Abstract
Objective
To assess the representativeness of the Heart Protection Study (HPS) and the Collaborative Atorvastatin Diabetes Study (CARDS) for incident statin users.
Design
A population-based analysis with linked register data.
Setting
Finland.
Population
56 963 patients with diabetes initiating statin use from 2005 to 2008.
Main outcome measures
We determined the proportions of real-world patients who fulfilled the eligibility criteria for HPS and CARDS trials and assessed the cardiovascular disease (CVD) event rates, assumed to reflect the background CVD risk, for those eligible and ineligible. We used descriptive statistics to identify the patient characteristics, lipid-lowering interventions and adherence to statin therapy.
Results
Of the real-world patients, 57% (N=32 582) fulfilled the eligibility criteria for HPS (DM) and 49% (N=20 499) of those without CVD for CARDS. The patients ineligible for HPS (DM) had a higher cumulative risk for CVD events than those eligible, whereas regarding CARDS the cumulative risks were of similar magnitude. The overall CVD event rates seemed to be comparable to those in the reviewed trials. Both trials were under-representative of women and users of antihypertensive agents and metformin. 27% and 29% of real-world patients had an initial statin dose corresponding to <20 mg of simvastatin. The proportions of patients who were deemed adherent were 57% in the real world and 85% in both trials.
Conclusions
Only half of the real-world patients would have qualified for the HPS (DM) and CARDS, limiting their representativeness for clinical practice. Women and users of antihypertensive agents and metformin were under-represented in both trials. These deviations reflect the changes in diabetes treatment over the years and are not expected to modify the average treatment effects of statins on CVD. Prescribing of lower statin doses in clinical practice than used in the trials and lower adherence may, however, attenuate the benefits in the real world.
Keywords: adherence, diabetes, representativeness, randomised controlled trial, statin
Strengths and limitations of this study.
This is the first study to assess the representativeness of the Heart Protection Study (HPS) and the Collaborative Atorvastatin Diabetes Study (CARDS) for real-world diabetes care.
We assessed various aspects potentially affecting the representativeness of the HPS and CARDS trials: the trial eligibility criteria, the participant characteristics, the statin interventions and adherence to statin therapy.
All trial eligibility criteria could not be assessed by registry data and we may have slightly underestimated or overestimated the proportion of those eligible for the trials.
Our assessment represents a case study in Finland; since the treatment practices vary between countries, similar evaluations of the representativeness of RCTs in other countries are needed.
Background
For persons with diabetes, statins are widely recommended to lower the risk of cardiovascular disease (CVD) events.1–5 The absolute risk of CVD events is higher among people with diabetes than among those without diabetes, and the risk is further increased in the presence of diabetes and prior CVD.6 7 The current European guidelines for dyslipidaemia and CVD prevention recommend statin therapy for nearly all patients with type 2 diabetes mellitus, as well as for those without CVD.1–3 Only patients under the age of 40 years, with newly diagnosed type 2 diabetes, and without clinical complications or other CVD risk factors, may be withheld from statin therapy.1 In practice, at least 80% of the patients with diabetes who begin taking a statin seem to have no established CVD.8 9
A large meta-analysis of 14 randomised controlled trials (RCTs) including only participants with diabetes showed a relative reduction of 21% for major vascular events (ie, coronary event, coronary revascularisation or stroke) per every 1 mmol/L reduction in low-density lipoprotein (LDL) cholesterol associated with statin therapy.10 This meta-analysis confirmed the benefits of statins in diabetic dyslipidaemia, as suggested by some RCTs. The two landmark trials providing evidence on the efficacy of statins in preventing CVD events in diabetes are the subanalysis of the Heart Protection Study, HPS (DM),11 and the the Collaborative Atorvastatin Diabetes Study (CARDS).12 The HPS (DM) included patients with diabetes, 51% of whom also had occlusive arterial disease.11 In the HPS (DM) trial, simvastatin (40 mg) reduced the rate of major vascular events by 22% during a mean follow-up of 4.8 years. In the CARDS trial, only patients with type 2 diabetes but without CVD were randomised and the trial reported a 37% relative reduction in major vascular events for atorvastatin (10 mg) for a median duration of 3.9 years.12 While the relative risk reductions for CVD events in diabetes associated with statin therapy seem to be broadly the same across various patient subgroups,11 the absolute benefits increase in line with the patients’ background CVD risk.11 13 14
The HPS (DM) and CARDS trials are commonly cited in clinical guidelines on statin use in diabetes,1 3 4 yet no studies have assessed their representativeness regarding real-world diabetes care. However, knowledge of their representativeness is essential for understanding the applicability of their findings.15 Previous research on the representativeness of statin trials has typically focused on demographic characteristics such as gender and age.16–19 We wanted to expand the current knowledge and studied various aspects possibly affecting the representativeness of the HPS (DM) and CARDS trials: the trial eligibility criteria, the participant characteristics, the statin interventions and adherence to statin therapy. We used data from a nationwide Finnish health register to characterise real-world patients with diabetes initiating statin use between 2005 and 2008. We divided the patients into those fulfilling and those not fulfilling the eligibility criteria of the HPS (DM) and CARDS trials. Second, to further evaluate the implications of the eligibility criteria on representativeness, we determined the occurrence of CVD events, which was assumed to reflect the background CVD risk, for the eligible and ineligible patients. Finally, we assessed the characteristics, lipid-lowering interventions and adherence to statins among the real-world patients with diabetes.
