Abstract
Primary malignant melanoma is a rare and aggressive neoplasm that originates from the proliferation of melanocytes. Although, it comprises 1.3% of all cancers, malignant melanoma of the oral cavity accounts for only 0.2-8% of all reported melanomas and occurs approximately 4 times more frequently in the oral mucosa of the upper jaw, usually on the palate or alveolar gingivae. Most of the mucosal melanomas are usually asymptomatic in early stages, and presents as pigmented patch or a mass delaying the diagnosis until symptoms of swelling, ulceration, bleeding, or loosening of teeth are noted. The prognosis is extremely poor, especially in advanced stages. Therefore, any pigmented lesion of undetermined origin should always be biopsied. We herewith report of two cases of oral malignant melanoma in a 60 and 75-year-old female.
Keywords: Gingiva, malignant melanoma, oral mucosa, palate, pigmented lesions
Introduction
Primary malignant melanoma of the mouth is an extremely rare tumor arising from the uncontrolled growth of melanocytes found in the basal layer of the oral mucous membrane. Its actual incidence in the population at present is unknown, but is estimated to vary widely between 0.2% and 8% of all melanomas and 1.3% of all cancers. It has a higher prevalence in blacks, Japanese, and Indians of Asia due to more frequent finding of melanin pigmentation in oral mucosa of these races.[1] Nearly 80% of oral malignant melanomas (OMM) arise in the mucosa of the upper jaws in elderly patients, with the majority occurring on keratinizing mucosa of the palate and alveolar gingivae. Other sites include mandibular gingiva, buccal mucosa, tongue, and floor of the mouth. Clinically, it is easy to diagnose them as these are pigmented ones and have irregular shape and outline. These are mostly asymptomatic and detected only when there is ulceration or hemorrhage of the overlying epithelium. The delayed detection may be the cause for the poor prognosis with a 5-year survival being between 10% and 25%.[1,2]
Case Reports
Case 1
A 60-year-old female patient reported to the Department of Oral Medicine and Radiology with the complaint of swelling and pain in the upper right posterior region of jaw since 1 month. Patient gave a history of an extraction of loose first maxillary molar on the right side after which she noticed increase in size of the swelling. Patient was a tobacco-mishri user since childhood. Past medical and family history was not significant.
Clinical examination revealed a diffuse extra oral swelling of about 7 × 6 cm extending horizontally from the ala of the nose to the pretragus region and vertically about from the infraorbital margin to the inferior border of the mandible. Intraorally, a large bluish black exophytic growth of size approximately 6 × 8 cm was seen extending from maxillary right lateral incisor to third molar region on buccal and palatal aspect crossing midline with ulcerations of overlying mucosa [Figure 1]. On palpation, it was firm and slightly tender. Right submandibular lymphnode was enlarged, firm, tender, and mobile. The provisional diagnosis of melanoma was made with a differential diagnosis of lymphoma and squamous cell carcinoma of the maxilla.
Figure 1.
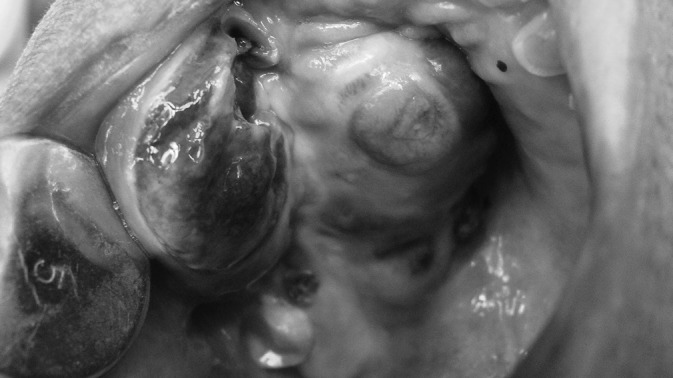
Clinical photograph of Case 1 showing exophytic bluish black growth in right maxilla and palate
Radiographic examination (orthopantomogram and paranasal view) [Figure 2] and CT scan was performed [Figures 3 and 4]. It revealed 9.1 × 6.8 × 6.2 cm sized large expansile, soft-tissue lesion involving right maxillary sinus, which caused destruction of its anterior walls. Cranially, it was seen up to the floor of the orbit without any bony destruction. Caudally, there was destruction of alveolar process of right maxilla with the loss of underlying teeth. Medially, the lesion was involving the lateral portion of hard palate on the right side and was extending into the right nasal cavity. Laterally, the lesion was involving the buccal space on the right side. Anteriorly the lesion caused the erosion of the anterior wall of the maxillary sinus and extended into the maxillofacial soft-tissue of the right side. Posteriorly, it was seen to extend the infratemporal fossa up to the anterior border of the right masseter muscle. Few enlarged lymph nodes were seen in the right submandibular region, largest measuring 16 × 11 mm in size.
