SUMMARY
The human immunodeficiency virus (HIV) associated tuberculosis (TB) epidemic remains an enormous challenge to TB control in countries with a high prevalence of HIV. In their 1999 article entitled ‘Will DOTS do it?’, De Cock and Chaisson questioned whether the World Health Organization’s DOTS Strategy could control this epidemic. Data over the past 10 years have clearly shown that DOTS is insufficient as a single TB control intervention in such settings because it does not address the fundamental epidemiological interactions between TB and HIV. Immunodeficiency is a principal river of this epidemic, and the solution must therefore include immune recovery using antiretroviral therapy (ART). Thus, in the era of global ART scale-up, we now ask the question, ‘Will ART do it?’ ART reduces the risk of TB by 67% (95%CI 61–73), halves TB recurrence rates, reduces mortality risk by 64–95% in cohorts and prolongs survival in patients with HIV-associated drug-resistant TB. However, the cumulative lifetime risk of TB in HIV infected individuals is a function of time spent at various CD4-defined levels of risk, both before and during ART. Current initiation of ART at low CD4 cell counts (by which time much HIV-associated TB has already occurred) and low effective coverage greatly undermine the potential impact of ART at a population level. Thus, while ART has proven a critical intervention for case management of HIV-associated TB, much of its preventive potential for TB control is currently being squandered. Much earlier ART initiation with high coverage is required if ART is to substantially influence the incidence of TB.
Keywords: HIV, TB, antiretroviral, control, incidence, mortality, Africa
INTRODUCTION
The Millennium Development Goals (MDGs) and associated World Health Organization (WHO) Stop TB targets for global tuberculosis (TB) control are to halt and start to reverse the rising incidence of TB and to halve the 1990 TB prevalence and death rates by 2015.1,2 Although progress is being made, these global targets are unlikely to be met.3 One of the major obstacles to meeting these targets is the HIV associated TB epidemic. HIV infection, the strongest known risk factor for TB, has fuelled explosive growth of the TB epidemic in areas with a high HIV burden over the past 25 years.
In 1999, De Cock and Chaisson posed the question ‘Will DOTS do it?’ with regard to the control of the HIV-associated TB epidemic.4 Since that time, the HIV epidemic has escalated hugely, and the associated increases in TB notification rates have clearly shown that DOTS is insufficient as a single control intervention. Over this time, the understanding of the epidemiological interaction of the HIV and TB epidemics has also grown and the need for additional interventions such as antiretroviral therapy (ART) and isoniazid preventive therapy (IPT; as part of the Three I’s strategy5) has become clear.
In the era of rapid scale-up of ART in resource limited settings, we now address a new question, ‘Will ART do it?’ In this paper we discuss the impact of ART within treatment cohorts on TB incidence rates, mortality and recurrence rates. We further examine the potential population-level impact of ART scale-up on the control of HIV-associated TB, including drug-resistant disease. We also consider whether there may be secondary benefits for non-HIV-infected individuals living in the same communities.
THE HIV-ASSOCIATED TB EPIDEMIC
Of the estimated global burden of 9.3 million new TB cases in 2007, 1.37 million (14.8%) were associated with HIV.3,6 Sub-Saharan Africa has borne the brunt of this co-epidemic, accounting for 79% of the global caseload of HIV-associated TB. As a result, TB notification rates have increased 3- to 5-fold since 1990 in many African countries, especially in the south of the continent, which is worst affected by the HIV epidemic and where pre-existing TB rates were high.6,7 TB is estimated to develop in approximately 1% of the population each year in South Africa and Swaziland. Much of this disease is among young adults aged 20–49 years of age,8,9 reflecting age-specific HIV prevalence rates.
South Africa alone accounts for approximately 25% of the annual global burden of HIV-associated TB, despite having just 0.7% of the global population.3 Notification rates in some poor South African communities worst affected by HIV have increased to over 2000 cases per 100 000 population,8,10 rates that are almost unprecedented in the era of short-course multidrug chemotherapy. Although TB notification rates continued to increase in South Africa and Swaziland in 2007, rates in many other African countries may have started to decline.3,6 If this reflects a true decline in TB incidence, this would most likely be due to reductions in HIV prevalence rather than as a direct result of any TB control interventions.6 However, much more rapid reductions in rates are needed to avert the huge accrual of mortality due to HIV associated TB.
An estimated 456 000 HIV-associated TB deaths occurred in 2007, accounting for approximately 23% of global acquired immune-deficiency syndrome (AIDS) related mortality.3 The estimated mortality rate attributable to HIV-TB in the WHO African region was between 20- and 60-fold higher than the rate in the five other WHO world regions. Rates were highest in the countries to the south of the continent, with South Africa alone accounting for almost 100 000 deaths in 2007.3 Several post-mortem studies conducted in sub-Saharan Africa have shown TB to be the most common HIV-associated pathology among patients dying in hospital with HIV/AIDS.11–14 TB was present in over one third of cadavers and in approximately one half of those with a pre-existing AIDS diagnosis. Thus, TB is a frequently unascertained cause of death among HIV-infected patients in sub-Saharan Africa.
The challenge of the HIV-associated TB epidemic in southern Africa has been compounded by the emergence of multi- and extensively drug-resistant (MDR and XDR) TB. South Africa is estimated to be the country with the fourth highest burden of MDR-TB in the world, although current accurate prevalence data are lacking for South Africa and sub-Saharan Africa as a whole.3 In South Africa, the intersection of the HIV and MDR- and XDR-TB epidemics have created a ‘perfect storm’ that threatens to further undermine TB control efforts.15 This was exemplified by an outbreak of MDR- and XDR-TB among HIV-infected patients at a district hospital in rural KwaZulu Natal Province in South Africa, in which 52 out of 53 patients died in a median of 16 days.16
The problem of HIV-associated TB is not confined to Africa. South-East Asia and India accounted for 11% of the global burden of cases in 2007, and HIV is also an important factor undermining TB control in the European Union and Latin America.6,17,18 The intersection of HIV and MDR-TB is also a particular challenge to TB control in countries of the former Soviet Union in eastern Europe.15 Thus, while much of this article focuses on sub-Saharan Africa, where the problem is most severe, the issues discussed are pertinent to settings wherever HIV has undermined TB control.
IMMUNODEFICIENCY: A KEY DRIVER OF THE EPIDEMIC
HIV has a critical impact on the host cell-mediated response to Mycobacterium tuberculosis.19 As a result, the risk of TB increases 2–3-fold within the first 2 years of HIV seroconversion20,21 and continues to rise as CD4 cell counts decrease.22,23 HIV-infected individuals with M. tuberculosis infection have an average annual risk of developing TB of approximately 10% per year,24 and yet this risk is highly dependent on the degree of immunodeficiency, the prevailing socio-economic conditions and the pressure of exogenous TB infection (Figure 1). Rates of HIV-associated TB approach 30% per year in those with the lowest CD4 cell counts living in Cape Town, South Africa.22 More advanced immunodeficiency is associated with increasing risk of extra-pulmonary, disseminated and sputum smear-negative TB and high mortality risk.26
Figure 1.
