Abstract
The sensitivity of chemical exchange saturation transfer (CEST) on glycosaminoglycans (GAG) in human knee cartilage (gagCEST) in vivo was evaluated at 3T and 7T field strengths. Calculated gagCEST values without accounting for B0 inhomogeneity (~0.6 ppm) were > 20%. After B0 inhomogeneity correction, calculated gagCEST values were negligible at 3T and ~6% at 7T. These results suggest that accurate B0 correction is a pre-requisite for observing reliable gagCEST. Results obtained with varying saturation pulse durations and amplitudes as well as the consistency between numerical simulations and our experimental results indicate that the negligible gagCEST observed at 3T is due to direct saturation effects and fast exchange rate. Since GAG loss from cartilage is expected to result in a further reduction in gagCEST, gagCEST method is not expected to be clinically useful at 3T. At high fields such as 7T, this method holds promise as a viable clinical technique.
Keywords: MRI, CEST, saturation pulse, Cartilage, GAG, gagCEST
INTRODUCTION
Osteoarthritis (OA) is one of the debilitating joint diseases of the musculoskeletal system. It affects more than 10% of adults and 70% of the population over the age of 65 years and has a significant negative impact on the quality of life of elderly individuals (1–3). While OA is now increasingly viewed as a metabolically active joint disorder of diverse etiologies, cartilage tissue degeneration is primarily implicated. It is generally believed that the initiating event of OA is predominantly due to loss of proteoglycans from the tissue (4). Proteoglycans (PG) are complex macromolecules that consist of proteins and polysaccharides. Aggrecan is the most common of these PG, and accounts for ~80–90% of the total PG. It consists of a protein core with a long extended domain to which many glycosaminoglycan (GAG) side chains are attached. Chondroitin sulfate (CS) is the predominant GAG molecule found in cartilage. In order for appropriate therapeutic intervention in OA, there is a critical need for diagnostic methods that quantify the early molecular changes in cartilage before the manifestation of morphological changes.
Since conventional magnetic resonance imaging (MRI) is neither proven sensitive nor accurate for the detection of early biochemical changes associated with the loss of PG, there have been several sophisticated MRI methods proposed to quantify these changes in vivo. These include sodium MRI, T1rho (T1ρ) MRI and delayed Gadolinium enhanced MRI contrast (dGEMRIC) (5–7). While sodium MRI is highly specific to PG, it requires special coils and hardware (multi-nuclear option). It is also associated with low SNR and requires ultrahigh fields. dGEMRIC on the other hand is a promising method that can be performed on standard clinical scanners. However, it has logistical issues such as long waiting periods following the injection of contrast agent. T1ρ MRI is a novel imaging method that has the ability to generate endogenous contrast that is sensitive to in vivo PG and collagen content(6). Recently, it has been shown that chemical exchange saturation transfer (CEST) of labile –OH protons on GAG with bulk water leads to a significant reduction of bulk water magnetization creating “gagCEST” (8). Using this approach significant gagCEST was reported from both ex vivo and in vivo cartilage without any systematic analysis of static magnetic field (B0) inhomogeneity (8).
It is well known that the B0 inhomogeneity would significantly affect the accuracy of the computed CEST values (9–11). B0 correction requires an estimate of local B0 variation. For B0 estimation, one can either use gradient echo MRI methods (12) or off-resonance saturation based methods (10,11,13). Recently published water saturation shift referencing (WASSR) approach (10) is also an off resonance saturation based method with optimized saturation pulses to provide only direct water saturation. For B0 correction, one can use either analytical expression in steady state saturation conditions (9) or interpolated (or fitted) z-spectral data (10,11,13). We have used interpolated WASSR data for B0 estimation.
In the current study, the feasibility of performing gagCEST on human cartilage in vivo was evaluated at 3T and 7T field strengths. Human cartilage gagCEST maps were computed before and after the correction of B0 inhomogeneity. Saturation pulse parameters were optimized to obtain maximal gagCEST in human cartilage and numerical simulations were performed to examine the effects of direct saturation (14) of water as well GAG –OH protons on the observed gagCEST.
