Abstract
Burkitt's lymphoma (BL) is the fastest growing malignancy of the lymphoreticular system to affect humans and has a potential ability to double in size every day. A case of maxillary sporadic BL (sBL) associated with neuro-orbital involvement in an Indian male is presented. sBL initially presented as maxillary swelling with no obvious dental and periodontal changes. Histological specimen from incisional biopsy revealed a round cell malignant tumor and immunohistochemistry reactions favored nonHodgkin's lymphoma consistent with BL. Four weeks later, patient presented with orbital involvement as diplopia, sixth cranial nerve palsy, and medial rectus palsy. Chemotherapy regimen according to LMB 89 protocol was started. During chemotherapy regimen patient showed bradycardia and Babinski response, suggestive of central nervous system involvement. sBL associated with orbital involvement is extremely rare and only seven cases have been reported. Our case showed unusual presentation; despite the aggressive tumor did not show any common clinical, radiological, and hematological findings. We also discussed the role of oral medicine specialist, importance of early diagnosis, and prompt referral in management of maxillary sBL.
Keywords: Burkitt's lymphoma, maxilla, nonHodgkin lymphomas, orbit
Introduction
Burkitt's lymphoma (BL) is a very high grade B cell neoplasm of the lymphoreticular system and is classified under nonHodgkin's lymphoma (NHL). It was first described and published by Denis Burkitt, termed the lesion “a sarcoma involving the jaws” by noticing the lesions on the faces of Central-African children.[1] According to its geographic distribution, incidence, magnitude, and risk factors there are three types of BL: Endemic BL, sporadic BL (sBL), and HIV infection associated BL. Endemic BL is seen in equatorial Africa and Papua New Guinea with estimated incidence rate of 5-15 cases/100,000 and mortality rates over 5.7/100,000.[2,3] Epstein-bar virus (EBV) is strongly associated in the development of endemic BL and cofactors may include equatorial humid weather of having malarial endemic, chromosomal abnormalities, immune defects, and protein energy deficits.[3,4] Endemic BL affects mainly facial skeleton in young children with peak incidence of 5-7 years, but can secondarily involve abdominal organs and bone marrow.[4] Central nervous system (CNS) involvement in endemic BL is reported in one-third of the cases and may lead to headaches, paraplegia, and cranial nerve defects.[5] Endemic BL shows good response to treatment.[6]
Sporadic BL is seen outside the African region in the rest of the world with estimated incidence rate of 0-8.8 cases in North America, 0-4.9 cases in far and Middle East Asia, and 0-4.6 cases in Europe/100,000. The Indian incidence varied from 0 to 1 case/100,000 with highest at Bangalore and lowest at Poona registries.[7,8] The incidence rates of NHL have risen all over the world including India in the last 30 years.[8] EBV is associated with only 20% of the cases. Time-space clustering studies suggested the combination of genetic susceptibility and specific undetermined environmental factors plays a role in the tumor formation.[1,4] sBL affects mainly abdominal organs, bone marrow infiltration, peripheral lymph node deposits, and Waldeyer's ring in older children and young adults with peak incidence of 10-12 years.[2,9,10] Head and neck involvement is seen in 10-30% of reported case series commonly in the form of cervical lymphadenopathy.[1,9,10] Facial bones and other extranodal sites of head and neck are involved in fewer than 10% of cases.[9] CNS involvement in sBL is uncommon.[2,11,12] Only seven cases of orbital involvement are reported worldwide until today in the literature.[13] We present the first reporting Indian case of maxillary sBL with neuro-orbital involvement.
Case Report
A 21-year-old Pharmacy Student was referred by his general dentist to the Department of Oral Medicine and Radiology for the evaluation of swelling in the right side cheek region of 2 months duration. The swelling was asymptomatic and showed a gradual increase in size to attain the present dimensions. The past medical and dental histories were noncontributory. Patient was in over all good health on general physical examination. Extraoral examination showed a mildly diffused, firm and nontender swelling in the right side maxillary region [Figure 1]. Single right submandibular lymphnode was palpable, measuring 1 × 2 cm in size, soft, tender, and nonadherent. Intraoral examination showed a firm, diffused, and nontender swelling on the right maxillary teeth bearing area measuring around 7 × 5 cm in size with anterioposterior extension from central incisor to second molar and mediolateral extension from midpalatine region to buccal sulcus [Figure 2]. Dental caries was seen in right maxillary first molar, but was not involving the pulp. No periodontal pockets and mobility of the teeth were seen apart from mild generalized gingivitis.
