic pain that were diametrically opposite to those of controls suggesting differences in motivational value assigned to the experimental stimulus. Whether these findings generalise to other salient stimuli, or influence the reinforcing effects of exogenous opioids await further investigation.
5. Future directions
The goals of healthcare are to improve comfort, psychosocial function, and longevity. Pain relief or comfort from opioid therapy does not necessarily lead to improvements in other aspects of health. Adverse effects can lead to significant physical deterioration and unintended respiratory depression and death can occur when opioid therapy is poorly monitored (Braden et al., 2010; Webster et al., 2011). Tolerance and opioid-induced hyperalgesia further limits the effectiveness of opioid-based analgesia in patients. Current animal data do show that tolerance and hyperalgesia are slow to develop in the presence of overt pathology that induces pain. However, the direct relevance of these mechanistic data for clinical practice remains to be established. Consequently, caution is being urged when initiating or escalating opioid therapy in patients for pain that is disproportionate to the disease process, or in the pain syndromes where pathology cannot as yet be demonstrated (Ngian et al., 2011). Addictive potential is now cited as a concern when opioids are used for treating chronic non-malignant pain (Dhalla et al., 2011; Okie, 2010). However, defining or diagnosing opioid misuse in patients with pain remains perplexing (Weissman and Haddox, 1989) and currently relies on behaviour that is also observed when pain is poorly treated (Bell and Salmon, 2009). Physicians are expected to distinguish between the use of opioids as ‘medications to relieve pain’, and the illegitimate use of opioids as ‘drugs to get high’(Fields, 2011). Unfortunately, there is no objective tool that can clarify motivation for opioid use in patients with pain. Opioids are intrinsically rewarding, but so is pain relief (Leknes et al., 2011) achieved through its use. Disambiguating these effects in patients with pain can be challenging. In-vivo human neuroimaging studies of opioid pharmacology now exist, but most data pertain to single dose opioids in healthy volunteers. There is a clear need for studies that are relevant to the complex issues clinicians face in prescribing long-term opioids for analgesia (Bruehl et al., 2013).
Contributor Information
Michael C. Lee, Email: michael.lee@ndcn.ox.ac.uk.
Irene Tracey, Email: irene.tracey@ndcn.ox.ac.uk.
References
- Adler L.J., Gyulai F.E., Diehl D.J., Mintun M.A., Winter P.M., Firestone L.L. Regional brain activity changes associated with fentanyl analgesia elucidated by positron emission tomography. Anesth. Analg. 1997;84:120–126. doi: 10.1097/00000539-199701000-00023. [DOI] [PubMed] [Google Scholar]
- Angst M.S., Clark J.D. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- Angst M.S., Koppert W., Pahl I., Clark D.J., Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Anissian L., Schwartz H.W., Vincent K., Vincent H.K., Carpenito J., Stambler N., Ramakrishna T. Subcutaneous methylnaltrexone for treatment of acute opioid-induced constipation: phase 2 study in rehabilitation after orthopedic surgery. J. Hosp. Med. 2012;7:67–72. doi: 10.1002/jhm.943. [DOI] [PubMed] [Google Scholar]
- Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9:463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Atlas L.Y., Whittington R.A., Lindquist M.A., Wielgosz J., Sonty N., Wager T.D. Dissociable influences of opiates and expectations on pain. J. Neurosci. 2012;32:8053–8064. doi: 10.1523/JNEUROSCI.0383-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azolosa J.L., Stitzer M.L., Greenwald M.K. Opioid physical dependence development: effects of single versus repeated morphine pretreatments and of subjects' opioid exposure history. Psychopharmacology (Berl) 1994;114:71–80. doi: 10.1007/BF02245446. [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Fields H.L., Apkarian A.V. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66:149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannister K., Dickenson A.H. Opioid hyperalgesia. Curr. Opin. Support. Palliat. Care. 2010;4:1–5. doi: 10.1097/SPC.0b013e328335ddfe. [DOI] [PubMed] [Google Scholar]
