Abstract
Background:
Periodontitis is a common inflammatory disease with complex and multi-factorial origin. Tobacco usage has shown its adverse effect on periodontal health. Various components within saliva not only protect the integrity of oral tissues, but also provide clues to local and systemic diseases and conditions. Salivary thiocyanate (SCN) has been shown to be a chemical indicator in smokers and smokeless tobacco users. Noninvasive nature of salivary testing has made it an attractive and effective alternative to blood and urine testing. Limited studies are there comparing and correlating the salivary SCN levels in smokers with chronic periodontitis (CP). However, no studies show correlation of salivary SCN among gutka chewers with CP.
Aims and Objectives:
The objective of the following study is to estimate, compare, and correlate the SCN levels in periodontally healthy, CP, smokers with CP and gutka chewers with CP subjects.
Materials and Methods:
Study includes 120 subjects with age 18-55 years, categorized as periodonally healthy (n = 30), CP (n = 30), smokers (n = 30), and gutka chewers (n = 30) with CP. Required clinical parameters such as gingival index, probing depth and clinical attachment loss were recorded and salivary SCN levels were estimated through ultraviolet-spectrophotometer.
Results:
Mean salivary SCN level were shown to be higher among smokers and gutka chewers with CP as compared to healthy and CP alone.
Conclusion:
The present study exhibited the significant increase in salivary SCN levels among smokers and gutka chewers when compared to others, concluding that the analysis of salivary SCN levels could be used as an adjunctive means of diagnosis.
Keywords: Chronic periodontitis, gutka chewers, salivary thiocyanate, smokers, ultraviolet-spectrophotometer
Introduction
Periodontitis is one of the common chronic inflammatory disease found in adults with multi-factorial origin that affects the periodontium.[1,2] The progression of the disease is dependent on the host response to pathogens that colonize the tooth surface. In addition to the direct etiological impact of bacteria, cigarette smoking and tobacco chewing is considered to be most important environmental risk factors for periodontitis.[3] Significantly higher loss of clinical attachment level and bone loss have been observed in smokers.[4,5,6] Periodontitis is more common and severe in smokers, characterized by deeper periodontal pockets, greater attachment loss, and more furcation defects.[7,8,9,10] The use of smokeless tobacco has shown its contribution in loss of clinical attachment level along with local gingival recession at the site of placement.[11,12]
Diagnosis of periodontal disease has been primarily based upon clinical and radiographic measures of periodontal tissue destruction. Saliva is regarded as one of the important factors in regulating oral health, with respect to both the volume produced and the ingredients it contains. It is a complex hypotonic aqueous solution containing proteins, peptides, enzymes, hormones, sugars, lipids, growth factors, and a variety of other compounds.[13] Human saliva has been increasingly investigated as an alternative to serum for a number of diagnostic purposes due to its non-invasive nature, with low risk of infection.[14] Therefore, it is expected that a considerable number of blood tests will be replaced by saliva tests in the near future.
Composition of tobacco smoke is a mixture of combustion gases in a suspension of particulate matter. The gas phase consists of carbon monoxide and hydrogen cyanide. Carbon monoxide, thiocyanate, nicotine, and cotinine are essential markers of smoke inhalation. Each cigarette delivers 30-200 μg of hydrogen cyanide into the mouth of the smokers.[15] Whereas, gutka is chewed slowly, and in this process its extract is absorbed locally as well as ingested to enter into the systemic circulation due to saliva. The use of gutka has been classified as carcinogenic to humans and also associated with oral diseases.[16,17,18]
Salivary thiocyanate (SCN) a metabolic product of cyanide, is an anion found in organic and inorganic compounds. SCN-ions are important because, they prevent toxic accumulations of hydrogen peroxide (H2O2) and hypochlorite (Ocl−), which may be carcinogenic or mutagenic. Therefore, insufficient levels of these antioxidant SCN-may provide inadequate protection from Ocl-, which intern would exacerbate inflammatory diseases, and rendering humans to diseases linked to myloperoxidase activity, including atherosclerosis, neurodegeneration and certain cancers (e.g., Gastric cancer, etc).[19]
SCN is a normal constituent of body fluids such as serum, saliva, urine, and tears. Diet and tobacco are its main sources in human body. Salivary SCN concentration in normal nonsmokers ranges from 0.5 to 2 mM with an average of 1 mM. However, heavy smokers may have salivary concentration range as high as 6 mM.[20,21,22]
The SCN present in the body fluids is partially because of detoxification of hydrogen cyanide in cigarette smokers.[23] The amount of SCN secretion in smokers is 200 mg/day more than nonsmokers.[24] This elevated level of SCN in the saliva of smokers may be responsible for excessive cancer risk of smokers through the nitrosation process,[25] which is a process of converting organic compounds into nitros derivatives which are potent carcinogens and teratogens.[26]
Though, there are numerous tobacco products in smoke of tobacco, SCN has a property to induce cancerous changes in epithelium. SCN is secreted in saliva and has a long half-life of 10-14 days in normal adults and is in continuous contact with epithelium through blood and saliva.[15,27]
Gutka is a popular commercially powdered product, consists of a number of ingredients, including tobacco, areca nut, slaked lime, and spices. It was introduced nearly more than 4 decades ago and is very commonly used in India.[28] Hence, it is important to know about the salivary SCN being liberated in different forms of tobacco consumed by humans.
