Abstract
Allogeneic hematopoietic stem cell transplantation (HSCT) is the recommended therapy for patients with relapsed AML despite little evidence showing a survival benefit for patients who undergo HSCT vs. chemotherapy alone.
Because a prospective randomized trial addressing this issue is unlikely to be conducted we retrospectively reviewed all patients given 1st salvage therapy for AML at M.D. Anderson Cancer Center from 1995–2004, focusing on patients given HSCT or chemotherapy without HSCT (a) as second salvage after 1st salvage failed to produce CR or (b) in 1st salvage-induced CR. Median survival in group (a) was 5.1 months for HSCT (n=84) vs. 2.3 months for chemotherapy (n=200, p=0.004) and, in group (b), was 11.7 months for HSCT (n=46) vs. 5.6 months for chemotherapy (n=66, p<0. 001). HSCT was associated with a survival benefit in each of 8 subgroups defined by age </≥50, high-risk cytogenetics or not, and treatment in 1st salvage-induced CR or 2nd salvage, and also in 5 of 6 subgroups defined by age </≥50 and 1st complete remission (CR1) duration (primary refractory, CR1 ≤36 weeks, CR1 >36 weeks). Our data suggest that HSCT is preferable to chemotherapy alone in these poor prognosis patients with particular benefit noted in patients <50.
Keywords: Relapsed acute myeloid leukemia, Hematopoietic stem cell transplant, Overall survival
INTRODUCTION
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with approximately 10,000 cases per year in the United States.1 While anthracycline + ara-C-containing chemotherapy is curative in some patients, approximately 80% will either relapse after achieving a 1st complete remission or have disease that is refractory to initial induction chemotherapy.2 Less than 30% of these patients will be alive 1 year from the time of relapse or failure of initial induction.3
Because of the extremely poor prognosis of patients with either relapsed or primary refractory AML, allogeneic hematopoietic stem cell transplant (HSCT) is often pursued as a curative treatment strategy. Several case series have reported survival rates of 25–40% at 2 or more years after HSCT for relapsed AML.3–5 Factors predicting favorable outcomes following HSCT include younger age, non high-risk cytogenetics, complete remission (CR) duration > 1 year, and the attainment of a second complete remission following salvage chemotherapy.3–5
These variables are also highly predictive of survival in AML patients who receive salvage chemotherapy rather than HSCT. This similarity suggests that HSCT and salvage chemotherapy are qualitatively similar, and that the favorable results reported with the former reflect patient selection bias. The optimal basis for testing this hypothesis would be a trial randomizing patients with relapsed or primary refractory AML and suitable transplant donors to receive either salvage chemotherapy or allogeneic HSCT. Because of the impracticality of this approach, we compared survival according to whether patients in prognostic subgroups defined by the above noted variables received HSCT or chemotherapy. Our analyses focus on two categories of patients: (1) those receiving HSCT or chemotherapy as second salvage therapy, i.e. after an initial (“1st salvage”) chemotherapy regimen failed to produce CR for relapsed or primary refractory AML and (2) those receiving HSCT or chemotherapy as post-remission therapy after 1st salvage chemotherapy produced CR in relapsed or primary refractory AML.
