Abstract
Background/Aims:
Colorectal cancer (CRC) is the second most common malignancy in the Saudi population, with an increasing incidence over the past 20 years. We aim to determine the baseline polyp as well as adenoma prevalence in a large cohort of patients and to find the possible age in which, if deemed appropriate, a CRC screening program should be initiated.
Patients and Methods:
A retrospective cohort study was conducted using an endoscopic reporting database of individuals seen at a major tertiary care university hospital (King Khalid University Hospital) in Riyadh, Saudi Arabia. Consecutive Saudi patients who underwent a colonoscopy between August 2007 and April 2012 were included. Patients were excluded if the indication for the colonoscopy was colon cancer, colonic resection, active colitis, active diverticulitis, inflammatory bowel disease, or if the patient was referred for polypectomy.
Results:
2654 colonoscopies were included in the study. The mean age of the study population was 50.5 years [standard deviation (SD) 15.9] and females represented 57.7%. The polyp detection rate in completed colonoscopies was 20.8% (95% CI: 19.2-22.5). Adenomas were found in 8.1% (95% CI: 7.1-9.1), while advanced adenomas were found in only 0.5% (95% CI: 0.2-0.7). Adenomas were found in the left side of the colon in 33.9%, followed by the rectum in 14.6%, ascending colon and cecum in 14.2%, transverse colon in 8.7%, and in multiple locations in 28.7%. Those with a prior history of polyps or CRC were more likely to have an adenoma at colonoscopy than those who did not (14.3% vs. 6.6%; P < 0.01). The adenoma prevalence varied between age groups and ranged from 6.2% to 13.6% with a higher proportion in older individuals; this trend was seen both in males (6.0-14.5%) and females (6.4-14.6%) as well as in those who had screening colonoscopies (6.3-18.4%). No age could be found at which a CRC screening program would be appropriate to initiate.
Conclusion:
The prevalence of polyps and adenomas in this cohort is less than that reported in the Western populations. But as this cohort included younger and symptomatic patients with only a small proportion undergoing screening, further studies in an asymptomatic population are needed.
Keywords: Adenoma, colon cancer, colonosocopy, early detection, endoscopy, epidemiology, polyp, prevalence, Saudi Arabia, tumor
Colorectal cancer (CRC) is one of the few diseases for which screening programs have shown to be efficacious in decreasing both the incidence as well as the mortality. Randomized controlled trials have demonstrated that repetitive fecal occult blood testing (FOBT) reduces the mortality from CRC by 16%, while once-only flexible sigmoidoscopy reduces CRC incidence and mortality by 18% and 28%, respectively[1]. Although complete colonoscopy, as opposed to flexible sigmoidoscopy, has a potentially higher impact on the reduction in the incidence and mortality from CRC, this is still to be proven in a randomized trial.[1]
CRC is the second most common malignancy in the Saudi population[2,3] and ranked as first among men and second[2,3] or third among women,[4] with a median age of 60 years for males and 58 years for females[4]. There is an increase in incidence of CRC observed in Saudi Arabia[5,6,7,8] from 1994-2003, where the age-standardized rate (ASR) per 100,000 population-years increased from 3.38-5.84, but it is still much lower than that reported for the United States of America (USA) in 2003, which was 30.49.[9] Data from the Saudi Cancer Registry reveal the ASR per 100,000 population-years in Saudi Arabia between 1994 and 2004 was 7.3,[4] whereas based on data from the International Agency for Research on Cancer, GLOBOCAN 2008, the ASR for the incidence for CRC per 100,000 population-years in Saudi Arabia was 12.1, mortality was 8.6 and the 5-year prevalence was 21.5.[2,3]
Whether the institution of a screening program for the general population is cost-effective and practical depends largely on the baseline prevalence of adenomas in that population, as well as the other factors related to the healthcare resources available.
Adenomas are the precursors of adenocarcinomas. Thus, a better understanding of the prevalence of adenomas in the general population would help clarify the efficacy of a CRC screening program. In this study, we aimed to determine the baseline polyp as well as adenoma prevalence in a large cohort of patients who underwent colonoscopies for various indications as well as opportunistic screening for CRC. We also aimed to define the possible age in which, if deemed appropriate, a CRC screening program should be initiated.