Taking into consideration the adverse effects of tobacco consumption, the present study was conducted aiming to investigate the salivary SCN levels in chronic periodontitis (CP) subjects, smokers and gutka chewers with CP and to compare and correlate these SCN levels with periodontitis. Our hypothesis is that an increase in consumption of these tobacco products can increase the clinical symptoms.
Materials and Methods
The present cross-sectional study comprised of 120 subjects ranging in age from 18 to 55 years, randomly selected from the out-patient department, Department of Periodontics, P.M.N.M Dental College and Hospital, Bagalkot, Karnataka, India. Patients were equally divided into four groups with 30 subjects in each group viz.; clinically healthy periodontium (Group I), CP subjects without any tobacco habits (Group II), smokers with CP (Group III) and gutka chewers with CP (Group IV).
Subjects were considered clinically healthy if probing pocket depth <3 mm. Periodontitis was defined as presence of at least seven teeth with probing pocket depth >5 mm. All subjects were systemically healthy with no medical conditions that would affect their participation in the study. Participants having CP showed no history of surgical therapy previously. Those patients who smoked more than five cigarettes a day since past several years were included in smoker's category. CP subjects who chew gutka, at least since 5 years were included in gutka chewer's category. The exclusion criterion was a history of any antimicrobial or anti-inflammatory medication within the previous 3 months for any reason, history of regular use of mouth washes.
All potential participants were explained concerning the need and design of the study. Written informed consent was obtained from all recruits. The study protocol was approved by the ethical committee of P.M.N.M. Dental College and Hospital, Bagalkot, Karnataka, India and in accordance with the Helsinki Declaration of 1975 as revised in 2000.
Clinical measurements
Gingival index (GI) (Loe and Silness-1963), probing depth (PD), clinical attachment loss (CAL) were recorded. PD and CAL were recorded to the nearest millimeter using the Williams graduated periodontal probe at four sites around each tooth (mesiobuccal, midbuccal, distobuccal and midlingual), excluding the third molars. One trained examiner obtained all the measurements so as to reduce intra-examiner variability.
Sample collection
All the saliva samples were collected before the clinical measurements were recorded, between 9 a.m. to 11 a.m. Stimulated whole saliva sample were obtained from these subjects and was stored in Eppendorf tubes at −20°C until analyzed.[22]
Biochemical analysis
Saliva samples were centrifuged at 3800 rpm for at least 10 min and salivary SCN concentration were estimated through ultraviolet (UV)-spectrophotometer.[29]
Thiocyanate determination
A volume of 0.5 ml of supernatant saliva obtained after centrifugation was mixed with 9.5 ml Fe(NO3)3 reagent; following reaction occurs:
Fe+3 (aq) + SCN (aq) → FeSCN2+ (aq)
This FeSCN2+ complex exhibit deep red color, which can be conveniently measured with UV spectrophotometry at 447 nm wavelengths. The FeSCN2+ concentration of saliva solution was calculated trough Lambert and Beers law.[29]
Statistical analysis
Using Statistical Package for Social Sciences version 12 (SPSS Inc. Chicago, USA 1999), data were analyzed. The patient's salivary SCN and clinical parameters were analyzed using one-way ANOVA and Kruskal-Wallis tests and in between comparison of all the four groups were carried out by using Scheff's multiple comparison test.
Results
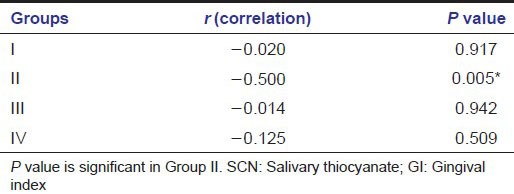
The mean SCN level are significantly higher in smokers 7.54817 (SD: 4.6195) followed by gutka chewers with CP 2.34551 (SD: 1.71345) as compared to healthy 1.97453 (SD: 1.58674) and CP subjects 1.57132 (SD: 1.41962) who do not use tobacco [Table 1].