Methods
Source of register data
We used the Diabetes in Finland (FinDM) database to identify patients with diabetes who initiated statin use between 2005 and 2008.20 The database was originally constructed for the monitoring of diabetes epidemiology, diabetes-related complications and diabetes care. It combines nationwide data from administrative health registers managed by the Finnish Social Insurance Institution (SII), the National Institute for Health and Welfare (THL) and Statistics Finland. The register data were linked on an individual basis using personal identification codes. The study group received a pseudonymous data set for its analyses. Persons with diabetes were identified as having been either reimbursed for purchases of drugs used in the treatment of diabetes (Anatomical Therapeutic Chemical code A10) or eligible for special reimbursement for medication costs due to diabetes or having a primary or secondary hospital discharge diagnosis for diabetes.20 The entitlement for special reimbursement is based on predefined criteria, a written certificate by the patient's treating physician and a review process conducted by the SII.
For all patients identified in the FinDM database, the data included information on all reimbursed prescription drug purchases in non-institutional settings (between 1994 and 2011), entitlements for special reimbursements due to severe chronic conditions such as coronary heart disease (CHD) (between 1964 and 2011), all hospitalisations (between 1969 and 2011), day surgical procedures (between 1994 and 2011) and outpatient hospital visits (between 1998 and 2011), with diagnostic information (ICD-8-9-10), admission and discharge dates and dates and causes of death (between 1971 and 2010).
We excluded women with gestational diabetes and persons having a single purchase for an antidiabetic drug or non-specific diabetes-related records only. The onset of diabetes refers to the first registration date of diabetes in any of the aforementioned registers. Accordingly, newly diagnosed diabetes was defined as diabetes registered 6 months or less before statin initiation. The type of diabetes was classified according to the type of antidiabetic medication purchased and the age of onset—patients under 40 years of age at the onset of diabetes with continuous prescription purchases for insulin and without purchases for medications that stimulate insulin secretion were defined as having type 1 diabetes mellitus and those not fulfilling these criteria were classified as having type 2 diabetes.
Ethical considerations
Permissions to collect data from the FinDM database were obtained from the maintainers of the registers.20 As a part of a larger diabetes research project, our study was approved by the ethics committee of the National Institute for Health and Welfare, Finland.
Study cohort
We identified all statin naïve patients with diabetes who made their first statin purchase (Anatomic Therapeutic Codes C10AA01-C10AA05, C10AA07, C10BA02) between 2005 and 2008. This period was chosen to capture recently treated patients and to attain a follow-up period comparable to those of the HPS and CARDS trials. The first statin purchase refers to no statins dispensed during the preceding 3 years.21 We defined the date of the first statin purchase as the cohort entry date for all individuals. The follow-up time was calculated from the cohort entry date until the first occurrence of the composite end point of a major cardiovascular event or censoring due to non-CVD-related death, permanent institutionalisation or 31 December 2010, whichever was first.
Major cardiovascular events
We chose a composite end point of CVD events as the outcome of interest, including acute myocardial infarction (as a primary or secondary discharge diagnosis of International Classification of Diseases (ICD)-10 I-21, I-22) and/or a coronary revascularisation procedure (procedure codes for coronary artery bypass grafting, angioplasty or stenting), stroke (as a primary or secondary discharge diagnosis of ICD-10, I-60, I-61, I-63) or CVD given as a primary cause of death (ICD-10 codes I-20–I-25, I-46, R96, R98, G45 and I-60–I-69).22 The validity of the Hospital Discharge Register and the Causes of Death Register for capturing CHD events and strokes is good.23–25 The index date for the event was either the hospital admission date or the date of out-of-hospital death.
Variables
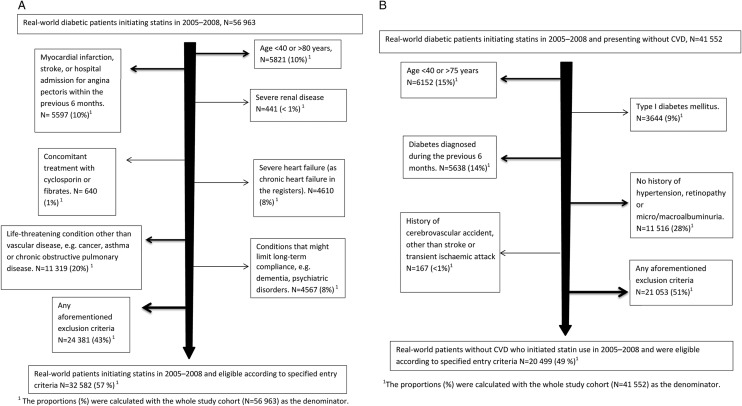
We extracted patient-level information on demographic characteristics, complications and prescribed medications related to diabetes, and cardiovascular comorbidities (table 1). Data were also extracted to cover the inclusion and exclusion criteria of the HPS11 13 and CARDS12 trials, as far as available in the registers (see figure 1A, B and online supplementary appendix table). For comorbidities, we used the corresponding ICD-10 codes captured in the Hospital Discharge Register. When available, additional data from the SII Special Reimbursement Register (including data on CHD, moderate or severe hypertension, chronic heart failure, chronic pulmonary diseases, malignancy, dementia and psychotic disorders) and the Prescription Register (purchases for specific medications used to treat hypertension, chronic pulmonary disorders, malignancies, dementia and psychotic disorders) were used. We collected data on comorbidities and vascular procedures from the 9 years preceding the cohort entry date, as well as data on prescriptions dispensed from the 4 months preceding this date. We further categorised the cohort members according to the presence of established CVD at the time of statin initiation, that is, applicable ICD-10 codes for CHD (ie, myocardial infarction, angina pectoris or unstable angina pectoris), stroke, transient ischaemic attack or peripheral vascular disease, surgical procedure codes for coronary revascularisation, amputation or other peripheral vascular procedure due to atherosclerosis or codes for special reimbursement due to CHD.