Figure 2.
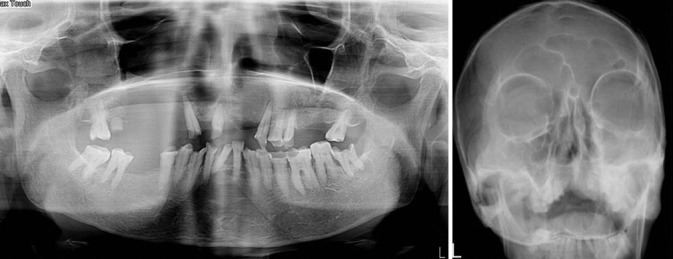
Orthopantomogram (a) and Paranasal (b) shows lesion in the right maxilla involving entire maxillary sinus
Figure 3.
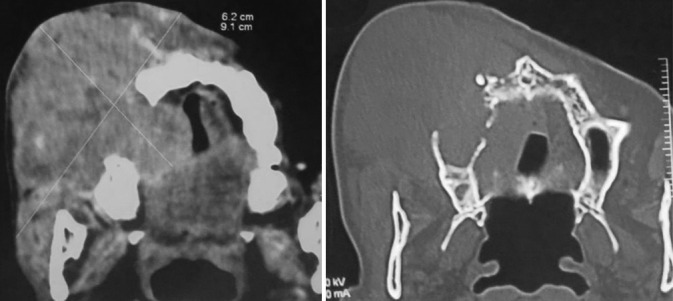
Axial CT scan (a) soft-tissue window and (b) bone window of Case 1
Figure 4.
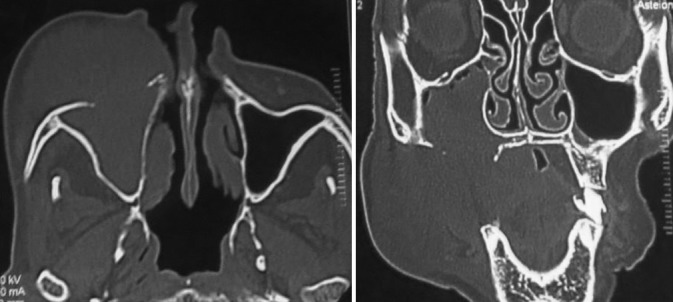
Axial (a) and coronal (b) section showing involvement of the right maxillary antrum with destruction of its anterior wall
An incisional biopsy was done. Histopathologically presence of numerous atypical melanocytes, spindle shaped and epitheloid cells arranged as loosely aggregated cords and presence of fine melanin pigmentation in the cells confirmed the diagnosis of melanoma. Immunohistochemical staining was done, which was positive for S-100 and HMB45. Radiotherapy was planned for the patient and was lost for the follow-up.
Case 2
A 75-year-old female reported to the Department of Oral Medicine and Radiology with a complaint of a painless swelling in the maxillary anterior region since past 6 months. On clinical examination, there was a presence of a multilobular bluish black growth on the right side of maxilla, involving gingiva and alveolar mucosa extending from upper right lateral incisor up to second premolar. There was also black discoloration of the alveolar mucosa in upper central incisors region and palatal mucosa of upper right first and second molar region. Overlying mucosa was ulcerated on the posterior aspect of the lesion [Figure 5]. The swelling was approximately 5 × 4 cm in size, firm and nontender. Two submandibular lymphnodes were palpable, firm, fixed, and nontender on right side. Intraoral periapical and occlusal radiographs showed irregular bone destruction with right maxilla extending from lateral incisor to second molar. It also revealed periodontal space widening from canine to first molar suggesting that a malignant process involved the periodontal space [Figure 6]. A clinical diagnosis of malignant melanoma was made.