PATIENT CHARACTERISTICS
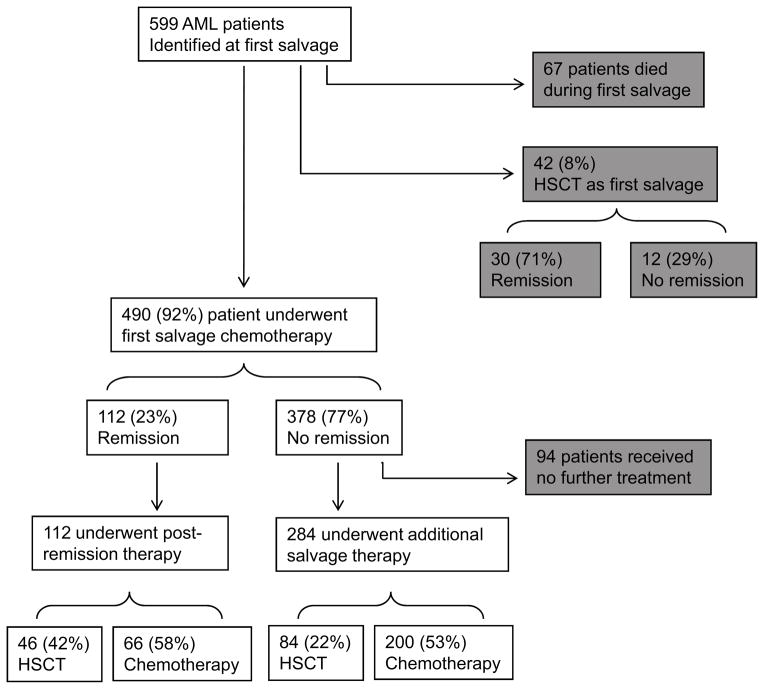
The starting point for our analyses were the 599 patients with relapsed or primary refractory AML, without inv(16), t(8;21), or t(15;17) who were treated by the Department of Leukemia at the M.D. Anderson Cancer Center between 1995 and 2004. After excluding the 67, patients who died during their 1st salvage therapy and the 42 who underwent HSCT as their 1st salvage therapy 490 patients remained. (Figure 1). Their median age was 59, and median 1st remission duration (CR1) was 22 weeks. High-risk cytogenetics (defined as -5, -7 or complex karyotype with ≥3 cytogenetic abnormalities) were present in 290 (59%). At the time of 1st salvage, median creatinine was 0.9 and median total bilirubin was 0.6 with no significant differences between patients who ultimately underwent HSCT and those who did not. The 1st salvage (S1) regimen contained high- or intermediate-dose ara-C in 383 (78%) of cases. One hundred twelve patients of the 490 patients (23%) achieved a CR with 1st salvage chemotherapy and 378 did not. This low CR rate is primarily due to the extremely poor prognosis of the group of patients being studied particularly their median CR1 being < 1 year.6 Ninety-four of the 378 received no treatment (HSCT or chemotherapy) after 1st salvage and we excluded these from subsequent analyses leaving 396 patients (112 who achieved CR, 284 who did not) in our final data set. These 396 included 46 who received HSCT in 1st-salvage induced CR, 66 who received chemotherapy in 1st-salvage induced CR, 84 who received HSCT as second salvage after failure of 1st salvage and 200 who received chemotherapy as second (and all subsequent) salvage after failure of 1st salvage. Thus 42% of the patients in 1st-salvage induced CR received an HSCT in this CR while 22% of patients in whom 1st salvage did not produce CR received HSCT as 2nd salvage. We compared survival from time of start of post-remission therapy in patients who did and did not receive HSCT and survival from start of 2nd salvage in patients who did and did not receive HSCT as second salvage.
Figure 1.
Flow chart of patient treatments. Of 599 evaluable patients, 67 died during 1st salvage chemotherapy and 42 underwent HSCT as their 1st salvage therapy (both shown in gray boxes). These groups were not used in this analysis. The remaining 490 patients were evaluable of whom 112 patients achieved CR (378 did not). After 1st salvage therapy 94 patients did not undergo further treatment (shown in gray box) and were subsequently excluded from further analysis leaving 396 patients for analysis.
Table 1 shows the conditioning regimens and stem cell sources for the 130 patients (33% of the 396) who underwent HSCT. The 67 patients transplanted between 2000–2004 were more likely to receive matched unrelated donor transplants and less likely to receive TBI. Despite these differences there was no difference in overall survival between patients receiving unrelated donor transplants vs. related donor transplants in both the 1st salvage induced CR subgroup (p=0.48) and the 2nd salvage subgroup (p=0.7). The 130 transplanted patients were a mean of 15 years younger than the 266 non-transplanted patients, were more likely to receive HSCT in CR rather than as 2nd salvage, and had on average a 10 weeks longer CR1 duration and a 6 week longer interval from 1st salvage to next therapy (HSCT or chemotherapy, table 2).
