Abstract
Background and Aims:
To systemically evaluate the efficacy of adjuvant vitamin E on the outcomes of nonalcoholic fatty liver disease (NAFLD) and/or nonalcoholic steatohepatitis (NASH) in children.
Materials and Methods:
We searched MEDLINE, PUBMED, EMBASE, the Cochrane Central Register Controlled Trials, and the Cochrane Database of Systematic Reviews over the period between January 1980 and September 2012 for the studies that examined the role of adjuvant vitamin E given at any dose or duration, alone or in combination with other interventions, on the outcome of pediatric NAFLD. The outcomes are alanine aminotransferase (ALT) normalization and histological improvement.
Results:
Five randomized trials were eligible to be included in our analysis, with a total of 270 participants. There was no statistically significant difference in the effect of adjuvant vitamin E on normalizing serum ALT [risk ratio (RR) =1.18, confidence interval (CI) =0.92-1.53, P = 0.77 for heterogeneity, I2 = 0%]. Sensitivity analysis showed that using higher doses of vitamin E, a longer duration of therapy or adding vitamin C did not change the effect on the measured outcome. Only two studies looked at histological changes as an outcome. We observed substantial heterogeneity between the two studies.
Conclusions:
Our meta-analysis did not find a significant effect of adjuvant vitamin E over placebo in normalizing serum ALT. Data on the long-term effect of adjuvant vitamin E on histological improvements in NAFLD patients are still lacking. Larger, well-designed randomized controlled trials (RCTs) in children with histological endpoints are still needed to answer this question.
Keywords: α-Tocopherol, antioxidants, steatohepatitis, steatosis
Nonalcoholic fatty liver disease (NAFLD) includes a spectrum of liver diseases that ranges from simple hepatic steatosis to steatohepatitis, which may subsequently progress to cirrhosis.[1] The prevalence of NAFLD has increased significantly in children along with the increasing prevalence of obesity. It has become the most common form of chronic liver disease in children in developed countries.[2]
NAFLD is recognized to be a multifactorial disease, which includes genetic, metabolic, and environmental factors; however, the precise etiology of NAFLD is poorly understood. The condition starts with the accumulation of triglycerides and free fatty acids within hepatocytes (steatosis), which is induced by insulin resistance (first hit). When the adaptive mechanisms of the hepatocytes fail to accommodate the accumulated free fatty acids, lipotoxicity occurs, which induces oxidative stress and inflammatory changes that progress to nonalcoholic steatohepatitis (NASH). This condition causes injury to the hepatocytes, fibrosis, and subsequently, cirrhosis.[3,4] As such, oxidative stress is increasingly considered to be the therapeutic target in NAFLD management through the use of antioxidant agents.
Currently, there is no definitive treatment for NAFLD; however, lifestyle changes (dietary and exercise programs) are the mainstay of the therapeutic interventions in NAFLD management, which have been shown to improve liver disease and resolve NASH changes in adults and children.[5,6] However, because of the issues regarding the long-term adherence to a strict lifestyle program, other pharmacological treatments have been attempted. These treatments include antioxidants, such as vitamins E and C, and insulin sensitizers, such as metformin, and lipid-lowering agents. Vitamin E is the most common antioxidant agent evaluated in NAFLD management. Its antioxidant activity is thought to be secondary to its effect on stabilizing the cell membranes by protecting the unsaturated fatty acids from lipid peroxidation and subsequent free radical generation which induce cell injury.[7]
Randomized controlled trials (RCTs) in adults and children have not uniformly demonstrated the beneficial effects of vitamin E on the long-term outcomes of NAFLD patients. The PIVENS trial, the largest adult trial, showed significant improvements in the aminotransferase levels, hepatic steatosis, lobular inflammation, and the total NAFLD activity score in the vitamin E group compared to the placebo group.[8] The TONIC trial, the largest pediatric trial, did not find significant differences between the vitamin E and placebo groups in improving alanine aminotransferase (ALT) levels. However, resolution of NASH was observed more in the vitamin E group compared to the placebo group, which was attributed primarily to the improvement in hepatocytes ballooning, but there were no differences in steatosis or lobular inflammation between the two groups.[9] Both these trials did not show any improvement in fibrosis. Mixed results were also reported from other small, open-labeled, and uncontrolled studies.