- Baron M.J., McDonald P.W. Significant pain reduction in chronic pain patients after detoxification from high-dose opioids. J. Opioid Manag. 2006;2:277–282. doi: 10.5055/jom.2006.0041. [DOI] [PubMed] [Google Scholar]
- Becerra L., Breiter H.C., Wise R., Gonzalez R.G., Borsook D. Reward circuitry activation by noxious thermal stimuli. Neuron. 2001;32:927–946. doi: 10.1016/s0896-6273(01)00533-5. [DOI] [PubMed] [Google Scholar]
- Bell K., Salmon A. Pain, physical dependence and pseudoaddiction: redefining addiction for 'nice' people? Int. J. Drug Policy. 2009;20:170–178. doi: 10.1016/j.drugpo.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Bencherif B., Fuchs P.N., Sheth R., Dannals R.F., Campbell J.N., Frost J.J. Pain activation of human supraspinal opioid pathways as demonstrated by [11C]-carfentanil and positron emission tomography (PET) Pain. 2002;99:589–598. doi: 10.1016/S0304-3959(02)00266-X. [DOI] [PubMed] [Google Scholar]
- Bingel U., Wanigasekera V., Wiech K., Ni Mhuircheartaigh R., Lee M.C., Ploner M., Tracey I. The effect of treatment expectation on drug efficacy: imaging the analgesic benefit of the opioid remifentanil. Sci. Transl. Med. 2011;3:70ra14. doi: 10.1126/scitranslmed.3001244. [DOI] [PubMed] [Google Scholar]
- Braden J.B., Russo J., Fan M.Y., Edlund M.J., Martin B.C., DeVries A., Sullivan M.D. Emergency department visits among recipients of chronic opioid therapy. Arch. Intern. Med. 2010;170:1425–1432. doi: 10.1001/archinternmed.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- British . British Pain Society; 2010. Opioids for Persistent Pain: Good Practice. [Google Scholar]
- Bruehl S., Apkarian A.V., Ballantyne J.C., Berger A., Borsook D., Chen W.G., Farrar J.T., Haythornthwaite J.A., Horn S.D., Iadarola M.J., Inturrisi C.E., Lao L., Mackey S., Mao J., Sawczuk A., Uhl G.R., Witter J., Woolf C.J., Zubieta J.K., Lin Y. Personalized medicine and opioid analgesic prescribing for chronic pain: opportunities and challenges. J. Pain. 2013;14:103–113. doi: 10.1016/j.jpain.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E., Gonzalez J.R., Maldonado R., Cabanero D., Puig M.M. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology. 2006;104:546–555. doi: 10.1097/00000542-200603000-00023. [DOI] [PubMed] [Google Scholar]
- Chen L., Malarick C., Seefeld L., Wang S., Houghton M., Mao J. Altered quantitative sensory testing outcome in subjects with opioid therapy. Pain. 2009;143:65–70. doi: 10.1016/j.pain.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou R., Ballantyne J.C., Fanciullo G.J., Fine P.G., Miaskowski C. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J. Pain. 2009;10:147–159. doi: 10.1016/j.jpain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Chou R., Fanciullo G.J., Fine P.G., Adler J.A., Ballantyne J.C., Davies P., Donovan M.I., Fishbain D.A., Foley K.M., Fudin J., Gilson A.M., Kelter A., Mauskop A., O'Connor P.G., Passik S.D., Pasternak G.W., Portenoy R.K., Rich B.A., Roberts R.G., Todd K.H., Miaskowski C. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J. Pain. 2009;10:113–130. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu L.F., Clark D.J., Angst M.S. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J. Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Colpaert F.C., Meert T., De Witte P., Schmitt P. Further evidence validating adjuvant arthritis as an experimental model of chronic pain in the rat. Life Sci. 1982;31:67–75. doi: 10.1016/0024-3205(82)90402-7. [DOI] [PubMed] [Google Scholar]
- Colpaert F.C., Tarayre J.P., Alliaga M., Bruins Slot L.A., Attal N., Koek W. Opiate self-administration as a measure of chronic nociceptive pain in arthritic rats. Pain. 2001;91:33–45. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- Comer S.D., Ashworth J.B., Sullivan M.A., Vosburg S.K., Saccone P.A., Foltin R.W. Relationship between rate of infusion and reinforcing strength of oxycodone in humans. J. Opioid Manag. 2009;5:203–212. doi: 10.5055/jom.2009.0022. [DOI] [PubMed] [Google Scholar]
