Abstract
Aim:
To comparatively evaluate the therapeutic efficacy of chlorhexidine (CHX) chips (Periocol-CG) and indigenous curcumin (CU) based collagen as adjuncts to scaling and root planning in the nonsurgical management of chronic periodontitis.
Materials and Methods:
A total of 120 sites from 60 patients presenting with chronic periodontitis (age group 25-55 years) of both sexes, with pocket depth of ≥5 mm with radiographic evidence of bilateral bone loss were earmarked for the study. A split mouth design was employed, and all the clinical parameters-plaque index, gingival index, probing pocket depth (PPD) and clinical attachment levels (CAL) were recorded at baseline, 1 month, 3 months, and 6 months. However, the microbiological parameters, i.e., N-benzoyl-DL-arginine-β-naphthylamide (BANA) test and microbial colony count were recorded at baseline, 3 months and 6 months postoperatively.
Results:
Significant reduction in plaque and gingival index scores were observed in both groups at the end of the study period, i.e., 6 months. The microbiological parameters (BANA test, microbial colony count), PPD and CAL levels also showed significant improvement in both groups. However, at the end of the study period CHX group showed greater improvement in all of these parameters compared to CU collagen group.
Conclusion:
Future directions of this study should include targeting the beneficial effects of these local drug delivery systems at varied concentrations so that they could be utilized to achieve the maximum beneficial therapeutic effects in the nonsurgical treatment of periodontal disease.
Keywords: Chlorhexidine gluconate, curcumin, gingivitis, local drug delivery, periodontal disease
Introduction
Periodontal disease is an infectious, inflammatory disease and its initiation and progression is significantly associated with overgrowth of certain pathogenic bacteria, liberation of bacterial toxins and inflammatory response of the host.[1] The microbial ecology of human periodontitis suggests therapies with antimicrobial agents. Highly organized bacterial populations form the apically advancing front of periodontal pockets in close proximity to connective tissue and alveolar bone destruction. Elimination or adequate suppression of putative periodontopathic microorganisms in the subgingival microbiota is essential for periodontal healing. Antimicrobial treatment in periodontics ranges from mechanical debridement of tooth surfaces and home plaque removal, to local and systemic delivery of antimicrobial agents.
Various investigators have advocated the use of antimicrobials, initially systemically and more recently topically for the management of chronic periodontitis.[2] With the recognition that antimicrobial agents could be useful as adjuncts in treating periodontal disease, investigators have sought to develop new ways of using these agents. One such approach is a local delivery of antimicrobial agents directly to the periodontal pocket. A local route of drug delivery (LDD) can attain 100 fold higher concentration of antimicrobial agent in sub-gingival sites compared with a systemic drug regimen.[3] LDD may employ antimicrobial agents not suitable for systemic administration, such as various broad spectrum antiseptic agents. In addition, local antibiotic placement also reduces the risk of drug resistant microbial populations in other sites.[4]
Curcumin (CU), a yellow pigment from Curcuma longa, is a major component of turmeric and is commonly used as a spice and food-coloring agent. It is also used as a cosmetic and in some medical preparations. The desirable preventive or putative therapeutic properties of CU have also been considered to be associated with its antioxidant,[5] anti-inflammatory,[6] antimicrobial[7] and chemopreventive[8] properties. The anti-inflammatory effect of CU is most likely mediated through its ability to inhibit cyclooxygenase-2 (COX-2), lipoxygenase (LOX), and inducible nitric oxide synthase (iNOS). COX-2, LOX, and iNOS are important enzymes that mediate inflammatory processes.[5] CU works to inhibit the activity and synthesis of the enzymes implicated in inflammation, such as, COX-2 and 5-LOX. Its anti-inflammatory action may also be attributed to inhibition of pro-inflammatory leukotrienes, postraglandins, and arachidonic acid, as well as to its neutrophil function during inflammatory states.[6]
On the other hand, chlorhexidine (CHX) gluconate is an effective bactericidal agent and broad spectrum antimicrobial drug. It has been extensively researched and is the gold standard antimicrobial drug in maintain periodontal health. CHX is safe and has an inherent advantage over antibiotics by not producing resistant microorganisms. Furthermore, it destroys all categories of microbes, not just bacteria, and there is little risk of development of opportunistic infections.
