Abstract
Problem
We sought to determine whether alternative complement activation fragment Bb (Bb) levels are elevated in the maternal, fetal, and placental blood in cases of severe preeclampsia (PE) compared with normotensive controls.
Method of study
This was a cross-sectional study of women admitted at ≥24 weeks gestation with or without severe PE. Maternal plasma was collected at the time of enrollment. Umbilical venous cord and intervillous space blood were collected at delivery. Plasma Bb levels were assessed using ELISA. Bb levels were compared between cases and controls.
Results
Median Bb levels were higher in the maternal plasma of severe PE subjects (n = 24) than in controls (n = 20), 1.45 ± 1.03 versus 0.65 ± 0.23 μg/mL, P < 0.001. In umbilical venous plasma, Bb levels were higher in severe PE subjects (n = 15) compared with controls (n = 15), 2.48 ± 1.40 versus 1.01 ± 0.57 μg/mL, P = 0.01.
Conclusion
Activation fragment Bb is increased in the maternal and umbilical venous blood of cases of severe PE when compared with normotensive controls. These data provide support for alternative complement pathway involvement in the pathogenesis of severe PE and demonstrate that alternative complement activation occurs not only in the maternal but also in the fetal compartment.
Keywords: Activation fragment Bb, alternative complement pathway, severe Preeclampsia
Introduction
Preeclampsia remains one of the major causes of perinatal mortality and morbidity worldwide,1 affecting 3–5% of all pregnancies.2 To date, the only curative treatment for PE is delivery, at times necessitating the delivery of a premature fetus. Previous investigators have demonstrated that suboptimal placentation leads to subsequent hemodynamic maladaptation in the first half of pregnancy resulting in systemic vascular dysfunction and manifestation of the clinical syndrome of PE in the second half of pregnancy.3,4 Numerous potential markers of preeclampsia and systemic vascular activation have been studied including multiple factors produced by the complement system.
The complement system consists of three activation pathways: the classical, lectin, and alternative pathway. There is significant communication between these pathways, and their biological functions are mediated through the production of activation fragments that initiate as well as amplify inflammation.5–7 Complement proteins are found in circulation, body fluids, and on the surface of tissues. The role of the complement system is to defend the host against pyogenic infections, provide communication between innate and adaptive immunity, and rid the system of immune complexes and inflammatory byproducts.5
Placental tissue necrosis or ischemia with subsequent reperfusion has been associated with problems in the early development of the placenta leading to events that potentially trigger complement activation.8 Activation of the complement system along with abnormal expression of angiogenic factors has been described during early pregnancy as a factor involved in the pathogenesis of PE.4,5,7 While not all studies have been conclusive with regard to the role of complement activation in PE,9 the classical complement system has been recognized in association with preeclampsia more often than not.10 More recently, the alternative complement system has been implicated in preeclampsia pathogenesis and qualified by elevation in complement pathway activation fragment Bb (Bb).11
Complement pathway activation fragment Bb (Bb) is a 65-kDa protein that constitutes the C3bBb convertase of the alternative complement pathway and serves as a marker of alternative complement activation, primarily in the circulation.12,13 If activation of alternative complement is part of the pathogenesis of PE instigated by the placenta in response to impaired placentation, we would predict that Bb levels would be elevated in the blood of subjects with the clinical syndrome of preeclampsia. Additionally, we would expect Bb levels to be higher at the maternal–placental interface.
Building on the discovery that early pregnancy maternal plasma Bb levels are elevated in women who are more likely to develop PE,7 we tested the hypotheses that Bb levels would be elevated at the time of diagnosis of pregnancies complicated by severe PE when compared with a normotensive control group. As we also sought to explore the impact of severe PE on the fetal alternative complement system, we measured Bb levels in umbilical cord plasma and placental intervillous space blood from severe PE subjects in comparison with a normotensive group. Thus, the objective of this study was to determine whether elevated levels of Bb are seen among women with the clinical syndrome of severe PE when compared with normotensive women using maternal, umbilical cord, and placental blood.