MATERIALS AND METHODS
Theoretical
In CEST experiments, frequency selective saturation of solute spins that are in exchange with solvent spins (e.g., water) leads to the transfer of saturated magnetization to the solvent thus decreasing the signal intensity of the solvent spins. Subsequently, longitudinal relaxation returns each nuclear spin system to its equilibrium values and eventually the system reaches a steady state. The steady state magnetization is given as:
| [1] |
where Msat∞ is the steady-state amplitude of the water proton magnetization during the irradiation of exchangeable solute spins; M0 is the amplitude of the water proton magnetization in the absence of saturation, k1 is the pseudo first order exchange rate constant, and T1w is the longitudinal relaxation time of water protons (14–16). This magnetization is then imaged to detect the CEST effect from solute nuclear spins. In order for the CEST effect to be efficiently observed, the slow to intermediate exchange condition (Δω>k) must be fulfilled where Δω is the chemical shift (or offset frequency) of the exchanging spins and k is the exchange rate. In general the CEST effect of the solute spins is computed using following equation:
| [2] |
where M0 is the water equilibrium magnetization, Msai (±Δω) are the water magnetizations obtained with saturation at a ‘+’ or ‘−’ Δω offset of the water resonance. In interpreting the CEST effect, other factors that play role are the amplitude and duration of the saturation pulse. These effects can be incorporated into a general solution obtainable from a theoretical analysis of a two-site exchange model in the presence of solute saturation. An analytical expression for the CEST effect can be derived (17–20) as:
| [3] |
where k is exchange rate (s−1 or Hz), α is an efficiency factor with α = 1 describing complete saturation (obtained with a sufficiently high amplitude saturation pulse), f is the fraction of exchangeable protons with respect to the total number of protons including water, R1w (=1/T1w) is the longitudinal relaxation rate of water protons and tsat is the length of the saturation pulse.
While Eq. [3] is useful in understanding the general CEST principles, its applicability is limited to solute selective steady state saturation only at +Δω offset frequency with no other saturation contaminations. In practice there is contamination from the DS of water to both Msat (±Δω) and from DS of solute protons while saturating at −Δω to Msat (−Δω). DS of water reduces the available bulk water protons for chemical exchange and reduces CEST contrast. DS of solute protons while saturating at −Δω additionally reduces water magnetization due to chemical exchange with the solute protons. In such cases to fully understand CEST and DS effects, one needs to resort to numerical simulations of Bloch-McConnell equations (17).
The effects of B0 and B1 variations on the observed CEST values are rather complex. Since the CEST asymmetry is based on the subtraction of images Msat (±Δω), any asymmetry created with local B0 variation will contaminate the observed CEST asymmetry. Hence, a very good estimate and correction of local B0 inhomogeneity is imperative to get accurate CEST asymmetry. B1 variations affect both the CEST effect (Eq. [3] provides a hint for this through the empirical factor α) as well as the amount of DS contamination.
CEST MR Sequence
The pulse sequence used in current study consists of a frequency selective saturation pulse train (user selected saturation offset frequency (Δω), saturation duration and B1rms) followed by a chemical shift selective fat saturation pulse and a segmented RF spoiled gradient echo (GRE) readout acquisition with centric phase encoding order. At the end of the GRE acquisition segments, a variable delay has been added to provide T1 recovery and keep the sequence under system RF safety limits. This sequence is flexible enough to be used for both the WASSR data acquisition as well as CEST imaging.
The saturation pulse train is composed of Hanning windowed rectangular pulses and delays between them. At 3T a 48 ms pulse with a 2 ms delay is used where as at 7T a 99.8 ms pulse with a 0.2 ms delay is used. The number of pulses in the train can be adjusted to provide variable saturation duration. The Hanning window shape and pulse duration were chosen based on MRI scanner hardware limits and minimal artifacts in phantom tests. The peak B1 of the Hanning windowed pulse is set to provide the required B1rms value. The saturation pulse excitation bandwidth (50%) is 10 Hz with a 1% bandwidth of 40 Hz (~0.28 ppm at 3T) for saturation train duration of 0.5 s. For longer saturation durations, these bandwidths are narrower.
Human Studies
The study was conducted under an approved Institutional Review Board protocol of the University of Pennsylvania. Five subjects were taken from a normal population in the age range of 28–40 yrs. Informed consent from each volunteer was obtained after explaining the study protocol. CEST imaging and z-spectrum acquisitions on the human knee were performed at 3T using an 18 cm diameter, eight-channel transmit–receive phased-array (PA) knee coil on a Siemens clinical scanner (Magnetom Tim Trio, Siemens Medical Solutions, Malvern, PA) and at 7T using the standard circularly polarized (CP) head coil on a Siemens 7T research scanner (Siemens Medical Solutions, Malvern, PA).
The actual study protocol consisted of the following steps: a localizer, WASSR, z-spectral or CEST acquisitions and B1 data collection. For WASSR acquisitions, Δω range of −1 to +1 ppm with step size of 0.05 ppm was used. For z-spectrum acquisitions, Δω range of −5 to +5 ppm with step size of 0.1 ppm was used. For CEST acquisitions, a limited Δω range required for B0 correction was used. This range was based on a quick inspection of dark regions in raw WASSR images (on scanner) at different WASSR saturation Δω. Typical Δω ranges used for CEST acquisitions were −1.7 ppm to −0.3 ppm and 0.3 ppm to 1.7 ppm with 0.1 ppm steps for a total of 30 images.