- Comer S.D., Sullivan M.A., Vosburg S.K., Kowalczyk W.J., Houser J. Abuse liability of oxycodone as a function of pain and drug use history. Drug Alcohol Depend. 2010;109:130–138. doi: 10.1016/j.drugalcdep.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton P., Charuvastra V.C., Kintaudi K., Ling W. Pain responses in methadone-maintained opioid abusers. J. Pain Symptom Manag. 2000;20:237–245. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- Conley K.M., Toledano A.Y., Apfelbaum J.L., Zacny J.P. Modulating effects of a cold water stimulus on opioid effects in volunteers. Psychopharmacology (Berl) 1997;131:313–320. doi: 10.1007/s002130050298. [DOI] [PubMed] [Google Scholar]
- Cooper Z.D., Sullivan M.A., Vosburg S.K., Manubay J.M., Haney M., Foltin R.W., Evans S.M., Kowalczyk W.J., Saccone P.A., Comer S.D. Effects of repeated oxycodone administration on its analgesic and subjective effects in normal, healthy volunteers. Behav. Pharmacol. 2012 Jun;23(3):271–279. doi: 10.1097/FBP.0b013e3283536d6f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortinez L.I., Brandes V., Munoz H.R., Guerrero M.E., Mur M. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br. J. Anaesth. 2001;87:866–869. doi: 10.1093/bja/87.6.866. [DOI] [PubMed] [Google Scholar]
- Craig A.D. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel–now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Dhalla I.A., Persaud N., Juurlink D.N. Facing up to the prescription opioid crisis. BMJ. 2011;343:d5142. doi: 10.1136/bmj.d5142. [DOI] [PubMed] [Google Scholar]
- Dickenson A.H., Kieffer B.L. Opioids: basic mechanisms. In: McMahon S., Koltzenburg M., Tracey I., Turk D.C., editors. Textbook of Pain. Elsevier; Philadelphia, PA: 2013. [Google Scholar]
- Doverty M., White J.M., Somogyi A.A., Bochner F., Ali R., Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- Eippert F., Finsterbusch J., Bingel U., Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- Ewan E.E., Martin T.J. Opioid facilitation of rewarding electrical brain stimulation is suppressed in rats with neuropathic pain. Anesthesiology. 2011;114:624–632. doi: 10.1097/ALN.0b013e31820a4edb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewan E.E., Martin T.J. Intracranial self-stimulation of the paraventricular nucleus of the hypothalamus: increased facilitation by morphine compared to cocaine. Anesthesiology. 2012;116:1116–1123. doi: 10.1097/ALN.0b013e3182518be3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields H.L. The doctor's dilemma: opiate analgesics and chronic pain. Neuron. 2011;69:591–594. doi: 10.1016/j.neuron.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestone L.L., Gyulai F., Mintun M., Adler L.J., Urso K., Winter P.M. Human brain activity response to fentanyl imaged by positron emission tomography. Anesth. Analg. 1996;82:1247–1251. doi: 10.1097/00000539-199606000-00025. [DOI] [PubMed] [Google Scholar]
- Fishbain D.A., Cole B., Lewis J.E., Gao J., Rosomoff R.S. Do opioids induce hyperalgesia in humans? An evidence-based structured review. Pain Med. 2009;10:829–839. doi: 10.1111/j.1526-4637.2009.00653.x. [DOI] [PubMed] [Google Scholar]
- Gardner E.L. What we have learned about addiction from animal models of drug self-administration. Am. J. Addict. 2000;9:285–313. doi: 10.1080/105504900750047355. [DOI] [PubMed] [Google Scholar]
- Guignard B., Bossard A.E., Coste C., Sessler D.I., Lebrault C., Alfonsi P., Fletcher D., Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- Harris R.E., Clauw D.J., Scott D.J., McLean S.A., Gracely R.H., Zubieta J.K. Decreased central mu-opioid receptor availability in fibromyalgia. J. Neurosci. 2007;27:10000–10006. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinl C., Drdla-Schutting R., Xanthos D.N., Sandkuhler J. Distinct mechanisms underlying pronociceptive effects of opioids. J. Neurosci. 2011;31:16748–16756. doi: 10.1523/JNEUROSCI.3491-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriksen G., Willoch F. Imaging of opioid receptors in the central nervous system. Brain. 2008;131:1171–1196. doi: 10.1093/brain/awm255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IASP . Part III: pain terms, a current list with definitions and notes on usage. In: Merskey M., Bogduk N., editors. Classification of Chronic Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. IASP Press; Seattle: 1994. pp. 209–214. [Google Scholar]