The aim of our s tudy is to complete a systematic review of the literature and perform a meta-analysis to determine the efficacy of adjuvant vitamin E supplementation (adjuvant to lifestyle changes program) on the outcomes of NAFLD and/or NASH in children.
MATERIALS AND METHODS
Study selection
The databases, MEDLINE, PUBMED, EMBASE, the Cochrane Central Register of Controlled Trials, the Cochrane Database of Systematic Reviews, and clinicaltrials. gov, were searched systematically for the time period between January 1980 and September 2012 by two independent reviewers. The search was limited to human studies and included both RCTs and observational studies that have at least two comparison arms. The search was limited to the pediatric age group (patients less than 18 years of age). The search was conducted using a combination of MeSH subject headings and text words as follows:
Firstly, MeSH terms and text words including fatty liver, nonalcoholic fatty liver disease, NAFLD, nonalcoholic steatohepatitis, NASH, steatosis, and steatohepatitis were entered
Secondly, we searched using the MeSH terms and text words, liver enzymes, aminotransferases, ALT and AST
Finally, we searched using the MeSH terms and text words, vitamin E, alpha-tocopherol, and antioxidants
We then combined the three searches
At the end, we used the “age filter” function in each database to limit our search results to the pediatric age groups (1-18 years).
In addition, we searched the reference lists of retrieved articles to find other potentially relevant articles. Also, we contacted experts in the field to inquire about any more published or unpublished trials.
Inclusion criteria
Types of studies
RCTs and observational studies that have at least two arms of comparison.
Types of participants
Children or adolescents younger than 18 years of age with a radiological and/or histological diagnosis of NAFLD/NASH that is not attributed to other causes of hepatic steatosis or steatohepatitis, such as viral hepatitis, autoimmune hepatitis (AIH), Wilson's disease, metabolic disease, or exposure to drugs that are known to induce steatosis, such as total parenteral nutrition (TPN).
Types of interventions
Vitamin E given at any dose or duration, alone or in combination with other interventions, versus a placebo or no intervention. Lifestyle interventions were considered as a co-intervention if applied equally in both arms.
Types of outcomes
Primary outcomes of interest included the following:
Effect of adjuvant vitamin E on the normalization of ALT levels
Effect of adjuvant vitamin E on histology improvement.
We decided to choose the percentage of patients with normalized ALT levels as an outcome (categorical outcome: yes/no) rather than the numerical ALT level changes (continuous outcome) because this more accurately reflects the effect compared to the level changes that tend to fluctuate over time and may just reflect the phenomenon of regression to the mean.
Secondary outcomes included any reported adverse events.
Data extraction and risk of bias assessment
Two independent authors (AS, AA) checked the titles and abstracts identified from the searches. We obtained the full texts of all the potentially relevant studies for the assessment. We selected the trials that satisfied our inclusion criteria and graded their methodological quality. Any disagreement was resolved by consensus. We excluded non-human studies, adult trials, observational studies that did not have a comparison group, and studies that did not report the outcomes of interest.
One author performed data extraction (AS) using a standardized form. This process was checked by the second author (AA). Data on the study design, sample size, participant characteristics [number of patients randomized, gender, age, and body mass index (BMI)], intervention characteristics (dose, duration, and route of administration) and the outcomes of interest were extracted.
The methodological quality of the studies was assessed using the Cochrane Risk of Bias Tool.[10] Each domain of the sex-domain tool was categorized as one of the following: Adequate, if low risk of bias; inadequate, if high risk of bias; or unclear, if uncertain risk of bias. Trials with adequate assessments in all the bias risk domains were considered as having low risk of bias and classified as high methodological quality. If one or more domains were judged as high risk of bias, then the study was regarded as a trial with high risk of bias and classified as low methodological quality.
Data analysis
We presented dichotomous outcomes as risk ratios (RR) with their 95% confidence intervals (CI). We used a fixed-effects model to calculate the pooled RR and 95% CI when the studies were sufficiently similar; however, we used a random effects model in the case of significant heterogeneity (i.e., having an I2 of 50% or more), as this model represents a more conservative approach.[11]
Because tests of heterogeneity may be insufficient to detect heterogeneity between studies when the number of studies is small, we also explored heterogeneity graphically and quantitatively using the I2 statistic. An I2 value of less than 25% was considered to have good homogeneity, a value of 25-50% to have reasonable homogeneity, and a value of >50% was considered to have significant heterogeneity. Then, we decided not to combine the results statistically.