With the recent advent of newer drugs and local drug delivery systems, the aim of the present study was formulated to compare the efficacy of an indigenously developed local drug delivery module, i.e., CU based collagen and compare it with the gold standard in nonsurgical periodontal therapeutics - namely CHX, in the form of a chip (Periocol CG).
Materials and Methods
The patients who participated in the study were selected from the outpatient pool of Department of periodontics, including both males and females in the age group of 25-50 years after obtaining ethical clearance from the institutional review board. Patients with chronic periodontitis with probing pocket depth (PPD) ≥5 mm, in contralateral sites with radiographic evidence of bone loss, free from any systemic disease, and who have not undergone any form of nonsurgical or surgical periodontal therapy in the last 6 months were considered as eligible for the study. Presence of overhanging restorations, antibiotics or any form of periodontal therapy in the previous 6 months, smoking habit, history of systemic disease, or cardiovascular diseases, which requires antibiotic prophylaxis, known allergy to CHX, pregnant and lactating women were excluded from the study.
The nature and design of the clinical trial was explained to patients, and consent was obtained for their participation in the prescribed proforma. The study used a split mouth design, wherein, two sites in the contralateral quadrants, which required periodontal treatment with PPDs ≥5 mm at baseline were chosen. A total of 120 sites from 60 patients were selected for the study. The study sites were randomly assigned to either CHX or CU groups using fair coin tossing method and subjected to double-blinded evaluation.
Impressions of the upper and lower arches were made using alginate impression material and casts were poured in dental stone. Acrylic stents were made with cold cure acrylic resin on each patient cast to fit over the occlusal one third of the teeth selected for the study. A groove was cut in the acrylic stent and hence that the probe could be inserted at a standardized point of entry into the pocket at recall visits. The pocket depth was measured using a pressure sensitive probe (Aesculap, Braun probe). The selected sites were grouped as CHX group (the sites who receive scaling and root planning along with CHX chip) and CU group (the sites who receive scaling and root planning along with CU in collagen).
Preparation of curcumin collagen sponge
Type I collagen was extracted from bovine archilles tendon using the procedure reported earlier, i.e., the clean archilles tendons were minced below 25°C and washed with cold distilled water.[9] The minced tissue was subjected to subsequent chemical and enzyme treatment and pure collagen was extracted. 1% w/v aqueous solution of pure type I collagen (in 0.1% 12 N HCL, pH 2.5) was prepared and agitated with 0.1% vol/vol nonionic wetting agent. The frothy mass was poured into Teflon trays and dried.[10] CU extract was re-dissolved in methanol and incorporated into the collagen sponge at the concentration of 50 mg/cm2. The sponge was allowed to dry in laminar airflow chamber, after which it was ready to use.[9] The CU collagen sponge undergoes faster resorption by enzymatic degradation in cases of exposition and resorption time varies between 8 and 12 weeks.
Before placement of LDD agents (CHX chips and CU collagen sponge) into the sulcus, the pockets were isolated, and surrounding areas dried up. The chips were grasped using forceps with the rounded edges away from the forceps and inserted into the periodontal pocket to its maximum depth until resistance has been felt. After the placement of the drugs in the sulcus, the treated sites were given a periodontal dressing to isolate the area and restrict the effect of drugs to the particular sites for at least a week. The patients were then advised not to use any chemical plaque control methods other than normal brushing and rinsing and not to floss in the test sites and adjacent interproximal areas to prevent dislodgement of the LDD agents.
The clinical parameters - plaque index (Silness and Loe, 1964), gingival index (Loe and Silness, 1963), PPD, and clinical attachment level (CAL) of the selected target site were recorded prior to the placement of the drug-at baseline, 1 month, 3 months and 6 months postoperatively. At all recall visits, only supra-gingival scaling was done that too, if necessary.
