Abstract
In all vertebrates sex determination is the step at which development of a testis or ovary is initiated in the bipotential gonad. Although Mus musculus and the red-eared slider turtle, Trachemys scripta, use different mechanisms to initiate organogenesis of the testis (the Y-linked gene, Sry, in the mouse vs. the incubation temperature of the egg in the turtle), the structure of the adult testis is strikingly similar. We have identified several cellular mechanisms involved in testis organogenesis in mouse. Here we investigated whether these cellular mechanisms are conserved in T. scripta downstream of the temperature-dependent switch. Cell tracing experiments indicated that the coelomic epithelium in T. scripta contributes precursors for Sertoli cells and interstitial cells as in mouse. However, we detect no male-specific mesonephric cell migration, a process required for the de novo testis cord-forming process in mouse. In contrast to mouse gonads, where no cord structure is discernible until after the divergence of testis development, we find that primitive sex cords continuous with the coelomic epithelium exist in all T. scripta gonads from the earliest bipotential stages examined. We conclude that typical testis architecture results from the maintenance and elaboration of primitive sex cords in T. scripta rather than the assembly of de novo structures as in mouse.
Keywords: Turtle, Sex determination, Gonad development, Testis, Ovary, Primordial germ cells
1. Introduction
Vertebrates use diverse mechanisms to determine sex. In mammals and birds, sex is determined genetically by the composition of the sex chromosomes. Mammals show male heterogamety (XY male and XX female) with a single gene Sry (Sex-determining Region of the Y chromosome) directing the primordial gonad to develop into a testis. In the absence of Sry, an ovary forms (Koopman et al., 1991; Lovell-Badge and Robertson, 1990). In contrast to mammals, birds have a female heterogamety system (ZZ male and ZW female) where dosage of the Z appears to be the sex-determining factor (Smith and Sinclair, 2001). However, many vertebrates do not rely on a genetic determinant. Environmental and social cues play directing roles in sex determination in many fish and reptiles. In crocodilians and most turtles, such as the red-eared slider turtle (Trachemys scripta), the incubation temperature of the egg determines the sex of the hatchlings (Wibbels et al., 1994).
Although the determinants that initiate divergent sexual development differ among vertebrates, the striking similarities in morphology and function of adult testes and ovaries suggest a conserved downstream mechanism for the formation of these organs. In support of this hypothesis, many genes known to be part of the sex-determining pathway in mammals have been found in other vertebrates. Homologues of mammalian Wt1, Sox9, Mis, Sf1, and Dmrt1 are expressed in the developing gonads of various marsupials, birds, turtles, fish, and alligators (Fleming et al., 1999; Kent et al., 1996; Moreno-Mendoza et al., 1999; Smith and Sinclair, 2001; Spotila et al., 1998; Western et al., 1999; Moreno-Mendoza et al., 2001; Torres-Maldonado et al., 2001).
Despite the fact that mouse and T. scripta have different sex-determining mechanisms, they show many similarities in gonad development. As in all vertebrates, mouse and turtle gonads arise as bipotential organs that are indistinguishable in male and female embryos. There is a critical window of time when the sex-determinant must be imposed upon the bipotential gonad to direct its fate. In mouse, expression of Sry must occur precisely between 10.5 and 11.5 dpc to initiate testis development. A delay in expression of Sry can lead to sex reversal of the XY gonad to the ovarian fate (Swain et al., 1998). In T. scripta, a temperature-dependent species, there is a window of time during which the sex of the embryo is sensitive to the sex-determination signal produced at different incubation temperatures. Incubation of eggs at 31 °C from stages 16 to 20 results in the ovarian fate. Testis fate is set when the eggs are incubated at 26 °C at similar stages (Wibbels et al., 1991). Temperature shifts or hormonal intervention during these temperature-sensitive stages leads to increased incidence of sex reversal (Crews, 1994).
The similar morphological structure in adult gonads, common gene expression profiles, bipotential status of embryonic gonads, and window of sensitivity to sex-determining signals all suggest that Mus musculus and T. scripta share common downstream mechanisms for organogenesis of the testis and ovary. However, a large volume of historical literature has described different structure for reptilian gonads at the initial bipotential stages (Allen, 1906; Vivien, 1959; Burns, 1961; Raynaud and Pieau, 1985; Wibbles et al., 1991). We were interested in analyzing whether similar or different mechanisms are employed to arrive at similar structure in adult organs. The answer to this question might provide a foundation to study the relationship of molecular signals and the processes of morphogenesis.
Recently, we began a comparative analysis of gonad development between the mouse and the turtle. In mouse, proliferation of Sertoli cell precursors is an essential process that must be initiated to direct the gonad to the male pathway (Schmahl et al., 2000). We found that proliferation of Sertoli cell precursors is also an underlying mechanism of the early size increase of male gonads in T. scripta (Schmahl et al., 2003). We have also identified two other cellular mechanisms that contribute to the organization of the testis in mouse: (1) multipotent cells from the coelomic epithelium divide, enter the gonad, and differentiate into somatic cell lineages (Karl and Capel, 1998); and (2) cells from the adjacent mesonephros migrate into the testis and contribute to endothelial and peritubular lineages (Martineau et al., 1997). In this study, we investigated whether these two mechanisms also contribute to the cellular and structural differentiation of the testis in T. scripta.
2. Materials and methods
2.1. Embryo collection and staging
Shipments of freshly laid eggs of red-eared slider turtles were obtained commercially from Kleibert Turtle Farms (Hammond, LA). Eggs were laid in a 24–48 h interval and thus the development of the embryos was generally synchronous. Eggs were incubated on moist vermiculite in a humidified incubator at either 26 or 31 °C. The progress of development was monitored by dissection of 1–2 eggs at regular intervals. Embryos were staged according to criteria established by Yntema (1968). Gonads were collected from 6–8 embryos at each specific stage of gonad development (from stage 16 to hatching) for each experiment.