Table 1.
Characteristics of the real-world patients with diabetes and initiating statin therapy and of the participants of the HPS-DM11 and CARDS12 trials
| Study population | HPS-DM11 | Real-world patients with diabetes (all) | CARDS12 (see online supplementary appendix) | Real-world patients with diabetes and without CVD* |
|---|---|---|---|---|
| Years of recruitment or cohort entry | 1994–1997 | 2005–2008 | 1997–2001 | 2005–2008 |
| Number of patients | 5963 | 56 963 | 2838 | 41 552 |
| Mean age in years (SD) | 62.1 (8.9) | 61.9 (12.4) | 61.5 (8.3) | 59.5 (12.0) |
| Min | 40 | 8 | 40 | 8 |
| Max | 80 | 98 | 75 | 96 |
| Age in years, N (%) | ||||
| <40 | 2384 (4) | 2266 (5) | ||
| 40–80 | 50 347 (88) | 37 622 (91) | ||
| ≥80 | 4232 (7) | 1664 (4) | ||
| Men, N (%) | 4147 (70) | 31 912 (56) | 972 (68) | 22 605 (54) |
| Cardiovascular disease, N (%) | 3051 (51) | 15 411 (27) | 0† | 0 |
| Coronary heart disease, N (%) | 1981 (33) | 11 054 (19) | 0† | 0 |
| Previous myocardial infarction, N (%) | 1125 (19) | 3742 (7) | 0† | 0 |
| Stroke, N (%) | NA | 3713 (7) | 0† | 0 |
| Peripheral arterial disease, N (%) | NA | 2046 (4) | 0† | 0 |
| Type I diabetes, N (%) | 615 (10) | 4463 (8) | 0 | 3644 (9) |
| Diabetes duration in years (SD) | 9.3 (8.9)‡ | 7.6 (8.5) | 7.9 (6.4) | 7.1 (8.2) |
| Diabetes duration ≤ 0.5 years, N (%) | NA | 7514 (13) | 0† | 5638 (14) |
| Retinopathy, N (%) | NA | 4569 (8) | 426 (30) | 3051 (7) |
| Microalbuminuria/macroalbuminuria, N (%) | NA | 124 (<1) | 172 (12) | 84 (<1) |
| Nephropathy, N (%) | 0† | 441 (<1) | 0† | 232 (<1) |
For the CARDS trial, only data for the arm treated with statins (N=1428) are presented.
*According to previous cardiovascular disease (CVD) status at baseline.
†Assumed on the basis of trial eligibility criteria.
‡Data for patients with type 2 diabetes.
CARDS, Collaborative Atorvastatin Diabetes Study; HPS-DM, Heart Protection Study-diabetes mellitus; NA, data not available.
Figure 1.
(A) Flow chart of real-world patients fulfilling a criterion for exclusion in the Heart Protection Study11 or fulfilling all eligibility criteria. (B) Flow chart of real-world patients fulfilling a criterion for exclusion in the Collaborative Atorvastatin Diabetes Study12 or fulfilling all eligibility criteria.
We calculated adherence to statin therapy for the cohort members as the truncated medication possession ratio (MPR).26 We assumed a dosage of one statin tablet per day and divided the total number of statin tablets dispensed by the total number of days of follow-up (since cohort entry to event occurrence or censoring). We calculated the MPR for all those followed for event occurrence, as well as separately for those surviving at the end of each year of follow-up. In both cases, we calculated the MPR for the period of interest and defined patients with good adherence to statin use as those with an MPR of ≥80%, a conventional cut-off value.27 28
To study dose titration, we calculated the daily statin doses as simvastatin equivalents,29 at statin initiation and at the end of 1 year of follow-up, for patients surviving for 365 days after the initiation and with at least one refill during the latter part of the follow-up year (ie, between 180 and 365 days after initiation).
Statistical analyses
We calculated the proportions of real-world patients meeting the eligibility criteria applied in the HPS (DM) trial and of those meeting any single criterion for exclusion (figure 1A). For both groups of patients deemed either eligible or ineligible, we estimated the cumulative hazard function for the composite end point using a Nelson-Aalen estimator (figure 2A) in a stratified survival analysis from the date of the first statin purchase until the date of the composite end point or censoring. As the CARDS trial included only patients without established CVD at cohort entry, we determined the denominator accordingly from the subgroup of real-world patients presenting without CVD (figure 1B) and performed similar analyses applying the eligibility criteria of the CARDS trial (figure 2B). We regarded the risk of CVD events during statin therapy as a function of the patient's background CVD risk and the effect of statins in reducing CVD, as performed by Van Staa et al.30 Statin therapy reduced the relative risk of major CVD events by 22% in the HPS (DM) trial11 and by 37% in the CARDS trial.12 If it is assumed that statins had a similar effect on our cohort members, the underlying CVD risks would have been 22–37% higher than those observed in this study.
Figure 2.
