Abstract
Background and Aims:
Egy-Score is a new noninvasive score for prediction of severe hepatic fibrosis in patients with chronic liver diseases. The aim of this study was to validate Egy-Score as a noninvasive score for predicting stage of hepatic fibrosis in a group of Egyptian chronic hepatitis C patients.
Patients and Methods:
One hundred Egyptian patients with chronic hepatitis C were enrolled. Mean age was 40.25 ± 9.39 years. They were subjected to CA19-9, alpha-2-macroglobulin, total bilirubin, platelet count and albumin, liver biopsy, and histopathological staging of hepatic fibrosis according to METAVIR scoring system as part of their assessment for treatment. Egy-Score was calculated according to the following formula: Egy-Score = 3.52 + 0.0063 × CA19-9 + 0.0203 × age + 0.4485 × alpha-2-macroglobulin + 0.0303 × bilirubin – 0.0048 × platelet – 0.0462 × albumin. Egy-Score results were correlated to the stage of hepatic fibrosis.
Results:
Egy-Score correlates positively with the stage of hepatic fibrosis (F0–F4). Egy-Score was able to differentiate significant hepatic fibrosis, severe hepatic fibrosis, and cirrhosis accurately. Cutoff values of Egy-Score were 2.91850 (for significant fibrosis), 3.28624 (for severe fibrosis), and 3.67570 (for cirrhosis). Sensitivity, specificity, and areas-under-ROC curve (AUROCs) were 75.8%, 68.42%, and 0.776 (for significant fibrosis “≥F2”), 91.67%, 77.63%, and 0.875 (for severe fibrosis “≥F3”), and 81.82%, 86.52%, and 0.874 (for cirrhosis “F4”), respectively.
Conclusion:
Egy-Score is a useful noninvasive panel of surrogate biomarkers that could accurately predict different stages of hepatic fibrosis in patients with chronic hepatitis C.
Keywords: Biomarkers, cirrhosis, fibrosis, HCV, noninvasive
Liver fibrosis (LF) is a kind of chronic damage of the liver and can lead to cirrhosis.[1] LF occurs in response to almost all causes of chronic liver injury.[2] An accurate assessment of the degree of fibrosis or presence of cirrhosis is critical both for the appropriate management of, and to provide prognosis for, patients with chronic hepatitis C infection (CHC).[3] Hepatic biopsy has traditionally been considered the standard procedure to define the stage of fibrosis, although sampling errors and interobserver variability are problems with the technique.[4] The limitations and the invasive nature of liver biopsy has encouraged extensive interest in the development of noninvasive tests to measure LF in patients with CHC.[5] Several noninvasive methods, ranging from serum marker assays to advanced imaging techniques, have been proved to be excellent tools for the evaluation of LF in patients with CHC.[6] Many blood tests have been proposed as alternatives to liver biopsy for identifying fibrosis or cirrhosis.[7] These blood tests are generally classified into direct and indirect markers for hepatic fibrosis.[8] Direct markers are molecules derived from extracellular matrix turnover reflecting the activity of the fibrotic process.[9] Indirect markers reflect alterations in hepatic functions and satisfy the request for a simple and easy to perform markers.[10] Most direct markers are not routinely requested for assessment of liver disease, whereas most indirect markers are routinely used and readily available. Examples of indirect markers include Prothrombin index, platelet count, and aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio.[11] Examples of direct markers include collagens (eg, procollagen I C-peptide, procollagen III N-peptide, type IV collagen and its fragments), glycoproteins and polysaccharides (eg, hyaluronic acid, laminin, tenascin, YKL-40), collagenases and their inhibitors (eg, metalloproteinases, tissue inhibitors of metalloproteinases), and cytokines (eg, transforming growth factor-β1and platelet-derived growth factor).[12] Due to poor accuracy of individual markers to assess LF, algorithms, or indices combining panels of markers have been developed.[13] Most commonly used panels include FibroTest,[14] AST-to-platelet ratio index (APRI),[15] FIB-4,[16] FORNs’ index,[17] HepaScore,[18] FibroMeters,[19] FibroIndex,[20] FibroSpect II,[21] and European liver fibrosis index (ELF).[22] These markers are initially developed and validated in CHC patients and are now being applied to other chronic liver diseases.[23] Noninvasive diagnostic approaches are not reliable to discriminate between the intermediate stages of fibrosis.[24] Therefore, we are still in need of new and more accurate biomarkers for assessing hepatic fibrosis. Egy-Score is a relatively new panel of biomarkers used for the assessment of the stage of hepatic fibrosis in patients with chronic liver diseases. It was initially studied in a heterogeneous group of patients (chronic hepatitis C, chronic hepatitis B, and autoimmune hepatitis). Egy-Score is a result of a regression equation based on six parameters (CA19-9, age, alpha-2-macroglobulin, total bilirubin, platelet count, and albumin).[25] Aim of the present study was to assess the performance of Egy-Score in staging hepatic fibrosis in a prospective cohort of Egyptian patients with CHC.