Knee imaging parameters were: slice thickness = 5 mm, flip angle = 10°, readout TR = 5.6 ms, TE = 2.7 ms, field of view = 140×140 mm2, matrix size = 192×192 with a segment size of 96. The whole sequence was repeated every 6 s at 3T and every 8 s at 7T for each Δω.
For WASSR acquisitions, a 0.2 s saturation pulse with B1rms of 0.13 µT was used in all cases. For complete z-spectral acquisitions, a 0.5 s saturation pulse with a B1rms of 2.2 µT was used with the same volunteer (n = 2) at 3T and 7T. For investigating the effects of saturation parameters, multiple CEST images were collected on two volunteers at both 3T and 7T using saturation pulses with B1rms of 18.5 Hz (0.4 µT) and 31 Hz (0.7 µT) over a duration range of 0.1–2.0 s, 62 Hz (1.4 µT) and 93 Hz (2.2 µT) over a duration range of 0.1 – 1.0 s and 124 Hz (2.9 µT) over a duration range of 0.1–0.5 s. At higher B1rms values, the signal to noise ratios of CEST images using longer duration pulses were too poor to measure reliable gagCEST.
CEST imaging was performed with 4 volunteers on both 3T and 7T using the imaging protocol as described above with a saturation duration of 0.5 s and B1rms of 2.2 µT.
Data analysis
All image processing and data analysis was performed using in-house programs written in MATLAB (version 7.5, R2007b). The cartilage section was manually segmented from the anatomical image and all data processing was performed only on this section. Acquired CEST data (at Δω = ±1.0 ppm) or z-spectral data (typically −5.0 to +5.0 ppm) were directly used to generate gagCEST maps or z-spectral asymmetry curves using Eq. [2] to get data without B0 correction. The mean and standard deviation of gagCEST or CEST asymmetry values were calculated over the small region of interest (ROI) drawn on cartilage region in the image. The gagCEST map was overlaid on one of the original anatomical images.
B0 and B1 Corrections
WASSR data acquired over the Δω range of + 1.0 to −1.0 ppm at steps of 0.05 ppm at each voxel are smoothed and interpolated using a cubic spline to generate data with a step size of 0.01 ppm. The Δω corresponding to the minimum of the interpolated data was used as the B0 value (δω) at each voxel (resolution = 0.01 ppm). Acquired CEST data (at offset frequencies, typically +0.3 to +1.7 ppm and −0.3 to −1.7 ppm) or z-spectral data (typically −5.0 to +5.0 ppm) were smoothed and interpolated using a cubic spline to generate data with a step size of 0.01 ppm. For B0 inhomogeneity correction, each voxel data value at Δω ppm was replaced by the interpolated data value from (Δω –δω) ppm. Either z-spectral asymmetry curves or CEST maps (based on the data from ±1.0 ppm) were generated using Eq. [2].
B1 field maps were obtained using a 2D single slice fast spin echo readout sequence with TE = 12 ms TR = 6 s, 128 × 128 image matrix. Two images were obtained using preparation square pulses with flip angles 30° and 60° (pulse duration = 0.3 ms). The 30° flip angle RF pulse amplitude was used as the reference B1 or B1ref. Flip angle (θ) maps were generated by solving following equation:
| [4] |
where S(θ) and S(2θ) denote voxel signals in an image with a preparation flip angle of θ and 2θ respectively. From the flip angle map, a B1 field map can be obtained using the relation, B1 = θ/(360τ). The coefficient B1/B1ref can be used if needed for B1 scaling of CEST values (21).
Simulations
Bloch McConnell equation solvers with two exchanging components (water and GAG) were written in MATLAB for analyzing the effects of CEST and DS at both 3T and 7T with the saturation pulse trains used in the experiments (14). Both the residual water magnetization affected only by DS without any CEST effects as well as the CEST asymmetry values contaminated with DS at different offset frequencies were calculated for the different saturation pulse durations and B1rms values used in the experiments.