PATIENTS AND METHODS
Data collection
A retrospective cohort study was conducted using an endoscopic reporting database of individuals seen at a major tertiary care university hospital (King Khalid University Hospital) in Riyadh, Saudi Arabia. The demographic data of consecutive Saudi patients who underwent a complete colonoscopy for all indications between August 2007 and April 2012 was collected retrospectively through the hospital information system (HIS), endoscopic e-reports, and a manual review of the files by two research assistants. The data collected included: Age, sex, symptoms, indication for the colonoscopy, quality of the bowel preparation, medication history, and comorbidities. For an incomplete colonoscopy, we used the definition given by the American Medical Association's (AMA) Current Procedural Terminology (CPT) coding system. According to this, incomplete colonoscopy is defined as “when performing an endoscopy on a patient who is scheduled and prepared for a total colonoscopy, if the physician is unable to advance the colonoscope beyond the splenic flexure, due to unforeseen circumstances.”. Whenever a colonoscopy was not completed, the reason for not completing the endoscopy was documented. The location of polyps was also documented. Patients who had any of the following as an indication for colonoscopy were excluded from this study: Colon cancer, colonic resection, active colitis, active diverticulitis, or inflammatory bowel disease (IBD). The indication of the colonoscopy was based on the documentation by the endoscopist on the electronic report form. The pathology reports were reviewed when a polypectomy was performed, and the poloyps were classified as adenomas or non-adenomas. Also, adenomas were classified as advanced if they were greater than 1 cm in size and/or had a villous component and/or had high-grade dysplasia.
The number ofgastroenterologists who staffed the endoscopy unit varied over the study period from four to nine, and the number of endoscopies performed in 2013 was about 7000 procedures.
No personal identification information or other personal identifiers such as address or hospital identification number were recorded to ensure patient confidentiality.
This study was approved ethically by the Internal Review Board (IRB) (study no. E-12-818) of King Khalid University Hospital.
Statistical analysis
Data analysis included descriptive statistics computed for continuous variables, including means, standard deviations (SDs), minimum and maximum values, as well as 95% confidence intervals (CIs). Frequencies are used for categorical variables.
When calculating the adenoma detection rate (ADR), the numerator included all colonoscopies where at least one polyp was found to be adenomatous, whether the pathology was tubular or villous or the polyp had high-grade dysplasia or adenocarcinoma.