Table 1.
| Conditioning |
|
|
|---|---|---|
| 1995–1999 (N=63) | 2000–2004 (N=67) | |
|
| ||
| N (%) | N (%) | |
| Reduced intensity | 28 (44%) | 30 (45%) |
| Fludarabine / Melphalan | 10 (16%) | 14 (21%) |
| Busulfan / Fludarabine | 1 (1%) | 16 (24%) |
| Miscellaneous | 17 (27%) | 0 (0%) |
| Ablative | 35 (56%) | 37 (55%) |
| TBI Containing | 15 (24%) | 2 (3%) |
| Busulfan / Cyclophosphamide | 20 (32%) | 12 (18%) |
| Busulfan / Fludarabine | 0 (0%) | 23 (34%) |
| Stem Cell Source | N (%) | N (%) |
|
| ||
| Matched Related | 42 (67%) | 30 (45%) |
| Matched Unrelated | 13 (21%) | 31 (46%) |
| Mis-matched Related | 7 (11%) | 3 (5%) |
| Mis-matched Unrelated | 0 | 2 (3%) |
| Cord Blood | 1 (1%) | 1 (1%) |
Baseline HSCT characteristics. The conditioning regimens and stem cell sources for the 130 allogeneic transplants performed after 1st salvage (S1) are shown above for the time periods 1995–1999 and 2000–2004. Over this time frame more matched unrelated donor transplants were performed. The conditioning regimens used also changed with fewer TBI containing regimens being employed and the majority of transplants being performed with busulfan / fludarabine based conditioning.
Table 2.
| Non-HSCT (N=360) | HSCT (N=130) | P-value | ||
|---|---|---|---|---|
| Mean (sd) | Mean (sd) | |||
|
|
||||
| Age | 59.5 (14.5) | 44.4 (14.4) | <0.0001 | |
| 1st CR duration in weeks | 31.1 (39.8) | 41.4 (43.2) | 0.0004 | |
| Delay time in weeks | 15.4 (23.2) | 21.6 (36.6) | <0.0001 | |
| N (%) | N (%) | |||
|
|
||||
| Cytogenetics | Unfavorable | 224 (62.2%) | 66 (50.8%) | |
| Intermediate | 136 (37.8%) | 64 (49.2%) | 0.03 | |
| 1st salvage-induced CR | Non-CR | 293 (81.4%) | 84 (64.6%) | |
| CR | 67 (18.6%) | 46 (35.4%) | 0.0002 | |
Wilcoxon test for continuous variables or Fisher exact test for categorical variables
Patient Characteristics according to salvage treatment group. For all evaluable patients treated for relapsed AML statistically significant (P<0.05) differences in age, CR1, and time between 1st salvage and subsequent treatment, proportion of patients with unfavorable cytogenetics, and treatment performed in 1st salvage induced CR were observed between the HSCT cohort and non-HSCT cohorts. These factors were included in the subsequent multiple subgroup analyses.