Microbiological analysis
The microbiological parameters, i.e., N-benzoyl-DL-arginine-β-naphthylamide (BANA) test and microbial colony count were performed at baseline, 3 and 6 months. The plaque samples were taken from the selected sites for bacteriological analysis. Subgingival plaque was analyzed for periodontopathic anerobic microorganisms from the samples collected from the selected sites by using BANA reagent strips. (BANA Met LLC, AnnArbor, Michigan, USA). After sampling the desired site, the upper portion of the matrix strip with the reagent was moistened with distilled water using an autoclaved sterile cotton pellet. The reagent strip was then folded and placed in an incubator for 15 min at 55°C.[11] The periodontopathic microorganisms capable of hydrolyzing the BANA reagent are Treponema denticola, Porphyromonas gingivalis, Tannerella forsythia and certain strains of Capnocytophaga species. These organisms are capable of producing trypsin like enzyme in significant amounts, which is responsible for the BANA reaction.[12]
For microbial colony count the sub-gingival plaque samples were collected from the sample site using gracey curettes and dispended into a sterile disposable plastic tube containing 5 ml of 0.9% saline. Mueller Hinton Agar plates were used for the microbial culturing. A 2 mm diameter loop was used for the smear preparation onto the glass plates. The loop was heated red hot prior to usage, and a loop full of the plaque sample with saline was smeared onto the agar. The periphery of the glass plate was avoided to prevent contamination with the water of condensation. The plates were then incubated at 37°C for 24 h, after which the numbers of colonies of microorganisms formed were counted.
Results
The results of the BANA test [Figure 1] showed a significant shift in the BANA positive sites to negative sites in both the groups by the end of the study period. At 3 months interval, both CHX and CU groups showed equal number of BANA positive sites (3.3%). However, at the 6 months interval CU group showed more number (10.0%) of BANA positive sites when compared with the CHX (0%) group. This shows that the number of BANA positive sites remained stable till the 3 months study period in both the CHX and CU groups, whereas there is a slight increase in the number of BANA positive sites in CU group by the end of the study period.
Figure 1.
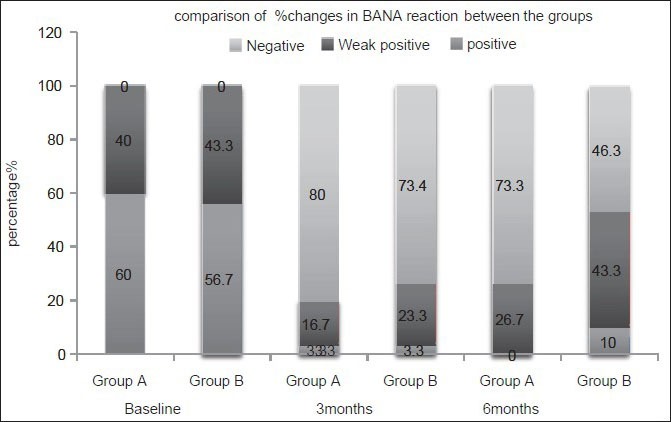
Analysis and comparison of the percentage changes in N-benzoyl-DL-arginine-b-naphthylamide test between the groups at different time points
In comparison, the mean difference in colony count in CHX group between baseline and 3 months was 27.2667 ± 8.545, which was statistically significant (P < 0.001). The mean difference in colony count between 3 months and 6 months was. 2667 ± 3.6477, which was not statistically significant (P = 0.692). In CU group, the mean difference in colony count between baseline and 3 months was 31.2 ± 8.4053, which was statistically significant (P < 0.001). The mean difference in colony count recurrence between 3 months and 6 months was 4.8333 ± 4.323 mm, which was also statistically significant (P < 0.001) [Figure 2].
Figure 2.