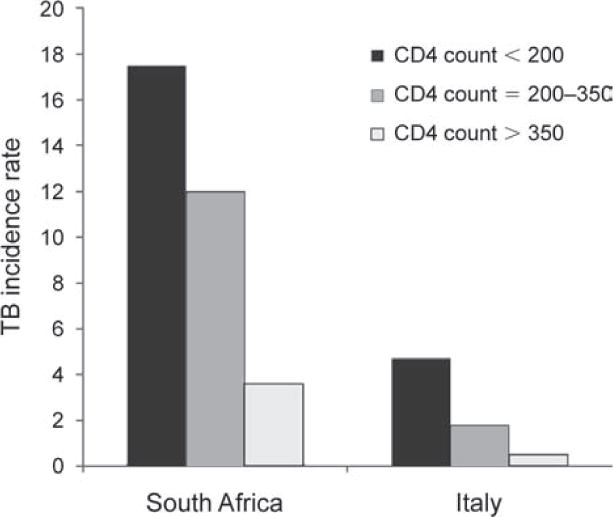
Tuberculosis (TB) incidence rates (cases/100 person-years) among human immunodeficiency virus infected patients prior to availability of antiretroviral therapy in South Africa and Italy. Rates are strongly associated with blood CD4 counts (cells/μl). Data adapted from Badri et al., 2002,25 and Antonucci et al., 1995.23
Early in the AIDS pandemic, patients with newly diagnosed HIV-associated TB were reported to have median CD4 counts of 200–350 cells/μl, which were generally higher than in patients with other serious opportunistic infections.27–29 However, more recent reports from Malawi and South Africa have reported that among patients with HIV-associated TB, median CD4 counts were 133 and 152 cells/μl, respectively.30,31 The fact that the majority of patients have advanced immunodeficiency has important implications for the potential preventive impact of ART. Whether differences between studies in median CD4 cell counts are real or a result of study design or due to other factors is not known. Such factors may include sampling biases, diagnostic criteria for TB, regional differences in the distributions of CD4 cell counts32 and maturation of the HIV epidemic over time.
WHY HAS DOTS BEEN INSUFFICIENT?
The WHO DOTS strategy has been the foundational TB control strategy globally over the past 15 years. Between 1995 and 2008, 36 million people were cured under DOTS worldwide and up to 6 million deaths were averted.17 However, the DOTS strategy has failed to control the African HIV-associated TB epidemic, even in countries considered to have ‘model’ programmes, such as Botswana, Tanzania and Malawi. Why has DOTS failed? Although poor programme implementation is undoubtedly a contributing factor in some countries and is a key driver of drug resistance, this is not the overriding reason. Rather, this relates to the fundamental epidemiological interaction between TB and HIV, as summarised in Table 1.
Table 1.
Reasons underlying the failure of the World Health Organization DOTS strategy to control TB in countries with high HIV prevalence
|
TB = tuberculosis; HIV = human immunodeficiency virus; LTBI = latent TB infection; AIDS = acquired immune-deficiency syndrome
The DOTS strategy aims to diagnose and effectively treat infectious pulmonary TB cases and thereby reduce onward transmission and secondary cases. Acquisition of HIV infection by individuals with M. tuberculosis infection is associated with a massive increase in TB risk from an approximately 10% lifetime risk to an approximately 10% risk each year.24 DOTS, however, does not prevent reactivation of latent TB infection (LTBI). When the HIV epidemic spread throughout sub-Saharan Africa, existing rates of LTBI were extremely high in many communities, with over two-thirds of adults in poor South African communities being infected.33 Thus, as HIV prevalence rates among antenatal women reached as high as 30% in South Africa in 2005, this served to fuel the fire of the existing TB epidemic.34
Another key issue is that DOTS does not reduce the very high susceptibility of HIV-infected individuals to infection or to the development of rapidly progressive disease following exogenous exposure.35 Thus, while DOTS may reduce TB transmission risk in the community, this may be outweighed many-fold by the greatly increased risk of rapidly progressive disease in HIV-infected people who are nevertheless exposed.36 As a result of this, rates of recurrent disease are also high.36
The major increases in incidence rates of HIV-associated TB may also further contribute to transmission, including in health care facilities where HIV infected patients tend to congregate for out- and in-patient care. Moreover, DOTS relies on passive case detection, waiting for patients to seek care and for clinicians to diagnosis TB. In settings where health services are weak, many cases are not detected and remain symptomatic and infectious for long periods of time, contributing to high rates of transmission.
In addition, DOTS is inadequate to reduce the risk of HIV-associated mortality, as many of the deaths are caused by bacterial sepsis, other infections and neoplasia.37 DOTS programmes have primarily focused on diagnosing and treating patients with sputum smearpositive TB, whereas the majority of HIV-associated TB is sputum smear-negative and is associated with high mortality risk.38 To compound all these factors, DOTS programmes have been severely strained and demoralised by the escalating caseload and poor outcomes, further increasing the challenge.
INTERNATIONAL PUBLIC HEALTH POLICY RESPONSE
As DOTS has proved to be insufficient as a single TB control intervention in settings with high HIV prevalence,4 more comprehensive strategies have had to be developed. Under the leadership of the WHO and the Stop TB Partnership, TB-HIV guidelines,39 a TB-HIV strategic framework40 and an interim TB-HIV policy41 were published between 2002 and 2004 to address the challenge of HIV-associated TB in the worst affected countries. These formulated an approach that used multiple interventions in combination with DOTS. These interventions aimed to reduce morbidity and mortality by HIV-testing TB patients and providing cotrimoxazole prophylaxis and ART for those testing positive.25,42,43 In addition, reductions in the burden of TB in HIV-infected individuals were targeted through TB preventive interventions such as IPT, intensified case finding and infection control, which were reconceptualised in 2008 as the ‘Three Is’ strategy.5
While the TB preventive interventions encompassed within the ‘Three Is’ strategy are extremely important, these do not alter the fundamental driver of this epidemic—progressive immunodeficiency. In hindsight, neither the interim TB-HIV policy41 nor the 2006 WHO Stop TB Strategy44 offered sufficient guidance with respect to the potentially crucial role of ART in TB prevention. The potential role of ART is the focus of the remainder of this article.
IMPACT OF ANTIRETROVIRAL THERAPY
Impact on TB incidence rates in cohorts
The initiation of ART leads to rapid functional recovery of mycobacteria-specific immune responses, with increasing lymphocyte proliferation and secretion of interferon-gamma by peripheral blood mononuclear cells stimulated ex vivo with mycobacterial antigens.43,45–47 This results in enhanced capacity to restrict mycobacterial growth.48 The rapidity of this restoration is illustrated by the fact that TB patients who develop immune reconstitution disease typically do so during the first few weeks after initiating ART.49,50 However, long-term recovery of TB-specific immune function is incomplete.43
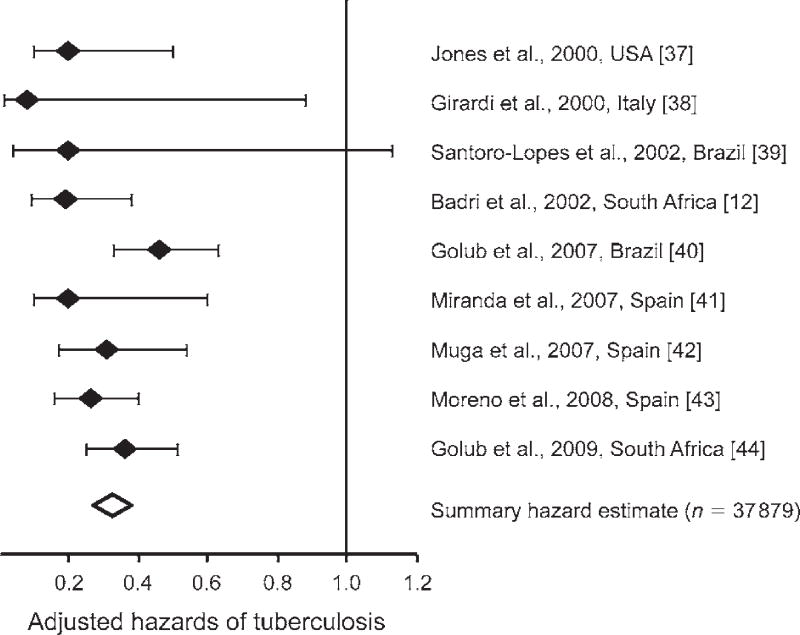
ART has a potent TB preventive effect.51 Even in the highest burden setting of South Africa, use of ART was associated with a TB risk reduction of approximately 80% among patients with a broad range of baseline CD4 cell counts and WHO stages of disease (Figure 2).25 Data from multiple other cohort studies in both high-income and resource-limited settings report TB risk reductions of 54–92% in adjusted analyses (Figure 3).51,52 In a meta-analysis of these studies, the summary estimate of this risk reduction was 67% (95%CI 61–73).52 Reductions in TB risk are similar among patients with either positive or negative tuberculin skin tests,53 suggesting an impact on risks of both endogenous reactivation disease and disease arising from exogenous exposure. ART also halves the risk of TB recurrence.54
Figure 2.