Figure 1.
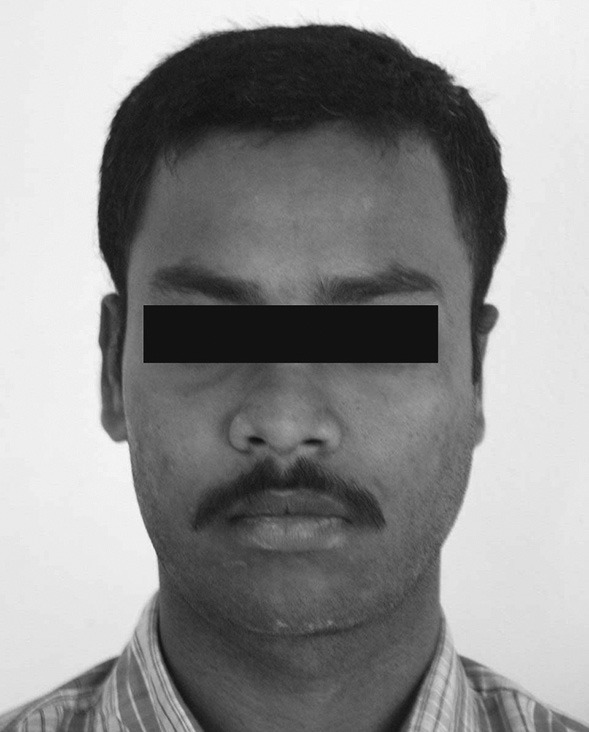
Extraoral photograph showing mildly diffused swelling on the right side maxillary region
Figure 2.
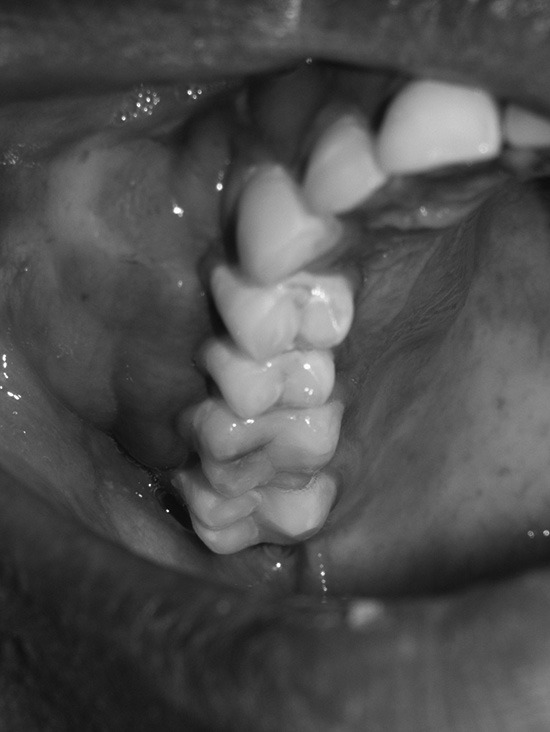
Intraoral photograph of showing diffused swelling on the right side maxillary teeth bearing area extending from central incisor to second molar
Intraoral periapical and maxillary occlusal radiographs showed intact lamina dura with mild diffused bony rarefactions [Figure 3] and panoramic radiograph didn’t show any boney abnormality [Figure 4]. Routine blood investigations were done and all the values were within normal limits apart from slightly raised erythrocyte sedimentation rate. Histological specimen from incisional biopsy revealed a round cell malignant tumor [Figure 5]. On immunohistochemistry (IHC) examination, many tumor cells showed positive reaction to CD79 and CD20 (L26), some tumor cells showed positive reaction to CD45 (leukocyte common antigen) and CD45RO (UCHL1), atypical tumor cells showed negative reaction to CD 3 and CD45RO (UCHL1), and CD 10 was inconclusive. Considering the histopathology, IHC reactions favored NHL consistent with BL [Figure 6].
Figure 3.