Characteristics of the test procedure [sensitivity, specificity, likelihood ratios, receiver operating characteristic (ROC) curve, and the area under the curve] were used to evaluate the optimal cut-off age for the initiation of a screening program if one was to be implemented.
We used the software STATA 11.2 (Stata Corp., College Station, TX, USA) in our analysis. A statistical significance threshold of P = 0.05 was adopted. No attempt at imputation was made for missing data.
RESULTS
Demographics and historical data
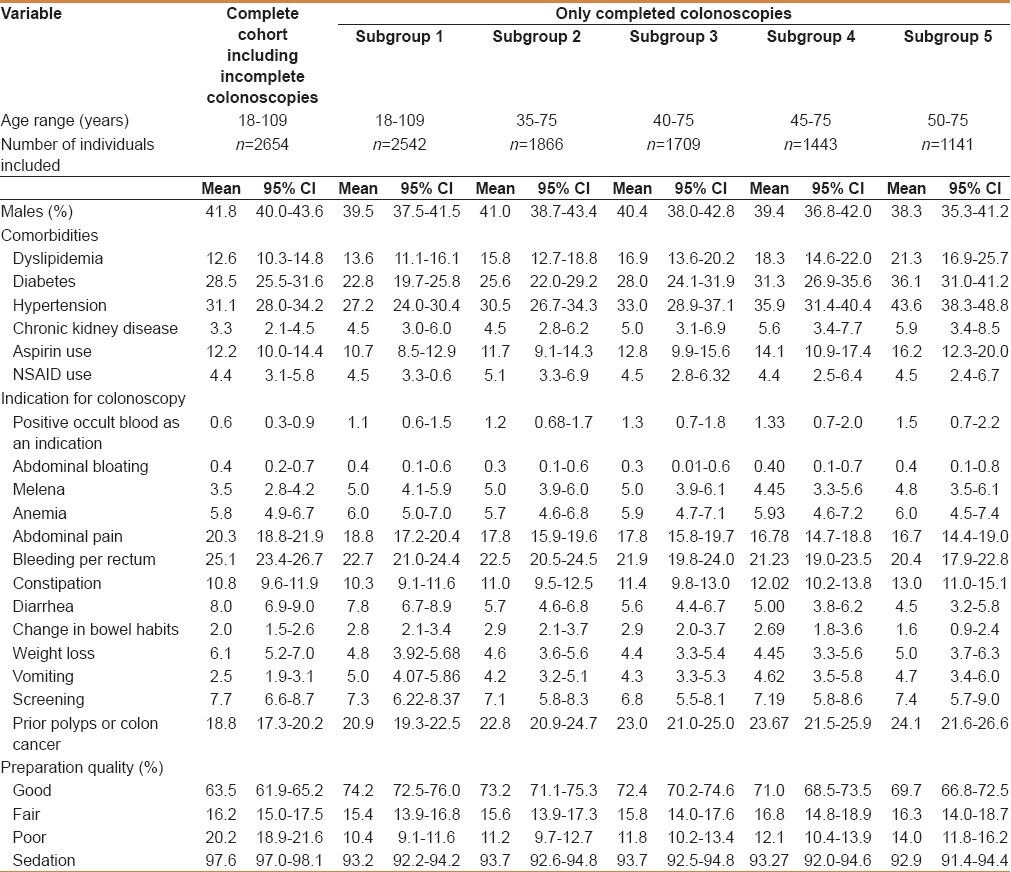
After applying our exclusion criteria, our cohort included 2654 colonoscopies. The mean age was 50.5 years (SD 15.9) and ranged from 18 to 109 years [Figure 1]. Females represented 57.7%, while males formed 42.3% of the study population. There was a history of a polyp removed in a prior colonoscopy in 18.3%, while screening was the indication in only 7.7%. Comorbidities included hypertension in 31.1%, diabetes in 28.5%, dyslipidemia in 12.6%, and chronic kidney disease in 3.3%. Aspirin was used by 12.2%, while other nonsteroidal anti-inflammatory drugs (NSAIDs) were used by 4.4% [Table 1]. The indications for colonoscopy included: Bleeding per-rectum (25.1%), abdominal pain (20.3%), constipation (10.8%), diarrhea (8%), screening (7.7%), weight loss (6.1%), anemia (5.8%), melena (3.5%), vomiting (2.5%), change in bowel habits (2%), positive occult blood test in stools (0.6%), and abdominal bloating (0.4%), while the remainder of the colonoscopies had other indications [Table 1]. Each variable is reported in Table 1 in different age strata that might be considered the potential ages for screening colonoscopy. There were 269 scopes that were performed for the same individuals during the study period.
Figure 1.
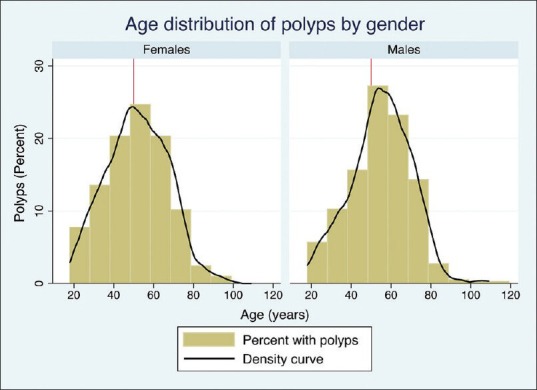
The age distribution of those who had polyps detected at colonoscopy
Table 1.
Characteristics of the study population stratified by age
Colonoscopy
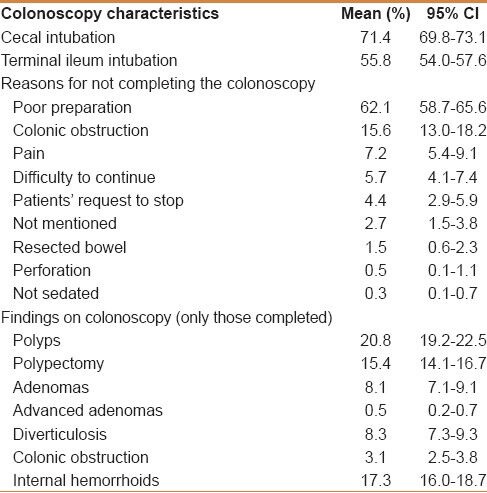
The bowel preparation protocol used had evolved over the study period, and in the latest protocol, a standard polyethylene glycol-electrolyte solution on the day before the colonoscopy was adopted. The quality of the preparation in the complete cohort was classified as good in 63.5%, fair in 16.2%, and poor in 20.2% [Table 1]. Sedation was used in the majority of the procedures (97.6%). The cecal intubation rate in the complete cohort was 71.4%, while the terminal ileum was intubated in 55.8%. Those having completed a colonoscopy were 2542 in number, whereas 112 were excluded. In those who had a good preparation quality, the cecal intubation rate was 92.5%. The reasons for an incomplete colonoscopy were poor preparation quality in 62.1%, colonic obstruction in 15.6%, pain in 7.2%, technical difficulty in 5.7%, while the rest of them were due to other reasons [Table 2].