2.2. Immunocytochemistry
Fresh or cultured gonads were fixed in 4% paraformaldehyde at 4 °C overnight. On the second day, gonads were processed and cut into 10 µm frozen sections as described (Karl and Capel, 1998). Sections were incubated for 1 h at room temperature in the blocking solution (10% goat serum, 0.1% Triton X-100 in PBS) followed by overnight incubation at 4 °C in primary antibodies diluted in the blocking solution. Sections were rinsed three times in PBS, and incubated in secondary antibodies diluted 1:500 in the blocking solution for 1 h at room temperature. Finally, sections were mounted in DABCO for confocal imaging using a Zeiss LSM 410 confocal microscope. The primary antibodies used were against laminin-1 (1:200, a rabbit polyclonal antibody, a gift from Harold Erickson), human Wilms’ Tumor (WT1, 1:100, a rabbit polyclonal antibody, Santa Cruz Biotechnology, Inc., C-19), and chicken VASA (1:200, a rabbit polyclonal antibody, a gift from Toshiaki Noce). The secondary antibodies were FITC- or Cy3-conjugated goat anti-rabbit antibody (1:500, Jackson Immunochemicals).
2.3. Cell tracing experiment
Turtle gonads were collected with the mesonephroi attached. Cell tracer (a mixture of 50 µM Mitotracker and 0.5 mg/ml 5-carboxytetramethylrhodamine, succinimidyl ester from Molecular Probes, Inc.) was pipetted onto the surface of the gonad as previously described (Darnell et al., 2000). Samples were washed in culture medium and then cultured on agar plates for 48 h at 31 or 26 °C (sex-specific temperatures consistent with the previous incubation temperature in ovo). Samples were fixed overnight in 4% paraformaldehyde at 4 °C and processed for immunocytochemistry.
2.4. Migration assay and organ culture
CD1 random-bred mouse strains (Charles River) and GFP transgenic mice (TgN(GFPU)5Nagy) (Hadjantonakis et al., 1998) were used for migration experiments. Genital ridges (gonad + mesonephros) from 11.25 – 11.5 dpc embryos (0.5 dpc = noon of the day when the vaginal plug was detected) were obtained. The sex of embryos was determined by detecting the presence of XX-specific Barr bodies in the amnion of individual embryos (Palmer and Burgoyne, 1991). We then separated the gonad from the mesonephros and assembled the recombinant explants as illustrated in Fig. 2. The explants were cultured at 32.5 °C with 5% CO2/95% air on a 1.5% agar block for 48 h in Dulbecco’s Minimal Eagle Medium (DMEM) without phenol red, supplemented with 10% fetal calf serum (Hyclone), 50 µg/ml ampicillin, and 25 µM HEPES. One micromole of a non-steroid aromatase inhibitor, letrazole (a gift from Allen Place) was added to the explant culture with turtle male gonads to ensure testis development at 32.5 °C. Control experiments were performed by culturing the explants with the same volume of 100% ethanol (1 µl, the solvent for letrazole). After 48 h culture, migration of GFP positive cells into the gonads was monitored using a Leica florescent stereoscope.
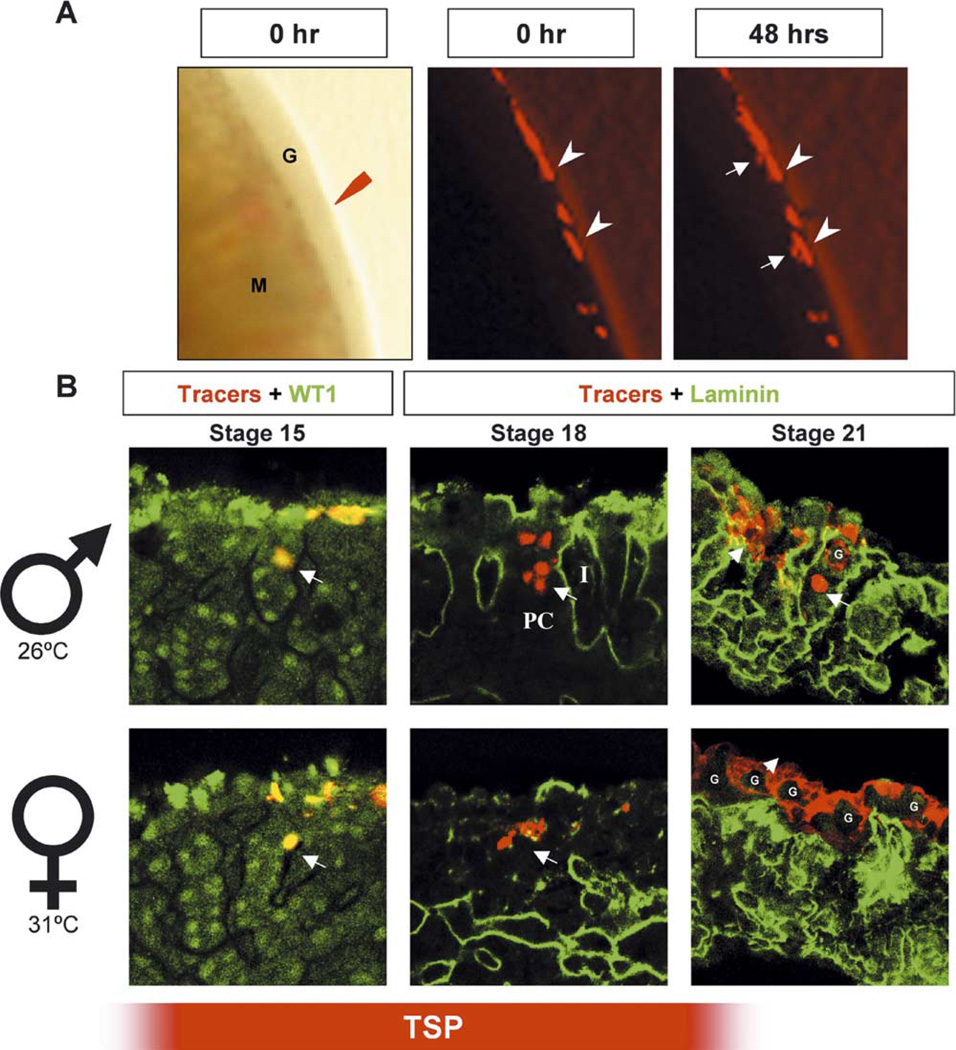
Fig. 2.