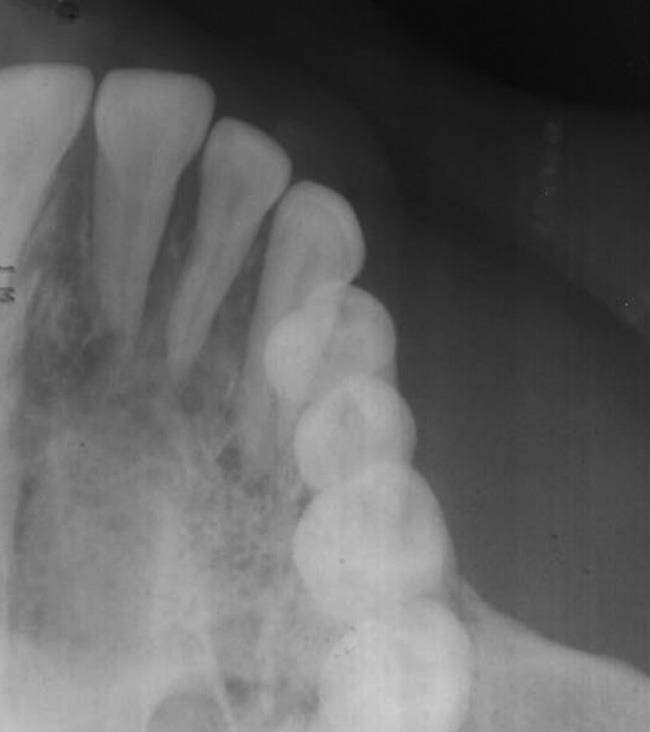
Maxillary occlusal radiograph showing mildly diffused rarefactions of the right side palatal bone
Figure 4.
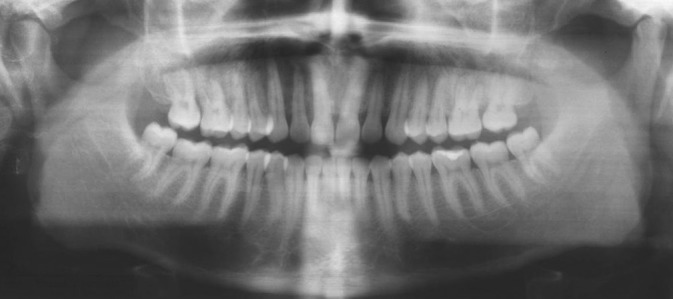
Orthopantomogram showing no bone changes in the right side maxillary region
Figure 5.
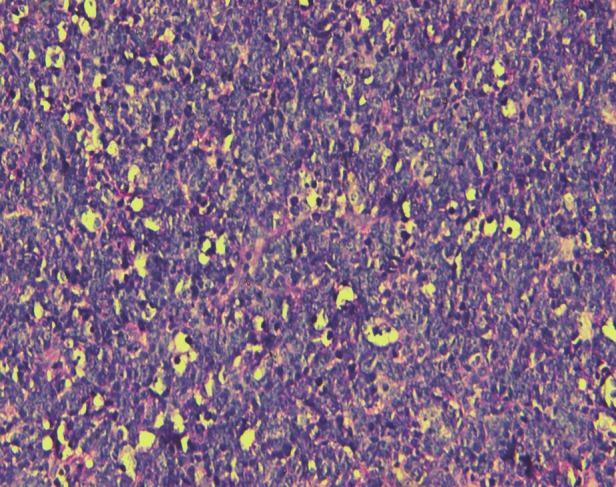
Photomicrograph stained with hematoxylin and eosin showing atypical lymphoid infiltrate in the connective tissue, ×10
Figure 6.
