Abstract
Background/Aims:
Hippo pathway plays a crucial role in cell proliferation, apoptosis, and tumorigenesis. This study aimed to investigate the expression of Hippo pathway components in the progression and metastasis of colorectal cancer (CRC).
Materials and Methods:
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to examine the mRNA expression levels of MST1, LATS2, YAP, TAZ, TEAD1, CDX2, and OCT4, and western blot (WB) was used to examine the protein expression levels of MST1, YAP, TEAD1, and CDX2 in 30 specimens of human colorectal adenomas, 50 pairs of human CRC tissues, and adjacent nontumorous tissues from CRC patients. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene in qRT-PCR.
Results:
The mRNA expression levels of MST1 and LATS2 showed an increasing tendency from CRC to adjacent nontumorous tissues (P < 0.001). Conversely, the mRNA expression levels of YAP, TAZ, TEAD, and OCT4 showed a decreasing tendency from CRC to adjacent nontumorous tissues (P < 0.001). MST1 protein was downregulated and YAP and TEAD1 proteins were upregulated in CRC (all P < 0.001). The mRNA and protein expression levels of CDX2 in CRC were significantly lower than those in colorectal adenomas and adjacent nontumorous tissues (P < 0.001), but there was no significant difference between the latter two groups (qRT-PCR, P = 0.113; WB, P = 0.151). Furthermore, statistical analysis showed that the expression levels of Hippo signal pathway components were associated with tumor differentiation, lymph node metastasis, and TNM stage.
Conclusion:
Hippo pathway is suppressed in the progression from colorectal adenomas to CRC and is associated with CRC progression and metastasis. This study suggests the components of Hippo pathway might be prognostic indicators for CRC patients.
Keywords: Colorectal adenomas, colorectal cancer, Hippo pathway, tumorigenesis
Colorectal cancer (CRC) was the third most commonly diagnosed cancer and the fourth most common cancer leading to death worldwide in 2008.[1] The prognosis of patients with CRC has improved during the past decades in many countries, probably because of increased application of colonoscopy with polypectomy.[2] As dysplastic adenomas are the most common form of premalignant precursor lesions, more and more studies focus on the adenoma-carcinoma sequence: Normal tissues → adenomas → carcinoma. The mechanisms of adenoma-carcinoma sequence are complicated, which include oncogenes, tumor-suppressor genes, and cancer stem cell.[3,4] The Hippo pathway was first discovered through genetic screens in Drosophila, and many components of the Hippo pathway are highly conserved from Drosophila to mammals, including mammalian STE20-like kinase 1/2 (MST1/2), salvador homolog 1 (SAV1), large tumor suppressor 1/2 (LATS1/2), Yes-associtated protein (YAP) and its paralog, transcriptional co-activator with PDZ-binding motif (TAZ), all of which form a kinase cascade. As a transcriptional co-activator, YAP or TAZ could combine with TEA domain family member 1 (TEAD1) in the nucleus to promote the expression of target genes. Model of the Hippo pathway in mammals is shown in Figure 1.[5] Recent studies have revealed that the Hippo pathway plays a critical role in cell growth, proliferation, apoptosis, organ size, and tumorigenesis. Meanwhile, the suppression of Hippo pathway has been reported in many cancers, such as breast, lung, and hepatocellular carcinoma (HCC).[6,7,8] However, few studies focus on the role of Hippo pathway in CRC systematically.
Figure 1.
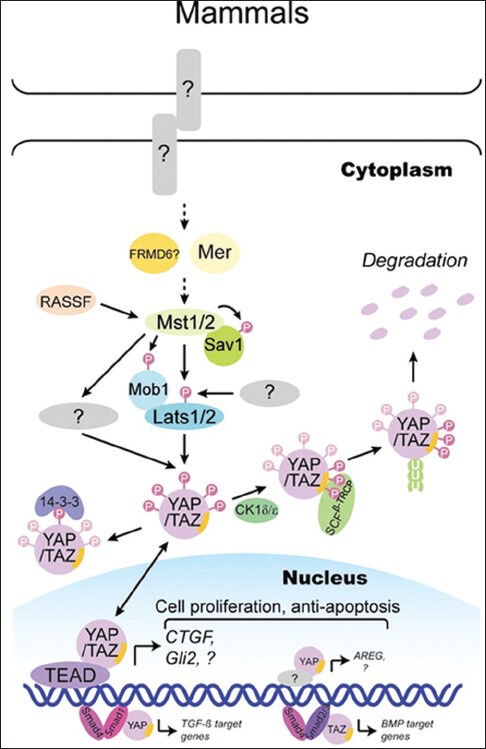
Model of the Hippo pathway in mammals
Caudal type homeobox transcription factor 2 (CDX2) and POU family transcription factor (OCT4) are identified as markers in embryonic stem (ES) cell. CDX2 is an intestine-specific protein which is critical to the intestinal development and differentiation, and previous studies indicate that the expression of CDX2 is reduced in CRC. OCT4 belongs to class 5 of POU family transcription factors, containing multiple transcription factors with pituitary-specific domain, octamer transcription factor domain, and neural Unc-86 transcription factor domain. Also, OCT4 suppresses the expression of CDX2 during embryogenesis, but there is no evidence demonstrating that CDX2 is suppressed by OCT4 in CRC.