Sensitivity analysis was performed to look for the effect of different doses of vitamin E (600 IU/day or more vs. less than 600 IU/day) and the effect of duration of the therapy (more or less than 1 year) on the outcomes of interest to explore if there is a dose- and/or duration-response effect. In addition, we did a sensitivity analysis to examine the effect of vitamin E alone or in combination with other antioxidants on the outcomes of interest. Finally, we did sensitivity analysis excluding the studies with “high risk of bias" to assess their influence on the outcomes. We planned to perform the quantitative analysis on an intention-to-treat basis. We had planned to examine the publication bias if an adequate number of studies were identified.
The meta-analysis was performed using RevMan V5® software.
RESULTS
Search results
In total, we identified 156 references: 115 through MEDLINE, PUBMED, and EMBASE; 31 through the Cochrane Central Register of Controlled Trials in The Cochrane Library; and 10 through the Cochrane Database of Systematic Reviews. Of these references, we identified 22 studies that examined the role of vitamin E in pediatric patients with NAFLD. Finally, we identified five completed trials[9,12,13,14,15] that fulfilled our inclusion criteria and included these in the analysis [Figure 1].
Figure 1.
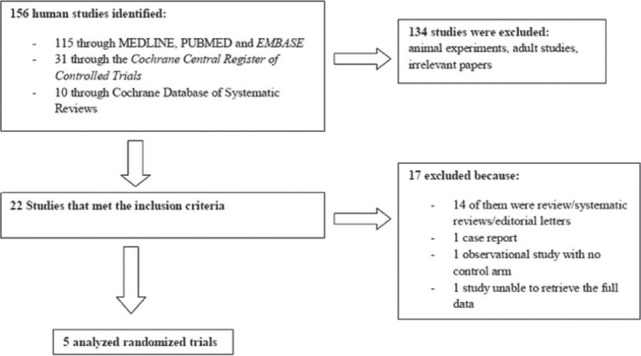
Flowchart of the search results
Nobili et al., published two trials.[13,14] The second trial was an open-label extension of the first, when the study was extended for another year to examine the effect of vitamin E on histological changes. We decided to extract the data about the first outcome (normalization of the ALT levels) from the first trial because it was of better quality with low risk of bias compared to the extension arm which was an open label. However, we decided to include only the histological data from the extension trial, recognizing its risk of bias. These five trials that satisfied our inclusion criteria included a total of 270 participants.
Description of the included studies
Two trials identified NAFLD/NASH cases based on elevated aminotransferases and ultrasound changes,[12,15] while the other two trials identified NAFLD/NASH based on elevated aminotransferases, ultrasound changes, and histology.[9,13]
All the included trials compared vitamin E to at least one control group. There were 2 three-arm studies: One compared vitamin E to metformin and a placebo,[9] while the other study compared non-blind vitamin E administration plus lifestyle intervention to a group with lifestyle intervention alone without medication (camp group) and a third group with no interventions at all (neither lifestyle program nor medication).[15]
The vitamin E dose and duration of the therapy varied considerably among the included studies [Table 1]. All the included studies, apart from the Nobili et al., trials, used vitamin E as a single antioxidant agent; however, Nobili et al., used vitamin C in combination with vitamin E as an adjuvant antioxidant agent.
Table 1.