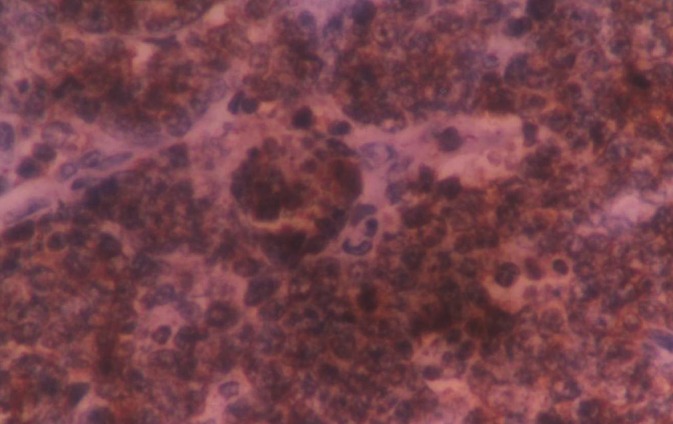
Photomicrograph showing positive reaction of many tumor cells to CD20 marker
Four weeks after, the incisional biopsy, patient presented to the department with further increase in the maxillary swelling and diplopia of the right eye. The patient was then referred to the Department of Medical Oncology of the Regional Cancer Center, Chennai for further evaluation and definitive management. On ophthalmic examination, patient had right side sixth cranial nerve and medial rectus palsy. Computed tomography (CT), axial section showed a tumor mass involving the right side maxilla, and sagittal section showed the mass extended to the inferior wall of the right orbit [Figure 7]. Ultrasonography (USG) of the abdomen and lymph nodes showed features of mild hydronephrosis and a lobulated hypoechoic mass measuring 7.8 × 7.3 × 3.5 cm with cystic spaces in the right iliac region, anterior to the right common iliac vessels suggested mass of nodal origin. Smear from bone marrow aspiration showed slightly hypercellular with 24% lymphocytes and 3% blast cells. Complete blood picture and biochemical analysis were done and all values were in normal limits apart from raised serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic-pyruvic transaminase (SGPT).
Figure 7.
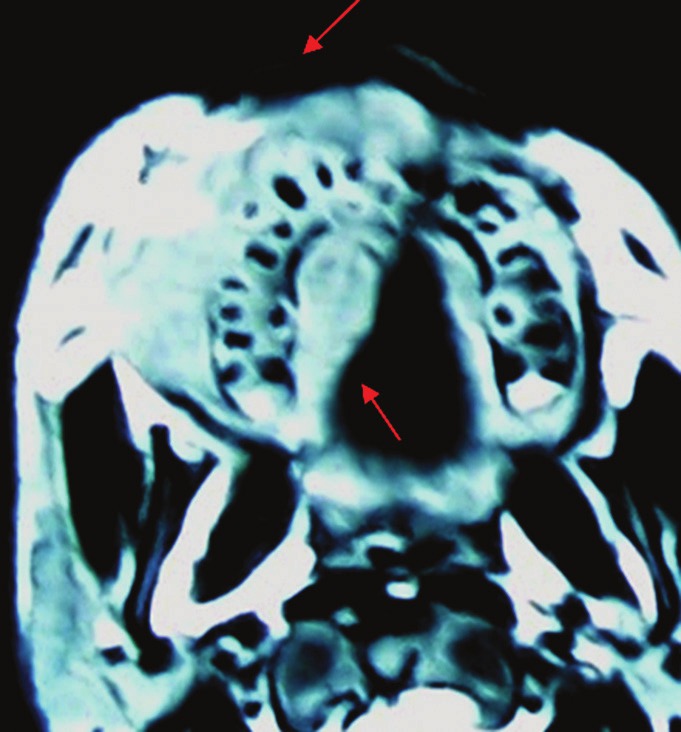
Computed tomography, axial section showing tumor mass involving the right side maxilla
The present case was classified as Group C (Any CNS involvement and/or bone marrow involvement) under French, American, British staging system for childhood B-Large and BL. Chemotherapy regimen was started according to LMB 89 protocol Group C. Patient received prephase cyclophosphamide, oncovin, prednisone (COP) followed by induction phase COP adriamycin, methotrexate (COPADM) I and II. Patient showed significant regression of both maxillary and abdominal mass with improvement of sixth nerve palsy, but developed severe neutropenia (neutrophils 0%, eosinophils 0%, monocytes 10.7%, lymphocytes 14.5%, and granulocytes 74.8%) which was rescued with granulocyte colony stimulating factor [Figure 8]. Patient received consolidation phase CYVE I and II (cytarabine (Ara-c), VP-16 (etoposide) followed by COPADM maintenance. Patient was inter-discharged at stable condition before next cycle of chemotherapy. Patient presented after 2 weeks with headache and sixth nerve palsy. Patient also showed bradycardia and Babinski response. Smear from cerebrospinal fluid showed few small groups of atypical lymphoid cells suggestive of CNS involvement. High dose chemotherapy regimen and radiotherapy was considered. However, patient died a week later from multisystem organ failure.
Figure 8.