Cell tracing experiments to identify the fate of the coelomic epithelium in turtle gonad development. (A) Turtle gonads from stages 15 to 23 (G) were collected with the mesonephros (M) attached. Fluorescent cell tracers (Mitotracker plus 5-carboxytetramethylrhodamine, succinimidyl ester, red in all sections) were applied to the surface of turtle gonads and the fate of labeled cells was followed after 48 h of culture. Pictures were taken either immediately (0 h, arrows) or 48 h after the application of cell tracers (arrowheads). After 48 h of culture, movement of labeled cells was observed (arrows). (B) Male or female gonads with tracer-labeled cells in red and WT1 staining in green (stage 15 shown as a representative sample) or male gonads with tracer-labeled cells in red and laminin staining in green (stages 18 and 21 shown as representative samples). Arrows, tracer-positive cells; G, primordial germ cells; PC, primitive cord; I, interstitium. The red bar on the bottom indicates the temperature sensitive period (TSP).
3. Results
3.1. Changes in cellular organization in the T.scripta gonad
Based on the previously described protocols (Bull and Vogt, 1981), we incubated eggs at either the male- (26 °C) or female- (31 °C) producing temperature until the embryos reached desired stages. Gonads (n = 6–8) were collected from each stage between stages 16 and 23 and at hatching (Wibbels et al., 1991). To reveal the cellular organization of the gonad, we performed immunocytochemistry on whole mount samples, using antibodies against laminin to outline the basal membrane of primitive sex cords (Fig. 1, green), and VASA to mark the primordial germ cells (PGCs) (Fig. 1, red) (Tsunekawa et al., 2000).
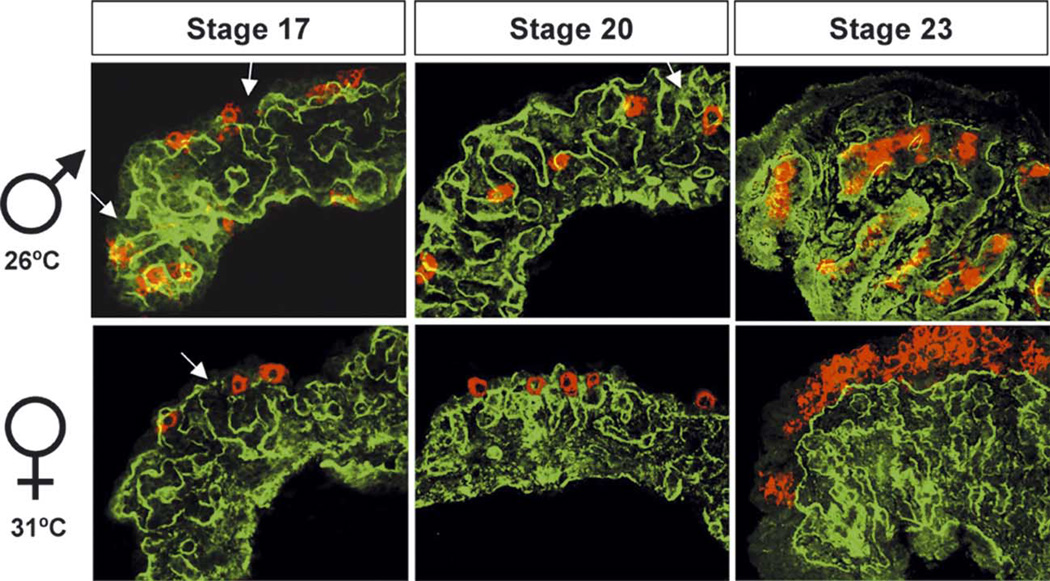
Fig. 1.
Changes in cellular organization in turtle gonad development. Male or female turtle gonads were collected from the temperature-sensitive stages (stages 16–19, only stage 17 shown as a representative sample), immediately after the temperature-sensitive stages (stages 20–21, only stage 20 is shown as a representative sample), and close to hatching (stage 22—hatching, only stage 23 is shown as a representative sample). Cellular organization was visualized by immunostaining for laminin (green) and VASA (red). Laminin outlined the basal membrane of primitive sex cords (green) and VASA marked the primordial germ cells (red). Arrows indicate the connections of primitive sex cords to the cortical region of the gonads. The temperature sensitive period is between stage 17 and 20.
Throughout the temperature-sensitive period, from stages 16 to 19, gonads from turtles incubated at both male- and female-producing temperatures were indistinguishable in structure (Fig. 1, only stage 17 is shown as a representative sample). On the surface of the gonad, VASA-positive PGCs were associated with the coelomic epithelium. PGCs were easily identified by their large nuclei surrounded by strong cytoplasmic VASA staining. Primitive sex cords outlined by laminin staining were randomly organized in the stroma of all gonads. The basal lamina of the primitive sex cords were often continuous with the basal lamina of the coelomic epithelium (Fig. 1, stage 17, arrows).
By the end of the temperature-sensitive period (Fig. 1, stage 20), in gonads incubated at the male-producing temperature, the sex cords became more elaborate, increased in size, yet remained in connection with the coelomic epithelium (Fig. 1, arrows). PGCs in male gonads were initially associated with the coelomic epithelium between stages 16 and 19, but were enclosed inside testis cords by stages 20 and 21. In contrast, at the female-producing temperature primitive sex cords decreased in size, while PGCs remained associated with the coelomic epithelium and never entered the interior of the gonad.
In male gonads from stage 22 to hatching (Fig. 1, only stage 23 is shown as representation), testis cords were well defined and surrounded by a distinct interstitium. Connections between the testis cords and coelomic epithelium were no longer present. The majority of the PGCs in male gonads were enclosed in testis cords while the cortical region diminished into a single cell layer. In contrast, at these stages there was a distinct cortex and medulla in the female gonad. The cortical region of female gonads became prominent as the primitive sex cords in the medullary region resolved to structures classically referred to as ovarian lacunae (Raynaud and Pieau, 1985) and separated from the cortical region by a continuous basal lamina. PGC numbers increased significantly in the cortical region of female gonads. No coelomic vessel (a structure characteristic of the mouse testis) was found in male or female turtle gonads at any stage of development.
3.2. Does the coelomic epithelium contribute to somatic precursor cells in T.scripta gonad development?
In the mouse, the coelomic epithelium contributes to precursors for the somatic cell lineages in both XY and XX gonads during the bipotential stage of development (Karl and Capel, 1998). To examine whether the coelomic epithelium in T. scripta gonads plays a similar role, we applied fluorescent cell tracers (Mitotracker + 5-carboxytetramethylrhodamine, succinimidyl ester) to the coelomic epithelium of turtle gonads dissected from stages 16 to 23. Labeled gonads were cultured with the mesonephros attached for 48 h (Fig. 2A). Samples were photographed either immediately (0 h, arrowheads) or 48 h after the application of cell tracers (Fig. 2A, arrows). After 48 h of culture the domain of labeled cells had visibly expanded suggesting that cells in the coelomic domain of the turtle gonad also contribute cells to the interior of the gonad (Fig. 2A, 48 h, arrows).