Characteristics of the included studies
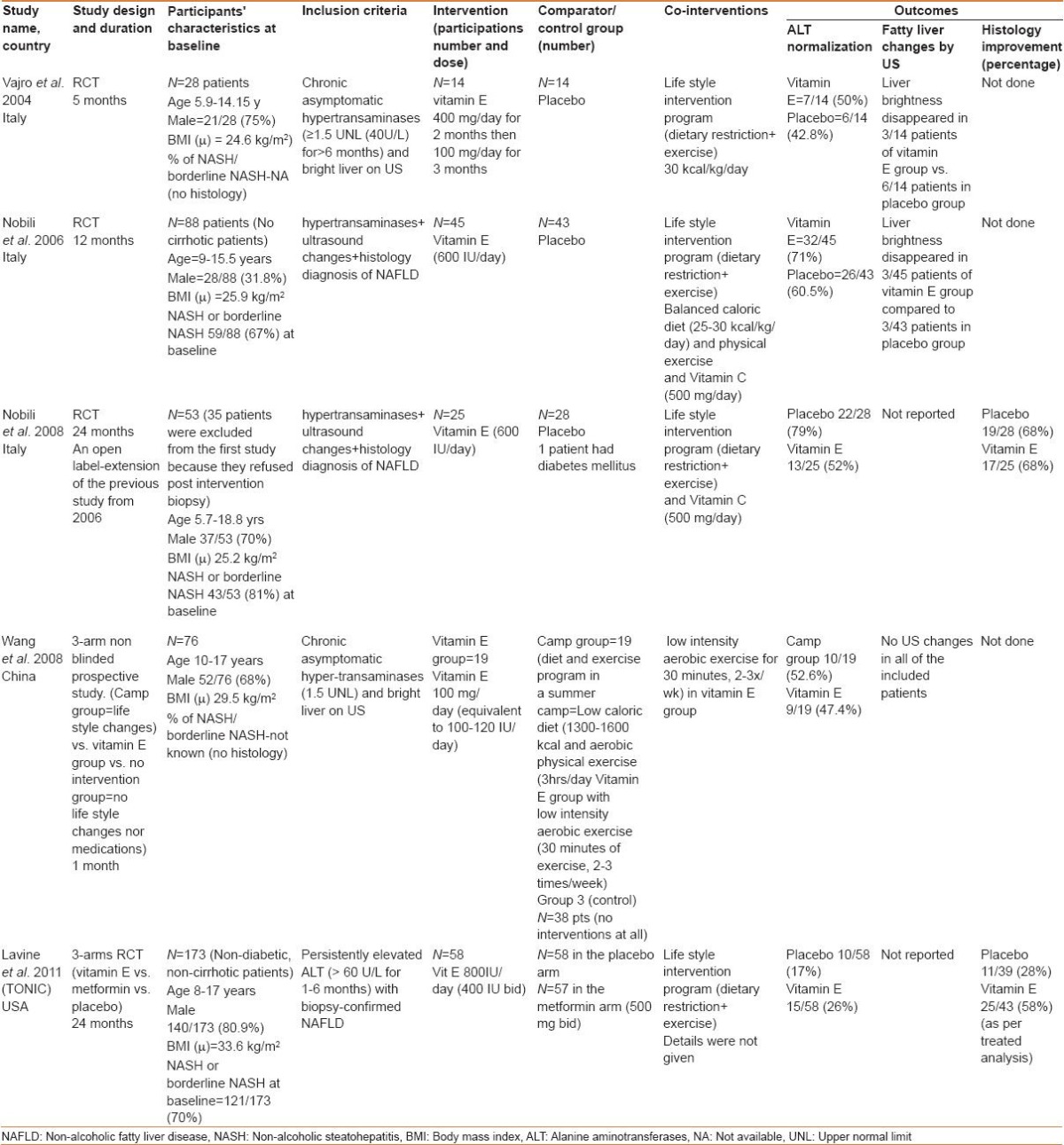
Most of the included studies were conducted over a year or less. Nobili et al., extended their first trial[13] for the second year to assess the histological changes, as an open-label extension.[14] The TONIC trial ran over 2 years.[9]
All the trials implemented a lifestyle intervention program (diet and exercise program) as a co-intervention that was applied for both the treatment and the control groups; however, these programs were not uniform among the included studies, with variable degrees of weight loss obtained at the end of each study.
All the studies reported data on aminotransferase changes; however, only two studies reported the data on pre-and post-treatment histology: The TONIC trial[9] and the extension arm of the Nobili trial.[14]
Table 1 summarizes the characteristic features of the included studies.
Excluded studies
A total of 17 studies were excluded from the analysis. Fourteen of them were reviews/systematic review articles. One study was excluded because there was no control arm and another study was a case report. We did not have access to the full text of one paper written in Chinese in which vitamin E was compared to a Chinese herbal medication.[16]
Assessment of the methodological quality of the included studies
The two investigators independently rated the methodological quality of the selected studies using the Cochrane Risk of Bias Tool. Only two trials were graded as high methodological trials.[9,13] These two trials met the criteria for random sequence generation, adequate allocation concealment, and adequate blinding of outcome assessment. All the included studies reported a full description of study withdrawals (see online Appendix A for the quality assessment of the included studies).