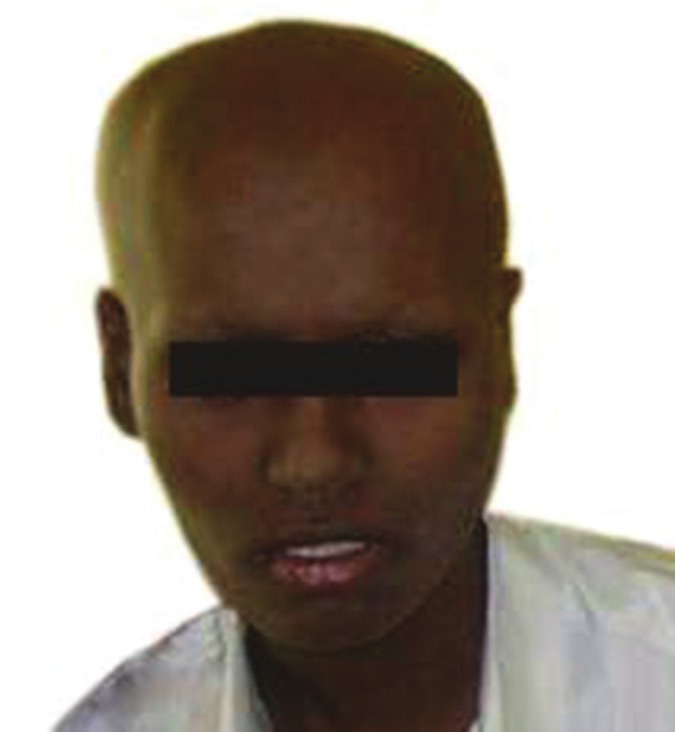
Extraoral photograph showing regressed maxillary swelling at the end of induction phase chemotherapy
Discussion
Sporadic BL is the fastest growing malignancy of the lymphoreticular system to affect humans with 80% of its cells undergoing mitosis at any point of time and has a potential ability to double in size every day.[1,5,14,15] The differential diagnosis of acute swelling in the maxilla as initial and solo presentation without local factors is extremely difficult. It is very important for the oral medicine specialist to be familiar with such clinically presented rarities in the maxilla such as Langerhan's disease, eosinophilic granuloma, osteosarcoma, rhabdomyosarcoma, multiple myeloma, leukemia's, malignant lymphomas or other rarest conditions possible because of the fact that they are the first one to come across and early diagnosis and prompt referral is imperative to prevent dissemination and to obtain favorable prognosis.
There are few cases of sBL involving extranodal sites of head and neck region are reported and the sites of involvement include maxilla, mandible, palate, cheeks, tongue, gingiva, lower lip, tonsils, nasopharynx, maxillary sinus, orbit, ethmoid, sphenoid, mastoid, occipital, frontal bone, and thyroid.[4,5,16,17,18] Clinical features of sBL may vary from no signs and symptoms to life-threatening airway obstruction according to the severity, anatomic location, and temporal presentation of the disease.[1] The clinical features in the jaws and surrounding structures may include facial swelling, exophytic growth, gingival hyperplasia, teeth mobility, teeth displacement, premature tooth eruption, pain, and/or sensory disturbances.[1,2,10,15,19] Pain is the most common presenting symptom and tooth pain as solo presentation secondary to dental tissue infiltrate is reported.[2,11,14,15] Mental nerve neuropathy as one of the clinical symptoms consists to pressure effect of the tumor has been described in the literature. Two reports of mental nerve neuropathy or “Numb chin syndrome” as only presentation of BL have been documented.[5,19] Our case showed unusual presentation; despite the aggressive maxillary tumor extending to orbit did not show any common presenting signs and symptoms such as tooth mobility, tooth displacement, pain, and sensory disturbances except facial swelling. Intraoral radiography showing break in the lamina dura around the teeth is one of the earliest radiological changes followed by numerous ill-defined small foci of radiolucent areas, which coalesce to form large radiolucent areas.[2,9,14,16] Radiological changes in the jaws may precede clinical involvement in sBL and some proposed radiological investigations for patients with unexplained features suspicious of sBL.[9,14] The present case did not show any marked radiological changes apart from mild diffused rarefactions on occlusal radiograph despite of extensive tumor suggests radiological features may not have preceded the clinical involvement in the present case. The most common abdominal site of involvement is bowel and adjacent mesentery followed by retroperitoneum, hepatomegaly, splenomegaly, ileocaecal region, uterus, ascites, and adrenal mass in descending order. USG of abdomen commonly shows a lobulated hyper-mixed echogenic mass.[6,20] The less common right iliac region is involved in our case and USG showed hypoechoic mass with cystic spaces. No cases of hypoechoic mass with cystic spaces at abdominal sites on USG were reported in the literature.