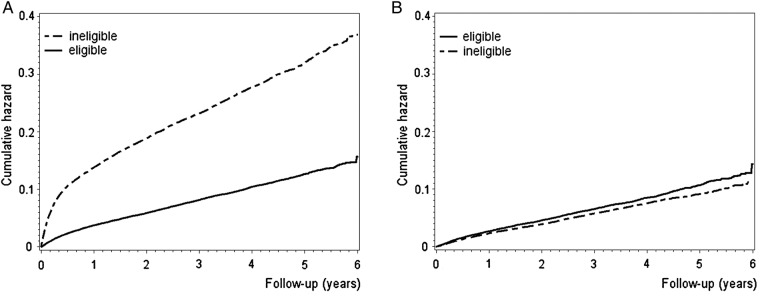
(A) Cumulative hazard for major vascular events among real-world patients with diabetes deemed eligible and ineligible for the Heart Protection Study.11 (B) Cumulative hazard for major vascular events among real-world patients with diabetes with no cardiovascular disease and deemed eligible and ineligible for the Collaborative Atorvastatin Diabetes Study.12
We used descriptive statistics to determine the patient characteristics and lipid-lowering interventions for all of the cohort members and also separately for those without CVD at statin initiation. We used the SAS release 9.2 program package (SAS Institute, Cary, North Carolina, USA) for all of the statistical analyses.
Results
Trial eligibility criteria and real-world patients
Altogether, 56 963 patients with diabetes initiated statin use between 2005 and 2008 in Finland. Most of them, 73% (N=41 552), had no apparent CVD at cohort entry. Fifty-seven per cent of the patients fulfilled the eligibility criteria of the HPS (DM) trial (figure 1A, see also online supplementary appendix table), and 49% of those with no established CVD fulfilled the criteria for the CARDS trial (figure 1B, see also see online supplementary appendix table). Patients with a recent CVD event were deemed ineligible for both trials.
Among the initiators, a major CVD event occurred for 8714 patients (15%) during a mean follow-up of 3.4 years. There were 2499 acute myocardial infarctions, 3163 strokes, 1856 coronary procedures and 1196 CVD deaths captured as first events in the registers. Altogether, 48 249 patients were censored: 2212 (4%) died of non-CVD-related causes, 942 (2%) were institutionalised and 45 095 (79%) reached the end of the study period (31 December 2010) without a major CVD event. For patients not meeting the eligibility criteria of the HPS (DM) trial, the cumulative risk of a CVD event occurring was two to three times higher compared to those who were eligible, whereas the cumulative risk for a CVD event occurring among the patients eligible or ineligible for the CARDS trial, all without established CVD, was of the same magnitude (figure 2).
Patient characteristics
Table 1 presents the descriptive characteristics for all of the real-world patients, as well as separately for those without CVD, for comparison with the data from the HPS (DM) and CARDS trials.
The respective data on medication use can be found in table 2.
Table 2.
Medications used by the real-world patients with diabetes and initiating statin therapy and those used by participants in the HPS-DM11 and CARDS12 trials
| Study population | HPS-DM11 | Real-world patients with diabetes (all) | CARDS12 (see online supplementary appendix) | Real-world patients with diabetes and without CVD* |
|---|---|---|---|---|
| Number of patients | 5963 | 56 963 | 2838 | 41 552 |
| Diabetes treatment, N (%) | ||||
| Diet only | NA (21) | 8325 (15) | 214 (15) | 5418 (13) |
| Insulin | NA (25†) | 15 564 (27) | 282 (20) | 10 874 (26) |
| Metformin | NA (31) | 34 606 (61) | 672 (47) | 26 716 (64) |
| Sulfonylurea | NA (42) | 16 312 (29) | 730 (51) | 11 059 (27) |
| Other oral agents | NA | 4363 (8) | NA | 3595 (9) |
| Treated hypertension or drugs lowering blood pressure, N (%)‡ | 2398 (40) | 42 885 (75) | 956 (67) | 28 818 (69) |
| Blood pressure lowering drugs, N (%) | ||||
| β-blockers | NA | 23 898 (42) | 219 (15) | 13 248 (32) |
| Calcium antagonists | NA | 13 686 (24) | 304 (21) | 8967 (22) |
| ACE inhibitors or angiotensin II receptor antagonists | NA | 32 683 (57) | 637 (45) | 22 557 (54) |
| Diuretics | NA | 11 942 (21) | 262 (18) | 6616 (16) |
For the CARDS trial, only data for the statin treated arm (N=1428) are presented.
*According to previous cardiovascular disease (CVD) status at baseline.
†Data for patients with type 2 diabetes.
‡Diuretics, β-blocking agents, calcium channel blockers and agents acting on the renin–angiotensin-pathway for the study cohort.
CARDS, Collaborative Atorvastatin Diabetes Study; HPS-DM, Heart Protection Study-diabetes mellitus; NA, data not available.
Compared with the HPS (DM) population, the real-world patients were less often male. The mean age was, however, well balanced, the proportions of those younger than 65 years being about 60%. The proportion of patients with CVD (or CHD) was lower (27% vs 51%), and the mean duration of diabetes was shorter (7.6 vs 9.3 years) among the real-world patients than in the HPS (DM) trial. Compared with the CARDS participants, real-world patients without established CVD were also less often male and were younger. Specifically, within the age bracket 40–75 years, 53% of the real-world patients (N=34 682) were younger than 60 years, the corresponding proportion being 39% in the CARDS trial. The mean duration of diabetes was well balanced between the real-world patients and the CARDS participants. Newly diagnosed diabetes was an exclusion criterion in the CARDS trial, whereas 14% of the real-world patients belonged to that group. Use of antihypertensive medication and metformin was more common, and the use of sulfonylureas less common, among the real-world patients than among participants in both trials.