Effects of the interventions on the primary outcomes
Effect of adjuvant vitamin E on normalization of serum ALT level
All the included trials reported changes in ALT. We included the data from the first trial performed by Nobili et al.,[13] because it was of an adequate methodological quality compared to the open-label extension. The trial by Nobili et al., and the TONIC trial showed a significant drop in the ALT levels in both the vitamin E and the placebo groups; however, this drop was similar in both arms. The study by Wang et al., showed a significant drop in the ALT levels in the vitamin E group and the lifestyle intervention group (camp group) compared to the no interventions group (no medication or lifestyle changes). This drop was more evident in the camp group compared to the vitamin E group. This study was the smallest and the shortest among the included studies. In addition, it had the highest risk of bias among all the included trials in the methodology assessment. Vajro et al., reported a comparable ALT drop in both groups; however, the beneficial effect of vitamin E was observed more in the patients who showed a better adherence to the lifestyle intervention programs compared to those who did not.
We pooled the data from these studies using the fixed-effects model. We found no significant difference in the proportion of the participants with normalized ALT levels between the vitamin E and the placebo groups (RR = 1.18, CI = 0.92-1.53, P = 0.77 for heterogeneity, I2 = 0%) [Figure 2].
Figure 2.
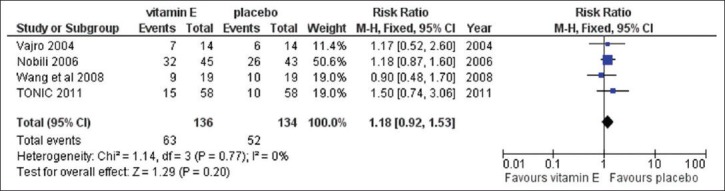
Analysis comparing the effect of vitamin E to the placebo for the proportion of participants with normalized ALT
Sensitivity analysis
We performed sensitivity analysis to examine the effect of the dose of vitamin E (below and above 600 IU/day), duration of the therapy (2 years/1 year), and addition of other antioxidant agents versus the placebo. We did not find any beneficial effect when a higher dose of vitamin E was used (600 IU/day or more) or when vitamin C was added. Moreover, there was no difference when a longer duration of therapy (2 years or more) was used. Excluding the studies with a high risk of bias did not change the results.
Effect of adjuvant vitamin E on histological improvement
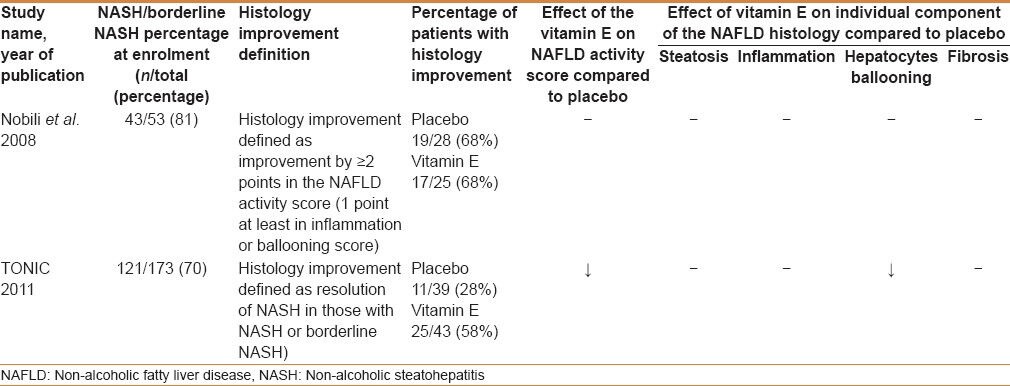
We found only two studies that looked at the histological changes pre- and post-intervention.[9,14] Nobili et al., reported NASH or borderline NASH in 81% of their patients at baseline, whereas the TONIC trial reported an incidence of 70%.
When we pooled the data from these two trials for meta-analysis, looking for the proportion of participants with histological improvement, we observed a substantial heterogeneity between the two studies (I2 = 80%). Therefore, it was not appropriate to combine their results statistically for this outcome.