- Iwai S., Kiguchi N., Kobayashi Y., Fukazawa Y., Saika F., Ueno K., Yamamoto C., Kishioka S. Inhibition of morphine tolerance is mediated by painful stimuli via central mechanisms. Drug Discov. Ther. 2012;6:31–37. [PubMed] [Google Scholar]
- Jensen K.B., Lonsdorf T.B., Schalling M., Kosek E., Ingvar M. Increased sensitivity to thermal pain following a single opiate dose is influenced by the COMT val(158)met polymorphism. PLoS One. 2009;4:e6016. doi: 10.1371/journal.pone.0006016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.K., Cunningham V.J., Ha-Kawa S., Fujiwara T., Luthra S.K., Silva S., Derbyshire S., Jones T. Changes in central opioid receptor binding in relation to inflammation and pain in patients with rheumatoid arthritis. Br. J. Rheumatol. 1994;33:909–916. doi: 10.1093/rheumatology/33.10.909. [DOI] [PubMed] [Google Scholar]
- Jones A.K., Watabe H., Cunningham V.J., Jones T. Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C]diprenorphine binding and PET. Eur. J. Pain. 2004;8:479–485. doi: 10.1016/j.ejpain.2003.11.017. [DOI] [PubMed] [Google Scholar]
- Kahan M., Mailis-Gagnon A., Wilson L., Srivastava A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 1: general population. Can. Fam. Physician. 2011;57 1257–1266, e1407–1218. [PMC free article] [PubMed] [Google Scholar]
- Kahan M., Wilson L., Mailis-Gagnon A., Srivastava A. Canadian guideline for safe and effective use of opioids for chronic noncancer pain: clinical summary for family physicians. Part 2: special populations. Can. Fam. Physician. 2011;57 1269–1276, e1419–1228. [PMC free article] [PubMed] [Google Scholar]
- Kendall S.E., Sjogren P., Pimenta C.A., Hojsted J., Kurita G.P. The cognitive effects of opioids in chronic non-cancer pain. Pain. 2010;150:225–230. doi: 10.1016/j.pain.2010.05.012. [DOI] [PubMed] [Google Scholar]
- King T., Vardanyan A., Majuta L., Melemedjian O., Nagle R., Cress A.E., Vanderah T.W., Lai J., Porreca F. Morphine treatment accelerates sarcoma-induced bone pain, bone loss, and spontaneous fracture in a murine model of bone cancer. Pain. 2007;132:154–168. doi: 10.1016/j.pain.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klega A., Eberle T., Buchholz H.G., Maus S., Maihofner C., Schreckenberger M., Birklein F. Central opioidergic neurotransmission in complex regional pain syndrome. Neurology. 2010;75:129–136. doi: 10.1212/WNL.0b013e3181e7ca2e. [DOI] [PubMed] [Google Scholar]
- Lasagna L., Von Felsinger J.M., Beecher H.K. Drug-induced mood changes in man. I. Observations on healthy subjects, chronically ill patients, and postaddicts. J. Am. Med. Assoc. 1955;157:1006–1020. doi: 10.1001/jama.1955.02950290026009. [DOI] [PubMed] [Google Scholar]
- Lee L.H., Irwin M.G., Lui S.K. Intraoperative remifentanil infusion does not increase postoperative opioid consumption compared with 70% nitrous oxide. Anesthesiology. 2005;102:398–402. doi: 10.1097/00000542-200502000-00024. [DOI] [PubMed] [Google Scholar]
- Leknes S., Lee M., Berna C., Andersson J., Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 2011;6:e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leknes S., Tracey I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008;9:314–320. doi: 10.1038/nrn2333. [DOI] [PubMed] [Google Scholar]