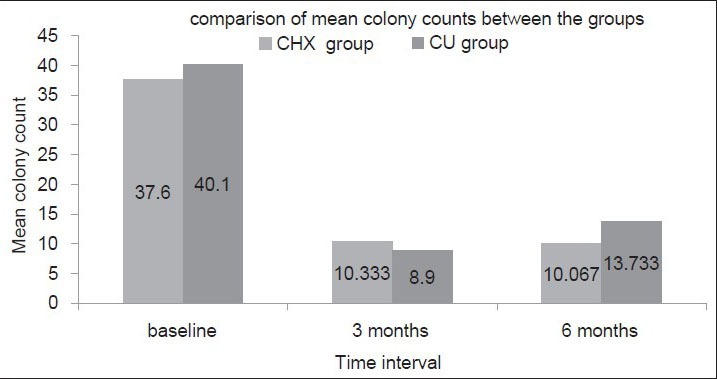
Analysis of the mean score colony count between the groups at different time intervals
At 3 months interval, the mean colony count was 10.333 ± 4.420 and 8.9 ± 4.02 for CHX and CU groups respectively, which was not statistically significant (P = 0.194). At 6 months, the mean colony count was 10.067 ± 5.711 and 13.733 ± 5.278 for CHX and CU groups respectively, which was statistically significant (P = 0.012) showing a positive response to CHX group.
In both CHX and CU groups, the various clinical parameters were assessed at baseline, 1 month, 3 months and 6 months, whereas the microbiological parameters were assessed at baseline, 3 months and 6 months after treatment.
In CHX group, the mean difference in the PPD scores between baseline and 1 month, 1 and 3 months, 3 and 6 months are 2.133 ± 0.730 mm, 0.767 ± 0.568 and 0.467 ± 0.571 mm respectively which were highly statistically significant (P < 0.001). In CU group, the mean difference in the PPD scores between baseline and 1 month, 1 and 3 months are 2.267 ± 0.828 mm and 0.661 ± 0.711, respectively which were highly statistically significant (P < 0.001). However, when the mean difference in PPD scores between 3 months and 6 months were compared. There was a slight increase in the scores in CU group [Figure 3].
Figure 3.
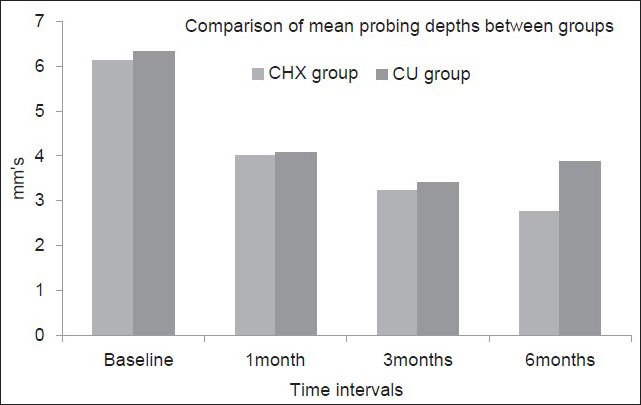
Comparison of the mean probing pocket depth scores between the groups at different time intervals
The mean difference of CAL scores between baseline and 1 month, 1 and 3 months, and 3 and 6 months were 2.167 ± 0.699 mm, 0.800 ± 0.714 and 0.367 ± 0.165 mm respectively in CHX group. Whereas the mean difference in CAL scores between baseline and 1 month, 1 and 3 months, and 3 and 6 months were 2.267 ± 0.828 mm, 0.661 ± 0.711 and 0.467 ± 0.730 mm, respectively in CU group [Figure 4].
Figure 4.
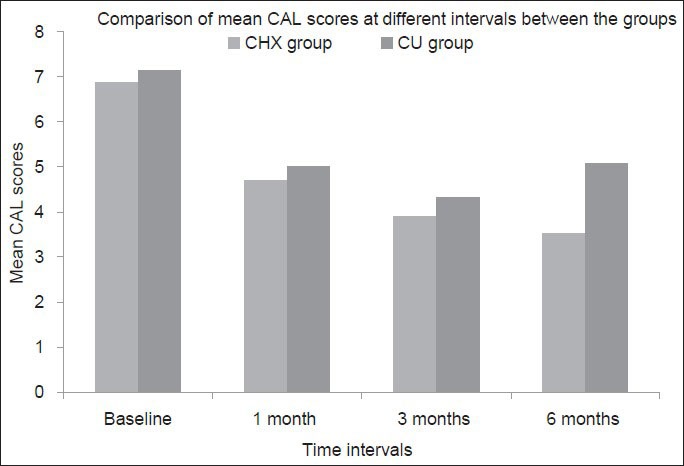
Comparison of the mean clinical attachment level scores between the groups at different time intervals
Intra group comparison of the mean PPD and CAL scores showed significant improvement in both the groups from baseline to 1 month. Intergroup comparison between the two groups, i.e., CHX and CU groups showed no statistical significant difference at baseline, 1 month and 3 months. However at the end of the study period, CHX group showed greater improvement in the PPD and CAL scores compared to CU group, which showed an increase in the scores (P < 0.001).