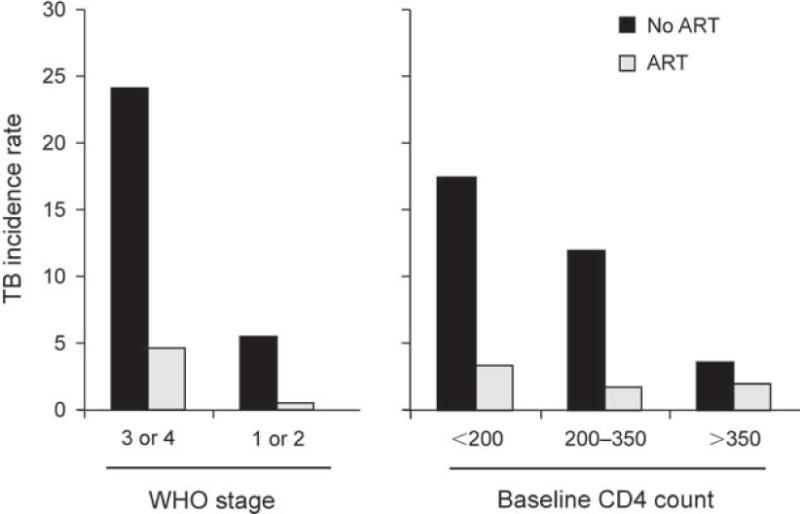
Tuberculosis (TB) incidence rates (cases/100 person-years) among human immunodeficiency virus infected patients in Cape Town, South Africa, prior to availability of antiretroviral therapy (ART) and during ART. Patients are stratified according to World Health Organization (WHO) stage of disease and baseline blood CD4 count (cells/μl). ART is seen to be associated with marked reductions in TB incidence rates. Data adapted from Badri et al., 2002.25
Figure 3.
Adjusted hazards of tuberculosis, comparing human immunodeficiency virus infected patients receiving antiretroviral therapy (ART) with those not receiving ART. Data from nine cohorts in Africa, Europe, and North and South America are presented together with a summary estimate derived from a meta-analysis. Data and figure adapted from Lawn et al., 2010.52 References cited in brackets pertain to reference 52.
The reduction in TB incidence rates during ART is time-dependent, with ongoing reductions during the first 2–3 years of treatment (Figure 4).55–59 These time-dependent changes in TB risk reflect the rate of ART-induced immune recovery, as the absolute current CD4 cell count at any given time during ART is a dominant factor associated with TB risk.60 A 10-fold reduction in TB risk is observed as CD4 cell counts increase from <100 to >500 cells/μl. In a South African cohort, TB incidence rates were extremely high during follow-up time accrued at CD4 counts of <200 cells/μl; an intermediate risk was observed at CD4 counts of 200–500 cells/μl and the lowest incidence rates were among those with current CD4 counts of >500 cells/μl (Figure 5).60 However, even among these patients with the highest CD4 cell counts, rates were approximately two-fold higher than rates among non-HIV-infected people living in the same community.
Figure 4.
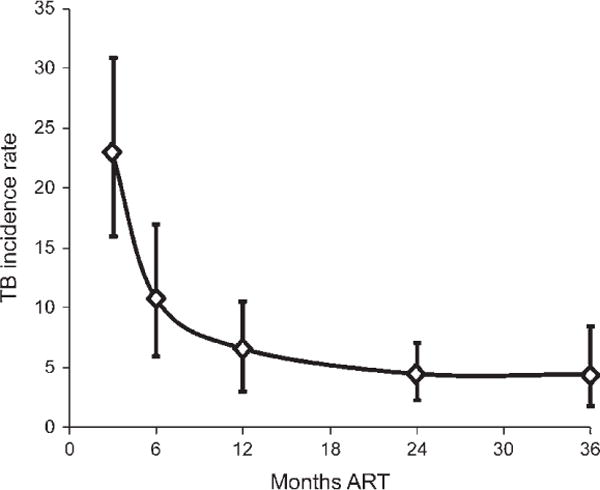
Tuberculosis (TB) incidence rates (cases per 100 person-years with 95% confidence intervals) during 36 months of antiretroviral therapy (ART) in a South African cohort. Rates are seen to continue to fall steeply during the first year of ART but reach a plateau between 2–3 years of ART at a rate of just under 5 cases/100 person-years. Data adapted from Lawn et al., 2006,59 and Lawn et al., 2009.60
Figure 5.
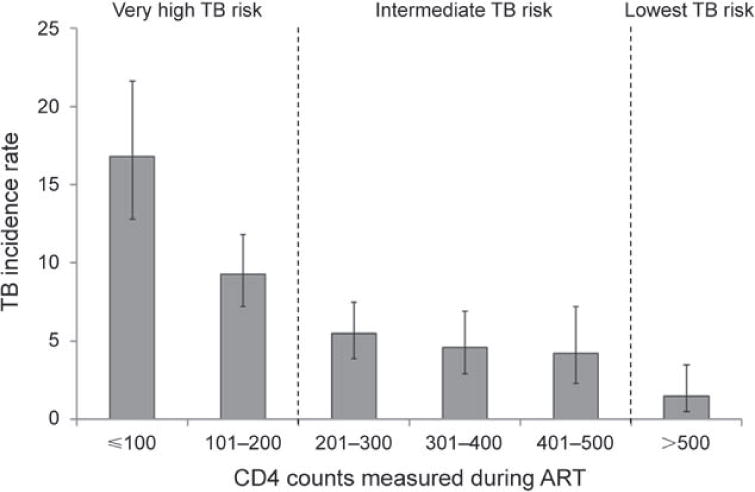
Tuberculosis (TB) incidence rates (cases/100 person-years, with 95% confidence intervals) in South African patients receiving antiretroviral therapy (ART). CD4 cell counts (cells/μl) were measured at baseline and every 4 months during ART. TB incidence rates stratified by updated CD4 cell counts during ART are presented. TB risk was extremely high while CD4 counts were <200 cells/μl, intermediate at counts of 200–500 cells/μl and lowest once CD4 counts exceeded a threshold of 500 cells/μl. Graph adapted from Lawn et al., 2009.60
Impact on mortality
Case-fatality rates are several-fold higher among HIV-infected TB patients than among those without HIV-coinfection, and are strongly related to the degree of immunodeficiency.26,61 In analyses adjusted for baseline patient characteristics, this risk is reduced by 64–95% by use of concurrent ART.51 The 2009 revision of the WHO guidelines for ART in resource-limited settings represents an important step forward in reducing mortality. These guidelines now recommend that all patients with HIV-associated TB should receive ART as soon as possible during the first 2–8 weeks of anti-tuberculosis treatment, regardless of CD4 cell count,62 consistent with the findings of randomised controlled trials in South Africa and Cambodia.63,64
The impact of ART on mortality from TB in individuals with HIV coinfection at a population level is therefore critically dependent upon achieving high rates of case ascertainment and subsequent linkage to ART care. This first requires high rates of HIV testing in TB patients, and yet just 22% of TB patients worldwide and 45% of those in sub-Saharan Africa were tested for HIV in 2008.3 Second, high rates of routine intensified TB case finding among those known to be living with HIV infection are also needed.5,65 However, just 4.1% of HIV-infected patients were reported to have undergone intensified TB case finding in 2008.3
ART and multidrug-resistant tuberculosis
Patients with HIV-associated drug-resistant TB have a high risk of mortality.15 ART is regarded as an essential component of their treatment,66 although there are few data that quantify their impact on outcomes. However, in a retrospective cohort of 82 patients with XDR-TB in South Africa, use of ART was associated with a 62% (95%CI 20–82) reduction in adjusted hazards of death.67
The role of ART for prevention of drug-resistant TB has not been studied. Data from the ongoing MDR- and XDR-TB epidemic in Tugela Ferry in rural KwaZulu Natal Province, South Africa, provide insights into the key role that ART could potentially play. Of 654 MDR- and XDR-TB cases treated in this setting between 2005 and 2007, over 90% were HIV-associated. Approximately two-thirds of patients had previously received treatment for TB, mostly within the preceding year,68 and molecular epidemiological data indicate that most drug-resistant cases were due to exogenous reinfection with a highly resistant strain following previous therapy for a different strain.69 Of note, median CD4 counts at diagnosis were just 87 cells/μl (interquartile range [IQR] 41–217) and 66 cells/μl (IQR 24–169), respectively, for patients with MDR- and XDR-TB.68 However, although many had received TB treatment in the preceding 12 months, only respectively 15% and 22% of these patients were receiving ART prior to their diagnosis of drug-resistant TB. The opportunity to start ART much earlier, and before the acquisition of MDR or XDR-TB, was therefore presumably missed in many of these cases. Had ART been started at the time they first entered clinical care, many subsequent cases of drug-resistant disease might have been prevented.