- Leppa M., Korvenoja A., Carlson S., Timonen P., Martinkauppi S., Ahonen J., Rosenberg P.H., Aronen H.J., Kalso E. Acute opioid effects on human brain as revealed by functional magnetic resonance imaging. Neuroimage. 2006;31:661–669. doi: 10.1016/j.neuroimage.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Li X., Angst M.S., Clark J.D. Opioid-induced hyperalgesia and incisional pain. Anesth. Analg. 2001;93:204–209. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- Liang D., Shi X., Qiao Y., Angst M.S., Yeomans D.C., Clark J.D. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol. Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luginbuhl M., Gerber A., Schnider T.W., Petersen-Felix S., Arendt-Nielsen L., Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth. Analg. 2003;96:726–732. doi: 10.1213/01.ANE.0000048086.58161.18. (table of contents) [DOI] [PubMed] [Google Scholar]
- Lyness W.H., Smith F.L., Heavner J.E., Iacono C.U., Garvin R.D. Morphine self-administration in the rat during adjuvant-induced arthritis. Life Sci. 1989;45:2217–2224. doi: 10.1016/0024-3205(89)90062-3. [DOI] [PubMed] [Google Scholar]
- Maarrawi J., Peyron R., Mertens P., Costes N., Magnin M., Sindou M., Laurent B., Garcia-Larrea L. Differential brain opioid receptor availability in central and peripheral neuropathic pain. Pain. 2007;127:183–194. doi: 10.1016/j.pain.2006.10.013. [DOI] [PubMed] [Google Scholar]
- Marsch L.A., Bickel W.K., Badger G.J., Rathmell J.P., Swedberg M.D., Jonzon B., Norsten-Hoog C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J. Pharmacol. Exp. Ther. 2001;299:1056–1065. [PubMed] [Google Scholar]
- Martin M., Hurley R.A., Taber K.H. Is opiate addiction associated with longstanding neurobiological changes? J. Neuropsychiatry Clin. Neurosci. 2007;19:242–248. doi: 10.1176/jnp.2007.19.3.242. [DOI] [PubMed] [Google Scholar]
- Mazzola L., Isnard J., Peyron R., Guenot M., Mauguiere F. Somatotopic organization of pain responses to direct electrical stimulation of the human insular cortex. Pain. 2009;146:99–104. doi: 10.1016/j.pain.2009.07.014. [DOI] [PubMed] [Google Scholar]
- Melichar J.K., Hume S.P., Williams T.M., Daglish M.R., Taylor L.G., Ahmad R., Malizia A.L., Brooks D.J., Myles J.S., Lingford-Hughes A., Nutt D.J. Using [11C]diprenorphine to image opioid receptor occupancy by methadone in opioid addiction: clinical and preclinical studies. J. Pharmacol. Exp. Ther. 2005;312:309–315. doi: 10.1124/jpet.104.072686. [DOI] [PubMed] [Google Scholar]
- Michna E., Blonsky E.R., Schulman S., Tzanis E., Manley A., Zhang H., Iyer S., Randazzo B. Subcutaneous methylnaltrexone for treatment of opioid-induced constipation in patients with chronic, nonmalignant pain: a randomized controlled study. J. Pain. 2011;12:554–562. doi: 10.1016/j.jpain.2010.11.008. [DOI] [PubMed] [Google Scholar]
- Mogil J.S. Animal models of pain: progress and challenges. Nat. Rev. Neurosci. 2009;10:283–294. doi: 10.1038/nrn2606. [DOI] [PubMed] [Google Scholar]
- Muscoli C., Doyle T., Dagostino C., Bryant L., Chen Z., Watkins L.R., Ryerse J., Bieberich E., Neumman W., Salvemini D. Counter-regulation of opioid analgesia by glial-derived bioactive sphingolipids. J. Neurosci. 2010;30:15400–15408. doi: 10.1523/JNEUROSCI.2391-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagi K., Pineyro G. Regulation of opioid receptor signalling: implications for the development of analgesic tolerance. Mol. Brain. 2011;4:25. doi: 10.1186/1756-6606-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M., Kishimoto Y., Ise Y., Yajima Y., Misawa K., Suzuki T. Direct evidence for the involvement of the mesolimbic kappa-opioid system in the morphine-induced rewarding effect under an inflammatory pain-like state. Neuropsychopharmacology. 2005;30:111–118. doi: 10.1038/sj.npp.1300527. [DOI] [PubMed] [Google Scholar]
- Ngian G.S., Guymer E.K., Littlejohn G.O. The use of opioids in fibromyalgia. Int. J. Rheum. Dis. 2011;14:6–11. doi: 10.1111/j.1756-185X.2010.01567.x. [DOI] [PubMed] [Google Scholar]
- Noble M., Treadwell J.R., Tregear S.J., Coates V.H., Wiffen P.J., Akafomo C., Schoelles K.M. Long-term opioid management for chronic noncancer pain. Cochrane Database Syst. Rev. 2010 doi: 10.1002/14651858.CD006605.pub2. CD006605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel B.G., Preibisch C., Wallenhorst T., Hummel T., Geisslinger G., Lanfermann H., Lotsch J. Differential opioid action on sensory and affective cerebral pain processing. Clin. Pharmacol. Ther. 2008;83:577–588. doi: 10.1038/sj.clpt.6100441. [DOI] [PubMed] [Google Scholar]