Intra group comparison of the plaque index [Figure 5] and gingival index scores [Figure 6] showed significant differences in both CHX and CU groups between different time intervals, i.e., baseline and 1 month, 1 and 3 months, 3 and 6 months (P < 0.05). However, intergroup comparison of the mean plaque index and gingival index scores at any time point in between both the groups showed no significant difference indicating the absence of bias between the two groups which could result due to poor patient compliance.
Figure 5.
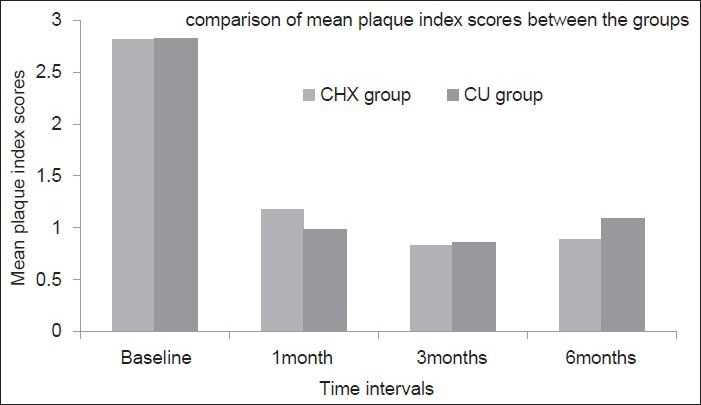
Comparison of the mean plaque index scores between the groups at different time intervals
Figure 6.
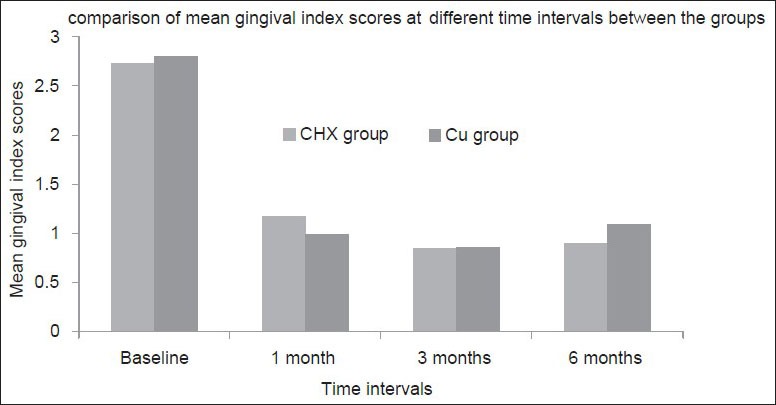
Comparison of the mean gingival index scores between the groups at different time intervals
Discussion
Several novel therapeutic approaches for the treatment of periodontal infections have been successfully applied,[13,14] including the use of scaling and root planning plus CHX chip.[15,16] Administration of CHX mouthwash has been confirmed as an effective antimicrobial agent, and different forms of CHX have been tested in periodontal treatment,[16,17,18,19] including mouthwashes, adhesive gels and chips, with varying results in clinical practice.[16,17,18,19]
The polyphenol natural product CU has been the subject of numerous studies over the past decades, which have identified and characterized the compound's pharmacokinetic, pharmacodynamic, and clinical pharmacological properties. In in vitro and in vivo model systems, CU displays potent pharmacological effects, by targeting many critical cellular factors, through a diverse array of mechanisms of action. Despite this tremendous molecular versatility, however, the clinical application of CU remains limited due to poor pharmacokinetic characteristics in human beings.[20,21,22] As CHX is the gold standard antiseptic for supragingival plaque control, the main aim of the present study was formulated to evaluate the efficacy of an indigenously developed CU based local drug delivery module and compare it with CHX in the form of periochip.