PATIENTS AND METHODS
Patient selection
Hundred treatment-naïve Egyptian patients with CHC were included in our observational study. They were prospectively recruited from Kasr Al-Aini Viral Hepatitis Center, Faculty of Medicine, Cairo University, between May 2011 and December 2012.
Investigations
All patients had positive hepatitis C virus antibody, positive HCV-RNA by PCR, negative ANA, negative HBsAg and HBcAb. Abdominal ultrasound was done for all subjects to assess liver disease and rule out any hepatic or pancreatic lesions.
Liver biopsy
As part of the assessment for treatment eligibility, percutaneous liver biopsies were taken from the right lobe of the liver of all patients using modified Menghini needle under ultrasound guidance. Liver biopsy specimens were fixed with formalin, embedded in paraffin, and stained with hematoxylin and eosin. Liver biopsies were examined by a single pathologist experienced in liver tissue, who was blinded to clinical and biochemical data of patients. All liver biopsy specimens were ≥20 mm in length and containing at least 11 portal tracts. Staging of hepatic fibrosis and grading of necroinflammatory activity was done according to METAVIR scoring system. Fibrosis score was staged on a five-point scale (F0 = no fibrosis, F1 = portal fibrosis without septa, F2 = few septa, F3 = numerous septa without cirrhosis, and F4 = cirrhosis). The activity score was graded according to the intensity of necroinflammatory lesions (A0 = no activity, A1 = mild activity, A2 = moderate activity, and A3 = severe activity).[26] Significant hepatic fibrosis, severe hepatic fibrosis, and cirrhosis were defined as a fibrosis stage ≥F2, ≥F3, and F4, respectively.
Egy-Score calculation
Patients’ sera were analyzed for (CA19-9, age, alpha-2- macroglobulin, total bilirubin, platelet count, and albumin) in the same day of performing liver biopsy. Egy-Score was calculated according to the original formula [1]: Egy-Score = 3.52 + 0.0063 × CA19-9 (U/mL) +0.0203 × age (year) +0.4485 × alpha-2-macroglobulin (g/L) +0.0303 × bilirubin (μmol/L)-0.0048 × platelet (K/μL)-0.0462 × albumin (g/L).
Consent and ethical aspects
Informed written consent from each patient and local ethical committee approval were available before starting data collection. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki.
Statistical analysis
Analysis of data was performed using SPSS 17 (Statistical Package for Scientific Studies) for Windows. Description of categorical variables was summarized by frequency counts and percentages. Continuous variables were summarized by means, medians, and standard deviations. Binary correlation was carried out by Spearman correlation test. Results were expressed in the form of correlation coefficient (r) and P values. Receiver-operating characteristic (ROC) curves were graphed to determine appropriate Egy-Score levels in predicting significant hepatic fibrosis (≥F2 METAVIR), severe hepatic fibrosis (≥F3 METAVIR), and cirrhosis (F4 METAVIR) that give optimal sensitivity, specificity, and positive and negative predictive values. All the hypotheses tested were two-sided and statistical significance was accepted at the 5% level.