Each trial had a different definition of histological improvement. The Nobili et al., trial defined histology improvement as an improvement by ≥2 points in the NAFLD activity score, whereas the TONIC trial defined it as a resolution of the NASH changes at the end of the study. Nobili et al., reported similar percentages of patients with histology improvement in both the placebo and vitamin E groups (68%), whereas the TONIC trial reported a higher percentage of histological improvement in the vitamin E group (58%) compared to the placebo group (28%). Table 2 summarizes the results of the NAFLD histological changes in both trials.
Table 2.
Effect of vitamin E on NAFLD histology
Secondary outcomes: Adverse events of vitamin E
No significant side effects were reported in the included studies. Vajro et al., reported one patient who had a significant increase in his ALT level (a five-fold increase) after starting vitamin E. This change was resolved after stopping the treatment.[12] The TONIC trial reported one patient who had mood changes and another patient who committed suicide; however, similar events were also observed in the placebo group.[9] Nobili et al., did not report any significant adverse events in the first trial or in the extension.[13,14] There were no reports of increased incidences of bleeding disorders in these trials, as had been reported previously in adult studies.[17]
Publication bias
We initially planned to use a funnel plot to assess the publication bias. However, because of the small number of the included studies, the funnel plot was inappropriate to assess for publication bias. It has been shown previously that up to five studies are too few to allow the detection of funnel-plot asymmetry.[18]
DISCUSSION
We identified five trials that reported the use of adjuvant vitamin E in pediatric patients with NAFLD. Changes in aminotransaminase levels have been shown to fluctuate over time and to poorly correlate with histological changes.[19,20] However, because it is a non-invasive and cheap surrogate marker for liver injury, ALT has been used in several studies as a primary outcome. Our meta-analysis did not find any significant beneficial effect of vitamin E in normalizing ALT, compared to the placebo. A longer duration of treatment, a higher dose of vitamin E, or the addition of vitamin C did not change the results in our sensitivity analysis.
There are no previously published systematic reviews or meta-analyses that examined the effect of adjuvant vitamin E on NAFLD using only pediatric data. To the best of our knowledge, our systematic review and meta-analysis is the first published study in the pediatric field. Our meta-analysis results are consistent with the results from other meta-analyses performed on adult patients, where no significant effect of vitamin E was found on the ALT levels among NAFLD/NASH patients.[21,22] Socha et al., published a meta-analysis that included data from two pediatric trials in addition to adult trials. They found no significant effect of vitamin E on the normalization of ALT levels. This meta-analysis did not include any studies that examined the effect of vitamin E on histological changes.[22]
The natural history of NAFLD in pediatric patients is not well understood because most of the studies on this disease were of short duration.[23] However, it is well documented that NAFLD in children has a distinct histological pattern compared to that seen in adults where more portal inflammation and less hepatocellular ballooning, lobular inflammation, and perisinusoidal fibrosis are observed. Consequently, data extrapolation from adult studies is not optimal for pediatric patients.[24]
We found two pediatric trials that assessed the effect of vitamin E on histological changes.[9,14] The Nobili et al., trial did not find a significant effect on the histological changes between the vitamin E and placebo groups, whereas the TONIC trial found a beneficial effect on hepatocyte ballooning. Both the trials did not find any effect on fibrosis. Both these trials had a small sample size, varied methodological quality, and a relatively short duration of therapy. There was a significant heterogeneity between these two trials; therefore, we did not proceed with the statistical analysis and could not make a definite conclusion about the beneficial effect of adjuvant vitamin E on histological changes on the basis of the identified trials.
RCTs in adults showed some evidence that vitamin E may improve the early histological features of NASH but not the features associated with advanced disease.[7] However, some of these meta-analyses reported significant limitations of the included trials, which have a clear impact on reaching a definite conclusion about the beneficial effect of vitamin E on histology changes.[21,22] Even if we assume that the results from adult studies are encouraging, we think that extrapolating from the adult data may not be appropriate for pediatric patients, keeping in mind that NAFLD histological changes in children may have distinctive patterns compared to the changes seen in adults.[24]
The trials that evaluated the effect of adjuvant vitamin E on pediatric NAFLD patients showed mixed results. These mixed results are partially due to the several limitations that these studies had. These included limited methodological quality of most of the included trials, small sample sizes, and using surrogate markers such as aminotransaminase levels and ultrasound changes as the primary endpoints rather than the histological changes. In addition, there was a considerable degree of discrepancies among the included studies in regard to the treatment protocols, including the doses and implementation of variable lifestyle intervention programs. The short and significantly variable durations of therapeutic intervention (1 month to 2 years) used in these trials do not allow for the proper examination of the long-term efficacy and safety of vitamin E. Moreover, lifestyle intervention programs were not consistent among the included studies. Variable degrees of weight loss were achieved at the end of each study, which may have had a major effect on the outcomes and should not be ignored.