Impact of ART scale-up on community rates of TB
While many data are available from treatment cohorts, few document the impact of ART scale-up at a population level. Early scale-up of ART in Rio de Janeiro, Brazil, was associated with a nearly 40% decrease in AIDS-related TB within several years, although this plateaued during ongoing scale-up.70 More comprehensive data are now emerging from studies of a community of approximately 15 000 people in a South African township with an extremely high burden of HIV-associated TB.8,71 Rapid scale-up of ART between 2002 and 2008 reached a coverage of 21% of all HIV-infected patients. During this period, HIV-associated TB case-fatality rates decreased from 23% in 2002 to 8% in 2008.72 ART scale-up was also temporally associated with a reduction in overall TB notification rates in the community from 2005 onwards, and these reductions were predominantly observed in the HIV-infected population pool. In addition, cross-sectional surveys conducted in this community in 2005 and 2008 showed a substantial reduction in the prevalence of undiagnosed HIV-associated TB during ART scaleup (Figure 6).73 These effects on the TB epidemic may have been dependent upon the unusual rapidity of ART scale-up, which resulted from an ART intervention study.74 If this were the case, sustaining this impact may depend upon maintaining a rate of scale-up that matches or exceeds the rate of HIV progression in the residual untreated HIV-infected patient pool.
Figure 6.
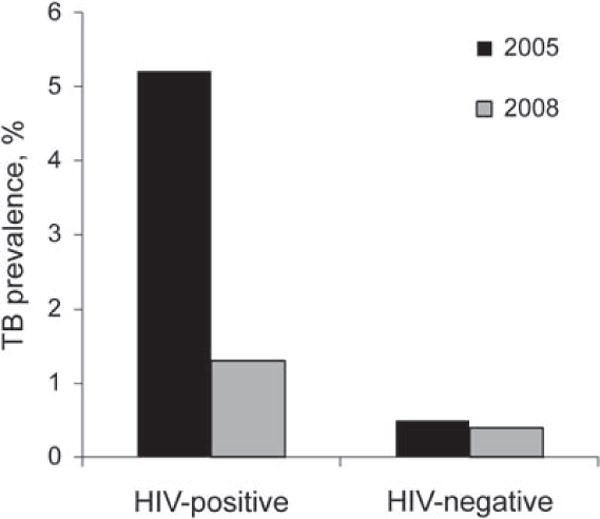
Changes in prevalence (%) of HIV-associated (HIV-positive) and non-HIV-associated (HIV-negative) tuberculosis (TB) in a community during rapid scale-up of antiretroviral therapy between 2005 and 2008. A 75% reduction in prevalence of HIV-associated TB was observed. Data adapted from Middelkoop et al., 2010.73
The impact of ART scale-up on TB transmission dynamics within communities is not known. However, most evidence indicates that the HIV-associated TB epidemic is not driving rates of TB infection or disease in the non-HIV-infected population.10,73,75,76 Thus, while ART is likely to reduce susceptibility to new infection, progressive primary disease and reactivation disease in HIV-infected people, it is unlikely to have substantial impact in the non-infected population pool, at least in the short-term.10 However, high ART coverage may ultimately provide a means of reducing HIV transmission within communities,77 resulting in longer-term reductions in susceptibility to TB.
LIMITATIONS OF ART FOR CONTROL OF HIV-ASSOCIATED TB
Although ART is associated with substantial reductions in TB incidence rates in treated cohorts, empirical observations and modelling studies suggest that, as currently implemented, ART may have a more limited long-term impact on TB rates at a population level.42 Multiple factors underlie this. At present, coverage with ART reaches only a limited proportion of the HIV-infected population who need it; achieving high coverage is likely to be resource-intensive and challenging.
Another critical factor limiting the impact of ART is the degree of immunodeficiency at which ART is initiated. Figure 7 illustrates how TB risk changes during the natural history of HIV progression—with TB rates rising steeply as CD4 counts decrease—and how rates decline following subsequent initiation of ART. Long-term cumulative TB risk will depend very strongly on the amount of time that patients spend at low CD4 cell counts before and during ART. Unfortunately, many HIV-infected patients first present to the health system either having already developed TB or with advanced immunodeficiency and a very high short-term risk of TB. Median CD4 counts of patients enrolling in ART programmes are typically in the range of 100–150 cells/μl;78 two thirds of such patients starting ART in two South African ART services had already had a TB diagnosis by the time of ART initiation.59,79 The TB preventive impact of earlier ART initiation was clearly demonstrated in a randomised controlled trial in Haiti.80 Patients randomised to start early ART (median CD4 count, 280 cells/μl) had a two-fold lower risk of TB compared to those receiving ART that was deferred until they had <200 cells/μl (median CD4 count, 166 cells/μl) over a median period of follow-up of 21 months.
Figure 7.
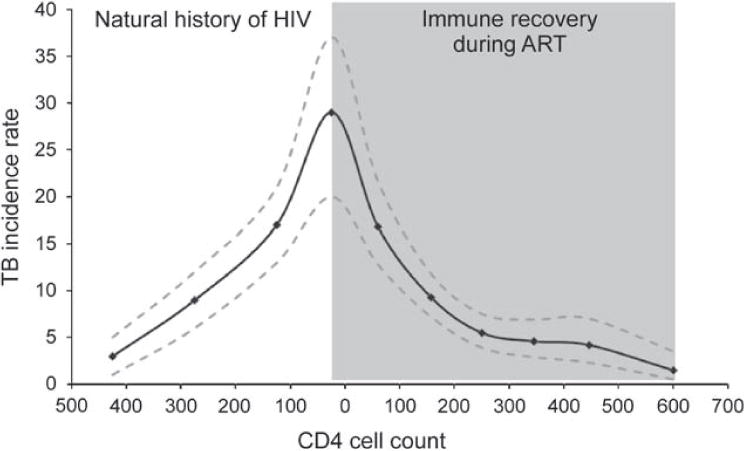
Graph showing increasing tuberculosis (TB) incidence rates (cases/100 person-years and 95% confidence intervals) with falling CD4 counts (cells/μl) in a South African cohort of HIV-infected patients prior to availability of antiretroviral therapy (ART; unshaded area) and the subsequent reduction in incidence rates with rising CD4 counts during ART (shaded area). The shape of the curve at the time of ART initiation will depend on the intensity of TB screening just prior to ART initiation. Cumulative TB risk will depend strongly on the period that patients spend at low CD4 counts both before and during ART. Data adapted from Holmes et al., 2006,22 and Lawn et al., 2009.60
Patients starting ART with low CD4 cell counts also remain at high TB risk until substantial CD4 cell count recovery has occurred (Figure 7). Eleven per cent of patients developed incident TB during the first year of ART in a South African cohort, adding to the high cumulative TB risk that had accrued pre-ART.59 Furthermore, during long-term ART, rates remain several-fold higher than rates among non-HIV-infected people living in the same community, with a rate of approximately 5% per year in a South African cohort (Figure 4). This may be due to immunological non-response,43 poor treatment adherence, ART failure and high ongoing exposure to TB both in the community and in health care settings. High ongoing TB risk may be sustained throughout the patient’s remaining lifespan. As life expectancy is greatly extended by ART, the cumulative lifetime risk of TB may remain very high indeed.