Figure 5.
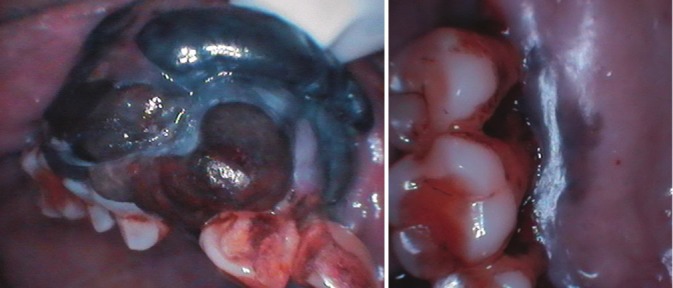
Clinical photograph of Case 2 showing a multilobular bluish black growth on the right side of maxilla, involving gingiva, and alveolar mucosa
Figure 6.
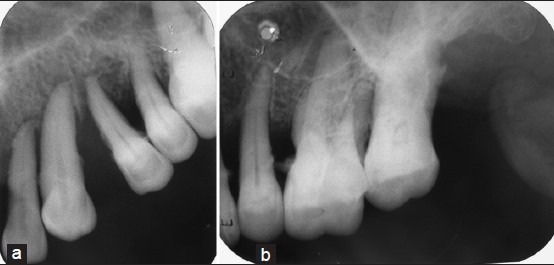
Intraoral periapical of maxillary right anterior (a) and posterior region (b) showing irregular bone destruction from lateral incisor to second molar with periodontal space widening from canine to first molar
Histopathological examination of incisional biopsy confirmed the diagnosis of malignant melanoma [Figure 7]. Patient refused other investigations as well as treatment and died after 1 year.
Figure 7.
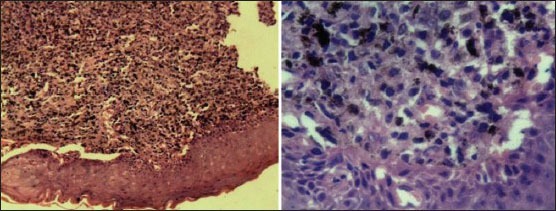
Histopathological section numerous atypical melanocytes, spindle shaped, and epithelioid cells with fine melanin pigmentation
Discussion
Melanocytes in the skin provide a protective function against the harmful effects of sun exposure. In oral mucosa, melanocytes are located in the tips and peripheries of the rete pegs. Melanocytes originate from the embryologic neural crest cells and migrate into the basal layer of epidermis. Melanocytes undergo malignant transformation to form melanoma cells, which are round or spindle cells with hyperchromatic nuclei. These malignant cells are devoid of dendritic processes and possess the ability of invasion to the superficial layers of epithelium and the underlying connective tissue.
The etiology of OMM is unknown in contrast to cutaneous melanoma which is linked to sun exposure.[3,4] Nevi are considered to be a potential source of some oral melanomas, but the sequence of events is poorly understood. Currently, most melanomas are thought to arise de novo.[3] The role of inhaled and ingested carcinogens as from tobacco use and chronic irritation from ill-fitting dentures in their pathogenesis has been suggested.
As neuroectodermal derivatives, melanocytes are known to migrate to the skin, retinal, uveal tract, and other ectodermally derived mucosae. Melanocytes migrate much less frequently to endodermally derived mucosae, such as nasopharynx, larynx, tracheobronchial tree, and esophagus; therefore, there is a lower frequency of melanoma in these locations.[2]
Oral melanoma is rare, and incidence rates of oral melanoma are not available. They are however, estimated to represent 1-2% of oral malignancies[5] and accounting for about 0.2-8% of all melanomas.[6,7] It is frequent in countries such as Japan, Uganda, and India.[5] This malignancy is a lesion of adulthood and may occur any time after the age of 30 years. The highest incidence is in the 5th decade of life (40-70 years).[5] A striking male predilection was seen in mucosal melanoma, with men being affected 3.5 times more frequently than women.[8] Our both of the cases were female patients.