Table 2.
Characteristics of colonoscopies in the complete cohort of patients, including those with an incomplete colonoscopy
Polyps and adenomas detected
The polyp detection rate in completed colonoscopies was 20.8%. When stratified by age, the polyp detection rate in completed colonoscopies and those with a good preparation ranged from 16.3% in those aged 35 to <40 years to as high as 31.8% in those aged 60 to <65 years [Table 3]. The polyp rates were higher in males in the age groups of 35 to <45 years than in females (17.4% vs. 15.3%; 19.4% vs. 15.1%), while in those older than 45 years, females had a higher proportion of polyps compared to males (23.5% vs. 15.6; 30.1% vs. 21.9%; 36.2% vs. 17.9%; 33.6% vs. 28.0%; 27.0% vs. 26.4%) in each age category, respectively. Also, the proportion of polyps in those who had screening as an indication was the lowest in those aged 60 to <65 years (20%), while it was the highest in the group aged 50 to <55 years (43.8%) [Table 3].
Table 3.
The proportion of polyps and adenomas in completed colonoscopies with a good quality stratified by age and gender as well as those who had screening as an indication for colonoscopy
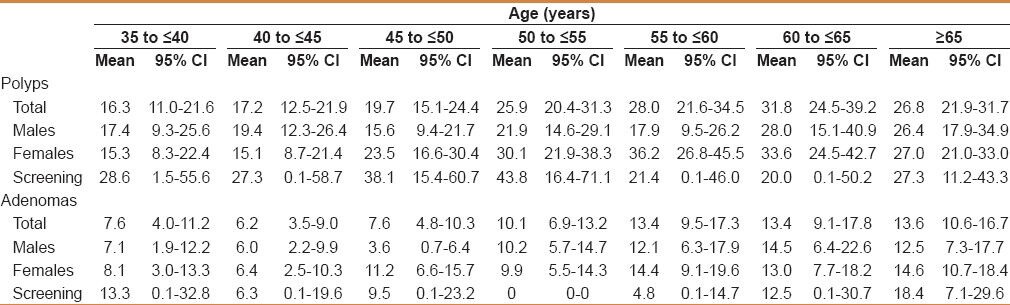
When stratified by age, adenomas ranged from 6.2% to 13.6% with a higher proportion in older individuals. This trend was seen in males (6.0-14.5%) and females (6.4-14.6%), as well as in those who had screening colonoscopies (6.3-18.4%) [Table 3].
Most of the polyps were found in the left side of the colon (32.7%), followed by the rectum (22.8%), the right side of the colon (14.5%), and the least were found in the transverse colon (9.6%), while 20.4% polyps were found in multiple locations [Table 4]. Adenomas had a similar distribution to that of polyps [Table 4], while advanced adenomas were found more frequently in the left side of the colon, followed by the rectum, then the transverse colon, and 42.1% were found in multiple locations [Table 4].
Table 4.
Location of polyps detected in the complete cohort

Those with a prior history of polyps or CRC were more likely to have adenoma at colonoscopy than those who did not (14.3% vs. 6.6%; P < 0.01).