STATISTICAL METHODS
Covariates were compared between the HSCT and chemotherapy groups using a Wilcoxon-Mann-Whitney test for quantitative variables7 or Fisher’s exact test8 for discrete variables. Unadjusted survival time probabilities were estimated using the method of Kaplan and Meier9 and between-group survival time comparisons were done using the log rank test.10
ANALYSIS
Initial Kaplan Meier survival analysis showed statistically longer survival in patients receiving HSCT (see below). Although multivariate analyses would typically be done to see if this difference reflected treatment (HSCT or chemotherapy) rather than a better underlying prognosis (table 2), multivariate analyses were complicated by the strong associations between treatment and the covariates shown in table 2. The strength of these associations was such that, for example, a longer time from 1st salvage to subsequent therapy (“delay time”) was essentially collinear with HSCT (p<0.001). Under these circumstances, the problem of collinearity makes multivariate regression models numerically unstable and their results unreliable. Rather than fitting such unreliable survival time regression models, instead we stratified patients into subgroups. Within each subgroup, Kaplan-Meier survival estimates were calculated and log-rank tests performed to compare overall survival (OS) between HSCT and non-HSCT groups.9,10 An overall comparison was also done, using a stratified log rank test.11
RESULTS
Unstratified survival for patients with relapsed or primary refractory AML by treatment type
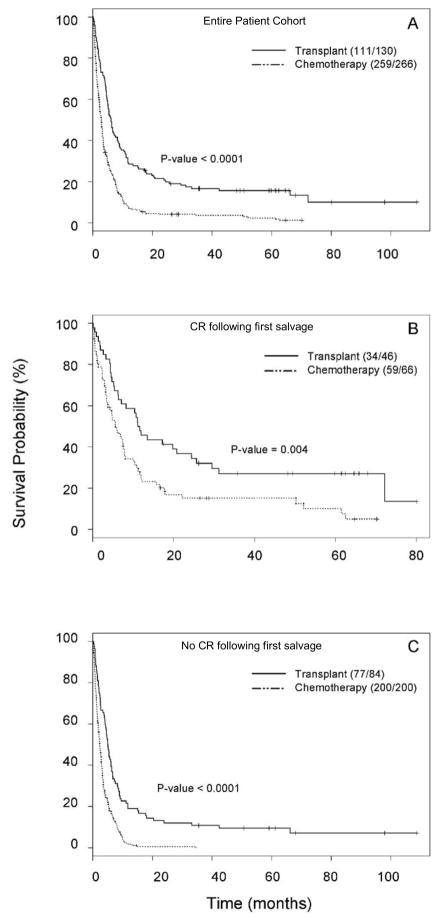
A log-rank test performed on the entire 396 patient cohort and Kaplan-Meier estimates revealed longer survival in patients who underwent HSCT (Figure 2a, p<0.0001)). This was true both in patients who had achieved a CR after 1st salvage and those who had not (Figures 2b and 2c respectively). In the CR group, median survival dated from start of post-remission therapy was 11.7 months for the HSCT group and 5.6 months for the chemotherapy group (p<0.001), and for patients receiving HSCT or chemotherapy as 2nd salvage median survival from start of second salvage was 5.1 months for the HSCT group and 2.3 months for the chemotherapy group (p=0.004).
Figure 2.
Overall survival of patient cohorts. Kaplan-Meier survival probability curves were generated to evaluate overall survival probability for the entire evaluable cohort. Figure 2A shows overall survival for the entire cohort according to treatment modality. Figure 2B shows overall survival in patients who received SCT in 1st-salvage –induced CR and figure 2C shows overall survival in patients who received SCT as 2nd salvage. In all 3 analyses a statistically significant survival advantage was observed in the HSCT cohorts.