To characterize the behavior of individual cells in the coelomic epithelium we labeled small groups of cells and cultured for 48 h at 26 or 31 °C. After culture, these gonads were fixed and sectioned for immunohistochemistry. Some sections (stages 15–17, stage 15 is shown as a representative sample) were stained with an antibody against WT1, a DNA-binding protein expressed at variable levels in most somatic cells of early gonads of many species including turtles (Kent et al., 1995; Pelletier et al., 1991; Schmahl et al., 2003). This antibody was useful because it distinguished invaginating somatic cells from invaginating germ cells (Fig. 2B, stage 15). Labeled somatic cells were observed in the interior of the gonad at all stages during the temperature-sensitive period and the pattern was similar in both sexes. In the representative image from gonads labeled at stage 15, labeled cells appear incorporated into the primitive cords, which can be distinguished by higher levels of WT1 staining. In some cases, we double labeled samples from these experiments with an antibody against laminin, which outlined the primitive cords. At stages 17–20, cells continued to move into the interior of the gonad in male and female samples (Fig. 2B, stage 18 is shown as a representative sample). After stage 20, a sexually dimorphic pattern emerged. Representative samples from stage 21, in which a large surface area was labeled, are shown (Fig. 2B). In both male and female gonads, tracers (red) labeled the cytoplasm of PGCs and somatic cells. In male gonads, labeled PGCs (G, large round cells, arrow) were found below the coelomic epithelium after 48 h of culture. These PGCs were enclosed in testis cords as indicated by staining for laminin (green). Somatic cells labeled with tracers either remained on the surface or invaded several cell layers below the surface of the gonad (smaller, non-spherical cells, arrowhead).
In female gonads, tracer-labeled PGCs, distinguished by their large size, remained associated with the coelomic epithelium and did not become incorporated into the primitive cords. Tracer-labeled somatic cells remained clustered and associated with PGCs at the surface (Fig. 2B, Stage 21, arrowhead).
3.3. Can turtle gonads induce mesonephric cell migration in a male-specific manner?
We have demonstrated in mouse that induction of cell migration from the mesonephros into the XY gonad is a male-specific event essential for the formation of testis cords (Capel et al., 1999; Martineau et al., 1997; Tilmann and Capel, 1999). To examine whether this cellular mechanism is conserved in turtle, we designed a series of recombinant cultures as illustrated in Table 1. We used a mesonephros from a transgenic mouse with ubiquitous expression of GFP as a source of migrating cells to test the ability of turtle gonads to induce migration in culture. We first conducted several control experiments to establish a feasible experimental design. Because the culture temperatures for turtle gonads (26 or 31 °C) were lower than for mouse gonads (37 °C), we determined whether culture at a lower temperature was permissive for normal mesonephric migration in mouse gonads. We found that 32.5 °C was the lowest temperature that allowed normal mesonephric migration into mouse gonads. However at 32.5 °C, all turtle gonads followed the female fate and developed into ovaries. Since the aim of these experiments was to determine whether there is a sex-specific ability of turtle gonads to induce mesonephric migration, we needed to establish conditions for male development at 32.5 °C. The addition of an aromatase inhibitor, letrazole, has been shown to block female development and maintain male development at the female-producing temperature (Jeyasuria et al., 1994; Place et al., 2001). We found that the addition of letrazole (1 µM) to cultures at 32.5 °C maintained male development at this female-producing temperature, based on morphological analysis (data not shown). To test if letrazole affected mesonephric migration in mouse, we repeated the mouse migration assay in the presence of letrazole (Table 1, column A). Letrazole had no effect on the induction of mesonephric cell migration in mouse. These control experiments ensured that the lower temperature and the letrazole treatment did not affect mouse mesonephric migration.
Table 1.
Experimental designs to test the ability of turtle gonads to induce mesonephric cell migration
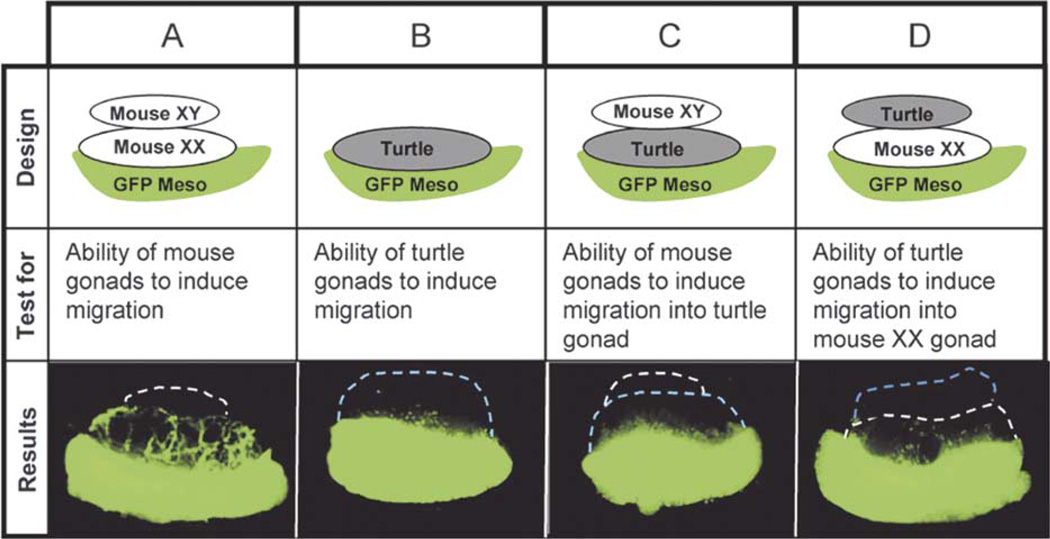 |
All tissue recombinants were cultured at 32.5 C for 48 h with or without the aromatase inhibitor Letrazole.