Materials and methods
This cross-sectional study utilized maternal, cord, and placental blood collected as part of the Placental Origins of Preeclampsia study approved by the Colorado Multiple Institutional Review Board. Study participants were recruited from the labor and delivery or antepartum service at The University of Colorado Hospital following admission between ≥24 and <42 weeks gestation. Cases were women in whom the diagnosis of severe PE was confirmed. Severe PE was defined as one or more of the following: systolic blood pressure ≥160 mm Hg and/or diastolic pressure ≥110 mm Hg; proteinuria of ≥5 g in a 24-h period or ≥3 + on urine dipstick, headache, visual disturbance (scotomata), epigastric pain, pulmonary edema, oliguria, or abnormal labs consistent with HELLP syndrome characterized by hemolysis (abnormal peripheral smear or lactate dehydrogenase ≥600 IU/L), elevated liver enzymes (aspartate aminotransferase or alanine aminotransferase ≥2 × normal), and low platelet count (platelets ≤100 × 103/μL).14 Fetal findings of intrauterine growth restriction (IUGR, estimated fetal weight <10th percentile for gestational age) or oligohydramnios (maximum vertical pocket of amniotic fluid <2 cm) were also used to classify a patient as having severe PE.15,16
Control subjects were normotensive women admitted at ≥24 to <42 weeks gestation for reasons other than evaluation of hypertension and/or proteinuria. Gestational age for all subjects was determined based on last menstrual period and/or correlation with the earliest ultrasound. Women with preterm premature rupture of the membranes (PPROM), known fetal anomalies, intra-amniotic or other active infection, chronic steroid use, and intrauterine fetal demise (IUFD) were excluded from both case and control groups. Intra-amniotic infection was diagnosed by the standard criteria of 2 or more of the following: temperature ≥38°C, maternal or fetal tachycardia, or uterine tenderness.17 Chronic steroid users included patients with autoimmune diseases, asthma, or other illness requiring oral or IV corticosteroids prior to the time of labor and delivery or antepartum admission. Informed written consent was obtained from each patient. The non-participation rate was 3.6%.
Following enrollment but prior to delivery, maternal blood was drawn, and the plasma assessed for complement activation fragment Bb. Umbilical cord blood and placental intervillous space (IVS) blood were collected at the time of delivery. In some cases, we were unable to obtain adequate cord blood and/or IVS blood for study. Therefore, study numbers vary based on whether it was possible to obtain maternal blood, cord blood, and/or IVS blood. Data were gathered on maternal age, medical and obstetrical history, pre-pregnancy body mass index (BMI), delivery history and mode of delivery, demographics, blood pressure, proteinuria, laboratory results, neonatal weight, and ultrasound information related to the pregnancy.
Sample Preparation and Assay for Complement Activation Fragment Bb
Following blood draw, each venous maternal and umbilical cord blood sample was centrifuged twice within 30 min, and the supernatant was removed, aliquoted, and placed in a freezer at −80°C.11 Intervillous space blood was suctioned directly from the IVS after removal of the basal plate of the placenta and then processed and stored in the same fashion. Complement activation fragment Bb was measured at the Complement Laboratory at National Jewish Health, Denver, CO. The assay employed a quantitative sandwich enzyme-linked immunosorbent assay (ELISA) that employs a monoclonal antihuman Bb as the capture antibody and an enzyme-conjugated goat antihuman Bb as the secondary antibody (Quidel, San Diego, CA, USA). Standards with known concentrations of Bb, and high and low plasma controls were run on each ELISA plate. The coefficient of variation for the in-house laboratory control was 10.2%. The technician performing the assay was blinded to any subject information. The normal range of Bb for non-pregnant controls in the laboratory is 0.00–0.83 μg/mL.
Statistical Analysis
Prism 5 (GraphPad Software, Inc., v5.0, San Diego, CA, USA) was used for all statistical analyses. Mann–Whitney U-test was used to assess maternal, umbilical cord, and IVS plasma Bb levels between cases and controls. Spearman correlation was used to evaluate maternal versus cord or placental blood Bb levels. For all analyses, significance was set at a P value of <0.05.
Results
Overall characteristics of the study subjects are presented in Table I. There were no statistical differences between the control and severe preeclamptic groups in relation to maternal age, parity, BMI, gestational age at initial blood draw, or mode of delivery. Race/ethnicity varied somewhat between the two groups. Controls delivered at a later gestational age and had a longer duration from blood draw to delivery. In the control group, 55% (n = 11) delivered preterm (<37 weeks gestation), while 67% (n = 16) of women with severe PE delivered preterm (P < 0.01). Characteristics of the severe preeclamptic population are presented in Table II.
Table I.