RESULTS
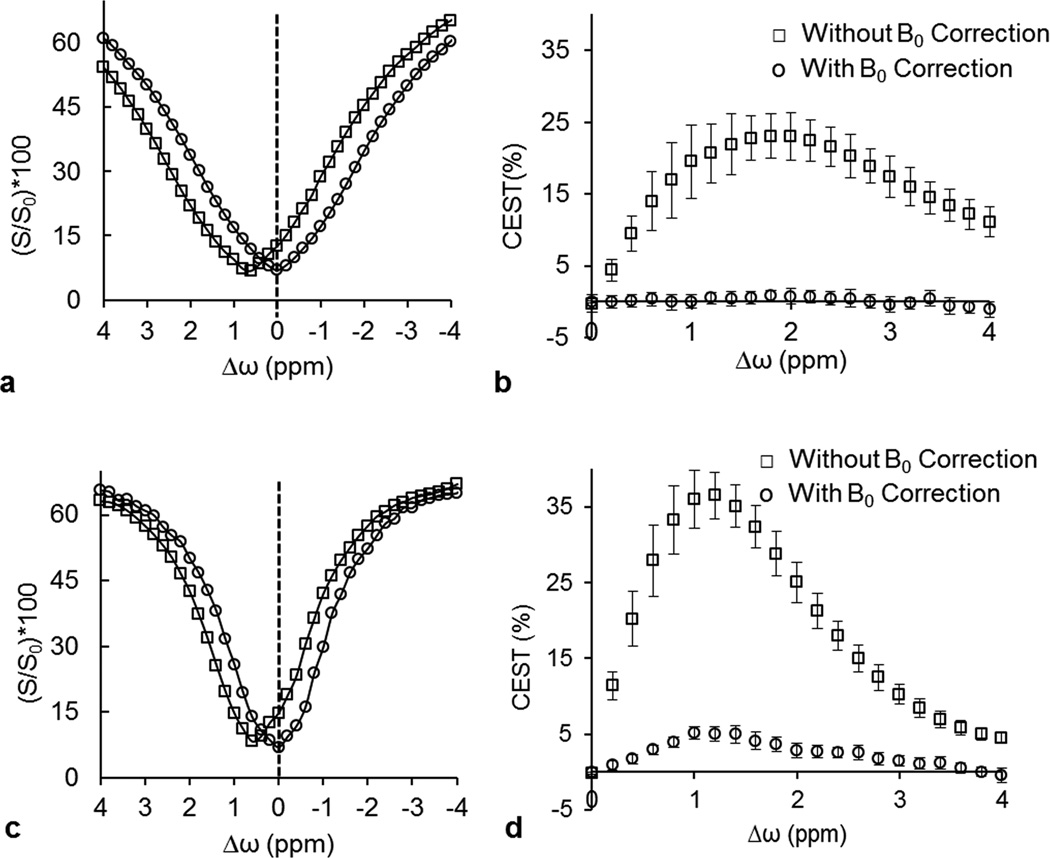
Figure 1 shows Z-spectra (a, c) and CEST asymmetry (b, d) plots of a small ROI from human knee cartilage before and after B0 inhomogeneity corrections from 3T and 7T. Without any corrections for B0 inhomogeneity, a clear shift (~0.6 ppm) in the Z-spectrum was observed in this ROI. This shift in the data is removed after correction for the B0 inhomogeneity. gagCEST calculated from the asymmetry plots generated without B0 correction show large effects (>20%) while after B0 correction the calculated gagCEST was negligible at 3T and ~6% at 7T. The error bars shown in figure 1 represent the standard deviation of the gagCEST values at each ppm over the ROI. A large number of voxels in corrected gagCEST map at 3T showed both positive and negative values at different Δω. Hence the gagCEST asymmetry derived through integration over an offset range around 1 ppm is also negligible. The effect of B1 inhomogeneity in the cartilage region was minor (<10%) in the current study at both 3T and 7T and hence no correction was necessary.
Figure 1.
Z-spectra (a, c) and CESTasym plots (b, d) of human knee cartilage without (□) and with (○) B0 inhomogeneity correction obtained at 3T and 7T respectively. Saturation pulse parameters were B1rms of 2.2 µT and duration of 0.5 s.