Lipid-lowering interventions
We give the distributions for statin doses at initiation and during the second half of the first year of follow-up as simvastatin equivalents in table 3.
Table 3.
Distributions (number and proportion) of statin doses as simvastatin equivalents at initiation and after 1 year of follow-up according to statin prescriptions redeemed by all patients with diabetes and by those without cardiovascular disease at statin initiation between 2005 and 2008 and surviving the following first year
| Real-world patients with diabetes (all) |
Real-world patients with diabetes and without cardiovascular disease |
|||
|---|---|---|---|---|
| Total number of patients | N=45 463 |
N=33 219 |
||
| Simvastatin (C10AA01) equivalent dose* | At initiation | After follow-up | At initiation | After follow-up |
| 5 mg | 142 (<1) | 185 (<1) | 105 (<1) | 142 (<1) |
| 10 mg | 12 180 (27) | 11 079 (24) | 9645 (29) | 8671 (26) |
| 20 mg | 23 671 (52) | 23 065 (51) | 17 803 (54) | 17 364 (52) |
| 40 mg | 7178 (16) | 8508 (19) | 4097 (12) | 5261 (16) |
| 80 mg | 2017 (4) | 2197 (5) | 1409 (4) | 1518 (5) |
| >80 mg | 225 (<1) | 368 (<1) | 120 (<1) | 216 (<1) |
*For equivalence, atorvastatin 10 mg=fluvastatin 80 mg=lovastatin 40 mg=pravastatin 40 mg=simvastatin 20 mg ≤rosuvastatin 5 mg.29
About one-quarter (27%) of all the real-world patients and 29% of those without CVD had a statin prescribed at a dose corresponding to <20 mg of simvastatin. These proportions did not change appreciably during the 1-year follow-up. Only 1% used fibrates at statin initiation (N=607 for all of the patients and N=406 for those without CVD).
For the real-world patients, the mean MPR was 72% for a mean follow-up of 3.4 years. The proportion of survivors with good adherence to statins (MPR ≥80%) decreased gradually from 63% (32 886/51 905) at the end of the first year of follow-up to 42% (4059/9626) at the end of the fifth year, the average proportion for all five study years being 57%. The corresponding proportions for the original HPS study participants were 89% and 82%, respectively, with an average proportion of 85%. In the CARDS trial, the proportion of participants with good adherence to lipid-lowering interventions was 90% for the first year, 78% for the fourth year (last available) and 85% on average.
Discussion
Limitations in representativeness for the real-world diabetes care were found when HPS (DM) and CARDS trials were compared with population level data. Approximately half of the real-world statin initiators with diabetes would have qualified for these two landmark randomised controlled trials. The background CVD risk, approximated by the cumulative risk for major CVD events after statin initiation, was either of the same magnitude or remarkably higher among those deemed ineligible in comparison with those eligible for the trials. Women were under-represented in both trials, and concomitant antihypertensive medications and metformin were more commonly used in the real-world setting. Furthermore, almost 30% of the real-world patients were prescribed statin doses that were lower than those used in the trials, and about 40% of the patients did not adhere to their statin therapy.
In RCTs, the legitimate aim of applying often strict eligibility criteria is to obtain a homogeneous trial population with minimum noise. This may, however, limit the representativeness of the RCTs for real-world clinical care.15 31–33 Yet, for the limitations in representativeness to have clinical impact, they would have to question the applicability of the trial findings for real-world clinical care. That is, they would have to modify the relative or absolute treatment effects of statins on CVD as they are translated to real-world settings from the trials.15 The proportions of patients deemed eligible in our study were similar to those recently reported in the UK for RCTs on novel oral anticoagulants (48–64%)34 and for the UK Prospective Diabetes Study (32–51%)35 but clearly higher than those for various other RCTs on intensive glucose lowering (4–36%).35 The background risk, reflected in the cumulative risk for CVD events after statin initiation among patients deemed ineligible, was either similar or remarkably higher as compared with those eligible. Correspondingly, the CVD event rate observed in the CARDS trial (about 10% in the placebo arm in 4 years)12 approximates the event rates observed in our study for those without CVD and deemed eligible or ineligible for CARDS. Absolute risk reduction associated with statin therapy in diabetes depends on the background risk for CVD events.11 13 14 Therefore, it seems reasonable to expect that the absolute risk reduction among all real-world patients with diabetes and without CVD has been of the same magnitude as that observed for patients with diabetes in CARDS. However, the event rate of the real-world patients meeting the eligibility criteria of the HPS (DM) trial was lower than that observed in the trial (about 20% in the placebo arm in 4 years).11 This may be explained by our finding on the larger proportion of real-world patients presenting without CVD at statin initiation. Also, the event rate was clearly higher for the patients deemed ineligible for HPS (DM). This, for comparison, may reflect the trial exclusion of high-risk patients with recent CVD events who are yet likely to benefit from intensive statin therapy.36 Still, the average event rate for all of the initiators, regardless of eligibility, was similar to the rate of major CVD events in the HPS (DM) trial (data not shown).