To test whether turtle gonads can induce migration of mouse mesonephric cells in a male-specific manner, we combined a turtle gonad with a GFP mouse mesonephros and cultured at 32.5 °C with or without letrazole. Turtle gonads consistently induced a small amount of migration in the presence (male-producing) or absence (female producing) of letrazole (Table 1, column B). These results could be due either to the fact that turtle gonads did not provide a fully compatible migration environment for mouse mesonephric cells (tissue incompatibility) or that turtle gonads did not produce a strong migration-inducing signal that could be recognized by mouse mesonephric cells. To test these possibilities, we assembled the recombinant cultures as illustrated in Table 1, columns C and D. In the first recombinant culture (Table 1, column C), we modified the experiment (Table 1, column A) by replacing the mouse XX gonad with a turtle gonad. Given that the mouse XY gonad can provide a proper signal, this experiment tested whether mouse mesonephric cells could migrate into the stroma of turtle gonads. However, the level of migration was not improved (compare Table 1, columns C with B) indicating that turtle gonads may not provide a compatible migration environment for mouse mesonephric cells.
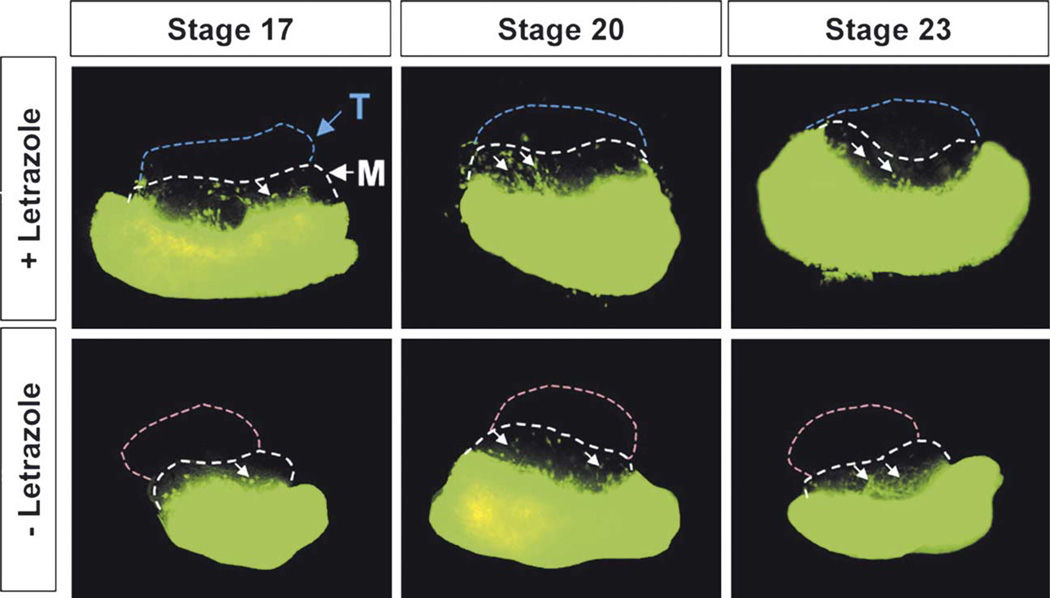
In the second recombinant culture (Table 1, column D), we replaced the mouse XY gonad in Table 1, column A with a turtle gonad. In this case, a mouse XX gonad provides a proper migration environment for mouse mesonephric cells. If turtle gonads produce a compatible signal, mesonephric cell migration should be induced. Here we found that migration was slightly improved. Turtle gonads from all stages (stages 17–23, only 17, 20, and 23 are shown as representative samples) induced mesonephric migration in the presence or absence of letrazole (Fig. 3A, arrows). However, migration was very diffuse and did not show the level or the characteristic pattern typical of mouse XY gonads (compared to Table 1, column A).
Fig. 3.
Recombinant explant culture to assay the ability of turtle gonads to induce mesonephric cell migration. As in Table 1, column D, a normal XX mouse gonad was recombined with a GFP mouse mesonephros (both tissues were collected at 11.5 dpc). A turtle gonad (from stage 16 to hatching, only stages 17, 20 and 23 are shown as representative samples) from either male- or female-producing temperature was added to the top of the XX mouse gonad followed by culture for 48 h at 32.5 °C with or without Letrazole, an aromatase inhibitor. T, turtle gonad; M, mouse gonad; arrows, migrating mouse GFP cells.
4. Discussion
In previous work we defined several cellular mechanisms in mouse testis morphogenesis. Based on the similarity of the adult testis structure between mouse and turtle, we investigated whether conserved mechanisms exist to control the formation of testis cords in both species. We first established a profile of testis cord formation in T. scripta by imaging laminin deposition in the basal lamina of testis cords, and following the movement of PGCs by VASA staining. Our immunocytochemical observations are consistent with a light microscope study reported by Wibbels et al. (1991), and with previous studies on other species where primitive sex cords have been reported at bipotential stages (for a comprehensive review of this literature see Allen, 1906; Vivien, 1959; Burns, 1961; Raynaud and Pieau, 1985). In contrast to early mouse gonads that show no cord-like structure at the bipotential stage, primitive sex cords were visible in all T. scripta gonads at the beginning of gonadogenesis and during temperature-sensitive stages. These primitive sex cords were visible throughout the gonad and their basal laminae were continuous with the basal lamina of the coelomic epithelium in some regions. After the bipotential stage, primitive sex cords disconnected from the coelomic epithelium and were maintained and expanded in the medulla of male gonads, whereas they flattened and decreased in size in female gonads.