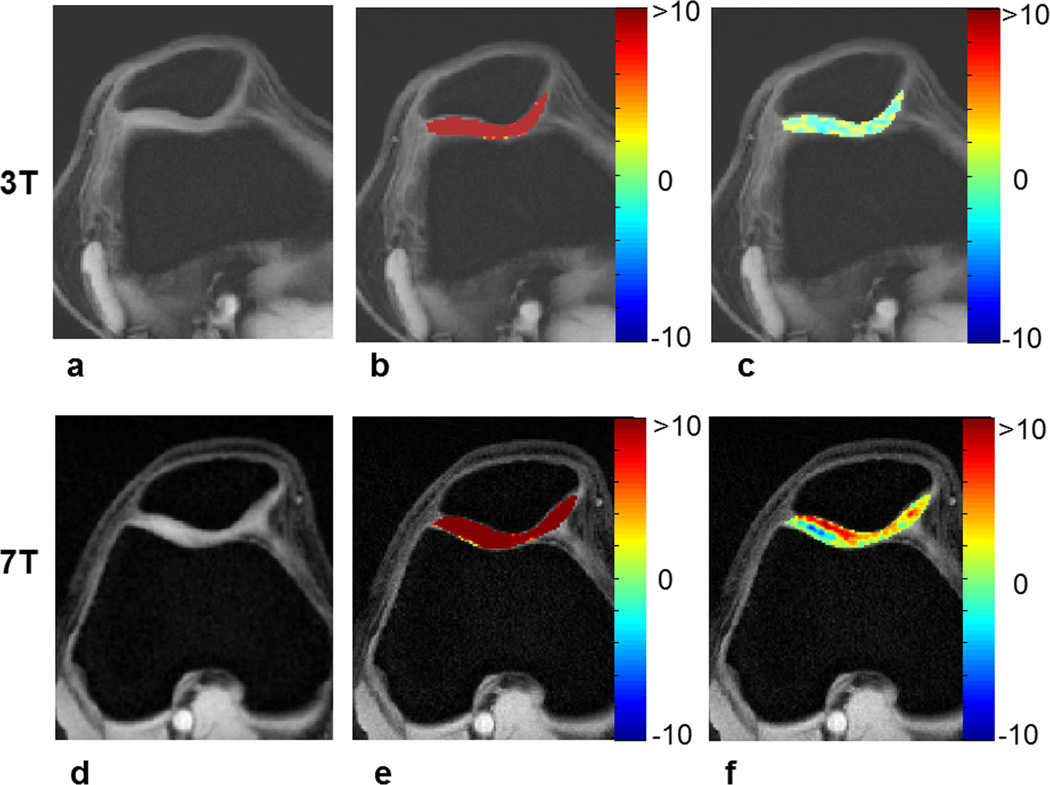
The top row of figure 2 shows a fat suppressed anatomical human knee image (a) and gagCEST maps (b, c) without and with B0 correction at 3T. Without any correction for B0 inhomogeneity, a >20% gagCEST was observed in cartilage (Fig. 2b). With B0 inhomogeneity corrections negligible gagCEST was observed. The corresponding images and maps from the same volunteer at 7T are shown in bottom row. After B0 correction ~6% gagCEST was observed.
Figure 2.
Top row contains fat suppressed anatomical image from knee cartilage (a), gagCEST maps of cartilage obtained without (b) and with correction (c) for B0 inhomogeneity at 3T. Corresponding images and maps from 7T are shown in the bottom row. Saturation pulse parameters were B1rms of 2.2 µT and duration of 0.5 s.
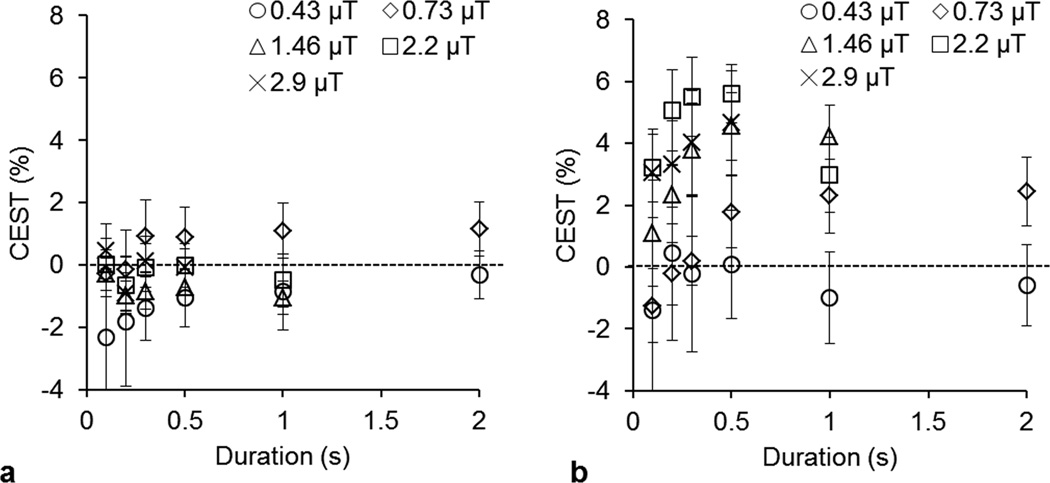
Figure 3 shows plots of knee cartilage gagCEST at varying saturation B1rms and durations obtained at both 3T (a) and 7T (b). Observed gagCEST was negligible at 3T for all B1rms and durations while at 7T the maximum gagCEST was observed at saturation B1rms of 2.2 µT and duration of 0.5 s.
Figure 3.
The saturation B1rms and duration dependence of gagCEST from human knee cartilage at 3T (a) and 7T (b). Different B1rms employed were: 0.43 µT(○), 0.73 µT(◊), 1.46 µT(Δ), 2.2 µT(□), and 2.9 µT(x).