Study characteristics of preeclamptic and normotensive pregnancies, based on maternal plasma
| Normotensive n = 20 |
Preeclamptic n = 24 |
|
|---|---|---|
| Maternal age (years) | 24.6 ± 5.9 | 28.0 ± 5.8 |
| Parity | ||
| Primiparous | 10 (50%) | 14 (58%) |
| Multiparous | 10 (50%) | 10 (42%) |
| Prepregnancy BMI | 24.9 ± 5.6 | 27.3 ± 7.3 |
| Race/Ethnicity | ||
| Non-Hispanic White | 9 (45%) | 9 (37%) |
| Black | 4 (20%) | 2 (8%) |
| Hispanic | 7 (35%) | 9 (37%) |
| Native American | 0 | 1(4%) |
| Asian/other | 0 | 3 (13%) |
| Gestational Age at blood draw (weeks) |
32.0 ± 5.4 | 32.8 ± 4.4 |
| Gestational Age at delivery (weeks)* |
36.0 ± 4.8 | 33.7 ± 3.9 |
| Duration blood draw to delivery (days)* |
10.7 ± 14 | 2.0 ± 2 |
| Delivered preterm (<37 weeks)* | 11 (55%) | 16 (67%) |
| Mode of delivery | ||
| Vaginal | 11(55%) | 12 (50%) |
| Cesarean | 9 (45%) | 12 (50%) |
| Labored* | 15 (75%) | 12 (50%) |
| Unlabored | 5 (25%) | 12 (50%) |
P ≤ 0.01,
all other comparisons NS.
Table II.
Characteristics of preeclamptic subjects
| n = 24 | |
|---|---|
| Blood pressure (mmHg) | |
| Systolic | 166 ± 20 |
| Diastolic | 103 ± 11 |
| Proteinuria (grams/24 hr) | 4.07 ± 3.2 |
| Symptoms | 17 (68%) |
| IUGR | 9 (36%) |
| Uncontrolled blood pressure | 5 (20%) |
| Chronic hypertension | 4 (16%) |
| Pregestational DM | 2 (8%) |
| HELLP syndrome | 2 (8%) |
| Oliguria | 1 (4%) |
| Oligohydramnios | 1 (4%) |
| Multiparous preeclamptic patients (n = 11) | |
| History of preeclampsia | 6 (55%) |
| History of eclampsia | 1 (9%) |
In accordance with our primary hypothesis, Bb levels were significantly higher in the plasma of subjects at the time of diagnosis of severe PE (n = 24) when compared with controls (n = 20), Fig. 1. As many of our severe preeclamptics were also delivered preterm, and preterm birth has also been associated with alternative complement activation,18 we evaluated maternal plasma Bb levels categorized by preterm (<37 weeks) and term (≥37 weeks) gestational age groups, Fig. 2. The greatest difference was between preterm normotensive and preterm severe preeclamptic groups. There was also a significant difference in Bb level between the control preterm (n = 11) versus term (n = 9) group but no difference between preterm and term severe preeclamptic subjects, Fig. 2.
Fig. 1.
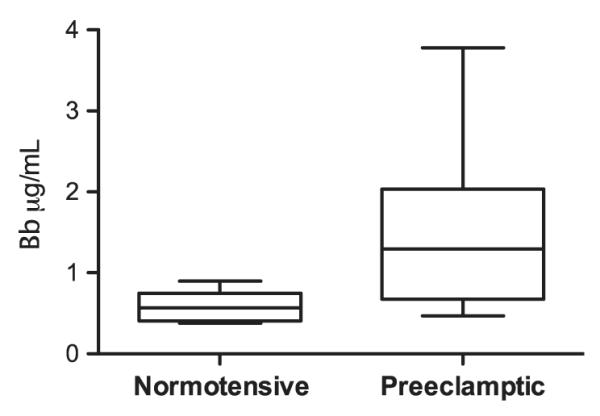
Maternal plasma Bb levels are higher in severe preeclamptic (n = 24) subjects than in normotensive controls (n = 20). Median Bb levels: 1.45 μg/mL severe preeclamptic versus 0.65 μg/mL controls, P ≤ 0.001. Data are median value, 25th and 75th percentiles (outer and inner bars), and minimum and maximum values (whiskers).
Fig. 2.