No significant side effects were reported in the included studies; however, it is important to recognize that these trials were small in size, short in duration, and were not sufficiently powered to look for adverse events.
It seems to be more logical to use combination therapy in managing these patients because NAFLD pathogenesis occurs in multiple steps. The beneficial effect of combination therapy has been observed in several adult trials.[25,26,27,28] The Nobili et al., trials used a combination of vitamin E and vitamin C, both of which acted primarily as antioxidants. However, there were no pediatric trials designed to examine the effect of combination therapy with different mechanisms, such as antioxidants and insulin sensitizers.
We acknowledge that our systematic review is not without limitations; our meta-analysis included a small number of trials with limited methodological quality. We did not have enough trials with reasonable homogeneity to examine the effect of vitamin E on the histological outcomes; therefore, we do not have a firm conclusion about this important outcome.
CONCLUSION
Our meta-analysis did not find a significant effect of adjuvant vitamin E over the placebo in normalizing serum ALT. Data on the long-term effects of adjuvant vitamin E on histological improvements in pediatric NAFLD patients are still lacking. In future, larger well-designed RCTs with adequate power and duration concentrating on the histological endpoints in children are still needed to answer this question. Thus far, lifestyle interventions (diet and exercise changes) are the only proven therapeutic intervention for NAFLD in children.
ACKNOWLEDGMENT
This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University.
APPENDIX
The quality assessment for the included studies
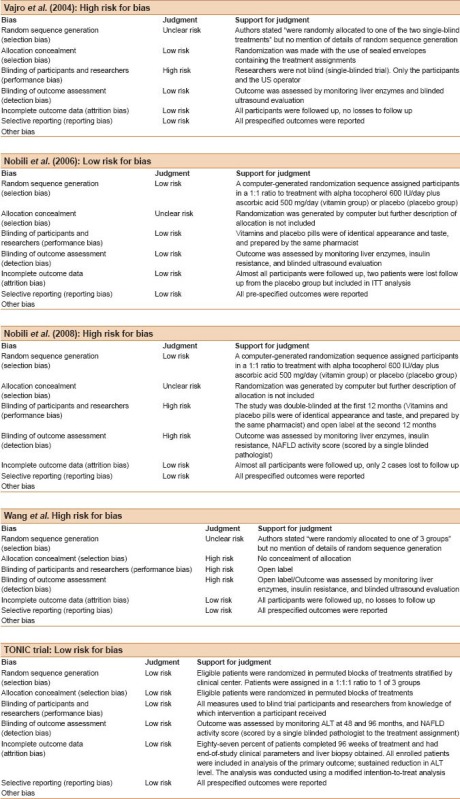
Footnotes
Source of Support: This work was supported by the College of Medicine Research Center, Deanship of Scientific Research, King Saud University
Conflict of Interest: None declared.