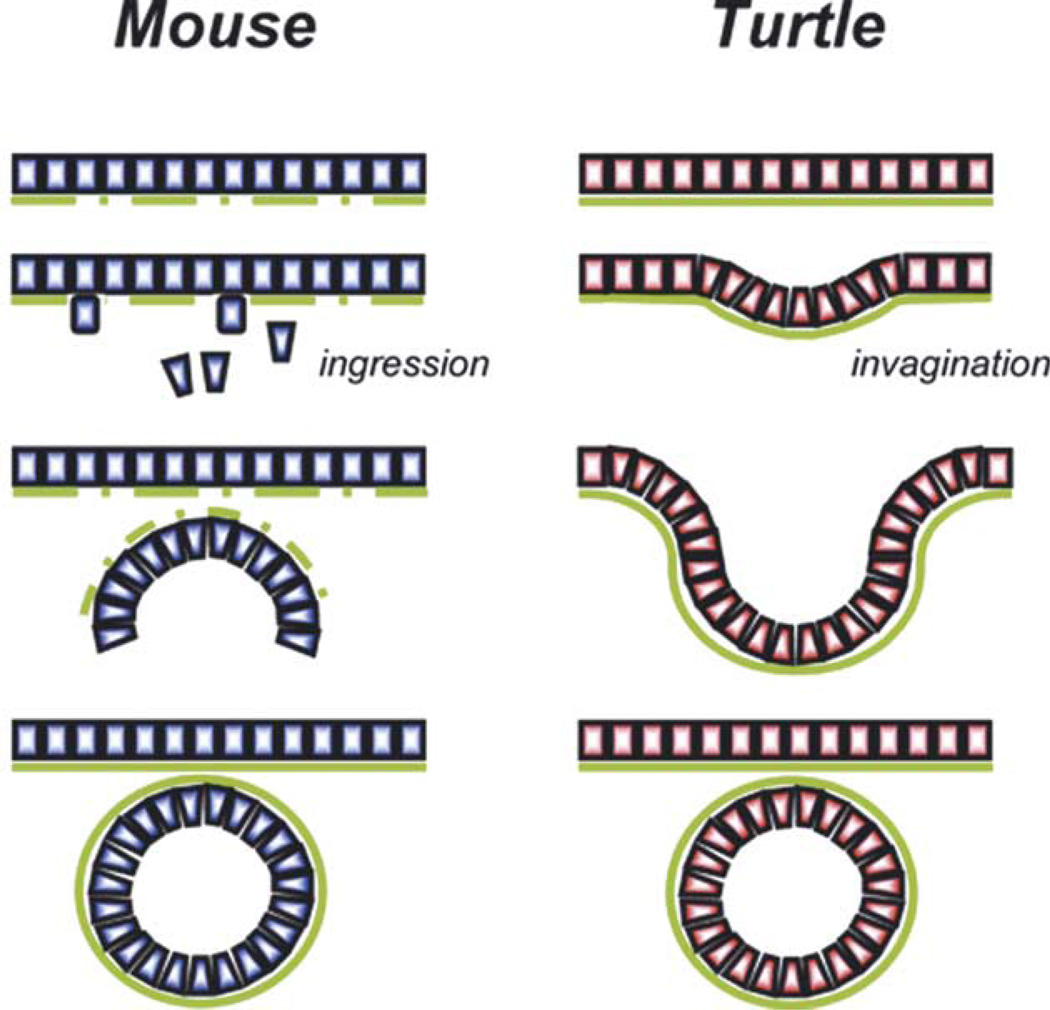
In both mouse and turtle male gonads, the coelomic epithelium gives rise to somatic cells in the primitive cords and probably to other somatic lineages outside the cords. In mouse, coelomic epithelial cells divide and individual daughter cells delaminate and move into the gonad (Karl and Capel, 1998). In T. scripta, when the epithelium is heavily labeled, marked cells tend to remain in groups associated with the original labeled populations. This pattern is similar at all stages examined and suggests an infolding of the coelomic epithelium rather than single cell ingressions. Fig. 4 illustrates two basic mechanisms of forming epithelial cords. We suggest that one is employed by mouse gonads, and the other by turtle: cords form in male mouse gonads by delamination of single cells, aggregation, and de novo assembly; whereas, in turtle gonads, primitive cords form in both sexes by invagination of the coelomic epithelium.
Fig. 4.
Model illustrating two basic mechanisms of forming epithelial cords. We suggest that testis cord formation in the mouse occurs by the mechanism at left while primitive cord formation in T. scripta occurs by the mechanism at right. Cord formation appears to occur in mouse by the de novo aggregation of individual cells that ingress into the gonad from the coelomic epithelium. In this case, the basal lamina must be reconstructed. In T. scripta, cord formation appears to occur by invagination of the coelomic epithelium, which eventually pinches off in male gonads to form freestanding cord structures. In this case, the basal lamina is continually intact.
Another striking difference between mouse and turtle gonads is the location of PGCs in bipotential gonads. PGCs in mouse are located in the interior of the gonad rather than at the coelomic surface as in T. scripta. We speculate that this difference is the consequence of different mechanisms of PGC migration. In mouse, PGCs migrate through the hindgut mesentery, traverse the mesonephros, and settle in the interior of the genital ridges between 10.5 and 11.5 dpc (Bendel-Stenzel et al., 1998). We do not know the migration path of turtle PGCs. However, the migration of PGCs in chick, another egg-laying vertebrate with similar gonadal structure to turtle, is well studied. Chick PGCs, derived from the germinal crescent area, circulate through the vascular system and extravagate from small vessels caudal to the vitelline artery. Chick PGCs then enter the neighboring thickened coelomic epithelium, which will become the definitive gonadal anlagen (Ukeshima et al., 1987; Tsunekawa et al., 2000). The close association of turtle PGCs with the coelomic epithelium suggests that their path of entry into the gonad may be very similar to chick. Although the initial location of PGCs in turtle gonads is different from that in the mouse gonad, the outcome is the same. In female turtle gonads, PGCs remain on the surface of the gonad and the primitive sex cords retract to the medulla. In contrast, as male turtle gonads develop, the primitive sex cords gradually envelop the PGCs and differentiate into testis cords. Perhaps this envelopment occurs through the connections between the epithelium and cord structures, it is not clear whether epithelial cells recruit PGCs or PGCs recruit epithelial cells into testis cords in male gonads. It will be important to approach this experiment by depleting turtle gonads of PGCs.
The formation of primitive sex cords in both sexes represents a mechanistic difference between mouse and turtle. In mouse the organization of testis cords is a male-specific process; cord structure is never seen in female gonads. Sry serves as a switch to initiate a cascade of molecular and cellular events that result in the assembly and formation of testis cord structures. One of the earliest cellular events during mouse gonadogenesis is the sex-specific cell migration from the mesonephros into the gonad. This event is downstream of Sry and has been shown to be essential for the organization of testis cords in mouse (Buehr et al., 1993; Martineau et al., 1997; Tilmann and Capel, 1999). Our efforts to test this possibility in T. scripta have resulted in only a minimal amount of mesonephric cell migration above background undermale and female producing conditions (Table 1, column D and Fig. 3A).While we cannot draw definitive conclusions from these negative experiments, these results suggest that sex-specific migration either does not occur or tissue/signal incompatibilities between the turtle and the mouse block their detection in our experiments. In support of the design of these experiments, xenografts between mouse and chick (Itasaki et al., 1996; Mitsiadis et al., 2003) suggest that tissue incompatibility between species is frequently not a problem with respect to cell mixing or molecular signals. Also consistent with the present findings, Moreno-Mendoza and colleagues cultured the gonad separated from the mesonephros during the bipotential period in the sea turtle, Lepidochelys olivacea, and showed that testis cord development was in no way dependent on the presence of the mesonephros (Moreno-Mendoza et al., 2001). Given the similar structure and development of the gonad in these two turtle species, the Moreno-Mendoza study would predict a failure to detect significant migration from the T. scripta mesonephros.