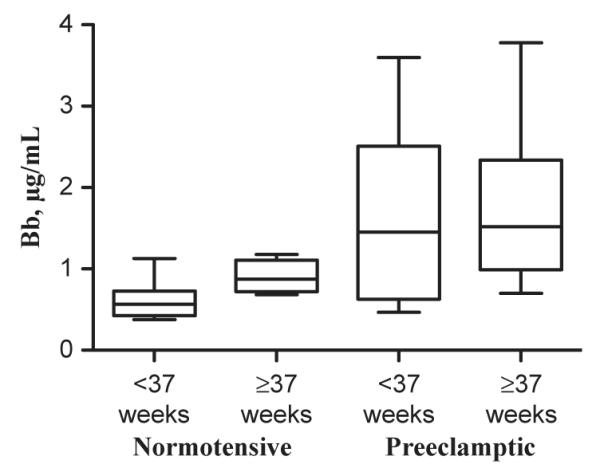
Maternal plasma Bb levels are higher in preterm (<37 weeks) and term (≥ 37 weeks) preeclamptic subjects (n = 12 preterm, n = 12 term) than controls (n = 11 preterm, n = 9 term). Median preterm normotensive Bb levels were lowest (0.57μg/mL) and significantly lower than term normotensive levels (0.88μg/mL, P = 0.02) as well as severe preeclamptic Bb levels at both gestational age groups, P = 0.0002. Data are median value, 25th and 75th percentiles (outer and inner bars), and minimum and maximum values (whiskers).
In investigating our secondary hypothesis, in which complement activation occurs in the fetal compartment, we examined venous cord blood for alternative complement activation. We found a significant difference when comparing the umbilical venous cord plasma Bb between the two groups (n = 15 control and n = 15 severe PE), Fig. 3. It is noteworthy that the median Bb level in severe preeclamptic cord blood was nearly 2.5 times that of control cord blood. Correlation analyses were then run to assess maternal versus umbilical cord Bb. There was a significant correlation between preeclamptic maternal and umbilical cord plasma Bb levels. The subject with the highest maternal plasma Bb (3.9 μg/mL, Fig. 4) was one of two subjects with HELLP syndrome. This correlation remains but is slightly less with removal of this subject. There was no correlation in the control group, Fig. 4.
Fig. 3.
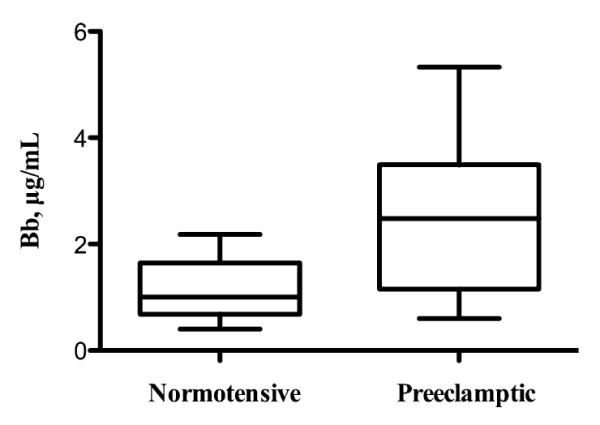
Median umbilical cord plasma Bb levels are significantly increased in severe preeclamptic cord blood (n = 15) compared to control cord blood (n = 15): 2.48 μg/mL preeclamptic versus 1.01 μg/mL control, P = 0.01. Data are median value, 25th and 75th percentiles (outer and inner bars), and minimum and maximum values (whiskers).
Fig. 4.
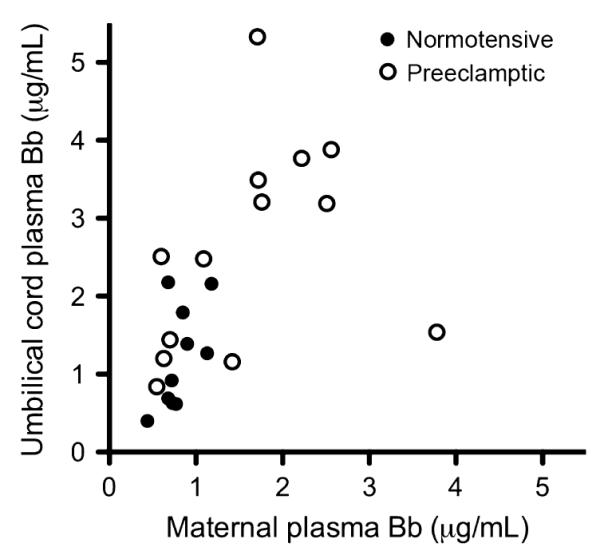
There is a correlation between maternal and umbilical cord venous plasma Bb in severe preeclamptic mothers and infants (r = 0.57, P = 0.04). There is no correlation in the normotensive control group (r = 0.44, P = 0.2).