Screening colonoscopies
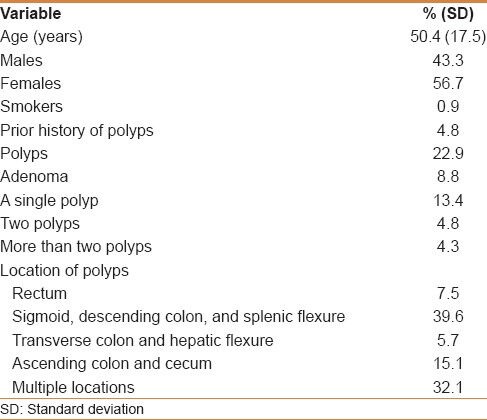
We looked at the subcategory of our study population that underwent screening colonoscopy, which represented 7.7%. The mean age for those who had screening was 50.4 years (SD 17.5), of whom 43.3% were males and 56.7% were females. Of those who had screening, 4.8% had a prior history of polyps that were removed. Only 22.9% of those who underwent screening colonoscopy had polyps and 8.8% had adenomas detected. A single polyp was detected in 13.4%, 4.8% had two polyps detected, and 4.3% had more than two polyps detected. These polyps were found in the sigmoid or descending colon in 39.6%, in multiple locations in 32.1%, in the ascending colon and cecum in 15.1%, in the rectum in 7.5%, and in the transverse colon in 5.7% [Table 5].
Table 5.
Subcategory of individuals who underwent a screening colonoscopy
ROC curve for the optimum age to perform colonoscopy for the detection of adenomas
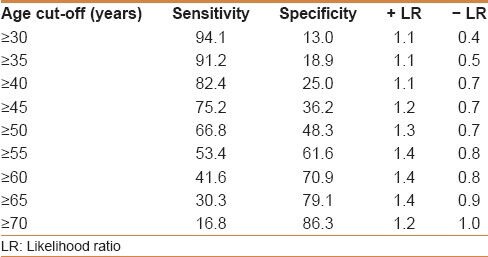
Age could not predict the occurrence of adenomas as the ROC model yielded an area under the ROC curve of 59% [Figure 2]. Different sensitivities, specificities, as well as positive and negative likelihood ratios are shown in Table 6.
Figure 2.
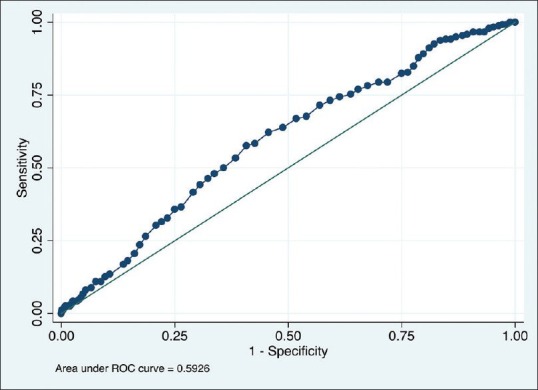
Receiver operating characteristics (ROC) curve for the optimum age to perform colonoscopy for the detection of adenomas. The area under the ROC curve was 0.59
Table 6.
Different age cut-offs with regard to sensitivity, specificity, and likelihood ratios for adenoma detection
DISCUSSION
CRC has become the most common cancer in males and the third common cancer in females in Saudi Arabia.[4] The burden of CRC in Saudi Arabia is predicted to increase.[9,10] Based on data from the Saudi Cancer Registry, the overall 5-year survival for CRC is 44.6%, and specifically, the 5-year survival for colon cancer was 40.9%, for rectosigmoid cancer was 34.8%, and for rectal cancers was 33.6%.[4] Furthermore, the 5-year survival for CRC based on the stage of disease was 63.3% for localized disease, 50.2% for those with regional disease, and 14.7% for patients with metastatic CRC.[4] CRC appears to affect males more than females in Saudi Arabia (66.1% vs. 33.9% respectively),[4] with females having a better overall survival when compared to males (50.6% vs. 41.0%, respectively).[4] Thus, there is a need to address the applicability of a national CRC screening program. One of the elements that need to be determined, if CRC screening is deemed applicable, would be the age at which CRC screening should be initiated. It would be required to determine the prevalence, histology, and age at which polyps are detected in the population. The prevalence of polyps in our cohort was 20.8%, while adenomas were found in only 8.1%, with not much difference between males and females. This is much less than that reported in other areas; in a large multicenter study from Italy, the median detection rates for polyp, neoplasia, and advanced neoplasia were 35%, 26%, and 13%, respectively.[11]
We believe that the low cecal intubation rate in our cohort is related mostly to the bowel preparation quality since in those with a good bowel preparation, the cecal intubation rate was 92.5%. The low cecal intubation rate reported could be attributed to the endoscopist opting to forgo the completion of the colonoscopy due to the poor colonoscopy preparation. This is evident from the cecal intubation rate being 76.7% in those with a fair bowel preparation and 50.2% in those with a poor preparation.