Subgroup analysis by age, cytogenetics and 1st Salvage Induced Remission Status
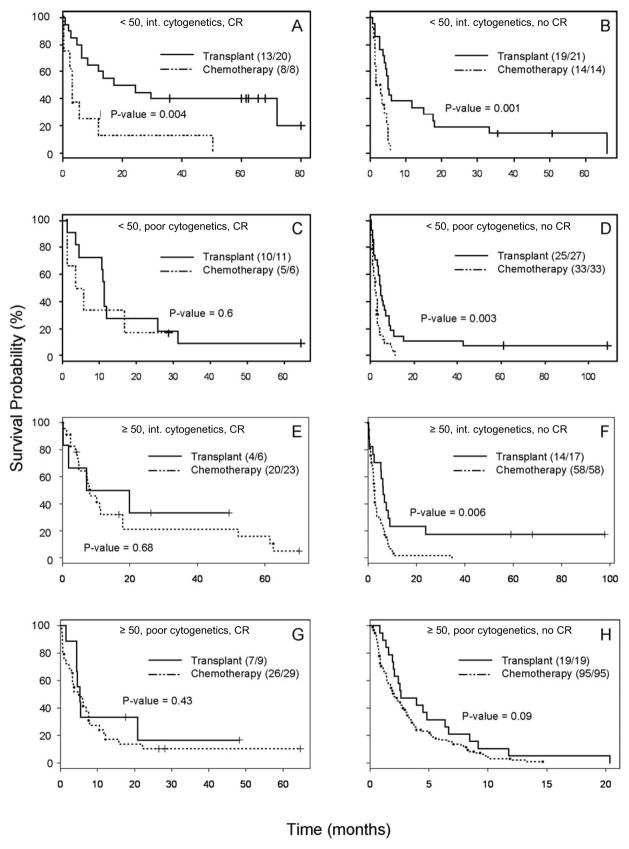
Because of the high degree of collinearity between treatment and non-treatment covariates (table 2) we used a multiple subgroup analysis strategy. Because of the limited number of patients a subgroup analysis accounting for all the covariates shown in table 2 was not feasible, producing 32 distinct subgroups. Hence initially the 396 patient cohort (figure 1) was divided into 8 subgroups based upon 3 dichotomous variables well known to be predictive of survival in these patients: age (<50 vs. ≥50), cytogenetics (intermediate vs. high-risk), and achievement of remission following 1st salvage therapy (yes vs. no). The resulting 8 Kaplan-Meier survival curves are shown in Figure 3, and their corresponding median survival times for patients who received HSCT or chemotherapy are shown in Table 3. HSCT had longer median survival in all of the 8 subgroups. These results were statistically significant (p < 0.05) in 4 of the 8 (2 of the 4 with more than the median subgroup size of 35 patients and 2 of the 4 with ≤35 patients). Application of a stratified log rank test to all 8 subgroups showed that survival in the entire HSCT treatment cohort was significantly longer compared to the entire chemotherapy alone group (p < 0.0001).
Figure 3.
First multiple subgroup analysis. Kaplan-Meier survival probability curves were generated for subgroups based upon remission status following 1st salvage, age, and cytogenetics as described in the heading for each curve. The letter heading for each curve matches the prognostic factors and survival data described in Table 3.
Table 3.
| N | Median Survival Time (95% CI) | P-value | ||
|---|---|---|---|---|
|
| ||||
| HSCT | Chemotherapy | |||
| A) Age<50, intermediate cytogenetics, 1st salvage induced CR (S1 CR) | 28 | 17.25 (8.29, NA) | 3.12 (2.36, NA) | 0.004 |
| B) Age<50, intermediate cytogenetics, no S1 CR | 35 | 5.15 (4.16, 18.03) | 2.36 (1.41, 5.15) | 0.001 |
| C) Age<50, unfavorable cytogenetics, S1 CR | 17 | 11.08 (10.43, NA) | 4.54 (1.15, NA) | 0.6 |
| D) Age<50, unfavorable cytogenetics, no S1 CR | 60 | 5.05 (3.90, 8.69) | 2.33 (1.51, 3.44) | 0.003 |
| E) Age≥50, intermediate cytogenetics, S1 CR | 29 | 13.5 (1.84, NA) | 8.1 (4.85, 17.9) | 0.68 |
| F) Age≥50, intermediate cytogenetics, no S1 CR | 75 | 6.26 (5.34, 23.87) | 2.66 (2.20, 3.48) | 0.006 |
| G) Age≥50, unfavorable cytogenetics, S1 CR | 38 | 5.21 (4.39, NA) | 4.92 (3.25, 7.9) | 0.43 |
| H) Age≥50, unfavorable cytogenetics, no S1 CR | 114 | 2.62 (2.07, 8.46) | 2.00 (1.48, 2.95) | 0.09 |
Overall survival comparison between HSCT cohort and non-HSCT cohort by multiple subgroup analyses for patients according to age, cytogenetics, and S1 CR status.