Several blood investigations are consistently altered with sBL include anemia, thrombocytopenia, SGOT and SGPT, alkaline phosphatase, serum lactic dehydrogenase (LDH), serum uric acid, and blood urea nitrogen.[4,17] Elevated serum LDH level is indicator for severity of tumor spread and also considered consistent with recurrent tumors.[4,17] Elevated serum uric acid is indicator for tumor necrosis.[4,17] In our case, the onset and progression of tumor was rapid with no notable elevated hematological indicators apart from increased SGOT and SGPT.
CNS involvement is uncommon in sBL and presented as Babinski response in our case[2,11,12] Babinski response is plantar extension reflex suggestive of disease of spinal cord and brain. Babinski response associates with sBL was not reported by previous authors in the literature. CNS Involvement in sBL indicates poor prognosis and manifestation of relapse.[9,17] PubMed electronic search showed only seven cases of sBL involving orbit. sBL with orbital involvement mainly affects older adults and our patient is youngest of all reported case.[13,21] The presenting orbital signs and symptoms include eye pain, eyelid swelling, chemosis, altered visual acuity, proptosis, ptosis, diplopia, restricted ocular motility, and afferent papillary defect.[13,21] The orbital involvement in our case presented initially as diplopia of the right eye followed by the right side sixth cranial nerve and medial rectus palsy. The present case is the first patient of sBL associated with sixth nerve and medial rectus palsy and was not reported previously in the literature. The prognosis of most of sBL with orbital involvement is poor and only one patient out of eight, including the present case was alive at 5 years posttreatment.[13,21] The combination of neuro-orbital involvement resulted in extremely poor prognosis in the present case.
The aggressive sBL patients may not necessarily show the common presenting clinical signs and symptoms, radiological findings, and varied laboratory panel as were reported in the literature, but not seen with our patient. The incidence of sBL is increasing worldwide; varied and aggressive presentations can be documented like neuro-orbital involvement in our case. An unexplained aggressive clinical presentation must have a high index of suspicion for sBL. A delayed treatment in the present case resulted in neuro-orbital dissemination contributed to poor prognosis essentials the importance of early diagnosis, and definitive care of sBL patients.
Acknowledgments
We thank Dr. S. Shirley, former consultant pathologist, regional cancer centre, Chennai for providing histopathology and immunohistochemistry photomicrographs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Jan A, Vora K, Sándor GK. Sporadic Burkitt's lymphoma of the jaws: The essentials of prompt life-saving referral and management. J Can Dent Assoc. 2005;71:165–8. [PubMed] [Google Scholar]
- 2.Valenzuela-Salas B, Dean-Ferrer A, Alamillos-Granados FJ. Burkitt's lymphoma: A child's case presenting in the maxilla. Clinical and radiological aspects. Med Oral Patol Oral Cir Bucal. 2010;15:e479–82. doi: 10.4317/medoral.15.e479. [DOI] [PubMed] [Google Scholar]
- 3.Orem J, Mbidde EK, Lambert B, de Sanjose S, Weiderpass E. Burkitt's lymphoma in Africa, a review of the epidemiology and etiology. Afr Health Sci. 2007;7:166–75. doi: 10.5555/afhs.2007.7.3.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton LL, McMillan CW, Webster WP. American Burkitt's lymphoma: A 10-year review and case study. Oral Surg Oral Med Oral Pathol. 1990;69:307–16. doi: 10.1016/0030-4220(90)90291-y. [DOI] [PubMed] [Google Scholar]