A statistically highly significant reduction in the PPD and CAL scores were observed in both the test groups from baseline to 3 months. In CHX group reduction was maintained till the end of the study period, i.e., 6 months, whereas CU group showed a mild deepening in probing depths, thus indicating a reversal of disease state. However both the therapies led to a significant reduction in the mean probing depth, CAL, plaque index and gingival index scores up to 3 months posttherapy. These results were in accordance with other studies that described the positive results of CHX plus scaling and root planing (SRP), where the subjects who received the combined therapy of SRP + CHX, however showed the greatest improvement in clinical parameters at 6 months posttherapy.[23] The positive results of CU can be attributed to the potent anti-inflammatory action of CU in periodontal disease.[23,24] While demonstrating no appreciable effect on alveolar bone resorption or p38 mitogen-activated protein kinases function, CU treatment appears to effectively inhibit transcriptional and translational expression of pro-inflammatory cytokines and dose-dependently attenuated nuclear factor-κB activation in the gingival tissue.[23] However the increase in the clinical parameters such as probing depth and clinical attachment loss in CU group can be attributed to the short term effect of the drug formulation which mandates the increase in the concentration of the drug in further research.
The findings of our study are also in partial accordance with previous studies which showed a significant improvement in CAL in both scaling and root planning plus CHX chip group and scaling and root planning alone group at 1 month, 3 months and at 6 months as compared to baseline.[25]
The BANA test has been successfully used in several clinical trials to evaluate changes in the subgingival microbiota after therapy.[26,27] This diagnostic technique detects the presence of arginine hydrolase, an enzyme produced by P. gingivalis, T. denticola and T. forsythia, three anaerobic species consistently associated with periodontal infections. Both groups showed a posttherapy reduction in the frequency of the anaerobic microorganisms detected by the BANA test. However, this reduction in the BANA positive sites was maintained in CHX group till the end of the study period, whereas in CU group there was a gradual increase in BANA positive sites to 10% within the period of 3-6 months. Comparison between the groups showed a statistically significant difference at the end of 6 months. These results were in accordance with the studies done earlier, which showed a significant reduction in BANA positive species in CHX mouth rinse group.[28]
Microbial culture showed a significant reduction in the number of colonies at the end of the test period in both groups. However there was a significant increase in the number of colonies in CU group in the period between 3 and 6 months, indicating that the therapeutic efficacy began to wave after 3 months. The results of both the microbiological and clinical parameters are in accordance with a similar study done to compare the efficacy of CHX and CU irrigation in sub-gingival sites where the CU irrigation showed a similar recurrence in clinical and microbiological parameters as compared to the CHX irrigation group.[29]
The duration of pocket exposure to the drug is the most important or critical factor in determining the efficacy of the treatment. The sustained exposure of the pocket environment to CHX for 3 days showed a short term antibacterial effect.[30] However a sustained 6-9 day exposure of pockets to the drug gave long lasting antibacterial and clinical results.[16,31] It can be stated that within the purview of the present study, it is obvious that local drug delivery when used in ideal situations, can definitely bring about significant changes in clinical parameters, reducing the necessity for surgical intervention. Further studies associating varied concentration of the drugs, stringent bacterial monitoring assessed after a longer duration would definitely lend credit to such data. Furthermore, it would help decide the frequency of intervals of re-treatment with the said drug formulations, thus aiding in cost-effective nonsurgical intervention of periodontal disease as and when the situations demand.