Table 1.
Mean±SD values of clinical parameters and salivary SCN levels among the study groups
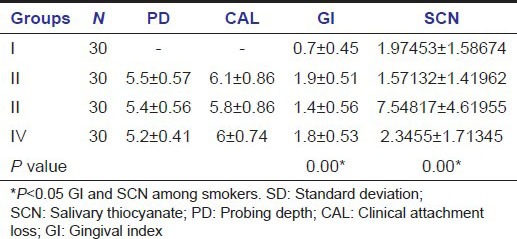
Significant difference was seen in the mean values of SCN of smokers with CP (P < 0.001) on Scheff's multiple comparisons test. There was no significant correlation between SCN with age, PD, CAL in any of the groups when seen group wise. There exist significant correlation between SCN, age, PD, and CAL if one ignores the groups [Table 2].
Table 2.
Scheff's multiple comparison test for SCN
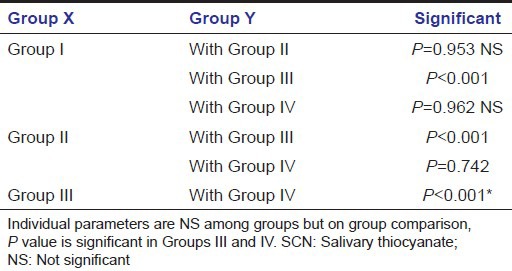
Correlation between gingival index and salivary thiocyanate
In group GI and SCN are negatively correlated and correlation is very poor. In Group II GI and SCN are negatively correlated, i.e., if SCN increases then GI decreases or if GI increases SCN decreases. Poor negative association exists between GI and SCN in Group III. In Group IV there is a negative correlation, but statistically not significant [Table 3].
Table 3.
Group correlation coefficient between SCN and GI
Discussion
The literature reveals the present study to be the first study to evaluate and compare the salivary SCN levels among gutka chewers with CP and with the smokers with CP as well as its correlation with CP, when compared with clinically healthy periodontium.
In the present study, high levels of salivary SCN were observed in gutka chewers with CP and levels were much higher in smokers with CP when compared to healthy and CP patients. The levels of salivary SCN in periodontally healthy and CP patients without any habits have no significant difference.
Previous reports have shown an inverse relationship between salivary SCN and gingival inflammation and plaque.[30,31] The level of salivary SCN is higher in tobacco users as compared with nontobacco users. Our results are in agreement with those reported in literature.[27,32]
In the present study, smokers with CP exhibited lower GI, PD and CAL as compared to gutka chewers with CP and CP subjects but higher GI, PD, and CAL when compared with healthy subjects. The study also showed that a negative correlation exists between clinical parameters and salivary SCN (Group I: r = −0.020, Group II: r = −0.500, Group III: r = −0.014, and Group IV: r = −0.125).
The negative and poor association between the GI of Group III and Group IV with SCN concludes that salivary defenses though compromised, the clinical symptoms occur at a slower rate. Therefore, a weak salivary immune system makes the periodontal ligament more susceptible to the disease process.
Salivary thiocyanate is responsible for neurological alterations (amblyopia, infant strabismus in children of smoking mothers) and endocrine alterations (increase in the incidence of nodular goiter) related to the smoking habit.[33,34,35] It is also one of the factors implicated in the delayed healing of smokers’ wounds.[36,37] Determining thiocyanate (SNC) levels in saliva is the most frequently used biochemical test for establishing the incidence or prevalence of tobacco consumption among adolescents.[32] The present and other studies indicate that whole saliva may contain simply measured indicators of SCN and may provide an important tool for treating as well as monitoring periodontitis. It also helps us understand the influence of local factors over the pathology. In long run further studies are required on large samples to determine the relationship between salivary SCN levels and periodontal disease in subjects using different forms of tobacco.
Conclusion
Since decades, there have been warnings against tobacco smoking and/or chewing and its harm to one's health. Therefore, there is a need to estimated and analyze the effects of tobacco usage in form of smoking as well as gutka chewing and detriments of its components.
As gutka chewing is harmful, the alarming scenario demands that health agencies and governmental organizations should launch awareness programs to inform and educate the public regarding the adverse health consequences and possible cancer risk associated with tobacco consumption. Salivary SCN not only helps us differentiate smokers and nonsmokers, it contributes in monitoring CP.
Hence, to conclude salivary SCN level stands out to be a useful marker of periodontal tissue destruction proving to be a promising diagnostic marker.