RESULTS
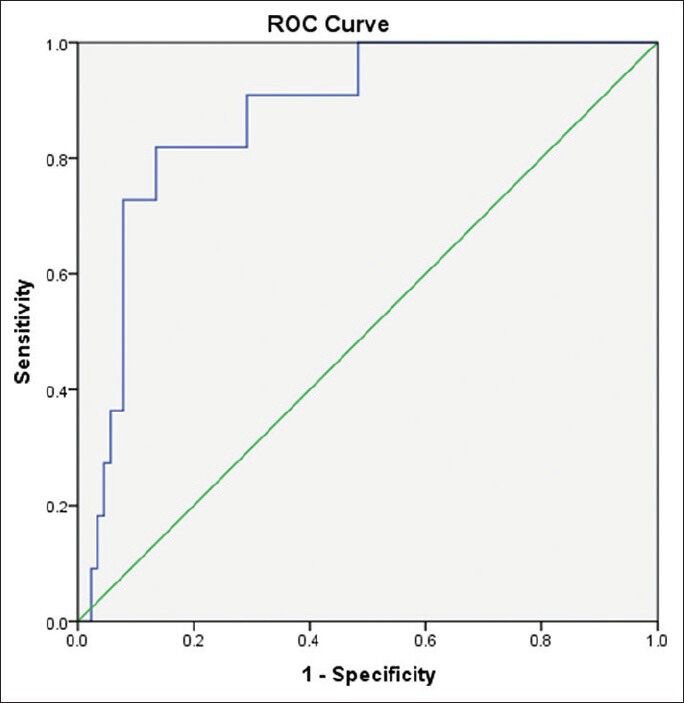
Our study included 100 treatment-naïve patients with chronic hepatitis C; 67 males and 33 females; their mean age ± SD was 40.25 ± 9.39. The mean values ± SD of the studied parameters were albumin, 41.89 ± 4.95 g/L; total bilirubin, 13.89 ± 3.60 μmol/L platelets, 201.58 ± 59.02 109/L; alpha-2-macroglobulin, 2.57 ± 0.54 g/L; and CA 19.9, 15.07 ± 16.64 U/mL) [Table 1]. Stages of hepatic fibrosis in biopsy specimens showed: 3 patients with F0 (3%), 35 patients with F1 (35%), 38 patients with F2 (38%), 13 patients with F3 (13%), and 11 patients with F4 (11%). Egy-Score was positively correlating to different stages of hepatic fibrosis (F0–F4) “r = 0.65, P < 0.001.” Different cutoff values of Egy-Score were tested for prediction of significant hepatic fibrosis (≥F2), severe hepatic fibrosis (≥F3), and cirrhosis (F4). Sensitivity, specificity, and positive and negative predictive values of different cutoff values of Egy-Score for detection of significant hepatic fibrosis (≥F2), severe hepatic fibrosis (≥F3), and cirrhosis (F4) are summarized in Tables 2–4, respectively. The most relevant cutoff values were 2.91850 for significant hepatic fibrosis (≥F2), 3.28624 for severe hepatic fibrosis (≥F3), and 3.67570 for cirrhosis (F4). The diagnostic value of the Egy-Score was assessed by ROC curve analysis, shown in Figures 1–3, which gave AUROCs of 0.776, 0.875, and 0.874 for the detection of significant hepatic fibrosis, severe hepatic fibrosis, and cirrhosis, respectively.
Table 1.
General characteristics of the study population
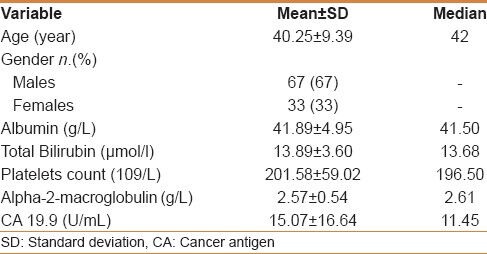
Table 2.