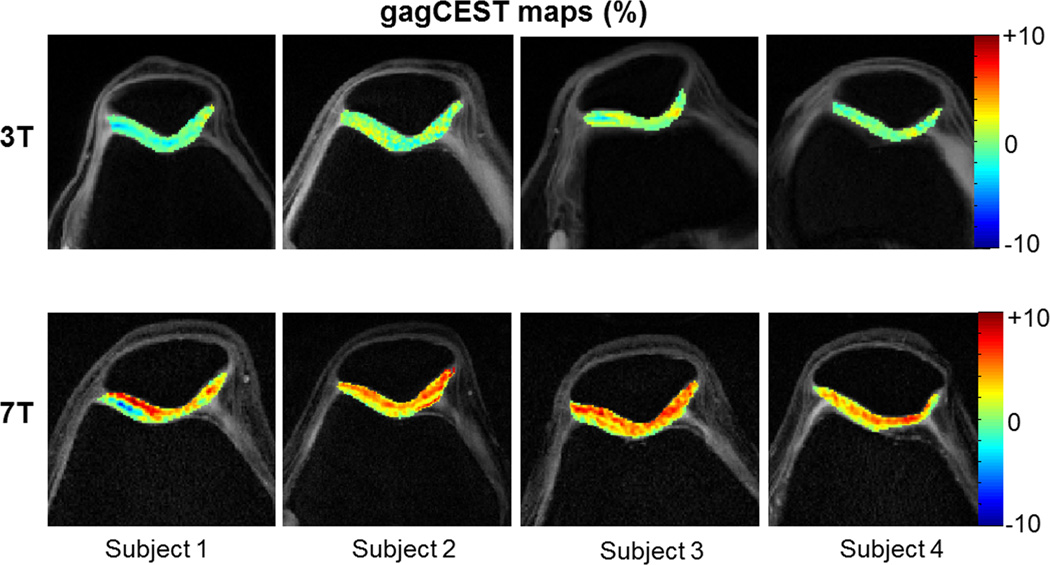
Figure 4 depicts corrected gagCEST images from the 4 healthy volunteers at 3T (a) and 7T (b). Again, the observed gagCEST at 3T is negligible while at 7T it is ~6%.
Figure 4.
Corrected knee gagCEST maps from the 4 volunteers at 3T (top row) and 7T (bottom row) for a saturation B1rms of 2.2 µT and duration of 0.5 s.
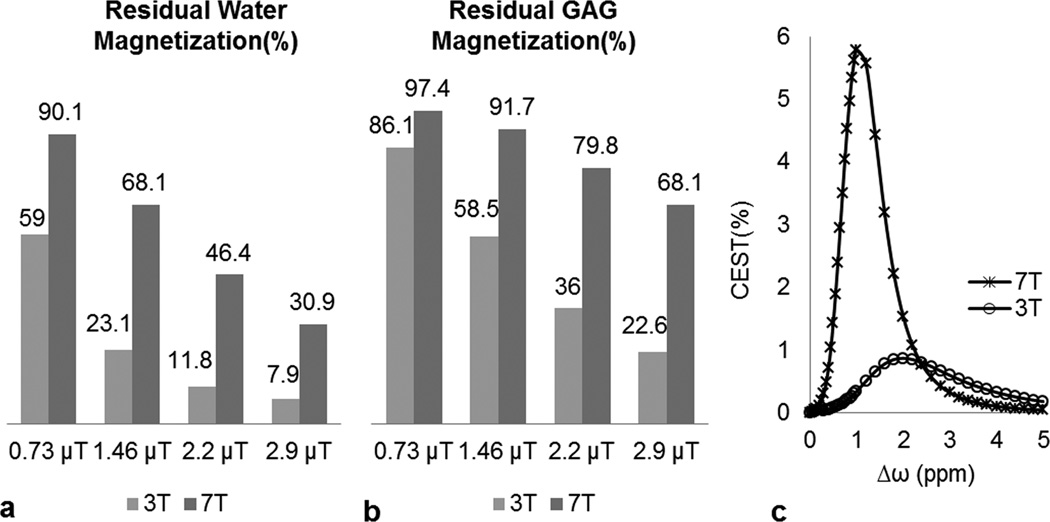
Figure 5 shows our simulation results at 3T and 7T: (a) the effect of water DS for a 0.5 s duration saturation pulse at different B1rms values. (b) The effect of DS of the GAG pool while saturating at −1 ppm for a 0.5 s duration saturation pulse at various B1rms values. The reductions in water and GAG magnetizations reduce the gagCEST sensitivity at 3T. (c) Simulated CEST asymmetry spectra for a saturation B1rms of 2.2 µT and duration of 0.5 s show that the theoretical gagCEST expected at 3T is 0.5% at 1.0 ppm while at 7T it is 5.8%. This is in line with the experimental results reported above.
Figure 5.