Subgroup analysis by age and CR1 Duration
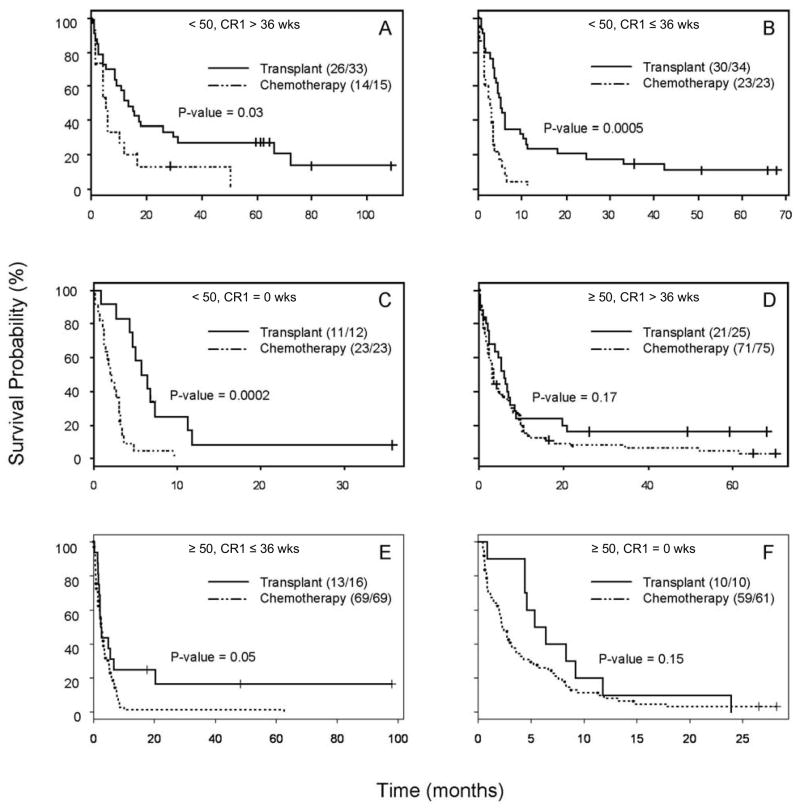
Additional analyses were performed to evaluate survival in the 6 subgroups defined by age (<50 vs. ≥50) and CR1 duration (primary refractory, CR1 duration ≤36 weeks, CR1 duration >36 weeks), with 36 weeks the overall median CR1 duration. The resulting 6 survival curves and associated overall survival data are shown in Figure 4 and Table 4 respectively. Median survival with HSCT was longer in 5 of the 6 groups, the exception being a 1-week advantage for chemotherapy in patients age ≥50 with 1st salvage-induced CR durations of < 36 weeks. The survival advantage observed in the HSCT groups were statistically significant in 3 of the 6 subgroups with non-significant survival advantages being observed in the ≥50 year old patients who were either primary refractory or had a CR1 duration of >36 weeks.
Figure 4.
Second multiple subgroup analysis. Kaplan-Meier survival probability curves were generated for subgroups based upon the known prognostic factors of age and CR1 duration as described in the heading for each curve. The letter heading for each curve matches the prognostic factors and survival data described in Table 4.
Table 4.
| N | Median Survival Time (95% CI) | P-value | ||
|---|---|---|---|---|
|
| ||||
| HSCT | Chemotherapy | |||
| A) Age<50, CR1 duration >36 weeks | 48 | 13.54 (8.69, 31.2) | 5.15 (4.0, 16.6) | 0.03 |
| B) Age<50, CR1 duration ≤36 weeks | 57 | 5.05 (4.29, 10.43) | 2.88 (1.44, 3.77) | 0.0005 |
| C) Age<50, CR1 duration =0 weeks | 35 | 5.71 (4.59, NA) | 1.84 (1.34, 3.15) | 0.0002 |
| D) Age≥50, CR1 duration >36 weeks | 100 | 6.13 (3.93, 8.98) | 3.48 (2.62, 6.0) | 0.17 |
| E) Age≥50, CR1 duration ≤36 weeks | 85 | 2.47 (1.9, NA) | 2.66 (1.97, 3.48) | 0.05 |
| F) Age≥50, CR1 duration =0 weeks | 71 | 5.85 (4.39, NA) | 2.26 (1.9, 3.57) | 0.15 |
Overall survival comparison between HSCT cohort and non-HSCT cohort by multiple subgroup analyses for patients according to age and CR1 duration.