The ADR is defined as the proportion of screening colonoscopies where at least one adenoma is detected.[12] In the Western population, the benchmark ADR for first time screening colonoscopies for individuals 50 years or older is 25% for males and 15% for females.[13] These cut-offs might be an underestimation of the true prevalence of adenomas in the population. A large cohort study found that as the ADR increased, the rate of interval adenocarcinomas detected between a screening colonoscopy and the next scheduled surveillance colonoscopy decreased.[14] Furthermore, a retrospective chart review from Mayo Clinic, Arizona, found that the ADR reached up to 42% for some gastroenterologists.[15]
In Oman, a study of patients referred for a colonoscopy to a major tertiary care hospital demonstrated that the ADR was 12.1%.[16] A series on individuals undergoing screening colonoscopy in China demonstrated that the ADR and advanced neoplasia rates were 26.1% and 10.5%, respectively.[17] A similar study from Thailand found the ADR to be 16.5%,[18] while a study from Korea in which individuals aged from 40 to 49 years were screened for CRC reported the ADR as 22.3%.[19] Thus, it seems that the adenomas are less prevalent in Asian populations compared to Western ones.
This distribution of adenomas as well as advanced adenomas in our study population, where the majority of adenomas were colonic, mostly left sided, followed by presence in locations such as rectum and then the other areas of colon [Table 4], is similar to the data by Mosli et al.,[7] as well as the distribution of CRC reported in the Saudi Cancer Registry, where colonic tumors formed 46.8%, tumors in rectum were 41.2%, and the rectosigmoid tumors comprised 12%.[4] Of interest, when one adds rectum and rectosigmoid tumors, these comprise 53.2% of all CRCs.
In this cohort, there was a trend for more adenomas being detected in males 9.1% (95% CI: 7.7-10.5) compared to females 6.7% (95% CI: 5.3-8.1), but it did not reach statistical significance. This variation between genders was also seen in the data reported by the International Agency for Research on Cancer, GLOBOCAN 2008, wherein the ASR for the incidence of CRC per 100,000 population-years was 14.3 for males compared to 9.7 for females.[3] Also, there was a trend toward an increase in the prevalence of adenomas with age [Table 3]; but at the same time, the prevalence in those aged less than 45 years was non-negligible (7.6% and 6.2%). This is in keeping with the report of Mosli et al.,[7] in which there was a significant proportion of patients with CRC younger than 45 years but the majority of CRC cases were older than 45 years of age from the years 2000 to 2006.[7]
Of note, the percentage of polyps and adenomas found in the relatively young age groups was more pronounced in those who had screening as an indication, where 28.6% had polyps and 13.3% had adenomas. This could be due to the fact that they were high-risk individuals as it is not common to screen this age group. Another possible explanation is that younger patients were more likely compliant with the bowel preparation, which improves visual spotting of polyps, as evident from the better bowel preparation quality in those younger than 35 years (70.0%) and older than 35 years (65.1%). Also, data from the Saudi Cancer Registry demonstrated that 20.9% of all cases of CRC were younger than 45 years of age.[7]
We found a higher proportion of adenomas in individuals who were undergoing colonoscopies with a prior history of adenomas or colon cancer (14.3% vs. 6.6%). This is in keeping with the literature, as demonstrated in a large population-based series from New Hampshire that there was a higher ADR in those who had surveillance colonoscopies compared to those who had screening colonoscopies (37% vs. 25%, respectively).[12]
One of the limitations in the study, however, is that we could not ascertain the duration between prior colonoscopy and the surveillance colonoscopy studied; if the time was short, then that would potentially decrease the ADR and the true prevalence might even be higher.[12] Indeed, the bowel preparation quality was less than optimum in a significant proportion of colonoscopies. This, in part, could be related to the patient population included as inpatients[20] as well as the fact that a significant proportion of patients were diabetics,[21] and both these factors are known to affect the preparation quality. Also, we had not adopted a split-dose regimen as opposed to the solution being administered a day before the colonoscopy. Furthermore, some variables that were missing and could have acted as confounders included obesity, history of smoking, and alcohol consumption. A large study from Korea demonstrated an increase in low-risk adenomas (OR 1.44; 95% CI: 1.23-1.69) as well as high-risk adenomas (OR 1.62; 95% CI: 1.09-2.41) in individuals who were obese, although they did not have metabolic syndrome, when compared to non-obese metabolically healthy individuals.[22] An association between alcohol and smoking history has also been demonstrated.[11] Also, there were colonoscopies that were performed more than once in some individuals of the studied population (10.1%); whether this would cause an increase or decrease in the ADR is not known since if these colonoscopies were unnecessarily repeated, that would decrease the ADR, while if they were truly indicated, that might cause an increase in the ADR.