- Okie S. A flood of opioids, a rising tide of deaths. N. Engl. J. Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- Ostrowsky K., Magnin M., Ryvlin P., Isnard J., Guenot M., Mauguiere F. Representation of pain and somatic sensation in the human insula: a study of responses to direct electrical cortical stimulation. Cereb. Cortex. 2002;12:376–385. doi: 10.1093/cercor/12.4.376. [DOI] [PubMed] [Google Scholar]
- Ozaki S., Narita M., Narita M., Iino M., Miyoshi K., Suzuki T. Suppression of the morphine-induced rewarding effect and G-protein activation in the lower midbrain following nerve injury in the mouse: involvement of G-protein-coupled receptor kinase 2. Neuroscience. 2003;116:89–97. doi: 10.1016/s0306-4522(02)00699-1. [DOI] [PubMed] [Google Scholar]
- Ozaki S., Narita M., Ozaki M., Khotib J., Suzuki T. Role of extracellular signal-regulated kinase in the ventral tegmental area in the suppression of the morphine-induced rewarding effect in mice with sciatic nerve ligation. J. Neurochem. 2004;88:1389–1397. doi: 10.1046/j.1471-4159.2003.02272.x. [DOI] [PubMed] [Google Scholar]
- Pattinson K.T., Rogers R., Mayhew S.D., Tracey I., Wise R.G. Pharmacological FMRI: measuring opioid effects on the BOLD response to hypercapnia. J. Cereb. Blood Flow Metab. 2007;27:414–423. doi: 10.1038/sj.jcbfm.9600347. [DOI] [PubMed] [Google Scholar]
- Pergolizzi J.V., Jr., Gharibo C., Passik S., Labhsetwar S., Taylor R., Jr., Pergolizzi J.S., Muller-Schwefe G. Dynamic risk factors in the misuse of opioid analgesics. J. Psychosom. Res. 2012;72:443–451. doi: 10.1016/j.jpsychores.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Petersen K.L., Jones B., Segredo V., Dahl J.B., Rowbotham M.C. Effect of remifentanil on pain and secondary hyperalgesia associated with the heat–capsaicin sensitization model in healthy volunteers. Anesthesiology. 2001;94:15–20. doi: 10.1097/00000542-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. Placebo and opioid analgesia– imaging a shared neuronal network. Science. 2002;295:1737–1740. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Ploghaus A., Narain C., Beckmann C.F., Clare S., Bantick S., Wise R., Matthews P.M., Rawlins J.N., Tracey I. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. J. Neurosci. 2001;21:9896–9903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploner M., Lee M.C., Wiech K., Bingel U., Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc. Natl. Acad. Sci. U. S. A. 2010;107:355–360. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portenoy R.K. Treatment of cancer pain. Lancet. 2011;377:2236–2247. doi: 10.1016/S0140-6736(11)60236-5. [DOI] [PubMed] [Google Scholar]
- Pud D., Cohen D., Lawental E., Eisenberg E. Opioids and abnormal pain perception: new evidence from a study of chronic opioid addicts and healthy subjects. Drug Alcohol Depend. 2006;82:218–223. doi: 10.1016/j.drugalcdep.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Rabiner E.A., Beaver J., Makwana A., Searle G., Long C., Nathan P.J., Newbould R.D., Howard J., Miller S.R., Bush M.A., Hill S., Reiley R., Passchier J., Gunn R.N., Matthews P.M., Bullmore E.T. Molecular and functional neuroimaging of human opioid receptor pharmacology. Mol. Psychiatry. 2011;16:785. doi: 10.1038/mp.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V., Rutkowski M.D., DeLeo J.A. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J. Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauf K., Vohra A., Fernandez-Jimenez P., O'Keeffe N., Forrest M. Remifentanil infusion in association with fentanyl-propofol anaesthesia in patients undergoing cardiac surgery: effects on morphine requirement and postoperative analgesia. Br. J. Anaesth. 2005;95:611–615. doi: 10.1093/bja/aei237. [DOI] [PubMed] [Google Scholar]