Both reviewed trials included fewer women and the use of antihypertensive medications and metformin was less common than in clinical practice. In addition, the HPS (DM) trial was under-representative regarding patients without CVD. Overall, the observations reflect a changing scenario of treatment over the years. Most of the placebo controlled statin RCTs, including CARDS and HPS (DM), recruited their participants with diabetes by the year 2001 or earlier.11 12 37–40 Since then, the diagnostic criteria for diabetes have broadened, and the recommendations for screening, nutrition and self-management have intensified.3–5 41 42 Statins are initiated in the earlier stages of the disease8 9 and the risk of CVD events associated with female gender is acknowledged.16 19 43 The role of metformin in the front line of therapy has become stronger,44–46 and the use of sulfonylureas has declined.44–46 This trend has been accompanied by improvements in blood pressure control44 45 47 and a decline in mortality in diabetes.48 The relative treatment effects of statins in CVD prevention in diabetes are constant regardless of gender, age, prior CVD, diabetes duration and the use of antihypertensive treatment.11 Therefore, the aforementioned deviations in patient characteristics and concomitant medications between the real-world clinical care and the reviewed trials are not expected to modify the relative treatment effects. However, the relative and absolute effects of statins vary with the type and dose of the statin intervention.11 12 Our findings support the common notion that the cumulative statin doses and adherence to statin therapy in the real world are lower than in the RCTs.27 49 50 This may attenuate the benefit and highlights the need for more appropriate implementation of evidence-based statin therapies in the real world.
Strengths and limitations
This is the first study to evaluate the representativeness of statin trials for real-world statin initiators with diabetes in terms of eligibility criteria, participant characteristics and medication use. The validity of our data for capturing real-world patients with pharmacologically treated diabetes in clinical practice in Finland is good.51 However, our study has some limitations owing to our reliance on register data. We have missed some patients with diabetes, especially among persons aged 65 years or more, who either had undiagnosed diabetes or who were on diet therapy only and had not received any hospital care.51 We had no data on liver function tests, cholesterol levels or smoking habits, and the actual date of diabetes onset was estimated using the first diabetes-related record in any of the registers. In addition, some complications, such as retinopathy, microalbuminuria and nephropathy, are likely to have been under-ascertained. Furthermore, the validity of our data on capturing clinically determined diabetes subtype has not been studied. Therefore, we were not able to define the eligibility criteria exactly in the same way that the reviewed trials had done, and we may have slightly underestimated or overestimated the proportions of eligible patients. While our dose assumption of one tablet per day is likely to reflect the prescribed statin dose,52 actual statin use could not be verified from the prescription register. Our study population included statin initiators only, and long-term users and non-users were excluded. Additionally, we focused on statin initiations from 2005 to 2008, and therefore our study populations may not reflect the current patients. In order to update the results, we analysed the characteristics of all statin initiators with diabetes in 2010 (N=12 541).We did not observe any appreciable changes in the age profile, proportion of men, proportion of those with medically treated hypertension or the proportion of patients with CVD, nor in the mean duration of diabetes (data not shown). However, the use of metformin was even more common (68%), and the use of other orally administered antidiabetic agents was of the same magnitude as that of sulfonylureas (12% and 13%, respectively). In addition, there was a shift towards higher statin doses: only one-fifth of the patients in 2010 initiated their statin use with a dose corresponding to <20 mg of simvastatin. Finally, diabetes care, including treatment of dyslipidaemia, is constantly evolving53 and our assessment represents a case study in Finland. Although the prevalence of statin use in diabetes in Finland seems to be at an average level,54 the treatment practices vary between countries.55 Thus, similar evaluations of the representativeness of RCTs with respect to the care of patients with diabetes in other countries are needed.
Conclusions
Only half of the real-world patients initiating statin use would have qualified for two landmark trials on statin use in persons with diabetes, the HPS (DM) and CARDS, limiting their representativeness for clinical practice. Women and users of antihypertensive agents and metformin were under-represented in both trials.
These deviations reflect the changes in diabetes treatment over the years and are not expected to modify the average treatment effects of statins on CVD. Prescribing of lower statin doses in clinical practice than those used in the reviewed trials and lower adherence may, however, attenuate the benefits in the real world when compared with the RCTs.
Supplementary Material
Acknowledgments
The authors thank the Social Insurance Institution, Finland and Orion Farmos Research Foundation for supporting this study. Georgianna Oja, ELS, is acknowledged for editing the language in this manuscript.
Footnotes
Contributors: PR designed the study, interpreted the data, drafted the article and is the guarantor of the study. RS designed the study, was involved with the acquisition of the data, designed the statistical analysis, interpreted the data and revised the article. MA designed the study, conducted the data analysis and revised the article. AH-S, RH, IK, TV and MJK designed the study, interpreted the data and revised the article.
Funding: This study was funded by a grant from the Social Insurance Institution (SII) (10/26/2007).
Competing interests: PR had support from the Social Insurance Institution of Finland and the Orion Farmos Research Foundation for the submitted work. RS, AH-S, TV, IK and RH had support from the Social Insurance Institution of Finland for the submitted work. RH had a financial relationship with the Academy of Finland and Hospital District of Southwest Finland within the previous 3 years. MJK had a financial relationship with the Social Insurance Institution of Finland within the previous 3 years. MA declares no competing interests.
Ethics approval: The ethics committee of the National Institute for Health and Welfare, Finland.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Additional data are available by emailing the corresponding author (PR).