Cutoff values of Egy-Score for detection of significant hepatic fibrosis (≥F2)
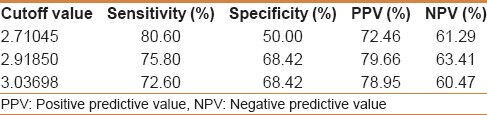
Table 4.
Cutoff values of Egy-Score for detection of cirrhosis (F4)

Figure 1.
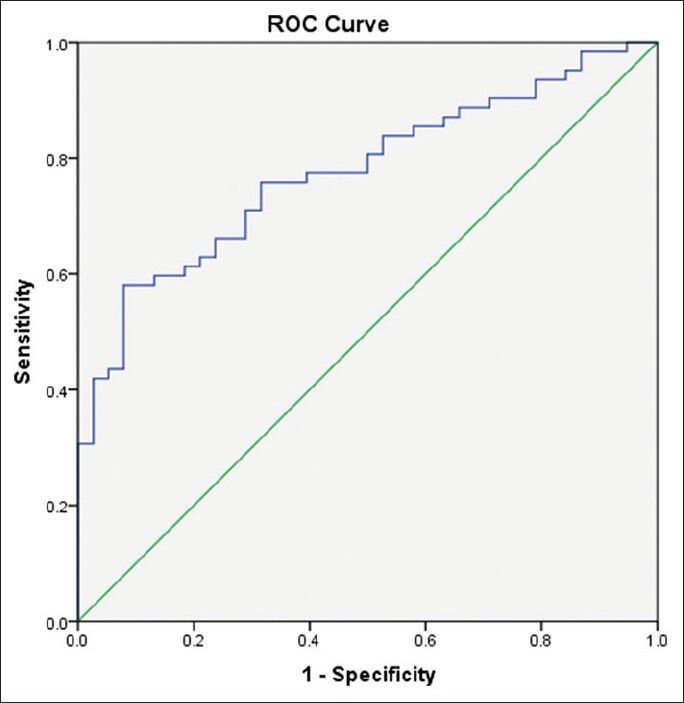
Receiver–operating curve for Egy-Score for detection of significant hepatic fibrosis (≥F2)
Figure 3.
Receiver-operating curve for Egy-Score for detection of severe hepatic fibrosis (F4)
Table 3.
Cutoff values of Egy-Score for detection of severe hepatic fibrosis (≥F3)
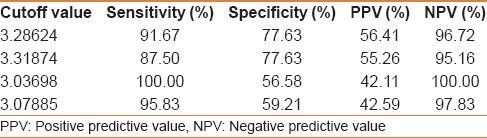
Figure 2.
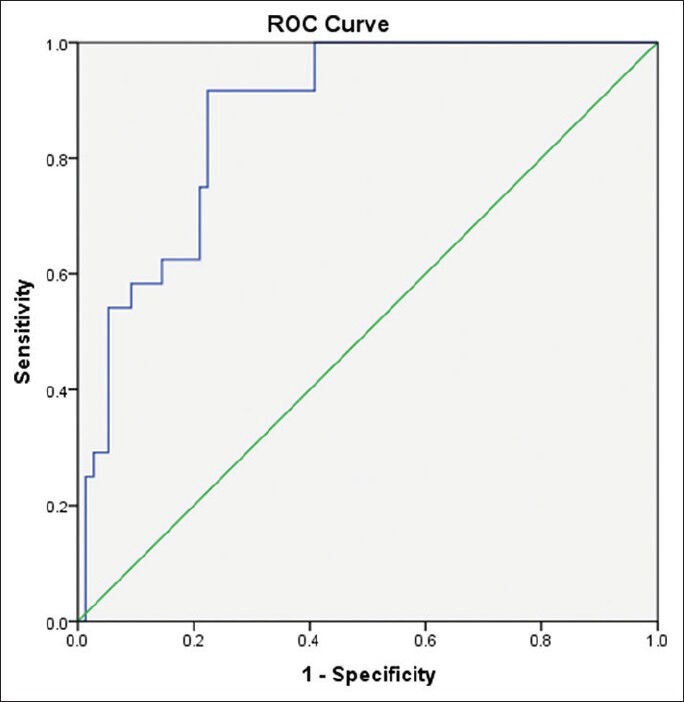
Receiver-operating curve for Egy-Score for detection of severe hepatic fibrosis (≥F3)
DISCUSSION
Noninvasive methods for assessment of hepatic fibrosis in CHC are growing and becoming more popular, especially with the development of more effective oral interferon-free regimens. The most important stages of hepatic fibrosis on which clinicians base their decisions for management of CHC patients are significant fibrosis and cirrhosis. In this study, we validated a relatively new panel of biomarkers “Egy-Score" for the noninvasive assessment of hepatic fibrosis and cirrhosis. Egy-Score showed good sensitivity, specificity, and positive and negative predictive values, and AUROCs for predicting significant fibrosis (≥F2), severe hepatic fibrosis (≥F3), and cirrhosis (F4). Our results showed less sensitivity and specificity in the detection of significant hepatic fibrosis (≥F2) than what is present in the original study of Egy-Score[25] that showed sensitivity of 79.4% and specificity of 79.3% in the detection of stages F0–F1. Also our results showed less sensitivity and better specificity for detection of severe hepatic fibrosis (≥F3) than what was reported in Egy-Score original study[25] that