Simulation results. (a) Residual water magnetization (%) after a 0.5 s duration saturation pulse train with different B1rms values applied at a 1 ppm offset symmetrically around water at 3T and 7T. (b) Residual GAG magnetization (%) after a 0.5 s duration saturation pulse train with different B1rms values applied at a −1 ppm offset from water at 3T and 7T. (c) gagCEST asymmetry plot simulations at 3T and 7T for a 0.5 s duration saturation pulse train with B1rms = 2.2 µT. Other simulation parameters: for water, concentration = 88 M (80% water fraction in cartilage), T1 = 1.2 s at 3T and 1.5 s at 7T, T2 = 0.038 s at 3T and 0.032 s at 7T. For GAG, exchange rate = 1000 Hz, concentration = 0.3 M, T1 = 1 s, T2 = 0.01 s and chemical shift = 1 ppm.
DISCUSSION
Given the geometry of knee and cartilage distribution, as shown in this study, there may be substantial B0 field variations within and around the cartilage. B0 inhomogeneity leads to a shift in the z-spectra and affects the magnitude of gagCEST observed at 1.0 ppm (Fig.1). This suggests that the significant gagCEST (>20%) reported earlier at 3T (8) is mainly due to the presence of B0 field inhomogeneity in the human cartilage. After B0 corrections, calculated gagCEST values were negligible at 3T and ~6% at 7T (Fig 2 & Fig. 4).
The saturation pulse amplitude (B1) dependency of gagCEST has been evaluated in in vivo cartilage (Fig. 3). These experimental results show that gagCEST stayed negligible when B1rms was varied between 0.4 to 2.9 µT at 3T and peaks at saturation B1rms of ~2.2 µT and duration of ~0.5 s with a value of ~6%. It is interesting to note that at lower B1rms values, we seem to be getting a small negative gagCEST. This is consistent with a nuclear Overhauser effect (NOE) induced water signal loss from GAG CH protons while saturating at −1.0 ppm. At higher B1rms values, this effect is suppressed. This has also been previously shown with in vitro data (8).
As seen from our simulation results (Fig. 5), one of the main reasons for the reduced efficiency of gagCEST at 3T seems to be DS effects leading to reduced water magnetization (when saturating at (±1 ppm) as well as chemical exchange with the reduced GAG magnetization when saturating at (−1 ppm). Another potential major cause for the gagCEST efficiency difference between 3T and 7T is that the reported –OH protons exchange rate kex of 1000 s−1 (22) is in fast exchange regime at 3T (kex > Δω (~800rad s−1)) whereas it is in slow exchange regime at 7T (kex <~ Δω(~1800 rad s−1)).
It is worthwhile to report the recent gagCEST study (23) in human knee cartilage performed at 7T has shown a peak value ~3% at 1.2 ppm. Since there is not enough information about the experimental parameters used in this study, it is difficult to compare our results with this study.
In summary, numerical simulations as well experimental results demonstrate that the DS effects of water and GAG are substantial contributors for negligible gagCEST observed after B0 correction in cartilage at 3T. Since GAG loss from cartilage is expected to result in a further reduction in gagCEST, this method is not expected to lead to accurate quantification of GAG content in healthy or degenerated cartilage at 3T. Given its magnitude (~6%) gagCEST at high fields such as 7T holds promise as a clinically viable technique.
ACKNOWLEDGEMENTS
This work was performed at an NIH-NCRR supported Biomedical Technology Research Center (P41RR002305) and was supported by NIAMS grant NIH-R01AR45404.
REFERENCES
- 1.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60(2):91–97. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best Pract Res Clin Rheumatol. 2006;20(1):3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Garstang SV, Stitik TP. Osteoarthritis: epidemiology, risk factors, and pathophysiology. Am J Phys Med Rehabil. 2006;85(11 Suppl):S2–S11. doi: 10.1097/01.phm.0000245568.69434.1a. quiz S12–14. [DOI] [PubMed] [Google Scholar]
- 4.Malemud CJ. Changes in proteoglycans in osteoarthritis: biochemistry, ultrastructure and biosynthetic processing. J Rheumatol Suppl. 1991;27:60–62. [PubMed] [Google Scholar]
- 5.Reddy R, Insko EK, Noyszewski EA, Dandora R, Kneeland JB, Leigh JS. Sodium MRI of human articular cartilage in vivo. Magn Reson Med. 1998;39(5):697–701. doi: 10.1002/mrm.1910390505. [DOI] [PubMed] [Google Scholar]
- 6.Regatte RR, Akella SV, Lonner JH, Kneeland JB, Reddy R. T1rho relaxation mapping in human osteoarthritis (OA) cartilage: comparison of T1rho with T2. J Magn Reson Imaging. 2006;23(4):547–553. doi: 10.1002/jmri.20536. [DOI] [PubMed] [Google Scholar]