References
- 1.The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J 2011;32:1769–818 [DOI] [PubMed] [Google Scholar]
- 2.The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). European guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur J Prev Cardiol 2012;4:585–667 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Clinical Excellence. Type 2 diabetes: the management of type 2 diabetes (update)—clinical guidelines 2008. http://www.nice.uk.com [Google Scholar]
- 4.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care 2013;36(Suppl 1):S11–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finnish Medical Society Duodecim. Diabetes (online) Käypä Hoito–suositus. (In Finnish, summary in English: Diabetes. Current care guideline). Working group appointed by the Finnish Medical Society Duodecim, the Finnish Society of Internal Medicine and the Medical Advisory Board of the Finnish Diabetes Society; Helsinki, 2011. http://www.kaypahoito.fi [Google Scholar]
- 6.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998;339:229–34 [DOI] [PubMed] [Google Scholar]
- 7.Schramm TK, Gislason GH, Køber L, et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation 2008;117:1945–54 [DOI] [PubMed] [Google Scholar]
- 8.Dominguez H, Schramm TK, Norgaard ML, et al. Initiation and persistence to statin treatment in patients with diabetes receiving glucose-lowering medications 1997–2006. Open Cardiovasc Med J 2009;3:152–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eliasson B, Svensson AM, Miftaraj M, et al. Clinical use and effectiveness of lipid lowering therapies in diabetes mellitus-an observational study from the Swedish National Diabetes Register. PLoS ONE 2011;6:e18744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cholesterol Treatment Trialistś (CTT) Collaborators. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–25 [DOI] [PubMed] [Google Scholar]
- 11.Collins R, Armitage J, Parish S, et al. Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet 2003;361:2005–16 [DOI] [PubMed] [Google Scholar]
- 12.Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685–96 [DOI] [PubMed] [Google Scholar]
- 13.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7–2212114036 [Google Scholar]
- 14.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials. Lancet 2010;376:1670–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atkins D, Chang S, Gartlehner G, et al. Assessing the Applicability of Studies When Comparing medical Interventions. Agency for Healthcare Research and Quality, January 2011. Methods Guide for Comparative Effectiveness Reviews. AHQR Publication No. 11-EHC019-EF. http://effectivehealthcare.ahrq.gov/ (accessed 6 May 2012) [PubMed] [Google Scholar]
- 16.Bartlett C, Doyal L, Ebrahim S, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technol Assess 2005;9:iii.– ix–x, 1–152 [DOI] [PubMed] [Google Scholar]
- 17.Konrat C, Boutron I, Trinquart L, et al. Underrepresentation of elderly people in randomised controlled trials. The example of trials of 4 widely prescribed drugs. PLoS ONE 2012;7:e33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bandyopadhyay S, Bayer AJ, O'Mahony MS. Age and gender bias in statin trials. QJM 2001;94:127–32 [DOI] [PubMed] [Google Scholar]
- 19.Wei L, Ebrahim S, Bartlett C, et al. Statin use in the secondary prevention of coronary heart disease in primary care: cohort study and comparison of inclusion and outcome with patients in randomised trials. BMJ 2005;330:821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sund R, Koski S. FinDM II. On the register-based measurement of the prevalence and incidence of diabetes and its long-term complications. A Technical Report Tampere: The Finnish Diabetes Association, 2009 [Google Scholar]
- 21.Korhonen MJ, Helin-Salmivaara A, Huupponen R. Dynamics of long-term statin therapy. Eur J Clin Pharmacol 2011;67:925–31 [DOI] [PubMed] [Google Scholar]
- 22.Winell KM, Pääkkönen R, Pietilä A, et al. Case fatality rates after first acute coronary syndrome in persons treated for type 2 diabetes show an improving trend. Diabetologia 2010;53:472–80 [DOI] [PubMed] [Google Scholar]
- 23.Pajunen P, Koukkunen H, Ketonen M, et al. The validity of the Finnish Hospital Discharge Register and Causes of Death Register data on coronary heart disease. Eur J Cardiovasc Prev Rehabil 2005;12:132–7 [DOI] [PubMed] [Google Scholar]
- 24.Tolonen H, Salomaa V, Torppa J, et al. The validation of the Finnish Hospital Discharge Register and Causes of Death Register data on stroke diagnoses. Eur J Cardiovasc Prev Rehabil 2007;3:380–5 [DOI] [PubMed] [Google Scholar]
- 25.Sund R. Quality of the Finnish Hospital Discharge Register: a systematic review. Scand J Public Health 2012;40:505–15 [DOI] [PubMed] [Google Scholar]
- 26.Leslie RS, Gwadry-Sridhar F, Thiebaud P, et al. Calculating medication compliance, adherence and persistence in administrative pharmacy claims databases. Pharm Programming 2009;1:13–19 [Google Scholar]
- 27.Benner J, Glynn R, Mogun H, et al. Long-term persistence in use of statin therapy in elderly patients. JAMA 2002;288:455–61 [DOI] [PubMed] [Google Scholar]
- 28.Perreault S, Dragomir A, Blais L, et al. Impact of better adherence to statin agents in the primary prevention of coronary artery disease. Eur J Clin Pharmacol 2009;65:1013–24 [DOI] [PubMed] [Google Scholar]
- 29. US Food and Drug Administration: Relative LDL-lowering Efficacy of Statin and Statin-based Therapies. http://www.fda.gov (accessed in Jul 2012)