- 5.Ugboko VI, Ndukwe KC, Adelusola KA, Durosinmi MA. Burkitt's lymphoma presenting as lower lip paraesthesia in a 24 year old Nigerian. Case report. Aust Dent J. 1999;44:58–60. doi: 10.1111/j.1834-7819.1999.tb00538.x. [DOI] [PubMed] [Google Scholar]
- 6.Bosco JI, Appaji L, Aruna K, Raghuram P, Rama Rao C, Vidya A. Clinical and radiological features of paediatric Burkitt's lymphoma – A four year study. Indian J Med Paediatr Oncol. 2007;28:14–7. [Google Scholar]
- 7.Cardy AH, Sharp L, Little J. Burkitt's lymphoma: A review of the epidemiology. Kuwait Med J. 2001;33:293–306. [Google Scholar]
- 8.Yeole BB. Trends in the incidence of Non-Hodgkin's lymphoma in India. Asian Pac J Cancer Prev. 2008;9:433–6. [PubMed] [Google Scholar]
- 9.Lund DI, Rodd H, Craig GT. Burkitt's lymphoma presenting with jaw lesions in a young white girl. Br J Oral Maxillofac Surg. 1997;35:438–41. doi: 10.1016/s0266-4356(97)90723-3. [DOI] [PubMed] [Google Scholar]
- 10.Ardekian L, Rachmiel A, Rosen D, Abu-el-Naaj I, Peled M, Laufer D. Burkitt's lymphoma of the oral cavity in Israel. J Craniomaxillofac Surg. 1999;27:294–7. doi: 10.1054/jcms.1999.0074. [DOI] [PubMed] [Google Scholar]
- 11.Nissenbaum M, Kaban LB, Troulis MJ. Toothache, paresthesia, and Horner syndrome: An unusual presentation of disseminated Burkitt's lymphoma. J Oral Maxillofac Surg. 2007;65:1395–401. doi: 10.1016/j.joms.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 12.Levine PH, Cho BR. Burkitt's lymphoma: Clinical features of North American cases. Cancer Res. 1974;34:1219–21. [PubMed] [Google Scholar]
- 13.Carmody J, Misra RP, Langford MP, Byrd WA, Ditta L, Vekovius B, et al. Orbital sporadic Burkitt lymphoma in an adult diabetic African American female and a review of adult orbital cases. Clin Ophthalmol. 2011;5:509–15. doi: 10.2147/OPTH.S16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui SH, Wong MH, Lam WY. Burkitt's lymphoma presenting as mandibular swelling – Report of a case and review of publications. Br J Oral Maxillofac Surg. 2000;38:8–11. doi: 10.1054/bjom.1999.0358. [DOI] [PubMed] [Google Scholar]
- 15.Patil K, Mahima VG, Jayanth BS, Ambika L. Burkitt's lymphoma in an Indian girl: A case report. J Indian Soc Pedod Prev Dent. 2007;25:194–9. doi: 10.4103/0970-4388.37018. [DOI] [PubMed] [Google Scholar]
- 16.Hanazawa T, Kimura Y, Sakamaki H, Yamaguchi A, Nagumo M, Okano T. Burkitt's lymphoma involving the mandible: Report of a case and review of Japanese cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:216–20. doi: 10.1016/s1079-2104(98)90429-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang MB, Strasnick B, Zimmerman MC. Extranodal American Burkitt's lymphoma of the head and neck. Arch Otolaryngol Head Neck Surg. 1992;118:193–9. doi: 10.1001/archotol.1992.01880020099022. [DOI] [PubMed] [Google Scholar]
- 18.Palled S, Bedre G, Muckaden MA, Ramadwar M, Bhagwat R, Banavali S, et al. Primary Burkitt's lymphoma of frontal bone: A rare presentation. J Clin Oncol. 2006;24:4521–2. doi: 10.1200/JCO.2006.06.2315. [DOI] [PubMed] [Google Scholar]
- 19.Martos-Díaz P, Bances-del-Castillo R, Vidal-Laso R, Mancha-de-la-Plata M, Cho-Lee GY, Naval-Gías L. Bilateral mental nerve neuropathy as the sole presenting symptom of Burkitt's Lymphoma. Med Oral Patol Oral Cir Bucal. 2009;14:e408–10. [PubMed] [Google Scholar]
- 20.Biko DM, Anupindi SA, Hernandez A, Kersun L, Bellah R. Childhood Burkitt lymphoma: Abdominal and pelvic imaging findings. AJR Am J Roentgenol. 2009;192:1304–15. doi: 10.2214/AJR.08.1476. [DOI] [PubMed] [Google Scholar]
- 21.Giuliari GP, Sadaka A, Cortez MA, Paniagua A. Orbital Burkitt's lymphoma: An aggressive presentation. Case Rep Ophthalmol Med 2012. 2012 doi: 10.1155/2012/354043. 354043. [DOI] [PMC free article] [PubMed] [Google Scholar]