Acknowledgments
Dr. Chandarshekar, Dr. Madhur Manak, Dr. Muddapure M, Dr. Parappa Sajjan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Giannobile WV, Beikler T, Kinney JS, Ramseier CA, Morelli T, Wong DT. Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontol 2000. 2009;50:52–64. doi: 10.1111/j.1600-0757.2008.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Position paper: Tobacco use and the periodontal patient. Research, Science and Therapy Committee of the American Academy of Periodontology. J Periodontol. 1999;70:1419–27. doi: 10.1902/jop.1999.70.11.1419. [DOI] [PubMed] [Google Scholar]
- 4.Grossi SG, Zambon JJ, Ho AW, Koch G, Dunford RG, Machtei EE, et al. Assessment of risk for periodontal disease. I. Risk indicators for attachment loss. J Periodontol. 1994;65:260–7. doi: 10.1902/jop.1994.65.3.260. [DOI] [PubMed] [Google Scholar]
- 5.Grossi SG, Genco RJ, Machtei EE, Ho AW, Koch G, Dunford R, et al. Assessment of risk for periodontal disease. II. Risk indicators for alveolar bone loss. J Periodontol. 1995;66:23–9. doi: 10.1902/jop.1995.66.1.23. [DOI] [PubMed] [Google Scholar]
- 6.Papapanou PN. Periodontal diseases: Epidemiology. Ann Periodontol. 1996;1:1–36. doi: 10.1902/annals.1996.1.1.1. [DOI] [PubMed] [Google Scholar]
- 7.Bolin A. Proximal alveolar bone loss in a longitudinal radiographic investigation. Swed Dent J Suppl. 1986;35:1–108. [PubMed] [Google Scholar]
- 8.Bergström J, Preber H. Tobacco use as a risk factor. J Periodontol. 1994;65:545–50. doi: 10.1902/jop.1994.65.5s.545. [DOI] [PubMed] [Google Scholar]
- 9.Norderyd O, Hugoson A, Grusovin G. Risk of severe periodontal disease in a Swedish adult population. A longitudinal study. J Clin Periodontol. 1999;26:608–15. doi: 10.1034/j.1600-051x.1999.260908.x. [DOI] [PubMed] [Google Scholar]
- 10.Mullally BH, Linden GJ. Molar furcation involvement associated with cigarette smoking in periodontal referrals. J Clin Periodontol. 1996;23:658–61. doi: 10.1111/j.1600-051x.1996.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 11.Robertson PB, Walsh M, Greene J, Ernster V, Grady D, Hauck W. Periodontal effects associated with the use of smokeless tobacco. J Periodontol. 1990;61:438–43. doi: 10.1902/jop.1990.61.7.438. [DOI] [PubMed] [Google Scholar]
- 12.Fisher MA, Taylor GW, Tilashalski KR. Smokeless tobacco and severe active periodontal disease, NHANES III. J Dent Res. 2005;84:705–10. doi: 10.1177/154405910508400804. [DOI] [PubMed] [Google Scholar]
- 13.Patil PB, Patil BR. Saliva: A diagnostic biomarker of periodontal diseases. J Indian Soc Periodontol. 2011;15:310–7. doi: 10.4103/0972-124X.92560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mandel ID. The diagnostic uses of saliva. J Oral Pathol Med. 1990;19:119–25. doi: 10.1111/j.1600-0714.1990.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 15.Benowitz NL. National Institute on Drug Abuse. Measurement in the analysis and treatment of smoking behavior. In: Grabowski J, Catherine SB, editors. The Use of Biological Fluid Samples in Assessing Tobacco Smoke Consumption. Research 48 Monograph Series. Maryland: National Institute On Drug Abuse; 1983. pp. 11–3. [Google Scholar]
- 16.Winn DM. Smokeless Tobacco or Health: An International Perspective. Bethesda: US Department of Health and Human Services, Public Health Service, National Institutes of Health, National Cancer Institute; 1993. Surveillance of and knowledge about cancer associated with smokeless tobacco use; pp. 11–8. [Google Scholar]
- 17.Basu R, Mandal S, Ghosh A, Poddar TK. Role of tobacco in the development of head and neck squamous cell carcinoma in an eastern Indian population. Asian Pac J Cancer Prev. 2008;9:381–6. [PubMed] [Google Scholar]