Conclusion
Within limits superimposed by a relatively small sample size, the results demonstrated that, both the groups (CHX and CU) produced a significant reduction in all the clinical and microbiological parameters. However, at the end of the study period, CHX group showed greater improvement. Coupled with the fact that various earlier preliminary studies suggest that the polyphenol is relatively safe for human administration, and CUs diverse molecular targeting capability may make it a true “go-to” agent for the prevention and therapy of various inflammatory and infectious diseases including periodontal disease. Future directions of this study should include targeting the beneficial effects of these local drug delivery systems at varied concentration so that they can be used to derive the maximum beneficial therapeutic effects in the nonsurgical treatment of periodontal disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34:235–49. [PubMed] [Google Scholar]
- 2.Carranza FA, Newman MG, Takei HH, Klokkevold PR. 10th ed. Missouri: Saunders Elsevier; 2006. Carranza's Clinical Periodontology; pp. 682–5. [Google Scholar]
- 3.Rams TE, Slots J. Local delivery of antimicrobial agents in the periodontal pocket. Periodontol 2000. 1996;10:139–59. doi: 10.1111/j.1600-0757.1996.tb00072.x. [DOI] [PubMed] [Google Scholar]
- 4.Soskolne WA. Subgingival delivery of therapeutic agents in the treatment of periodontal diseases. Crit Rev Oral Biol Med. 1997;8:164–74. doi: 10.1177/10454411970080020501. [DOI] [PubMed] [Google Scholar]
- 5.Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–2. doi: 10.1016/0006-2952(76)90421-4. [DOI] [PubMed] [Google Scholar]
- 6.Menon VP, Sudheer AR. Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol. 2007;595:105–25. doi: 10.1007/978-0-387-46401-5_3. [DOI] [PubMed] [Google Scholar]
- 7.Negi PS, Jayaprakasha GK, Mohan Rao Jagan L, Sakariah KK. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J Agric Food Chem. 1999;47:4297–300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 8.Campbell FC, Collett GP. Chemopreventive properties of curcumin. Future Oncol. 2005;1:405–14. doi: 10.1517/14796694.1.3.405. [DOI] [PubMed] [Google Scholar]
- 9.Sripriya R, Rafiuddinahmed MD, Seghal PK, Jayakumar R. Influence of laboratory ware related changes in conformational and mechanical properties of collagen. J Appl Polym Sci. 2003;87:2186–92. [Google Scholar]
- 10.Sripriya R, Kumar MS, Sehgal PK. Improved collagen bilayer dressing for the controlled release of drugs. J Biomed Mater Res B Appl Biomater. 2004;70:389–96. doi: 10.1002/jbm.b.30051. [DOI] [PubMed] [Google Scholar]
- 11.Fenno JC, Lee SY, Bayer CH, Ning Y. The opdB locus encodes the trypsin-like peptidase activity of Treponema denticola. Infect Immun. 2001;69:6193–200. doi: 10.1128/IAI.69.10.6193-6200.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bretz WA, Loesche WJ. Characteristics of trypsin-like activity in subgingival plaque samples. J Dent Res. 1987;66:1668–72. doi: 10.1177/00220345870660111301. [DOI] [PubMed] [Google Scholar]
- 13.Cobb CM. Non-surgical pocket therapy: Mechanical. Ann Periodontol. 1996;1:443–90. doi: 10.1902/annals.1996.1.1.443. [DOI] [PubMed] [Google Scholar]
- 14.Socransky SS, Haffajee AD. Dental biofilms: Difficult therapeutic targets. Periodontol 2000. 2002;28:12–55. doi: 10.1034/j.1600-0757.2002.280102.x. [DOI] [PubMed] [Google Scholar]
- 15.Azmak N, Atilla G, Luoto H, Sorsa T. The effect of subgingival controlled-release delivery of chlorhexidine chip on clinical parameters and matrix metalloproteinase-8 levels in gingival crevicular fluid. J Periodontol. 2002;73:608–15. doi: 10.1902/jop.2002.73.6.608. [DOI] [PubMed] [Google Scholar]
- 16.Soskolne WA, Heasman PA, Stabholz A, Smart GJ, Palmer M, Flashner M, et al. Sustained local delivery of chlorhexidine in the treatment of periodontitis: A multi-center study. J Periodontol. 1997;68:32–8. doi: 10.1902/jop.1997.68.1.32. [DOI] [PubMed] [Google Scholar]