‘TEST AND TREAT’ STRATEGY FOR TB CONTROL
Mathematical modelling studies suggest that widespread implementation of ART early in the course of HIV infection may have a major effect on control of the HIV-associated TB epidemic at a population level.81 The so-called ‘test and treat’ strategy77 would require a high frequency of HIV testing in the population so that people might be tested on average on an annual basis, for example. Those testing positive would then initiate ART immediately, regardless of the CD4 cell count at the time of diagnosis. Such a strategy would theoretically reduce HIV-associated TB via two mechanisms. Early ART would reduce the time patients accrued at low CD4 cell counts with high risk of TB (Figure 7). Second, HIV-infected individuals receiving early ART would be less likely to transmit HIV, thereby reducing HIV prevalence in the community.77
The potential impact of the implementation of such a strategy in South Africa is shown in modelling analyses (Figure 8), for which the methods are described in detail elsewhere.81 Assuming that coverage increases to 95% and that 8% of patients are lost to care in the first 6 months of ART and 3% per year thereafter, annual HIV testing and immediate initiation of ART would reduce TB incidence by 50% within 5 years of full implementation, and would reduce HIV-associated TB by 95% within 40 years. The rapid, short-term decline in HIV-associated TB is a consequence of the immediate reduction in the risk of TB following immune reconstitution once people start ART, while the slower, long-term decline in TB results from reduced HIV transmission and the consequent slow reduction in the number of HIV-positive people alive and on ART. Figure 8 also shows the impact on TB notification rates if ART were to be started at CD4 counts of 200, 350 or 500 cells/μl. The later ART is started, the lower the impact on TB rates.
Figure 8.
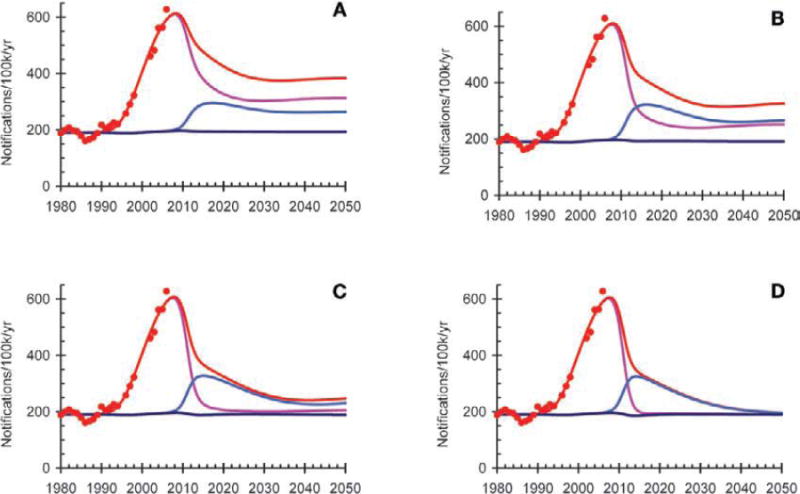
Mathematical modelling to estimate the impact of antiretroviral therapy (ART) on tuberculosis (TB) notification rates in South Africa. The model is fitted to the HIV epidemic curve and the TB notification rates for the country. Each graph shows TB notification rates among HIV-negative individuals (dark blue line), HIV-infected people not on ART (pink line), HIV-positive people on ART (light blue line) and the whole population (red line). The model assumes that people are tested for HIV on average once a year and start ART when their CD4 count falls to <200 cells/μl (A), <350 cells/μl (B) or 500 cells/μl (C), or as soon as they test positive for HIV (D).
This model has been applied to nine countries in sub-Saharan Africa for which good data on trends in HIV prevalence and TB notification rates are available.81 If people are started on ART 5, 2 or 1 years after HIV seroconversion, it is estimated that the HIV-associated TB incidence rates in 2050 would be reduced by 66% (range 57–80), 95% (range 93–96) and 97.7% (range 96.9–98.2), respectively.81
Another major advantage in early start of ART may be the reduction in risk of TB transmission to other HIV-infected individuals. This may be particularly important with regard to the drug-resistant TB epidemic. For example, the MDR- and XDR-TB epidemic in rural KwaZulu Natal appears to be characterised by nosocomial and community-based transmission of disease to HIV-infected patients with advanced immunodeficiency.16,68 An aggressive policy of community-wide HIV testing and rapid initiation of ART in those found to be eligible could potentially reduce the pool of patients who are highly susceptible to acquisition of drug-resistant infection and rapid progression to drug-resistant TB disease.
Regardless of arguments concerning the feasibility of the ‘test and treat’ strategy, the overall message is clear. The earlier ART is started, the greater the potential for prevention of HIV-associated TB, including drug-resistant disease. Field trials assessing the efficacy, feasibility, safety, impact and cost of the ‘test and treat’ strategy are needed. In the meantime, the upward revision of the WHO guidelines in 2010 to recommend ART for all HIV-infected patients with CD4 counts <350 cells/μl is an important development.62
ART AS A CENTRAL COMPONENT OF A COMPREHENSIVE PUBLIC HEALTH STRATEGY
In this article, we have considered the two important roles of ART in addressing the HIV-associated TB epidemic: 1) management of patients with HIV-associated TB to reduce case fatality, and 2) primary and secondary TB prevention to reduce disease incidence rates. With regard to the former, the 2010 revision of the WHO ART guidelines recommends ART for all patients with a diagnosis of HIV-associated TB, regardless of CD4 cell count.62 This is an important development. Effective implementation will be critically dependent upon achieving high rates of case ascertainment through a two-fold strategy of regular intensified TB case finding in HIV-infected patients and provider-initiated HIV testing and counselling in TB patients.5,65,82 An appropriate package of care for patients with HIV-associated TB that includes TB treatment, cotrimoxazole and ART greatly reduces mortality risk.83
With regard to the control of TB incidence rates at a population level, however, it is clear that ART as currently implemented is not a ‘magic bullet’. The impact of ART scale-up on TB control would be likely to be much greater, however, if ART were initiated earlier in the disease course and with high coverage. Moreover, it is clear that it should be used as a central element within a comprehensive public health approach that incorporates DOTS, massive scale-up of HIV testing,84 IPT, intensified case finding and infection control as synergistic and complementary interventions (Table 2).85
Table 2.
A comprehensive public health approach to control of the HIV-associated TB incidence rates at a population level
|
HIV = human immunodeficiency virus; TB = tuberculosis; VCT = voluntary counselling and testing; ART = antiretroviral treatment.