- Reznikov I., Pud D., Eisenberg E. Oral opioid administration and hyperalgesia in patients with cancer or chronic nonmalignant pain. Br. J. Clin. Pharmacol. 2005;60:311–318. doi: 10.1111/j.1365-2125.2005.02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivat C., Vera-Portocarrero L.P., Ibrahim M.M., Mata H.P., Stagg N.J., De Felice M., Porreca F., Malan T.P. Spinal NK-1 receptor-expressing neurons and descending pathways support fentanyl-induced pain hypersensitivity in a rat model of postoperative pain. Eur. J. Neurosci. 2009;29:727–737. doi: 10.1111/j.1460-9568.2009.06616.x. [DOI] [PubMed] [Google Scholar]
- Samaha A.N., Robinson T.E. Why does the rapid delivery of drugs to the brain promote addiction? Trends Pharmacol. Sci. 2005;26:82–87. doi: 10.1016/j.tips.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T. Variations in brain volume and regional morphology associated with chronic pain. Curr. Rheumatol. Rep. 2008;10:467–474. doi: 10.1007/s11926-008-0077-7. [DOI] [PubMed] [Google Scholar]
- Scott D.J., Stohler C.S., Egnatuk C.M., Wang H., Koeppe R.A., Zubieta J.K. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch. Gen. Psychiatry. 2008;65:220–231. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Seymour B., O'Doherty J.P., Koltzenburg M., Wiech K., Frackowiak R., Friston K., Dolan R. Opponent appetitive-aversive neural processes underlie predictive learning of pain relief. Nat. Neurosci. 2005;8:1234–1240. doi: 10.1038/nn1527. [DOI] [PubMed] [Google Scholar]
- Simonnet G., Rivat C. Opioid-induced hyperalgesia: abnormal or normal pain? Neuroreport. 2003;14:1–7. doi: 10.1097/00001756-200301200-00001. [DOI] [PubMed] [Google Scholar]
- Sprenger T., Valet M., Boecker H., Henriksen G., Spilker M.E., Willoch F., Wagner K.J., Wester H.J., Tolle T.R. Opioidergic activation in the medial pain system after heat pain. Pain. 2006;122:63–67. doi: 10.1016/j.pain.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Thomas J., Karver S., Cooney G.A., Chamberlain B.H., Watt C.K., Slatkin N.E., Stambler N., Kremer A.B., Israel R.J. Methylnaltrexone for opioid-induced constipation in advanced illness. N. Engl. J. Med. 2008;358:2332–2343. doi: 10.1056/NEJMoa0707377. [DOI] [PubMed] [Google Scholar]
- Tompkins D.A., Campbell C.M. Opioid-induced hyperalgesia: clinically relevant or extraneous research phenomenon? Curr. Pain Headache Rep. 2011;15:129–136. doi: 10.1007/s11916-010-0171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey I. Nociceptive processing in the human brain. Curr. Opin. Neurobiol. 2005;15:478–487. doi: 10.1016/j.conb.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Tracey I. Getting the pain you expect: mechanisms of placebo, nocebo and reappraisal effects in humans. Nat. Med. 2010 Nov;16(11):1277–1283. doi: 10.1038/nm.2229. [DOI] [PubMed] [Google Scholar]
- Tracey I., Mantyh P.W. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. [DOI] [PubMed] [Google Scholar]
- Urch C.E., Donovan-Rodriguez T., Gordon-Williams R., Bee L.A., Dickenson A.H. Efficacy of chronic morphine in a rat model of cancer-induced bone pain: behavior and in dorsal horn pathophysiology. J. Pain. 2005;6:837–845. doi: 10.1016/j.jpain.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Vaccarino A.L., Marek P., Kest B., Ben-Eliyahu S., Couret L.C., Jr., Kao B., Liebeskind J.C. Morphine fails to produce tolerance when administered in the presence of formalin pain in rats. Brain Res. 1993;627:287–290. doi: 10.1016/0006-8993(93)90332-h. [DOI] [PubMed] [Google Scholar]
- van Dorp E.L., Kest B., Kowalczyk W.J., Morariu A.M., Waxman A.R., Arout C.A., Dahan A., Sarton E.Y. Morphine-6beta-glucuronide rapidly increases pain sensitivity independently of opioid receptor activity in mice and humans. Anesthesiology. 2009;110:1356–1363. doi: 10.1097/ALN.0b013e3181a105de. [DOI] [PubMed] [Google Scholar]
- Wager T.D., Scott D.J., Zubieta J.K. Placebo effects on human mu-opioid activity during pain. Proc. Natl. Acad. Sci. U. S. A. 2007;104:11056–11061. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner K.J., Sprenger T., Kochs E.F., Tolle T.R., Valet M., Willoch F. Imaging human cerebral pain modulation by dose-dependent opioid analgesia: a positron emission tomography activation study using remifentanil. Anesthesiology. 2007;106:548–556. doi: 10.1097/00000542-200703000-00020. [DOI] [PubMed] [Google Scholar]