- 7.Tiderius CJ, Olsson LE, Leander P, Ekberg O, Dahlberg L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 2003;49(3):488–492. doi: 10.1002/mrm.10389. [DOI] [PubMed] [Google Scholar]
- 8.Ling W, Regatte RR, Navon G, Jerschow A. Assessment of glycosaminoglycan concentration in vivo by chemical exchange-dependent saturation transfer (gagCEST) Proc Natl Acad Sci U S A. 2008;105(7):2266–2270. doi: 10.1073/pnas.0707666105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun PZ, Farrar CT, Sorensen AG. Correction for artifacts induced by B-0 and B-1 field inhomogeneities in pH-Sensitive chemical exchange saturation transfer (CEST) Imaging. Magnetic Resonance in Medicine. 2007;58(6):1207–1215. doi: 10.1002/mrm.21398. [DOI] [PubMed] [Google Scholar]
- 10.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magn Reson Med. 2009;61(6):1441–1450. doi: 10.1002/mrm.21873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stancanello J, Terreno E, Castelli DD, Cabella C, Uggeri F, Aime S. Development and validation of a smoothing-splines-based correction method for improving the analysis of CEST-MR images. Contrast Media Mol I. 2008;3(4):136–149. doi: 10.1002/cmmi.240. [DOI] [PubMed] [Google Scholar]
- 12.Webb P, Spielman D, Macovski A. Inhomogeneity Correction for Invivo Spectroscopy by High-Resolution Water Referencing. Magnetic Resonance in Medicine. 1992;23(1):1–11. doi: 10.1002/mrm.1910230102. [DOI] [PubMed] [Google Scholar]
- 13.Zhou J, Payen JF, Wilson DA, Traystman RJ, van Zijl PC. Using the amide proton signals of intracellular proteins and peptides to detect pH effects in MRI. Nat Med. 2003;9(8):1085–1090. doi: 10.1038/nm907. [DOI] [PubMed] [Google Scholar]
- 14.Sherry AD, Woods M. Chemical exchange saturation transfer contrast agents for magnetic resonance imaging. Annu Rev Biomed Eng. 2008;10:391–411. doi: 10.1146/annurev.bioeng.9.060906.151929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward KM, Aletras AH, Balaban RS. A new class of contrast agents for MRI based on proton chemical exchange dependent saturation transfer (CEST) J Magn Reson. 2000;143(1):79–87. doi: 10.1006/jmre.1999.1956. [DOI] [PubMed] [Google Scholar]
- 16.Ward KM, Balaban RS. Determination of pH using water protons and chemical exchange dependent saturation transfer (CEST) Magn Reson Med. 2000;44(5):799–802. doi: 10.1002/1522-2594(200011)44:5<799::aid-mrm18>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 17.Woessner DE, Zhang S, Merritt ME, Sherry AD. Numerical solution of the Bloch equations provides insights into the optimum design of PARACEST agents for MRI. Magn Reson Med. 2005;53(4):790–799. doi: 10.1002/mrm.20408. [DOI] [PubMed] [Google Scholar]
- 18.van Zijl PC, Jones CK, Ren J, Malloy CR, Sherry AD. MRI detection of glycogen in vivo by using chemical exchange saturation transfer imaging (glycoCEST) Proc Natl Acad Sci U S A. 2007;104(11):4359–4364. doi: 10.1073/pnas.0700281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McMahon MT, Gilad AA, Zhou J, Sun PZ, Bulte JW, van Zijl PC. Quantifying exchange rates in chemical exchange saturation transfer agents using the saturation time and saturation power dependencies of the magnetization transfer effect on the magnetic resonance imaging signal (QUEST and QUESP): Ph calibration for poly-L-lysine and a starburst dendrimer. Magn Reson Med. 2006;55(4):836–847. doi: 10.1002/mrm.20818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou J, Wilson DA, Sun PZ, Klaus JA, Van Zijl PC. Quantitative description of proton exchange processes between water and endogenous and exogenous agents for WEX, CEST, and APT experiments. Magn Reson Med. 2004;51(5):945–952. doi: 10.1002/mrm.20048. [DOI] [PubMed] [Google Scholar]
- 21.Haris M, Cai K, Singh A, Hariharan H, Reddy R. In vivo mapping of brain myo-inositol. Neuroimage. 2011;54(3):2079–2085. doi: 10.1016/j.neuroimage.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hills BP, Cano C, Belton PS. Proton Nmr Relaxation Studies of Aqueous Polysaccharide Systems. Macromolecules. 1991;24(10):2944–2950. [Google Scholar]
- 23.Schmitt B, Zbyn S, Stelzeneder D, Jellus V, Paul D, Lauer L, Bachert P, Trattnig S. Cartilage Quality Assessment by Using Glycosaminoglycan Chemical Exchange Saturation Transfer and (23)Na MR Imaging at 7 T. Radiology. 2011;260(1):257–264. doi: 10.1148/radiol.11101841. [DOI] [PubMed] [Google Scholar]