Early HIV diagnosis prior to the development of TB is needed to permit primary TB prevention. With the expansion of HIV testing services in most African countries, a substantial proportion of HIV-infected persons could discover their HIV status while still asymptomatic. It will be crucial to link such persons as early as possible to quality assured CD4 testing and to ongoing care so that those with CD4 cell counts of <350 cells/μl can initiate ART without delay. Studies will need to be done to determine whether the CD4 cell count threshold for ART initiation should continue to be moved upwards, and, indeed, whether CD4 cell count testing is required at all, in line with the ‘test and treat’ approach.
ART and IPT are both effective interventions for preventing HIV-associated TB, but work via complementary mechanisms, through restoration of antimycobacterial immune function, killing of the organism and prevention of re-infection during the course of chemoprophylaxis.52 IPT reduces TB risk by 32% overall and by 64% (95%CI 39–78) in the subset of patients with positive tuberculin skin tests,86 and is the key TB preventive intervention prior to ART eligibility. However, among patients first presenting with low CD4 counts in high TB prevalence settings, reliable exclusion of active TB is more difficult, fewer patients are eligible for IPT or have a positive tuberculin skin test, and waning immune function may limit the durability of IPT effect. In such patients, ART is the primary intervention needed to reduce mortality, and this will also reduce TB incidence rates.
TB rates during ART remain higher than background, however, and emerging data suggest that there is an additive preventive effect among patients who receive a combination of ART and IPT.52,53,87 Thus, proposed new WHO guidelines recommend using both interventions. The rationale, evidence and practicalities of combining these two interventions have been reviewed in detail elsewhere.52 ART and IPT can only be delivered effectively and safely in the context of effective intensified TB case finding and infection control. Thus, collectively, ART and the WHO three Is strategy5 form highly complementary and additive interventions for the prevention of HIV-associated TB.52,85
In conclusion, prevention of HIV-associated TB requires the effective implementation of several complementary interventions used in combination with ART. However, unless there is a significant shift towards initiation of ART at much higher CD4 cell counts than is currently happening, much of the potential of ART for TB prevention will be squandered. Much bolder approaches to ART implementation are required.
Acknowledgments
SDL is funded by the Wellcome Trust, London, UK. RW is funded in part by the National Institutes of Health (NIH) through a CIPRA grant 1U19AI053217-01 and R01 grant (AI058736-01A1). REC is supported by NIH grant AI01637 and by the Bill and Melinda Gates Foundation.
References
- 1.United Nations. The Millennium Development Goals report 2008. New York, NY, USA: United Nations; 2008. http://www.un.org/millenniumgoals/. Accessed February 2011. [Google Scholar]
- 2.World Health Organization. Global plan to stop TB 2006–2015. Geneva, Switzerland: WHO; Stop TB Partnership. http://www.stoptb.org/global/plan/ Accessed February 2011. [Google Scholar]
- 3.World Health Organization. Epidemiology, strategy, financing. Geneva, Switzerland: WHO; 2009. Global tuberculosis control 2009. (WHO/HTM/TB/2009.411). http://www.who.int/tb/publications/global_report/2009/en/index.html Accessed February 2011. [Google Scholar]
- 4.De Cock KM, Chaisson RE. Will DOTS do it? A reappraisal of tuberculosis control in countries with high rates of HIV infection. Int J Tuberc Lung Dis. 1999;3:457–465. [PubMed] [Google Scholar]
- 5.World Health Organization. Report of a joint WHO HIV/AIDS and TB Department meeting, 2008. Geneva, Switzerland: WHO; 2008. WHO Three I’s meeting. http://www.who.int/hiv/pub/meetingreports/WHO_3Is_meeting_report.pdf Accessed February 2011. [Google Scholar]
- 6.Lawn SD, Churchyard G. Epidemiology of HIV-associated t uberculosis. Curr Opin HIV AIDS. 2009;4:325–333. doi: 10.1097/COH.0b013e32832c7d61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaisson RE, Martinson NA. Tuberculosis in Africa: combating an HIV-driven crisis. N Engl J Med. 2008;358:1089–1092. doi: 10.1056/NEJMp0800809. [DOI] [PubMed] [Google Scholar]
- 8.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis. 2006;42:1040–1047. doi: 10.1086/501018. [DOI] [PubMed] [Google Scholar]
- 9.Richards SB, St Louis ME, Nieburg P, et al. Impact of the HIV epidemic on trends in tuberculosis in Abidjan, Cote d’Ivoire. Tubercle Lung Dis. 1995;76:11–16. doi: 10.1016/0962-8479(95)90572-3. [DOI] [PubMed] [Google Scholar]
- 10.Middelkoop K, Bekker LG, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis. 2008;47:349–355. doi: 10.1086/589750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lucas SB, Hounnou A, Peacock C, et al. The mortality and pathology of HIV infection in a West African city. AIDS. 1993;7:1569–1579. doi: 10.1097/00002030-199312000-00005. [DOI] [PubMed] [Google Scholar]
- 12.Rana F, Hawken MP, Meme HK, et al. Autopsy findings in HIV-1-infected adults in Kenya. J Acquir Immune Defi c Syndr Hum Retrovirol. 1997;14:83–85. doi: 10.1097/00042560-199701010-00017. [DOI] [PubMed] [Google Scholar]
- 13.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997–1998. Int J Tuberc Lung Dis. 2002;6:55–63. [PubMed] [Google Scholar]
- 14.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: a postmortem study. PLoS Med. 2010;7:e1000296. doi: 10.1371/journal.pmed.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 16.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. A short update to the 2009 report. Geneva, Switzerland: WHO; 2009. Global tuberculosis control. http://www.who.int/tb/publications/global_report/2009/update/tbu_9.pdf Accessed February 2011. [Google Scholar]
- 18.Lazarus JV, Olsen M, Ditiu L, Matic S. Tuberculosis-HIV coinfection: policy and epidemiology in 25 countries in the WHO European region. HIV Med. 2008;9:406–414. doi: 10.1111/j.1468-1293.2008.00567.x. [DOI] [PubMed] [Google Scholar]
- 19.Lawn SD, Butera ST, Shinnick TM. Tuberculosis unleashed: the impact of human immunodefi ciency virus infection on the host granulomatous response to Mycobacterium tuberculosis. Microbes Infect. 2002;4:635–646. doi: 10.1016/s1286-4579(02)01582-4. [DOI] [PubMed] [Google Scholar]
- 20.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. J Infect Dis. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 21.Glynn JR, Murray J, Bester A, Nelson G, Shearer S, Sonnenberg P. Effects of duration of HIV infection and secondary tuberculosis transmission on tuberculosis incidence in the South African gold mines. AIDS. 2008;22:1859–1867. doi: 10.1097/QAD.0b013e3283097cfa. [DOI] [PubMed] [Google Scholar]
- 22.Holmes CB, Wood R, Badri M, et al. CD4 decline and incidence of opportunistic infections in Cape Town, South Africa: implications for prophylaxis and treatment. J Acquir Immune Defi c Syndr. 2006;42:464–469. doi: 10.1097/01.qai.0000225729.79610.b7. [DOI] [PubMed] [Google Scholar]
- 23.Antonucci G, Girardi E, Raviglione MC, Ippolito G. Risk factors for tuberculosis in HIV-infected persons. A prospective cohort study. The Gruppo Italiano di Studio Tubercolosi e AIDS (GISTA) JAMA. 1995;274:143–148. doi: 10.1001/jama.274.2.143. [DOI] [PubMed] [Google Scholar]