DISCUSSION
For at least the last 30 years physicians have debated the relative merits of allogeneic HSCT vs. chemotherapy without HSCT in various scenarios (CR produced by initial AML treatment, 1st salvage, CR produced by 1st salvage, 2nd salvage, with the latter 2 scenarios examined here). The length of the debate reflects the relatively small medical (as opposed to statistical) differences between the two alternatives and the difficulty in fundamentally addressing the question through a trial randomizing patients with identified donors between HSCT and chemotherapy. As an alternative to randomization, studies have often used multivariate analyses to ask whether, after accounting for known prognostic covariates, one treatment was superior to another. However the issue of collinearity between treatment (HSCT vs. chemotherapy) and patient covariates known to strongly predict survival time made us hesitant to carry out multivariate regression analyses here, prompting us to compare HSCT and chemotherapy in each of several “comparable” sub-groups using log-rank tests and Kaplan-Meier plots within each subgroup, with comparability based on the covariates shown in table 2. These simple analyses suggest that HSCT is preferable to chemotherapy in the majority of patients about to receive 2nd salvage for active AML or in whom 1st salvage has produced a CR, including patients < and ≥ age 50, with high-risk or other cytogenetics, and with varying durations of 1st CR. It should be noted however, that the survival benefit for patient cohorts ≥50 years old did not reach statistical significance (p<0.05) in most circumstances. While the survival benefit in this older population is less clear in this study, recent reports on the efficacy of reduced intensity conditioning in the elderly may point to improved outcomes in the group, compared to the outcomes in our study cohort, half of whom underwent HSCT before 2000.12
An important question is whether the covariates in table 2 were in fact prognostic in our patients, as reported in other series. In general this appears to be the case. For example, a comparison of high-risk vs. non high-risk cytogenetics in otherwise similar groups (groups A vs. C, B vs. D, E vs. G and F vs. H in table 3) indicates that high-risk cytogenetics was strongly associated with worse survival regardless of whether patients received HSCT or chemotherapy. Patients did better when treated in 1st-salvage induced CR rather than in second salvage (Groups A vs. B, C vs. D, E vs. F, G vs. H) independent of treatment, as they did when their CR duration was greater rather than less than 36 weeks (A vs. B, D vs. E, table 4).
The time from 1st salvage therapy until next treatment (i.e. “delay time”) strongly correlated with treatment type (HSCT vs. chemotherapy), and it is possible that the longer “delay time” in the HSCT group led to a selection of patients with a generally more indolent AML. Our survival analyses minimized the effect of “delay time” by measuring survival from the time of subsequent therapy. Regardless, the clinical significance of “delay time” is likely minor when the survival outcomes for patients transplanted in CR are compared to the outcomes for all other groups (e.g. transplanted not in CR, not transplanted). As a whole, our data support the urgency in proceeding to allogeneic transplant as soon as possible to either perform a transplant while the patient is in CR or to at least perform a transplant while the patient has minimal disease burden, and toxicity from salvage chemotherapy. This approach is often complicated by unrelated donor searches, which can lengthen a time to subsequent therapy by months. Given the extremely poor outcomes for all of the non-transplant patient cohorts in our study alternative donor strategies should be investigated to reduce the time to transplant and hopefully allow an increased percentage of transplants to be performed in CR.13,14
Differences in outcome such as seen in figure 2 may of course reflect not only effects of treatment or the effects of known prognostic covariates (table 2) but also differences in unknowable or unquantifiable factors. Principal among these factors in our patients was selection bias, operating such that patients with donors might not have been transplanted because of fear that they would do poorly. This possibility is typically addressed by comparing outcomes in patients with and without donors regardless of whether the former received HSCT. The retrospective nature of our analysis did not permit a donor vs. no donor comparison. However, the fact that 33% of our patients (130/396) received an HSCT, including 42% of those in 1st salvage-induced CR and 22% as second salvage, suggests that the effect of selection bias was not overwhelming, particularly given that during the time encompassed by this study the use of unrelated donors was not as common as it is today.