- 18.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Szép S, Lu Z. The antioxidant role of thiocyanate in the pathogenesis of cystic fibrosis and other inflammation-related diseases. Proc Natl Acad Sci U S A. 2009;106:20515–9. doi: 10.1073/pnas.0911412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tenovuo J. Ed I. II. Boca Raton, FL: CRC; 1989. Human Saliva: Clinical Chemistry and Microbioloy; pp. 55–91. [Google Scholar]
- 21.Pruitt KM, Tenovuo J. New York: Dekker; 1985. The Lactoperoxidase System: Chemical and Biological Significance; pp. 101–12. [Google Scholar]
- 22.Rai B, Kharb S, Anand SC. Salivary enzymes and thiocynate: Salivary markers of periodontitis among smokers and non-smokers; a pilot study. Adv Med Dent Sci. 2007;1:1–4. [Google Scholar]
- 23.Johnson WR, Hale RW, Nedlock JW, Grubbs HJ, Powell DH. The distribution of products between mainstream and side-stream smoke. Tob Sci. 1973;17:141–4. [Google Scholar]
- 24.Joergensen KA, Lawerson SO. Studies on the reaction of nitrosyl cation with thiocyanate anion using energy weighted overlap and ab initio calculations. J Am Chem Soc. 1984;106:4687–91. [Google Scholar]
- 25.Calabrese EJ. United Kingdom: Lewis Publishers; 1991. Multiple Chemical Interactions; pp. 160–1. [Google Scholar]
- 26.Ward MH. Too much of a good thing. Nitrate from nitrogen fertilizers and cancer? Rev Environ Health. 2009;24:357–63. doi: 10.1515/reveh.2009.24.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tenovuo J, Mäkinen KK. Concentration of thiocyanate and ionizable iodine in saliva of smokers and nonsmokers. J Dent Res. 1976;55:661–3. doi: 10.1177/00220345760550042001. [DOI] [PubMed] [Google Scholar]
- 28.Changrani J, Gany FM, Cruz G, Kerr R, Katz R. Paan and gutka use in the United States: A pilot study in Bangladeshi and Indian-Gujarati immigrants in New York city. J Immigr Refug Stud. 2006;4:99–110. doi: 10.300/J500v04n01_07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahti M, Vilpo J, Hovinen J. Spectrophotometric determination of tiocyanate in human saliva. J Chem Educ. 1999;76:1281–2. [Google Scholar]
- 30.Rosin M, Kramer A, Bradtke D, Richter G, Kocher T. The effect of a SCN-/H2O2 toothpaste compared to a commercially available triclosan-containing toothpaste on oral hygiene and gingival health – A 6-month home-use study. J Clin Periodontol. 2002;29:1086–91. doi: 10.1034/j.1600-051x.2002.291207.x. [DOI] [PubMed] [Google Scholar]
- 31.Jalil RA, Ashley FP, Wilson RF, Wagaiyu EG. Concentrations of thiocyanate, hypothiocyanite, ‘free’ and ‘total’ lysozyme, lactoferrin and secretory IgA in resting and stimulated whole saliva of children aged 12-14 years and the relationship with plaque accumulation and gingivitis. J Periodontal Res. 1993;28:130–6. doi: 10.1111/j.1600-0765.1993.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 32.Tsuge K, Kataoka M, Seto Y. Cyanide and thiocyanate levels in blood and saliva of healthy adult volunteers. J Health Sci. 2000;46:343–50. [Google Scholar]
- 33.Costagliola C, Cotticelli L, Menzione M, Rinaldi M, Russo S, Rinaldi E. Red cell reduced glutathione and tobacco smoke-induced optic neuropathy. Metab Pediatr Syst Ophthalmol. 1990;13:96–8. [PubMed] [Google Scholar]
- 34.Fukayama H, Nasu M, Murakami S, Sugawara M. Examination of antithyroid effects of smoking products in cultured thyroid follicles: Only thiocyanate is a potent antithyroid agent. Acta Endocrinol (Copenh) 1992;127:520–5. doi: 10.1530/acta.0.1270520. [DOI] [PubMed] [Google Scholar]
- 35.Hakim RB, Tielsch JM. Maternal cigarette smoking during pregnancy. A risk factor for childhood strabismus. Arch Ophthalmol. 1992;110:1459–62. doi: 10.1001/archopht.1992.01080220121033. [DOI] [PubMed] [Google Scholar]
- 36.Mosely LH, Finseth F. Cigarette smoking: Impairment of digital blood flow and wound healing in the hand. Hand. 1977;9:97–101. doi: 10.1016/s0072-968x(77)80001-6. [DOI] [PubMed] [Google Scholar]
- 37.Silverstein P. Smoking and wound healing. Am J Med. 1977;93(Suppl 1A):22–4. doi: 10.1016/0002-9343(92)90623-j. [DOI] [PubMed] [Google Scholar]