- Wanigasekera V., Lee M.C., Rogers R., Hu P., Tracey I. Neural correlates of an injury-free model of central sensitization induced by opioid withdrawal in humans. J. Neurosci. 2011;31:2835–2842. doi: 10.1523/JNEUROSCI.5412-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera V., Lee M.C., Rogers R., Kong Y., Leknes S., Andersson J., Tracey I. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc. Natl. Acad. Sci. U. S. A. 2012;109:17705–17710. doi: 10.1073/pnas.1120201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waxman A.R., Arout C., Caldwell M., Dahan A., Kest B. Acute and chronic fentanyl administration causes hyperalgesia independently of opioid receptor activity in mice. Neurosci. Lett. 2009;462:68–72. doi: 10.1016/j.neulet.2009.06.061. [DOI] [PubMed] [Google Scholar]
- Webster L.R., Cochella S., Dasgupta N., Fakata K.L., Fine P.G., Fishman S.M., Grey T., Johnson E.M., Lee L.K., Passik S.D., Peppin J., Porucznik C.A., Ray A., Schnoll S.H., Stieg R.L., Wakeland W. An analysis of the root causes for opioid-related overdose deaths in the United States. Pain Med. 2011;12(Suppl. 2):S26–S35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- Weeks J.R. Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science. 1962;138:143–144. doi: 10.1126/science.138.3537.143. [DOI] [PubMed] [Google Scholar]
- Weissman D.E., Haddox J.D. Opioid pseudoaddiction – an iatrogenic syndrome. Pain. 1989;36:363–366. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- Willoch F., Schindler F., Wester H.J., Empl M., Straube A., Schwaiger M., Conrad B., Tolle T.R. Central poststroke pain and reduced opioid receptor binding within pain processing circuitries: a [11C]diprenorphine PET study. Pain. 2004;108:213–220. doi: 10.1016/j.pain.2003.08.014. [DOI] [PubMed] [Google Scholar]
- Wise R.G., Rogers R., Painter D., Bantick S., Ploghaus A., Williams P., Rapeport G., Tracey I. Combining fMRI with a pharmacokinetic model to determine which brain areas activated by painful stimulation are specifically modulated by remifentanil. Neuroimage. 2002;16:999–1014. doi: 10.1006/nimg.2002.1146. [DOI] [PubMed] [Google Scholar]
- Wise R.G., Tracey I. The role of fMRI in drug discovery. J. Magn. Reson. Imaging. 2006;23:862–876. doi: 10.1002/jmri.20584. [DOI] [PubMed] [Google Scholar]
- Wise R.G., Williams P., Tracey I. Using fMRI to quantify the time dependence of remifentanil analgesia in the human brain. Neuropsychopharmacology. 2004;29:626–635. doi: 10.1038/sj.npp.1300364. [DOI] [PubMed] [Google Scholar]
- Wu C.L., Raja S.N. Treatment of acute postoperative pain. Lancet. 2011;377:2215–2225. doi: 10.1016/S0140-6736(11)60245-6. [DOI] [PubMed] [Google Scholar]
- Younger J.W., Chu L.F., D'Arcy N.T., Trott K.E., Jastrzab L.E., Mackey S.C. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacny J.P., McKay M.A., Toledano A.Y., Marks S., Young C.J., Klock P.A., Apfelbaum J.L. The effects of a cold-water immersion stressor on the reinforcing and subjective effects of fentanyl in healthy volunteers. Drug Alcohol Depend. 1996;42:133–142. doi: 10.1016/0376-8716(96)01274-4. [DOI] [PubMed] [Google Scholar]
- Zelaya F.O., Zois E., Muller-Pollard C., Lythgoe D.J., Lee S., Andrews C., Smart T., Conrod P., Vennart W., Williams S.C., Mehta M.A., Reed L.J. The response to rapid infusion of fentanyl in the human brain measured using pulsed arterial spin labelling. MAGMA. 2012;25:163–175. doi: 10.1007/s10334-011-0293-4. [DOI] [PubMed] [Google Scholar]
- Zollner C., Mousa S.A., Fischer O., Rittner H.L., Shaqura M., Brack A., Shakibaei M., Binder W., Urban F., Stein C., Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J. Clin. Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta J.K., Smith Y.R., Bueller J.A., Xu Y., Kilbourn M.R., Jewett D.M., Meyer C.R., Koeppe R.A., Stohler C.S. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–315. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]