We could not determine if every polyp detected on colonoscopy was removed. Some endoscopists depend on the morphological features of polyps on regular white light endoscopy; in some situations, narrow band imaging as well as the size of the polyp prior to its removal might lead them to forgo removing the polyps if it is believed that it is a hyperplastic polyp. This approach might potentially decrease the ADR as demonstrated in a study where even when the gastroenterologists were trained in pit pattern recognition, the accuracy, sensitivity, and specificity of in vivo diagnoses of these polyps were 76.6%, 78.1%, and 73.4%, respectively,[23] and the image-based recommendations for post-polypectomy surveillance were correct in only 69.5% of cases.[23]
One of the factors which might have decreased the adenoma detection in our cohort is that the mean age of the studied population was relatively young (50.5 years), as higher rates of adenomas have been demonstrated in those older than 50 years of age.
We could not determine the optimal age at which screening for CRC would be beneficial. This is most probably due to a proportion of young individuals in our cohort having adenomas [Table 3]. This issue can also be seen in the relatively high proportion of young individuals who developed CRC, based on the Saudi Cancer Registry.[7] One way to tackle this issue is to characterize specific risk factors for developing CRC at a relatively younger age and offer that subgroup CRC screening, devising a cut-off for the remainder of the population.
In conclusion, although the proportion of those who had screening as an indication for colonoscopy in our cohort was low, this study sheds some light on the prevalence of adenomas in those undergoing colonoscopies for various indications as well as opportunistic screening, and adds to the data that would determine if screening for CRC is a need and would be efficacious in Saudi Arabia. A prospective study addressing the modality and efficacy of CRC screening in Saudi Arabia is needed to answer some of the remaining questions.
Footnotes
Source of Support: The authors extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding of this research through the Research Group Project number RGP-VPP-279
Conflict of Interest: None declared.
REFERENCES
- 1.Garborg K, Holme O, Loberg M, Kalager M, Adami HO, Bretthauer M. Current status of screening for colorectal cancer. Ann Oncol. 2013;24:1963–72. doi: 10.1093/annonc/mdt157. [DOI] [PubMed] [Google Scholar]
- 2.Bray FR ME, Ferlay J. Estimates of global cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 3.Ferlay J SH, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. [accessed on 25/10/2013]. GLOBOCAN 2008 v2.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Available from: http://globocan.iarc.fr . [Google Scholar]
- 4.Al-Ahwal MS, Shafik YH, Al-Ahwal HM. First national survival data for colorectal cancer among Saudis between 1994 and 2004: What's next? BMC Public Health. 2013;13:73. doi: 10.1186/1471-2458-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayyub MI, Al-Radi AO, Khazeindar AM, Nagi AH, Maniyar IA. Clinicopathological trends in colorectal cancer in a tertiary care hospital. Saudi Med J. 2002;23:160–3. [PubMed] [Google Scholar]
- 6.Mosli MH, Al-Ahwal MS. Does the increasing trend of colorectal cancer incidence in jeddah reflect a rise in the kingdom of saudi arabia? Asian Pac J Cancer Prev. 2012;13:6285–8. doi: 10.7314/apjcp.2012.13.12.6285. [DOI] [PubMed] [Google Scholar]
- 7.Mosli MH, Al-Ahwal MS. Colorectal cancer in the Kingdom of Saudi Arabia: Need for screening. Asian Pac J Cancer Prev. 2012;13:3809–13. doi: 10.7314/apjcp.2012.13.8.3809. [DOI] [PubMed] [Google Scholar]