Following preliminary analyses of our maternal and umbilical cord plasma samples and noting increased Bb in both maternal and fetal samples, we began to assess blood at the intervillous space (IVS), to determine whether the placenta was the site of alternative complement activation and therefore would be the site of greatest Bb levels in cases of severe PE versus normotensive controls. We assessed Bb levels in the IVS of the placentas of control (n = 8) versus severe preeclamptic (n = 3) subjects using ELISA. There was no difference between normotensive and severe preeclamptic plasma Bb levels at the IVS, nor was there a correlation between maternal plasma Bb and IVS plasma Bb in either group, data not shown.
Discussion
In this study, we demonstrate that alternative complement pathway activity is increased in the maternal and umbilical cord blood of women and fetuses with the clinical syndrome of severe preeclampsia at the time of diagnosis when compared with normotensive controls. Our results suggest that alternative complement activation, as evidenced by factor Bb levels, occurs both within the maternal and fetal compartments. Based on our limited IVS data, alternative complement activation may not occur centrally in the placenta, but rather occurs in the systemic circulation of both the mother and fetus. Activation of the complement system along with abnormal expression of angiogenic factors has been described during early pregnancy as a factor involved in the pathogenesis of PE.4,5,7 Whether factors produced by the placenta, such as angiogenic factors, are secreted into the maternal and fetal circulations that initiate complement activation systemically is yet to be determined. The correlation that we found between maternal and fetal plasma Bb levels in preeclamptic subjects suggests that a common process may be occurring.
Factor Bb levels were evaluated in the maternal plasma of preeclamptic subjects, healthy pregnant patients, and non-pregnant women by Derzsy et al. with no difference among the three study groups.19 In contrast to our study, their preeclamptic population was comprised predominantly of term gestations, and both mild and severe preeclamptics were included. In our study, the majority of subjects were preterm with severe preeclampsia, likely representing a more serious form of the disease. Exaggerated alternative complement pathway activation and expression of Bb may represent a difference in the pathogenesis of early severe preeclampsia versus mild term preeclampsia.
Elevation in Bb in women more likely to experience a spontaneous preterm birth or other adverse pregnancy outcome has also been reported.18,20 Overall, Bb levels in our study were lower in the preterm normotensive group than either our pre-term or term preeclamptic groups. Additionally, alternative complement pathway activation in the umbilical cord blood of fetuses of severe preeclamptic subjects was markedly higher than controls irrespective of gestational age at delivery. Our findings suggest that alternative complement levels are much higher in relation to severe preeclampsia than they may be in relation to spontaneous preterm birth.
Limitations of this study include limited umbilical cord and IVS blood from some subjects, especially in cases of prematurity. Two subjects who had maternal blood draw remote from the date of delivery developed an intra-amniotic infection. Their umbilical cord blood and IVS blood were excluded. Additionally, we had an inadequate sample size to explore the effect of demographic characteristics or mode of delivery on Bb levels. In particular, obesity in conjunction with elevated Bb levels increases the risk of PE beyond elevated Bb alone.21 This information may further elucidate specific groups of women at risk of severe preeclampsia related to abnormal alternative complement activation.
Our data support the hypothesis that alternative complement activation is increased at the time of clinical diagnosis of severe preeclampsia. Further studies will be required to determine whether incorporation of maternal plasma Bb levels in clinical cases where the diagnosis of preeclampsia is uncertain or clouded may improve our ability to diagnose this disorder. These studies also raise the question of what the impact of alternative complement system activation may be on the neonate. Determining whether alternative complement activation is part of the pathogenesis or the response to preeclampsia warrants further investigation, but our studies highlight that, in relationship to complement, PE impacts both the maternal and fetal compartments.
Acknowledgements
This work was supported in part by NIH/NCATS Colorado CTSI Grant Number UL1 TR000154. Contents are the authors’ sole responsibility and do not necessarily represent official NIH views. Additional funding was provided by a University of Colorado Denver Department of Obstetrics and Gynecology Academic Enrichment Fund Grant (Hoffman) and National Institutes of Health/NICHD R01 HD60723 (Winn).