showed sensitivity of 95.9% and specificity of 69.8% in detection of stages F0-F2. The differences between our results and original Egy-Score study results may be due to using different cutoff values. We used the cutoff 2.91850 for significant hepatic fibrosis, and the cutoff 3.28624 for severe hepatic fibrosis rather than 3 for significant hepatic fibrosis and 4 for severe hepatic fibrosis suggested in the original study. Widely used patented and nonpatented noninvasive panels for assessment of hepatic fibrosis (FibroTest, FibroMeters, HepaScore, and APRI) were prospectively compared in a study on 1307 patients, the results of that study showed AUROCs ranging from 0.72-0.78 for detection of significant fibrosis and from 0.77-0.86 for detection of cirrhosis.[27] In our study, Egy-Score showed similar AUROC for detection of significant hepatic fibrosis (0.776) and cirrhosis (0.874) similar to what was reported for known patented and nonpatented scores. Limitations of our study included the following: Sample size was hundred patients only, so cutoff values need to be validated in a prospective multicenter larger cohort. Validation in other chronic liver diseases, in treatment contexts and comparison with other noninvasive methods, namely, biomarkers panels (eg, APRI, FORNS, FIB-4, and FibroTest) and radiological methods (eg, FibroScan) should be also done.
CONCLUSION
Egy-Score showed good sensitivity, specificity, positive and negative predictive values, and over all accuracy for detecting different stages of hepatic fibrosis and cirrhosis in patients with chronic hepatitis C.
Footnotes
Source of Support: The manuscript was presented in meeting of the Asia Pacific association of liver diseases, Singapore, Saturday - June 08, 2013 08:30-17:30.
Conflict of Interest: None declared.