We suggest that, as a result of the fact that primitive sex cords exist in all early turtle gonads, de novo cord formation is not required during testis organogenesis. The male-producing temperature must maintain, rather than initiate, the structure of the primitive cords. Cell migration may not be required for primitive cord formation in turtles, or it may occur in both XX and XY gonads at very early stages prior to primitive cord formation.
In conclusion, T. scripta and M. musculus show similarities and differences in several cellular mechanisms of gonadogenesis. In both species the coelomic epithelium seems to be a source of somatic cells in the gonad. However, in contrast to mouse, the signaling and cellular mechanisms that initially establish cord structure are not sex-specific in T. scripta. These events must occur in both sexes at early stages when the bipotential gonads first form. Subsequent steps in the male pathway serve to maintain and elaborate testis cord structure, resulting in highly homologous cord structure between mouse and turtle by the end of the sex determination period. This represents a significant difference in strategy to achieve a very similar morphological outcome. The reason for this species difference is not yet clear, but it could be a consequence of the initial location of turtle PGCs in the coelomic epithelium rather than in the interior of the gonad as in the mouse. Several signaling pathways have now been identified in mouse (FGF9, PDGF, DHH, and WNT4) that are believed to be involved in orchestrating the cellular organization of the testis and ovary (Colvin et al., 2001; Brennan et al., 2003; Vainio et al., 1999; Yao et al., 2002). A comparative analysis of these pathways in T. scripta should provide information about how molecular signals are employed to direct the cellular organization processes that lead to organogenesis.
Acknowledgements
We thank Harold Erickson for generously providing the antibody against laminin and Allen Place for providing the aromatase inhibitor. We are particularly grateful to Toshiaki Noce and Naoki Tsunekawa for their kind gift of an antibody against VASA, which made much of this work possible. We thank Doug Coveney for his comments on the manuscript and Iordan Batchvarov, Fernando Pierucci-Alves, and Louise Reynard for their excellent technical support during the course of this work. We would also particularly like to thank Claude Pieau for kindly providing much of the classic literature cited in this manuscript. This work was supported by a grant to BC from the National Science Foundation, and a fellowship to H.H.Y. from the Lalor Foundation.
References
- Allen BM. The embryonic development of the rete-cords and sex-cords of Chrysemys. American Journal of Anatomy. 1906;5:79–94. [Google Scholar]
- Bendel-Stenzel M, Anderson R, Heasman J, Wylie C. The origin and migration of primordial germ cells in the mouse. Seminars in Cell and Developmental Biology. 1998;9:393–400. doi: 10.1006/scdb.1998.0204. [DOI] [PubMed] [Google Scholar]
- Brennan J, Tilmann C, Yao H, Capel B. Pdgfra mediates testis cord organization and fetal Leydig cell development in the XY gonad. Genes and Development. 2003;17:800–810. doi: 10.1101/gad.1052503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehr M, Gu S, McLaren A. Mesonephric contribution to testis differentiation in the fetal mouse. Development. 1993;117:273–281. doi: 10.1242/dev.117.1.273. [DOI] [PubMed] [Google Scholar]
- Bull JJ, Vogt RC. Temperature-sensitive periods of sex determination in Emydid turtles. Journal of Experimental Zoology. 1981;218:435–440. doi: 10.1002/jez.1402180315. [DOI] [PubMed] [Google Scholar]
- Burns RK. Role of hormones in the differentiation of sex. In: Young WC, editor. Sex and Internal Secretions. Vol. 1. Baltimore, MD: Williams and Wilkins; 1961. pp. 76–158. [Google Scholar]
- Capel B, Albrecht KH, Washburn LL, Eicher EM. Migration of mesonephric cells into the mammalian gonad depends on Sry. Mechanisms of Development. 1999;84:127–131. doi: 10.1016/s0925-4773(99)00047-7. [DOI] [PubMed] [Google Scholar]
- Colvin JS, Green RP, Schmahl J, Capel B, Ornitz DM. Male-to-female sex reversal in mice lacking fibroblast growth factor 9. Cell. 2001;104:875–889. doi: 10.1016/s0092-8674(01)00284-7. [DOI] [PubMed] [Google Scholar]
- Crews D. Temperature, steroids, and sex determination. Journal of Endocrinology. 1994;142:1–8. doi: 10.1677/joe.0.1420001. [DOI] [PubMed] [Google Scholar]
- Darnell DK, Garcia-Martinez V, Lopez-Sanchez C, Yuan S, Schoenwolf GC. Dynamic labeling techniques for fate mapping, testing cell commitment, and following living cells in avian embryos. Methods in Molecular Biology. 2000;135:305–321. doi: 10.1385/1-59259-685-1:305. [DOI] [PubMed] [Google Scholar]
- Fleming A, Wibbels T, Skipper JK, Crews D. Developmental expression of steroidogenic factor 1 in a turtle with temperature-dependent sex determination. General and Comparative Endocrinology. 1999;116:336–346. doi: 10.1006/gcen.1999.7360. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mechanisms of Development. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Itasaki N, Sharpe J, Morrison A, Krumlauf R. Reprogramming Hox expression in the vertebrate hindbrain: influence of paraxial mesoderm and rhombomere transposition. Neuron. 1996;16:487–500. doi: 10.1016/s0896-6273(00)80069-0. [DOI] [PubMed] [Google Scholar]
- Jeyasuria P, Roosenburg WM, Place AR. Role of P-450 aromatase in sex determination of the diamondback terrapin, Malaclemys terrapin. Journal of Experimental Zoology. 1994;270:95–111. doi: 10.1002/jez.1402700111. [DOI] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Developmental Biology. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Kent J, Coriat AM, Sharpe PT, Hastie ND, van Heyningen V. The evolution of WT1 sequence and expression pattern in the vertebrates. Oncogene. 1995;11:1781–1792. [PubMed] [Google Scholar]