Primary oral melanomas are initially asymptomatic and usually not noticed by the patients which contribute to the delay in diagnosis. Most lesions appear as new lesions from apparently normal mucosa, whereas about 30-50% are preceded by oral pigmentations for several months or even years.[3,9,10] In the late course of the disease, pain, ulceration, and bleeding may be present. Oral lesions may be uniformly brown or black or show variation in color with black, brown, grey, purple and red shades or depigmentation.[8] About 10% of cases are amelanotic. At presentation, approximately 13-20% patients have lymph node metastasis.
The ABCDE criteria used in the clinical diagnosis of cutaneous melanoma may also be used for OMM. These are: Asymmetry in the shape of the lesion, border irregularities, color variation, diameter >6 mm and evolving changes in the lesion over time are characteristic criteria.[3]
Involvement of jaw bones by melanoma radiographically is rare. However, when they do involve the bone, they are indistinguishable from any other lytic malignant tumor or osteomyelitis. Determination of the melanoma stage is important for planning appropriate treatment and assessing prognosis. A simple tumor-node-metastasis classification for malignant melanoma is as follows.[8]
Stage I: Primary tumor present only (N0M0)
Level I: Pure in situ melanoma without evidence of invasion or in situ melanoma with “microinvasion”
Level II: Invasion up to the lamina propria
Level III: Deep skeletal tissue invaging into the skeletal muscle, bone, or cartilage.
Stage II: Tumor metastatic to regional lymph nodes (N1M0)
Stage III: Tumor metastatic to distant sites (M1).
CT and MRI studies should be undertaken to explore the exact extent of lesion and regional metastasis to the submandibular and cervical lymph nodes. Incisional biopsy is the method of choice for diagnosis. The relatively high incidence of malignant versus benign melanotic lesions suggest that melanotic lesions of the oral mucosa should be assessed with some care.[5]
Histologically, the presence of high density of atypical melanocytes (usually larger than the normal melanocytes and having varying degrees of nuclear pleomorphism and hyperchromatism) are seen in the epithelial and connective tissue junction, in the biopsy of melanotic lesions of the oral mucosa are suspicious of OMM.[11]
The mucosal melanomas can show two principal patterns: An in situ pattern (15%) in which the neoplasm is limited to the epithelium and the epithelial-connective tissue interface (junctional), and an invasive pattern (30%) in which the neoplasm is found within the supporting connective tissue. A combined pattern (55%) of invasive melanoma with an in situ component is typical for most advanced lesion.[7]
More than 95% of the lesions are anti S-100 antigen positive and more specific markers include HMB45, Melan-A and antityrosinase.[7]
Differential diagnosis of melanoma includes oral melanotic macule, smoking associated melanosis, medication induced melanosis (antimalarial drugs and minocycline), melanoplakia, Cushing's syndrome, postinflammatory pigmentation, melanoacanthoma, melanotic nevi of oral mucosa, blue nevi, Addison's disease, Peutz-Jeghers syndrome, amalgam tattoo, Kaposi's sarcoma, physiologic pigmentations, and many other conditions sharing the same macroscopic characters.[12] Moreover, it is necessary that OMM should be differentiated from other malignant entities, such as poorly differentiated carcinoma and large anaplastic lymphoma.[11] Amelanotic malignant melanoma without radial growth phase may be misdiagnosed as epulis or squamous cell carcinoma.[12]
Traditionally, wide surgical excision with adequate negative margins with or without neck dissection is the treatment of choice for malignant melanoma, with radiotherapy and chemotherapy as adjunctive treatment methods. In case of OMM, if the disease is localized it can be controlled by radiotherapy, in contrast to cutaneous melanoma. Chemotherapy and immunotherapy may play a role to prevent distant metastasis.[13]
Dacarbazine (DTIC) and interferon-alpha-2b are used for chemotherapeutical and immunotherapical treatments with the first line of drug being DTIC, alone or in combination with nimustine hydrochloride and vincristine.[4] or different combinations of bacillus Calmette-Guerin and recombinant interlukin-2.