- 24.Corbett EL, Watt CJ, Walker N, et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 25.Badri M, Wilson D, Wood R. Effect of highly active antiretroviral therapy on incidence of tuberculosis in South Africa: a cohort study. Lancet. 2002;359:2059–2064. doi: 10.1016/S0140-6736(02)08904-3. [DOI] [PubMed] [Google Scholar]
- 26.Ackah AN, Coulibaly D, Digbeu H, et al. Response to treatment, mortality, and CD4 lymphocyte counts in HIV-infected persons with tuberculosis in Abidjan, Cote d’Ivoire. Lancet. 1995;345:607–610. doi: 10.1016/s0140-6736(95)90519-7. [DOI] [PubMed] [Google Scholar]
- 27.Theuer CP, Hopewell PC, Elias D, Schecter GF, Rutherford GW, Chaisson RE. Human immunodefi ciency virus infection in tuberculosis patients. J Infect Dis. 1990;162:8–12. doi: 10.1093/infdis/162.1.8. [DOI] [PubMed] [Google Scholar]
- 28.Perronne C, Ghoubontni A, Leport C, Salmon-Ceron D, Bricaire F, Vilde JL. Should pulmonary tuberculosis be an AIDS-defining diagnosis in patients infected with HIV? Tubercle Lung Dis. 1992;73:39–44. doi: 10.1016/0962-8479(92)90078-X. [DOI] [PubMed] [Google Scholar]
- 29.Mukadi Y, Perriens JH, St Louis ME, et al. Spectrum of i mmunodeficiency in HIV- infected patients with pulmonary tuberculosis in Zaire. Lancet. 1993;342:143–146. doi: 10.1016/0140-6736(93)91346-n. [DOI] [PubMed] [Google Scholar]
- 30.Teck R, Ascurra O, Gomani P, et al. WHO clinical staging of HIV infection and disease, tuberculosis and eligibility for antiretroviral treatment: relationship to CD4 lymphocyte counts. Int J Tuberc Lung Dis. 2005;9:258–262. [PubMed] [Google Scholar]
- 31.Kaplan R, Bekker L-G, Caldwell J, et al. HIV prevalence, CD4 count distribution and case fatality for TB patients treated in primary health care facilities in Cape Town. Durban, South Africa. 2nd South African TB Conference; May 2010; [Abstract #306] [Google Scholar]
- 32.Williams BG, Korenromp EL, Gouws E, Schmid GP, Auvert B, Dye C. HIV infection, antiretroviral therapy, and CD4+ cell count distributions in African populations. J Infect Dis. 2006;194:1450–1458. doi: 10.1086/508206. [DOI] [PubMed] [Google Scholar]
- 33.Wood R, Liang H, Wu H, et al. Changing prevalence of tuberculosis infection with increasing age in high-burden townships in South Africa. Int J Tuberc Lung Dis. 2010;14:406–412. [PMC free article] [PubMed] [Google Scholar]
- 34.Abdool Karim SS, Churchyard GJ, Abdool Karim Q, Lawn SD. HIV infection and tuberculosis in South Africa: an urgent need to escalate the public health response. Lancet. 2009;374:921–933. doi: 10.1016/S0140-6736(09)60916-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326:231–235. doi: 10.1056/NEJM199201233260404. [DOI] [PubMed] [Google Scholar]
- 36.Sonnenberg P, Murray J, Glynn JR, Shearer S, Kambashi B, Godfrey-Faussett P. HIV-1 and recurrence, relapse, and re-infection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–1693. doi: 10.1016/S0140-6736(01)06712-5. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg AE, Lucas S, Tossou O, et al. Autopsy-proven causes of death in HIV-infected patients treated for tuberculosis in Abidjan, Cote d’Ivoire. AIDS. 1995;9:1251–254. doi: 10.1097/00002030-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 38.Getahun H, Harrington M, O’Brien R, Nunn P. Diagnosis of smear-negative pulmonary tuberculosis in people with HIV infection or AIDS in resource-constrained settings: informing urgent policy changes. Lancet. 2007;369:2042–2049. doi: 10.1016/S0140-6736(07)60284-0. [DOI] [PubMed] [Google Scholar]
- 39.World Health Organization. Guidelines for implementing collaborative TB and HIV programme activities. Geneva, Switzerland: WHO; 2003. (WHO/CDS/TB/2003.319. WHO/HIV/2003.01). http://www.who.int/tb/publications/2003/en/index1.html Accessed February 2011. [Google Scholar]
- 40.World Health Organization. Strategic framework to decrease the burden of TB/HIV. Geneva, Switzerland: WHO; 2002. (WHO/CDS/TB/2002.296. WHO/HIV_AIDS/2002.2). http://www.who.int/tb/publications/who_cds_tb_2002_296/en/index.html Accessed February 2011. [Google Scholar]
- 41.World Health Organization. Interim policy on collaborative TB/HIV activities. Geneva, Switzerland: WHO; 2004. (WHO/HTM/TB/2004.330. WHO/HTM/HIV/2004.1). http://whqlibdoc.who.int/hq/2004/WHO_HTM_TB_2004.330_eng.pdf Accessed February 2011. [Google Scholar]
- 42.Williams BG, Dye C. Antiretroviral drugs for tuberculosis control in the era of HIV/AIDS. Science. 2003;301:1535–1537. doi: 10.1126/science.1086845. [DOI] [PubMed] [Google Scholar]
- 43.Lawn SD, Bekker LG, Wood R. How effectively does HAART restore immune responses to Mycobacterium tuberculosis? Implications for tuberculosis control. AIDS. 2005;19:1113–1124. doi: 10.1097/01.aids.0000176211.08581.5a. [DOI] [PubMed] [Google Scholar]
- 44.Stop TB Partnership and World Health Organization. Building on and enhancing DOTS to meet the TB-related Millennium Development Goals. Geneva, Switzerland: WHO; 2006. The Stop TB Strategy. (WHO/HTM/TB/2006.368). [Google Scholar]
- 45.Li TS, Tubiana R, Katlama C, Calvez V, Ait MH, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 46.Wendland T, Furrer H, Vernazza PL, et al. HAART in HIV-infected patients: restoration of antigen-specific CD4 T-cell responses in vitro is correlated with CD4 memory T-cell reconstitution, whereas improvement in delayed type hypersensitivity is related to a decrease in viraemia. AIDS. 1999;13:1857–1862. doi: 10.1097/00002030-199910010-00007. [DOI] [PubMed] [Google Scholar]
- 47.Foudraine NA, Hovenkamp E, Notermans DW, et al. Immunopathology as a result of highly active antiretroviral therapy in HIV-1-infected patients. AIDS. 1999;13:177–184. doi: 10.1097/00002030-199902040-00005. [DOI] [PubMed] [Google Scholar]
- 48.Kampmann B, Tena-Coki GN, Nicol MP, Levin M, Eley B. Reconstitution of antimycobacterial immune responses in HIV-infected children receiving HAART. AIDS. 2006;20:1011–1018. doi: 10.1097/01.aids.0000222073.45372.ce. [DOI] [PubMed] [Google Scholar]
- 49.Lawn SD, Myer L, Bekker LG, Wood R. Tuberculosis-associated immune reconstitution disease: incidence, risk factors and impact in an antiretroviral treatment service in South Africa. AIDS. 2007;21:335–341. doi: 10.1097/QAD.0b013e328011efac. [DOI] [PubMed] [Google Scholar]
- 50.Lawn SD, Bekker LG, Miller RF. Immune reconstitution disease associated with mycobacterial infections in HIV-infected individuals receiving antiretrovirals. Lancet Infect Dis. 2005;5:361–373. doi: 10.1016/S1473-3099(05)70140-7. [DOI] [PubMed] [Google Scholar]
- 51.Lawn SD, Kranzer K, Wood R. Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin Chest Med. 2009;30:685–699. doi: 10.1016/j.ccm.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lawn SD, Wood R, De Cock KM, Kranzer K, Lewis JJ, Churchyard GJ. Antiretrovirals and isoniazid preventive therapy in the prevention of HIV-associated tuberculosis in settings with limited health-care resources. Lancet Infect Dis. 2010;10:489–498. doi: 10.1016/S1473-3099(10)70078-5. [DOI] [PubMed] [Google Scholar]
- 53.Samandari T, Mosimaneotsile B, Agizew T, et al. Randomized placebo-controlled trial of 6 vs. 36 months isoniazid preventive therapy for HIV-infected adults in Botswana. Program and abstracts of the 17th Conference on Retroviruses and Opportunistic Infections; 16–19 February 2010; San Francisco, CA, USA. Abstract no 104LB. http://retroconference.org/2010/Abstracts/39555.htm Accessed March 2011. [Google Scholar]