REFERENCES
- 1.Della Corte C, Alisi A, Saccari A, De Vito R, Vania A, Nobili V. Nonalcoholic fatty liver in children and adolescents: An overview. J Adolesc Health. 2012;51:305–12. doi: 10.1016/j.jadohealth.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Mencin AA, Lavine JE. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2011;14:151–7. doi: 10.1097/MCO.0b013e328342baec. [DOI] [PubMed] [Google Scholar]
- 3.Alkhouri N, Dixon LJ, Feldstein AE. Lipotoxicity in nonalcoholic fatty liver disease: Not all lipids are created equal. Expert Rev Gastroenterol Hepatol. 2009;3:445–51. doi: 10.1586/egh.09.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patton HM, Lavine JE, Van Natta ML, Schwimmer JB, Kleiner D, Molleston J, et al. Clinical correlates of histopathology in pediatric nonalcoholic steatohepatitis. Gastroenterology. 2008;135:1961–71.e2. doi: 10.1053/j.gastro.2008.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51:121–9. doi: 10.1002/hep.23276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nobili V, Marcellini M, Devito R, Ciampalini P, Piemonte F, Comparcola D, et al. NAFLD in children: A prospective clinical-pathological study and effect of lifestyle advice. Hepatology. 2006;44:458–65. doi: 10.1002/hep.21262. [DOI] [PubMed] [Google Scholar]
- 7.Adinolfi LE, Restivo L. Does vitamin E cure nonalcoholic steatohepatitis? Expert Rev Gastroenterol Hepatol. 2011;5:147–50. doi: 10.1586/egh.11.27. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675–85. doi: 10.1056/NEJMoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: The TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Higgins JPT, Green S, editors. The Cochrane Collaboration; 2011. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] www.cochrane-handbook.org . [Google Scholar]
- 11.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 12.Vajro P, Mandato C, Franzese A, Ciccimarra E, Lucariello S, Savoia M, et al. Vitamin E treatment in pediatric obesity-related liver disease: A randomized study. J Pediatr Gastroenterol Nutr. 2004;38:48–55. doi: 10.1097/00005176-200401000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Nobili V, Manco M, Devito R, Ciampalini P, Piemonte F, Marcellini M. Effect of vitamin E on aminotransferase levels and insulin resistance in children with non-alcoholic fatty liver disease. Aliment Pharmacol Ther. 2006;24:1553–61. doi: 10.1111/j.1365-2036.2006.03161.x. [DOI] [PubMed] [Google Scholar]
- 14.Nobili V, Manco M, Devito R, Di Ciommo V, Comparcola D, Sartorelli MR, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: A randomized, controlled trial. Hepatology. 2008;48:119–28. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 15.Wang CL, Liang L, Fu JF, Zou CC, Hong F, Xue JZ, et al. Effect of lifestyle intervention on non-alcoholic fatty liver disease in Chinese obese children. World J Gastroenterol. 2008;14:1598–602. doi: 10.3748/wjg.14.1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu F. Clinical study on prevention efficacy of Jianpi Huatan Fang in treating non-alcoholic fatty liver disease in children. Zhongguo Zhong Yao Za Zhi. 2012;37:2465–8. [PubMed] [Google Scholar]
- 17.Schürks M, Glynn RJ, Rist PM, Tzourio C, Kurth T. Effects of vitamin E on stroke subtypes: Meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702. doi: 10.1136/bmj.c5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutton AJ, Duval SJ, Tweedie RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta-analyses. BMJ. 2000;320:1574–7. doi: 10.1136/bmj.320.7249.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manco M, Alisi A, Nobili V. Risk of severe liver disease in NAFLD with normal ALT levels: A pediatric report. Hepatology. 2008;48:2087–8. doi: 10.1002/hep.22631. [DOI] [PubMed] [Google Scholar]
- 20.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: Present and future. Hepatology. 2007;46:582–9. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 21.Lirussi F, Azzalini L, Orando S, Orlando R, Angelico F. Antioxidant supplements for non-alcoholic fatty liver disease and/or steatohepatitis. Cochrane Database Syst Rev. 2007;1:CD004996. doi: 10.1002/14651858.CD004996.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children: A systematic review. J Pediatr Gastroenterol Nutr. 2009;48:587–96. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- 23.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: A follow-up study for up to 20 years. Gut. 2009;58:1538–44. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwimmer JB, Behling C, Newbury R, Deutsch R, Nievergelt C, Schork NJ, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–9. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 25.Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2004;2:1107–15. doi: 10.1016/s1542-3565(04)00457-4. [DOI] [PubMed] [Google Scholar]
- 26.Harrison SA, Torgerson S, Hayashi P, Ward J, Schenker S. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol. 2003;98:2485–90. doi: 10.1111/j.1572-0241.2003.08699.x. [DOI] [PubMed] [Google Scholar]
- 27.Dufour JF, Oneta CM, Gonvers JJ, Bihl F, Cerny A, Cereda JM, et al. Randomized placebo-controlled trial of ursodeoxycholic acid with vitamin e in nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2006;4:1537–43. doi: 10.1016/j.cgh.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 28.Foster T, Budoff MJ, Saab S, Ahmadi N, Gordon C, Guerci AD. Atorvastatin and antioxidants for the treatment of nonalcoholic fatty liver disease: The St Francis Heart Study randomized clinical trial. Am J Gastroenterol. 2011;106:71–7. doi: 10.1038/ajg.2010.299. [DOI] [PubMed] [Google Scholar]