Most oral melanomas are large at presentation and have a poor prognosis than cutaneous melanoma. The reported 5 years survival rate for OMM has ranged from 4.5%-29% with a median survival rate of 18.5 months after initial diagnosis.[10] In general, the survival rates are poor and worse for those with metastasis.
Prevention and screening for OMM include annual evaluation of pigmented lesions of oral mucosa. Early diagnosis is promoted by careful oral examination and early biopsy of pigmented and suspicious nonpigmented masses along with advanced surgical techniques and considerations to chemotherapy, radiotherapy, immunotherapy, and combined therapy may help in improving the prognosis of patients with OMM.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bhullar RP, Bhullar A, Vanaki SS, Puranik RS, Sudhakara M, Kamat MS. Primary melanoma of oral mucosa: A case report and review of literature. Dent Res J (Isfahan) 2012;9:353–6. [PMC free article] [PubMed] [Google Scholar]
- 2.Femiano F, Lanza A, Buonaiuto C, Gombos F, Di Spirito F, Cirillo N. Oral malignant melanoma: A review of the literature. J Oral Pathol Med. 2008;37:383–8. doi: 10.1111/j.1600-0714.2008.00660.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajendran R. A Textbook of Oral Pathology. Philadelphia: Saunders; 2006. Benign and malignant tumours of oral cavity; pp. 86–229. [Google Scholar]
- 4.Umeda M, Shimada K. Primary malignant melanoma of the oral cavity – Its histological classification and treatment. Br J Oral Maxillofac Surg. 1994;32:39–47. doi: 10.1016/0266-4356(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 5.Steidler NE, Reade PC, Radden BG. Malignant melanoma of the oral mucosa. J Oral Maxillofac Surg. 1984;42:333–6. doi: 10.1016/0278-2391(84)90116-2. [DOI] [PubMed] [Google Scholar]
- 6.Garzino-Demo P, Fasolis M, Maggiore GM, Pagano M, Berrone S. Oral mucosal melanoma: A series of case reports. J Craniomaxillofac Surg. 2004;32:251–7. doi: 10.1016/j.jcms.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Rapini RP, Golitz LE, Greer RO, Jr, Krekorian EA, Poulson T. Primary malignant melanoma of the oral cavity. A review of 177 cases. Cancer. 1985;55:1543–51. doi: 10.1002/1097-0142(19850401)55:7<1543::aid-cncr2820550722>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 8.Meleti M, Leemans CR, Mooi WJ, Vescovi P, van der Waal I. Oral malignant melanoma: A review of the literature. Oral Oncol. 2007;43:116–21. doi: 10.1016/j.oraloncology.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Rapidis AD, Apostolidis C, Vilos G, Valsamis S. Primary malignant melanoma of the oral mucosa. J Oral Maxillofac Surg. 2003;61:1132–9. doi: 10.1016/s0278-2391(03)00670-0. [DOI] [PubMed] [Google Scholar]
- 10.Strauss JE, Strauss SI. Oral malignant melanoma: A case report and review of literature. J Oral Maxillofac Surg. 1994;52:972–6. doi: 10.1016/s0278-2391(10)80083-7. [DOI] [PubMed] [Google Scholar]
- 11.González-García R, Naval-Gías L, Martos PL, Nam-Cha SH, Rodríguez-Campo FJ, Muñoz-Guerra MF, et al. Melanoma of the oral mucosa. Clinical cases and review of the literature. Med Oral Patol Oral Cir Bucal. 2005;10:264–71. [PubMed] [Google Scholar]
- 12.Tanaka N, Mimura M, Ogi K, Amagasa T. Primary malignant melanoma of the oral cavity: Assessment of outcome from the clinical records of 35 patients. Int J Oral Maxillofac Surg. 2004;33:761–5. doi: 10.1016/j.ijom.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Hashemi Pour MS. Malignant melanoma of the oral cavity: A review of literature. Indian J Dent Res. 2008;19:47–51. doi: 10.4103/0970-9290.38932. [DOI] [PubMed] [Google Scholar]