REFERENCES
- 1.Mak TM, Huang YP, Zheng YP. Liver fibrosis assessment using transient elastography guided with real-time B-mode ultrasound imaging: A feasibility study. Ultrasound Med Biol. 2013;39:956–66. doi: 10.1016/j.ultrasmedbio.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134:1670–81. doi: 10.1053/j.gastro.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez HC, Jafri SM, Gordon SC. Role of liver biopsy in the era of direct-acting antivirals. Curr Gastroenterol Rep. 2013;15:307. doi: 10.1007/s11894-012-0307-z. [DOI] [PubMed] [Google Scholar]
- 4.Roca B, Resino E, Torres V, Herrero E, Penades M. Interobserver discrepancy in liver fibrosis using transient elastography. J Viral Hepat. 2012;19:711–5. doi: 10.1111/j.1365-2893.2012.01608.x. [DOI] [PubMed] [Google Scholar]
- 5.Lai M, Afdhal NH. Editorial: Staging liver fibrosis in hepatitis C: A challenge for this decade. Am J Gastroenterol. 2011;106:2121–2. doi: 10.1038/ajg.2011.343. [DOI] [PubMed] [Google Scholar]
- 6.Trifan A, Stanciu C. Checkmate to liver biopsy in chronic hepatitis C? World J Gastroenterol. 2012;18:5514–20. doi: 10.3748/wjg.v18.i39.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: A systematic review. Ann Intern Med. 2013;158:807–20. doi: 10.7326/0003-4819-158-11-201306040-00005. [DOI] [PubMed] [Google Scholar]
- 8.Trifan A, Stanciu C. Checkmate to liver biopsy in chronic hepatitis C? World J Gastroenterol. 2012;18:5514–20. doi: 10.3748/wjg.v18.i39.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu T, Wang X, Karsdal MA, Leeming DJ, Genovese F. Molecular serum markers of liver fibrosis. Biomark Insights. 2012;7:105–17. doi: 10.4137/BMI.S10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attallah AM, Abdallah SO, Attallah AA, Omran MM, Farid K, Nasif WA, et al. Diagnostic value of fibronectin discriminant score for predicting liver fibrosis stages in chronic hepatitis C virus patients. Ann Hepatol. 2013;12:44–53. [PubMed] [Google Scholar]
- 11.Castera L. Non-invasive assessment of liver fibrosis in chronic hepatitis C. Hepatol Int. 2011;5:625–34. doi: 10.1007/s12072-010-9240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grigorescu M. Noninvasive biochemical markers of liver fibrosis. J Gastrointestin Liver Dis. 2006;15:149–59. [PubMed] [Google Scholar]
- 13.Papastergiou V, Tsochatzis E, Burroughs A. Non-invasive assessment of liver fibrosis. Ann Gastroenterol. 2012;25:218. [PMC free article] [PubMed] [Google Scholar]
- 14.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T MULTIVIRC Group. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: A prospective study. Lancet. 2001;357:1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 15.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 16.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. APRICOT Clinical Investigators. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 17.Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986–92. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 18.Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: An accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem. 2005;51:1867–73. doi: 10.1373/clinchem.2005.048389. [DOI] [PubMed] [Google Scholar]
- 19.Calès P, Boursier J, Oberti F, Hubert I, Gallois Y, Rousselet MC, et al. FibroMeters: A family of blood tests for liver fibrosis. Gastroenterol Clin Biol. 2008 Sep;32(6 Suppl 1):40–51. doi: 10.1016/S0399-8320(08)73992-7. [DOI] [PubMed] [Google Scholar]
- 20.Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. FibroIndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology. 2007;45:297–306. doi: 10.1002/hep.21520. [DOI] [PubMed] [Google Scholar]
- 21.Poordad FF. FIBROSpect II: A potential noninvasive test to assess hepatic fibrosis. Expert Rev Mol Diagn. 2004;4:593–7. doi: 10.1586/14737159.4.5.593. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. European Liver Fibrosis Group. Serum markers detect the presence of liver fibrosis: A cohort study. Gastroenterology. 2004;127:1704–13. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 23.Srygley FI, Patel K. Noninvasive assessment of liver fibrosis in chronic hepatitis C infection. Curr Hepat Rep. 2008;7:164–72. [Google Scholar]
- 24.Gangadharan B, Bapat M, Rossa J, Antrobus R, Chittenden D, Kampa B, et al. Discovery of novel biomarker candidates for liver fibrosis in hepatitis C patients: A preliminary study. PLoS One. 2012;7:e39603. doi: 10.1371/journal.pone.0039603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alboraie MA, Afifi ME, Elghamry FG, Shalaby HA, Elshennawy GE, Abdelaziz AA, et al. Egy-Score predicts severe hepatic fibrosis and cirrhosis in Egyptians with chronic liver diseases. A pilot study. Hepat Mon. 2013;13:e10810. doi: 10.5812/hepatmon.10810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289–93. doi: 10.1002/hep.510240201. [DOI] [PubMed] [Google Scholar]
- 27.Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: A multicenter prospective study (the FIBROSTIC study) J Hepatol. 2010;53:1013–21. doi: 10.1016/j.jhep.2010.05.035. [DOI] [PubMed] [Google Scholar]