- 30.van Staa TP, Smeeth L, Ng ESet al. The efficiency of cardiovascular risk assessment: do the right patients get statin treatment? Heart 2013;99:1597–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rothwell PM. External validity of randomised controlled trials: ‘to whom do the results of this trial apply?’ Lancet 2005;365:82–93 [DOI] [PubMed] [Google Scholar]
- 32.Van Spall HG, Toren A, Kiss A, et al. Eligibility criteria of randomized controlled trials published in high-impact general medical journals: a systematic sampling review. JAMA 2007;297:1233–40 [DOI] [PubMed] [Google Scholar]
- 33.Britton A, McKee M, Black N, et al. Threats to applicability of randomised trials: exclusions and selective participation. J Health Serv Res Policy 1999;4:112–21 [DOI] [PubMed] [Google Scholar]
- 34.Lee S, Monz BU, Clemens A, et al. Representativeness of the dabigatran, apixaban and rivaroxaban clinical trial populations to real-world atrial fibrillation patients in the United Kingdom: a cross-sectional analysis using the General Practice Research Database. BMJ Open 2012;14, 2:pii:e001768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saunders C, Byrne CD, Guthrie B, et al. External validity of randomized controlled trials of glycemic control and vascular disease: how representative are participants? Diabet Med 2013;30:300–8 [DOI] [PubMed] [Google Scholar]
- 36.Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504 [DOI] [PubMed] [Google Scholar]
- 37.Pyörälä K, Pedersen TR, Kjekshus J, et al. Cholesterol lowering with simvastatin improves prognosis of diabetic patients with coronary heart disease: a subgroup analysis of the Scandinavian Simvastatin Survival Study (4S). Diabetes Care 1997;20:614–20 [DOI] [PubMed] [Google Scholar]
- 38.Goldberg RB, Mellies MJ, Sacks FM, et al. Cardiovascular events and their reduction with pravastatin in diabetic and glucose-intolerant myocardial infarction survivors with average cholesterol levels: subgroup analyses in the cholesterol and recurrent events (CARE) trial. Circulation 1998;98:2513–19 [DOI] [PubMed] [Google Scholar]
- 39.Knopp RH, d'Emden M, Smilde JG, et al. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006; 29:1478–85 [DOI] [PubMed] [Google Scholar]
- 40.Sever PS, Poulter NR, Dahlöf B, et al. Reduction in cardiovascular events with atorvastatin in 2,532 patients with type 2 diabetes: Anglo-Scandinavian Cardiac Outcomes Trial-lipid-lowering arm (ASCOT-LLA). Diabetes Care 2005;28:1151–7 [DOI] [PubMed] [Google Scholar]
- 41.American Diabetes Association. Nutrition recommendations and interventions for diabetes—2006. Diabetes Care 2006;29:2140–57 [DOI] [PubMed] [Google Scholar]
- 42.American Association of Diabetes Educators. Position statement. intensive diabetes management: implications of the DCCT and UKPDS. Diabetes Educ 2002;28:735–40 [DOI] [PubMed] [Google Scholar]
- 43.Kuusisto J, Laakso M. Update on type 2 diabetes as a cardiovascular disease risk equivalent. Curr Cardiol Rep 2013;15:331. [DOI] [PubMed] [Google Scholar]
- 44.Charlton J, Latinovic R, Gulliford MC. Explaining the decline in early mortality in men and women with type 2 diabetes: a population-based cohort study. Diabetes Care 2008;9:1761–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mann DM, Woodward M, Ye F, et al. Trends in medication use among US adults with diabetes mellitus: glycemic control at the expense of controlling cardiovascular risk factors. Arch Intern Med 2009;169:1718–20 [DOI] [PubMed] [Google Scholar]
- 46.González EL, Johansson S, Wallander MA, et al. Trends in the prevalence and incidence of diabetes in the UK: 1996-2005. J Epidemiol Community Health 2009;63:332–6 [DOI] [PubMed] [Google Scholar]
- 47.Vehko T, Manderbacka K, Arffman M, et al. Changing patterns of secondary preventive medication among newly diagnosed coronary heart disease patients with diabetes in Finland: a register-based study. Scand J Public Health 2010;38:317–24 [DOI] [PubMed] [Google Scholar]
- 48.Gulliford MC, Charlton J. Is relative mortality of type 2 diabetes mellitus decreasing? Am J Epidemiol 2009;169:455–61 [DOI] [PubMed] [Google Scholar]
- 49.Donnelly LA, Doney ASF, Morris AD, et al. Long-term adherence to statin treatment in diabetes. Diabet Med 2008;25:850–5 [DOI] [PubMed] [Google Scholar]
- 50.Perreault S, Blais L, Lamarre D, et al. Persistence and determinants of statin therapy among middle-aged patients for primary and secondary prevention. Br J Clin Pharmacol 2005;59:564–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sund R, Harno K, Ranta S, et al. Evaluation of case inclusion in two population-based diabetes registers. Fin J eH eW 2010;2:136–46 [Google Scholar]
- 52.Lesen E, Sandstrom TZ, Carlsten A, et al. A comparison of two methods for estimating refill adherence to statins in Sweden: the RARE project. Pharmacoepidemiol Drug Saf 2011;20: 1073–9 [DOI] [PubMed] [Google Scholar]
- 53.Rydén L, Grant PJ, Anker SD, et al. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 2013;34:3035–87 [DOI] [PubMed] [Google Scholar]
- 54.Kiivet R, Sund R, Linna M, et al. Methodological challenges in international performance measurement using patient-level administrative data. Health Policy 2013;112:110–21 [DOI] [PubMed] [Google Scholar]
- 55.Stone MA, Charpentier G, Doggen K, et al. Quality of care of people with type 2 diabetes in eight European countries. Diabetes Care 2013;36:2628–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.