Use of the M.D. Anderson database allowed us to capture the outcome of chemotherapy without HSCT in a well-defined group of patients. Thus, this study is the first to compare survival in patients with AML in 1st salvage induced CR or about to receive 2nd salvage for active AML according to whether patients received HSCT or chemotherapy without HSCT. Despite the limitations of a retrospective study noted above, our data suggest that HSCT is the preferable option in the great majority of these patients, particularly those <50 years old and judged healthy enough to receive HSCT. Indeed, the extremely poor outcomes for patients <50 years old who did not undergo transplant when considered in light of recent reports on the efficacy of cord blood transplantation would suggest that this strategy should also be considered.13,14 Nonetheless the prognosis of patients with relapsed AML remains poor, and novel investigational approaches should still be vigorously pursued in these patients.
Footnotes
AUTHORSHIP
P.M.A. performed research, compiled and analyzed data, and wrote the paper. M.d.L. analyzed data and assisted with writing. S.P. compiled patient data. W.Q. and X.W. analyzed data. P.F.T. analyzed data and assisted with writing. S.G., F.R., H.K., and R.C. assisted with analysis and writing. E.E. designed the research and assisted with data analysis and writing.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 2.Yanada M, Garcia-Manero G, Borthakur G, Ravandi F, Kantarjian H, Estey E. Potential cure of acute myeloid leukemia : analysis of 1069 consecutive patients in first complete remission. Cancer. 2007 doi: 10.1002/cncr.23112. [DOI] [PubMed] [Google Scholar]
- 3.Breems DA, Van Putten WL, Huijgens PC, et al. Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol. 2005;23:1969–1978. doi: 10.1200/JCO.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 4.Michallet M, Thomas X, Vernant JP, et al. Long-term outcome after allogeneic hematopoietic stem cell transplantation for advanced stage acute myeloblastic leukemia: a retrospective study of 379 patients reported to the Societe Francaise de Greffe de Moelle (SFGM) Bone Marrow Transplant. 2000;26:1157–1163. doi: 10.1038/sj.bmt.1702690. [DOI] [PubMed] [Google Scholar]
- 5.Wong R, Shahjahan M, Wang X, et al. Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant. 2005;11:108–114. doi: 10.1016/j.bbmt.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Estey EH. Treatment of relapsed and refractory acute myelogenous leukemia. Leukemia. 2000;14:476–479. doi: 10.1038/sj.leu.2401568. [DOI] [PubMed] [Google Scholar]
- 7.Randles R, Wolfe D. Introduction to the Theory of Nonparametric Statistics. John Wiley. 1979;1:1. [Google Scholar]
- 8.Fisher R. On the interpretation of χ2 from contingency tables, and the calculation of P. Journal of the Royal Statistical Society. 1922;85:87–94. [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimator from incomplete observations. J American Statistical Association. 1958;53:457–481. [Google Scholar]
- 10.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;60:163–170. [PubMed] [Google Scholar]
- 11.Jennison C, Turnbull B. Group Sequential Methods with Applications to Clinical Trials. Boca Raton: Chapman and Hall/CRC; 2000. [Google Scholar]
- 12.de Lima M, Anagnostopoulos A, Munsell M, et al. Nonablative versus reduced-intensity conditioning regimens in the treatment of acute myeloid leukemia and high-risk myelodysplastic syndrome: dose is relevant for long-term disease control after allogeneic hematopoietic stem cell transplantation. Blood. 2004;104:865–872. doi: 10.1182/blood-2003-11-3750. [DOI] [PubMed] [Google Scholar]
- 13.Atsuta Y, Suzuki R, Nagamura-Inoue T, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008;14:282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]