- Kent J, Wheatley SC, Andrews JE, Sinclair AH, Koopman P. A male-specific role for SOX9 in vertebrate sex determination. Development. 1996;122:2813–2822. doi: 10.1242/dev.122.9.2813. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the murine primary testis determining gene, Tdy. Development. 1990;109:635–646. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- Martineau J, Nordqvist K, Tilmann C, Lovell-Badge R, Capel B. Male-specific cell migration into the developing gonad. Current Biology. 1997;7:958–968. doi: 10.1016/s0960-9822(06)00415-5. [DOI] [PubMed] [Google Scholar]
- Mitsiadis TA, Cheraud Y, Sharpe P, Fontaine-Perus J. Development of teeth in chick embryos after mouse neural crest transplantations. Proceedings of the National Academy of Sciences USA. 2003;100:6541–6545. doi: 10.1073/pnas.1137104100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Mendoza N, Harley VR, Merchant-Larios H. Differential expression of SOX9 in gonads of the sea turtle Lepidochelys olivacea at male- or female-promoting temperatures. Journal of Experimental Zoology. 1999;284:705–710. doi: 10.1002/(sici)1097-010x(19991101)284:6<705::aid-jez12>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Moreno-Mendoza N, Harley VR, Merchant-Larios H. Temperature regulates SOX9 expression in cultured gonads of Lepdochelys olivacea, a species with temperature with temperature sex. Developmental Biology. 2001;229:319–326. doi: 10.1006/dbio.2000.9952. [DOI] [PubMed] [Google Scholar]
- Palmer S, Burgoyne PS. XY follicle cells in the ovaries of XO/XY and XO/XY/XYY mosaic mice. Development. 1991;111:1017–1020. doi: 10.1242/dev.111.4.1017. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Schalling M, Buckler AJ, Rogers A, Haber DA, Housman D. Expression of the Wilms’ tumour gene WT1 in the murine urogenital system. Genes and Development. 1991;5:1345–1356. doi: 10.1101/gad.5.8.1345. [DOI] [PubMed] [Google Scholar]
- Place AR, Lang J, Gavasso S, Jeyasuria P. Expression of P450(arom) in Malaclemys terrapin and Chelydra serpentina: a tale of two sites. Journal of Experimental Zoology. 2001;290:673–690. doi: 10.1002/jez.1118. [DOI] [PubMed] [Google Scholar]
- Raynaud A, Pieau C. Embryonic development of the genital system. In: Gans C, editor. Biology of the Reptilia. Vol. 15. Wiley, New York: 1985. pp. 150–174. [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Yao HH, Pierucci-Alves F, Capel B. Colocalization of WT1 and cell proliferation revels conserved mechanisms in temperature-dependent sex determination. Genesis. 2003;35:193–201. doi: 10.1002/gene.10176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Sinclair AH. Sex determination in the chicken embryo. Journal of Experimental Zoology. 2001;290:691–699. doi: 10.1002/jez.1119. [DOI] [PubMed] [Google Scholar]
- Spotila L, Spotila J, Hall S. Sequence and expression analysis of Wt1 and Sox9 in the red-eared slider turtle, Trachemys scripta. Journal of Experimental Zoology. 1998a;281:417–427. doi: 10.1002/(sici)1097-010x(19980801)281:5<417::aid-jez7>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Swain A, Narvaez V, Burgoyne P, Camerino G, Lovell-Badge R. Dax1 antagonizes Sry action in mammalian sex determination. Nature. 1998;391:761–767. doi: 10.1038/35799. [DOI] [PubMed] [Google Scholar]
- Tilmann C, Capel B. Mesonephric cell migration induces testis cord formation and Sertoli cell differentiation in the mammalian gonad. Development. 1999;126:2883–2890. doi: 10.1242/dev.126.13.2883. [DOI] [PubMed] [Google Scholar]
- Torres-Maldonado L, Moreno-Mendoza N, Landa A, Merchant-Larios H. Timing of SOX9 downregulation and female sex determination in gonads of the sea turtle Lepidochelys olivacea. Journal of Experimental Zoology. 2001;290:498–503. doi: 10.1002/jez.1093. [DOI] [PubMed] [Google Scholar]
- Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T. Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development. 2000;127:2741–2750. doi: 10.1242/dev.127.12.2741. [DOI] [PubMed] [Google Scholar]
- Ukeshima A, Kudo M, Fujimoto T. Relationship between genital ridge formation and settlement site of primordial germ cells in chick embryos. Anatomical Record. 1987;219:311–314. doi: 10.1002/ar.1092190312. [DOI] [PubMed] [Google Scholar]
- Vainio S, Heikkila M, Kispert A, Chin N, McMahon A. Female development in mammals is regulated by Wnt-4 signaling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vivien JH. Reactivite particuliere du cortex gonadique et de l’epithelium du canal de muller a l’action des hormones sexuelles chez le jeune male d’Emys Leprosa s, taite apres l’eclosion. Archives d’Anatomie Microscopique et de Morphologie Experimentale. 1959;48:297–311. [Google Scholar]
- Western P, Harry JL, Graves J, Sinclair A. Temperature-dependent sex determination: upregulation of SOX9 expression after commitment to male development. Developmental Dynamics. 1999;214:171–177. doi: 10.1002/(SICI)1097-0177(199903)214:3<171::AID-AJA1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Wibbels T, Bull JJ, Crews D. Chronology and morphology of temperature-dependent sex determination. Journal of Experimental Zoology. 1991;260:371–381. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- Wibbels T, Bull JJ, Crews D. Temperature-dependent sex determination: a mechanistic approach. Journal of Experimental Zoology. 1994:71–78. doi: 10.1002/jez.1402600311. [DOI] [PubMed] [Google Scholar]
- Yao HH, Whoriskey W, Capel B. Desert Hedgehog/Patched 1 signaling specifies fetal Leydig cell fate in testis organogenesis. Genes and Development. 2002;16:1433–1440. doi: 10.1101/gad.981202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yntema CL. A series of stages in the embryonic development of Chelydra serpentina. Journal of Morphology. 1968;125:219–251. doi: 10.1002/jmor.1051250207. [DOI] [PubMed] [Google Scholar]