- 8.Aljebreen AM. Clinico-pathological patterns of colorectal cancer in Saudi Arabia: Younger with an advanced stage presentation. Saudi J Gastroenterol. 2007;13:84–7. doi: 10.4103/1319-3767.32183. [DOI] [PubMed] [Google Scholar]
- 9.Ibrahim EM, Zeeneldin AA, El-Khodary TR, Al-Gahmi AM, Bin Sadiq BM. Past, present and future of colorectal cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol. 2008;14:178–82. doi: 10.4103/1319-3767.43275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim E, Bin SB, Banjar L, Awadalla S, Abomelha MS. Current and future cancer burden in Saudi Arabia: Meeting the challenge. Hematol Oncol Stem Cell Ther. 2008;1:210–5. doi: 10.1016/s1658-3876(08)50006-9. [DOI] [PubMed] [Google Scholar]
- 11.Ricci E, Hassan C, Petruzziello L, Bazzoli F, Repici A, Di Giulio E. Inter-centre variability of the adenoma detection rate: A prospective, multicentre study. Dig Liver Dis. 2013 doi: 10.1016/j.dld.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 12.Anderson JC, Butterly LF, Goodrich M, Robinson CM, Weiss JE. Differences in Detection Rates of Adenomas and Serrated Polyps in Screening Versus Surveillance Colonoscopies, Based on the New Hampshire Colonoscopy Registry. Clin Gastroenterol Hepatol. 2013 doi: 10.1016/j.cgh.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex DK, Petrini JL, Baron TH, Chak A, Cohen J, Deal SE, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Kaminski MF, Regula J, Kraszewska E, Polkowski M, Wojciechowska U, Didkowska J, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 15.Boroff ES, Gurudu SR, Hentz JG, Leighton JA, Ramirez FC. Polyp and adenoma detection rates in the proximal and distal colon. Am J Gastroenterol. 2013;108:993–9. doi: 10.1038/ajg.2013.68. [DOI] [PubMed] [Google Scholar]
- 16.Ashktorab H, Brim H, Al-Riyami M, Date A, Al-Mawaly K, Kashoub M, et al. Sporadic colon cancer: Mismatch repair immunohistochemistry and microsatellite instability in Omani subjects. Dig Dis Sci. 2008;53:2723–31. doi: 10.1007/s10620-007-0189-3. [DOI] [PubMed] [Google Scholar]
- 17.Leung WK, Tang V, Lui PC. Detection rates of proximal or large serrated polyps in Chinese patients undergoing screening colonoscopy. J Dig Dis. 2012;13:466–71. doi: 10.1111/j.1751-2980.2012.00621.x. [DOI] [PubMed] [Google Scholar]
- 18.Aswakul P, Prachayakul V, Lohsiriwat V, Bunyaarunnate T, Kachintorn U. Screening colonoscopy from a large single center of Thailand-something needs to be changed? Asian Pac J Cancer Prev. 2012;13:1361–4. doi: 10.7314/apjcp.2012.13.4.1361. [DOI] [PubMed] [Google Scholar]
- 19.Choi YS, Suh JP, Lee DS, Youk EG, Lee IT, Lee SH, et al. Colonoscopy screening for individuals aged 40-49 years with a family history of stomach cancer in Korea. Int J Colorectal Dis. 2010;25:443–7. doi: 10.1007/s00384-009-0855-3. [DOI] [PubMed] [Google Scholar]
- 20.Ness RM, Manam R, Hoen H, Chalasani N. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–802. doi: 10.1111/j.1572-0241.2001.03874.x. [DOI] [PubMed] [Google Scholar]
- 21.Chung YW, Han DS, Park KH, Kim KO, Park CH, Hahn T, et al. Patient factors predictive of inadequate bowel preparation using polyethylene glycol: A prospective study in Korea. J Clin Gastroenterol. 2009;43:448–52. doi: 10.1097/MCG.0b013e3181662442. [DOI] [PubMed] [Google Scholar]
- 22.Yun KE, Chang Y, Jung HS, Kim CW, Kwon MJ, Park SK, et al. Impact of body mass index on the risk of colorectal adenoma in a metabolically healthy population. Cancer Res. 2013;73:4020–7. doi: 10.1158/0008-5472.CAN-12-3477. [DOI] [PubMed] [Google Scholar]
- 23.Schachschal G, Mayr M, Treszl A, Balzer K, Wegscheider K, Aschenbeck J, et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut. 2013 doi: 10.1136/gutjnl-2013-304562. [DOI] [PubMed] [Google Scholar]