- 17.Paolantonio M, D’Ercole S, Pilloni A, D’Archivio D, Lisanti L, Graziani F, et al. Clinical, microbiologic, and biochemical effects of subgingival administration of a X anthan-based chlorhexidine gel in the treatment of periodontitis: A randomized multicenter trial. J Periodontol. 2009;80:1479–92. doi: 10.1902/jop.2009.090050. [DOI] [PubMed] [Google Scholar]
- 18.Gupta R, Pandit N, Aggarwal S, Verma A. Comparative evaluation of subgingivally delivered 10% doxycycline hyclate and xanthan-based chlorhexidine gels in the treatment of chronic periodontitis. J Contemp Dent Pract. 2008;9:25–32. [PubMed] [Google Scholar]
- 19.Rusu D, Benta A, Necker A. Non-surgical periodontal therapy using a novel chlorhexidine based xanthan gel; a split mouth study. Int Poster J Dent Oral Med. 2005;7:286–91. [Google Scholar]
- 20.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 21.Sharma RA, Gescher AJ, Steward WP. Curcumin: The story so far. Eur J Cancer. 2005;41:1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 22.Shishodia S, Sethi G, Aggarwal BB. Curcumin: Getting back to the roots. Ann N Y Acad Sci. 2005;6:206–17. doi: 10.1196/annals.1352.010. [DOI] [PubMed] [Google Scholar]
- 23.Guimarães MR, de Aquino SG, Coimbra LS, Spolidorio LC, Kirkwood KL, Rossa C., Jr Curcumin modulates the immune response associated with LPS-induced periodontal disease in rats. Innate Immun. 2012;18:155–63. doi: 10.1177/1753425910392935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guimarães MR, Coimbra LS, de Aquino SG, Spolidorio LC, Kirkwood KL, Rossa C., Jr Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J Periodontal Res. 2011;46:269–79. doi: 10.1111/j.1600-0765.2010.01342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mizrak T, Güncü GN, Caglayan F, Balci TA, Aktar GS, Ipek F. Effect of a controlled-release chlorhexidine chip on clinical and microbiological parameters and prostaglandin E2 levels in gingival crevicular fluid. J Periodontol. 2006;77:437–43. doi: 10.1902/jop.2006.050105. [DOI] [PubMed] [Google Scholar]
- 26.Bretz WA, Lopatin DE, Loesche WJ. Benzoyl-arginine naphthylamide (BANA) hydrolysis by Treponema denticola and/or Bacteroides gingivalis in periodontal plaques. Oral Microbiol Immunol. 1990;5:275–9. doi: 10.1111/j.1399-302x.1990.tb00425.x. [DOI] [PubMed] [Google Scholar]
- 27.Syed SA, Gusberti FA, Loesche WJ, Lang NP. Diagnostic potential of chromogenic substrates for rapid detection of bacterial enzymatic activity in health and disease associated periodontal plaques. J Periodontal Res. 1984;19:618–21. doi: 10.1111/j.1600-0765.1984.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 28.Faveri M, Gursky LC, Feres M, Shibli JA, Salvador SL, de Figueiredo LC. Scaling and root planing and chlorhexidine mouthrinses in the treatment of chronic periodontitis: A randomized, placebo-controlled clinical trial. J Clin Periodontol. 2006;33:819–28. doi: 10.1111/j.1600-051X.2006.00994.x. [DOI] [PubMed] [Google Scholar]
- 29.Gottumukkala SN, Koneru S, Mannem S, Mandalapu N. Effectiveness of sub gingival irrigation of an indigenous 1% curcumin solution on clinical and microbiological parameters in chronic periodontitis patients: A pilot randomized clinical trial. Contemp Clin Dent. 2013;4:186–91. doi: 10.4103/0976-237X.114874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friedman M, Golomb G. New sustained release dosage form of chlorhexidine for dental use. I. Development and kinetics of release. J Periodontal Res. 1982;17:323–8. doi: 10.1111/j.1600-0765.1982.tb01160.x. [DOI] [PubMed] [Google Scholar]
- 31.Stabholz A, Sela MN, Friedman M, Golomb G, Soskolne A. Clinical and microbiological effects of sustained release chlorhexidine in periodontal pockets. J Clin Periodontol. 1986;13:783–8. doi: 10.1111/j.1600-051x.1986.tb00882.x. [DOI] [PubMed] [Google Scholar]