References
- 1.McMaster MT, Zhou Y, Fisher SJ. Abnormal placentation and the syndrome of preeclampsia. Semin Nephrol. 2004;24:540–547. doi: 10.1016/s0270-9295(04)00124-x. [DOI] [PubMed] [Google Scholar]
- 2.Dekker G, Robillard PY. Pre-eclampsia: is the immune maladaptation hypothesis still standing? An epidemiological update J Reprod Immunol. 2007;76:8–16. doi: 10.1016/j.jri.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Sibai B, Dekker G, Kupferminc M. Pre-eclampsia. Lancet. 2005;365:785–799. doi: 10.1016/S0140-6736(05)17987-2. [DOI] [PubMed] [Google Scholar]
- 4.Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Walport MJ. Complement. First of two parts. N Engl J Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 6.Salmon JE, Girardi G, Holers VM. Complement activation as a mediator of antiphospholipid antibody induced pregnancy loss and thrombosis. Ann Rheum Dis. 2002;61(Suppl 2):ii46–ii50. doi: 10.1136/ard.61.suppl_2.ii46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holers VM, Girardi G, Mo L, Guthridge JM, Molina H, Pierangeli SS, Espinola R, Xiaowei LE, Mao D, Vialpando CG, Salmon JE. Complement C3 activation is required for antiphospholipid antibody-induced fetal loss. J Exp Med. 2002;195:211–220. doi: 10.1084/jem.200116116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caucheteux SM, Kanellopoulos-Langevin C, Ojcius DM. At the innate frontiers between mother and fetus: linking abortion with complement activation. Immunity. 2003;18:169–172. doi: 10.1016/s1074-7613(03)00028-1. [DOI] [PubMed] [Google Scholar]
- 9.Mellembakken JR, Hogasen K, Mollnes TE, Hack CE, Abyholm T, Videm V. Increased systemic activation of neutrophils but not complement in preeclampsia. Obstet Gynecol. 2001;97:371–374. doi: 10.1016/s0029-7844(00)01179-0. [DOI] [PubMed] [Google Scholar]
- 10.Haeger M, Unander M, Bengtsson A. Complement activation in relation to development of preeclampsia. Obstet Gynecol. 1991;78:46–49. [PubMed] [Google Scholar]
- 11.Lynch AM, Murphy JR, Byers T, Gibbs RS, Neville MC, Giclas PC, Salmon JE, Holers VM. Alternative complement pathway activation fragment Bb in early pregnancy as a predictor of preeclampsia. Am J Obstet Gynecol. 2008;198:385, e1–9. doi: 10.1016/j.ajog.2007.10.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pavlov IY, De Forest N, Delgado JC. Specificity of EIA immunoassay for complement factor Bb testing. Clin Lab. 2011;57:225–228. [PubMed] [Google Scholar]
- 13.Kolb WP, Morrow PR, Tamerius JD. Ba and Bb fragments of factor B activation: fragment production, biological activities, neoepitope expression and quantitation in clinical samples. Complement Inflamm. 1989;6:175–204. doi: 10.1159/000463093. [DOI] [PubMed] [Google Scholar]
- 14.Haddad B, Barton JR, Livingston JC, Chahine R, Sibai BM. HELLP (hemolysis, elevated liver enzymes, and low platelet count) syndrome versus severe preeclampsia: onset at < or=28.0 weeks’ gestation. Am J Obstet Gynecol. 2000;183:1475–1479. doi: 10.1067/mob.2000.106975. [DOI] [PubMed] [Google Scholar]
- 15.Sibai BM, Barton JR. Expectant management of severe preeclampsia remote from term: patient selection, treatment, and delivery indications. Am J Obstet Gynecol. 2007;196:514, e1–9. doi: 10.1016/j.ajog.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Haddad B, Sibai BM. Expectant management in pregnancies with severe pre-eclampsia. Semin Perinatol. 2009;33:143–151. doi: 10.1053/j.semperi.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 17.Newton ER, Prihoda TJ, Gibbs RS. Logistic regression analysis of risk factors for intra-amniotic infection. Obstet Gynecol. 1989;73:571–575. [PubMed] [Google Scholar]
- 18.Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, Dong Z, Chaiworapongsa T, Kim SK, Ogge G, Pacora P, Yeo L, Hassan SS. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63:318–330. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2011;47:1500–1506. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354, e1–8. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lynch AM, Murphy JR, Gibbs RS, Levine RJ, Giclas PC, Salmon JE, Holers VM. The interrelationship of complement-activation fragments and angiogenesis-related factors in early pregnancy and their association with pre-eclampsia. BJOG. 2010;117:456–462. doi: 10.1111/j.1471-0528.2009.02473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