- 54.Golub JE, Durovni B, King BS, et al. Recurrent tuberculosis in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2008;22:2527–2533. doi: 10.1097/QAD.0b013e328311ac4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Girardi E, Sabin CA, d’Arminio MA, et al. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 56.Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–2116. doi: 10.1097/01.aids.0000194808.20035.c1. [DOI] [PubMed] [Google Scholar]
- 57.Brinkhof MW, Egger M, Boulle A, et al. Tuberculosis after initiation of antiretroviral therapy in low-income and high-income countries. Clin Infect Dis. 2007;45:1518–1521. doi: 10.1086/522986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moore D, Liechty C, Ekwaru P, et al. Prevalence, incidence and mortality associated with tuberculosis in HIV-infected patients initiating antiretroviral therapy in rural Uganda. AIDS. 2007;21:713–719. doi: 10.1097/QAD.0b013e328013f632. [DOI] [PubMed] [Google Scholar]
- 59.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 60.Lawn SD, Myer L, Edwards D, Bekker LG, Wood R. Shortterm and long-term risk of tuberculosis associated with CD4 cell recovery during antiretroviral therapy in South Africa. AIDS. 2009;23:1717–1725. doi: 10.1097/QAD.0b013e32832d3b6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mukadi YD, Maher D, Harries A. Tuberculosis case fatality rates in high HIV prevalence populations in sub-Saharan Africa. AIDS. 2001;15:143–152. doi: 10.1097/00002030-200101260-00002. [DOI] [PubMed] [Google Scholar]
- 62.World Health Organization. Recommendations for a public health approach (2010 revision) Geneva, Switzerland: WHO; 2010. Antiretroviral therapy for HIV infection in adults and adolescents. http://www.who.int/hiv/pub/arv/adult/en/index.html Accessed August 2010. [PubMed] [Google Scholar]
- 63.Abdool Karim SS, Naidoo K, Grobler A, et al. Timing of initiation of antiretroviral drugs during tuberculosis therapy. N Engl J Med. 2010;362:697–706. doi: 10.1056/NEJMoa0905848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blanc F-X, Sok T, Laureillard D, et al. Significant enhancement in survival with early (2 weeks) vs late (8 weeks) initiation of highly active antiretroviral treatment (HAART) in severely immunosuppressed HIV-infected adults with newly diagnosed tuberculosis. Vienna, Austria. XVIII International AIDS Society Conference; July 2010; [Abstract THLBB1] [Google Scholar]
- 65.Kranzer K, Houben RM, Glynn JR, Bekker LG, Wood R, Lawn SD. Yield of HIV-associated tuberculosis during intensified case finding in resource-limited settings: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:93–102. doi: 10.1016/S1473-3099(09)70326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Scano F, Vitoria M, Burman W, Harries AD, Gilks CF, Havlir D. Management of HIV-infected patients with MDR- and XDRTB in resource-limited settings. Int J Tuberc Lung Dis. 2008;12:1370–1375. [PubMed] [Google Scholar]
- 67.Dheda K, Shean K, Zumla A, et al. Early treatment outcomes and HIV status of patients with extensively drug-resistant tuberculosis in South Africa: a retrospective cohort study. Lancet. 2010;375:1798–1807. doi: 10.1016/S0140-6736(10)60492-8. [DOI] [PubMed] [Google Scholar]
- 68.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181:80–86. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 69.Andrews JR, Gandhi NR, Moodley P, et al. Exogenous re-infection as a cause of multidrug-resistant and extensively drug-resistant tuberculosis in rural South Africa. J Infect Dis. 2008;198:1582–1589. doi: 10.1086/592991. [DOI] [PubMed] [Google Scholar]
- 70.Pacheco AG, Durovni B, Cavalcante SC, et al. AIDS-related tuberculosis in Rio de Janeiro, Brazil. PLoS ONE. 2008;3:e3132. doi: 10.1371/journal.pone.0003132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wood R, Middelkoop K, Myer L, et al. Undiagnosed tuberculosis in a community with high HIV prevalence: implications for tuberculosis control. Am J Respir Crit Care Med. 2007;175:87–93. doi: 10.1164/rccm.200606-759OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Middelkoop K, Wood R, Myer L, et al. Widespread ART is associated with decline in TB prevalence. Cape Town, South Africa. 5th International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; July 2009; [Abstract WELBB105] [Google Scholar]
- 73.Middelkoop K, Bekker LG, Myer L, et al. Antiretroviral program associated with reduction in untreated prevalent tuberculosis in a South African township. Am J Respir Crit Care Med. 2010;182:1080–1085. doi: 10.1164/rccm.201004-0598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sanne I, Orrell C, Fox MP, et al. Nurse versus doctor management of HIV-infected patients receiving antiretroviral therapy (CIPRA-SA): a randomised non-inferiority trial. Lancet. 2010;376:33–40. doi: 10.1016/S0140-6736(10)60894-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Egwaga SM, Cobelens FG, Muwinge H, Verhage C, Kalisvaart N, Borgdorff MW. The impact of the HIV epidemic on tuberculosis transmission in Tanzania. AIDS. 2006;20:915–921. doi: 10.1097/01.aids.0000218557.44284.83. [DOI] [PubMed] [Google Scholar]
- 76.Corbett EL, Charalambous S, Fielding K, et al. Stable incidence rates of tuberculosis (TB) among human immunodeficiency virus (HIV) negative South African gold miners during a decade of epidemic HIV-associated TB. J Infect Dis. 2003;188:1156–1163. doi: 10.1086/378519. [DOI] [PubMed] [Google Scholar]
- 77.Granich RM, Gilks CF, Dye C, De Cock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 78.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 2010;24:1273–1280. doi: 10.1097/QAD.0b013e328339e20e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Severe P, Juste MA, Ambroise A, et al. Early versus standard antiretroviral therapy for HIV-infected adults in Haiti. N Engl J Med. 2010;363:257–265. doi: 10.1056/NEJMoa0910370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Williams BG, Granich R, De Cock KM, Glaziou P, Sharma A, Dye C. Antiretroviral therapy for tuberculosis control in nine African countries. Proc Nat Acad Sci USA. 2010;107:19485–19489. doi: 10.1073/pnas.1005660107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.World Health Organization, Joint United Nations Programme on HIV/AIDS. Guidance on provider-initiated HIV testing and counselling in health facilities. Geneva, Switzerland: WHO/UNAIDS; 2007. http://www.who.int/hiv/pub/guidelines/9789241595568_en.pdf Accessed February 2011. [Google Scholar]
- 83.Harries AD, Zachariah R, Lawn SD. Providing HIV care for co-infected tuberculosis patients: a perspective from sub-Saharan Africa. Int J Tuberc Lung Dis. 2009;13:6–16. [PubMed] [Google Scholar]
- 84.Lugada E, Millar D, Haskew J, et al. Rapid implementation of an integrated large-scale HIV counseling and testing, malaria, and diarrhea prevention campaign in rural Kenya. PLoS ONE. 2010;5:e12435. doi: 10.1371/journal.pone.0012435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet. 2010;375:1906–1919. doi: 10.1016/S0140-6736(10)60409-6. [DOI] [PubMed] [Google Scholar]
- 86.Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV-infected persons. Cochrane Database Syst Rev. 2010(1):CD000171. doi: 10.1002/14651858.CD000171.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]