Our study aimed to investigate the expression of Hippo pathway components in the progression from colorectal adenomas to CRC, and we wondered whether CDX2 and OCT4 are the target genes of YAP or TAZ which combine with TEAD1. In addition, we assessed the correlation of the clinicopathologic characteristics in CRC patients.
MATERIALS AND METHODS
Patients and tissue specimens
This study was approved by the ethics committee of Qingdao University Medical College and obtained the consent from the patients. Fifty pairs of CRC and adjacent nontumorous tissues were obtained from the patients who had undergone surgical operation and 30 specimens of colorectal adenoma tissues were obtained from the patients undergoing polypectomy at Affiliated Hospital of Qingdao University during 2012. Adjacent nontumorous tissues were dissected more than 5 cm away from the cancer edge. All the patients with CRC did not receive preoperative chemoradiotherapy. The specimens, which were verified by a pathologist, were snap-frozen in liquid nitrogen after resection and stored at −80°C.
Quantitative real-time polymerase chain reaction
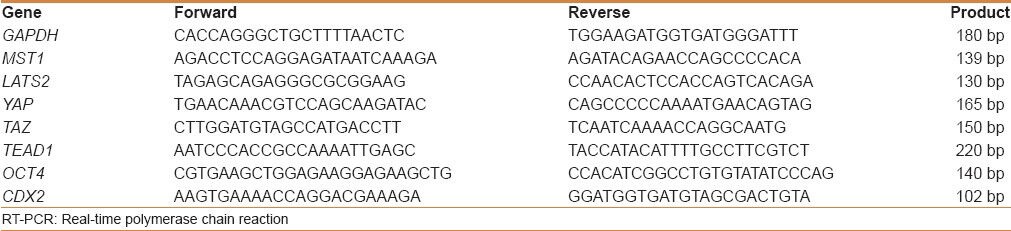
Quantitative real-time polymerase chain reaction (qRT-PCR) was used to determine the expression levels of Hippo pathway components in CRC (n = 50), adjacent nontumorous tissues (n = 50), and colorectal adenomas (n = 30). Total RNA was extracted from the specimens using Trizol reagent (Invitrogen, Carlsbad, USA) according to the manufacturer's protocol and RNA quality was determined by measurements of OD26/OD280 and analysis on an agarose gel. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the housekeeping gene for loading control. All the sequences of primers are listed in Table 1. The cDNA synthesis was carried out using TaKaRa PrimerScript™ RT-PCR. The qRT-PCR was performed with SYBR® Premix Ex Taq™ II (TaKaRa) according to the manufacturer's protocol by Roche LightCyle Real-time PCR (Roche, Basel, Switzerland). Reactions for all assays were carried out in a total volume of 20 μl (containing 2 × SYBR II 10 μl, forward primer 1.6 μl, reverse primer 1.6 μl, ddH2 O 4.8 μl, cDNA 2 μl) with the following amplification steps: An initial denaturation at 95°C for 30 s followed by 40 cycles of denaturation at 95°C for 5 s and annealing at 60°C for 30 s. The 2−ΔΔCT method was used to calculate the relative expression levels. The group with adjacent nontumorous tissues was used as a normal standard for normalization and comparison for each gene. Three separate experiments were performed for each clone.
Table 1.
The sequence of primers used in RT-PCR
Western blot analysis
Western blot (WB) analysis was used to determine the expression levels of the components of the Hippo pathway in CRC (n = 50), adjacent nontumorous tissues (n = 50), and colorectal adenomas (n = 30). Total proteins were extracted from the tissues which were grinded at low temperature using a mixture of phenylmethylsulfonyl fluoride or phenylmethanesulfonylfluoride (PMSF) and radio immunoprecipitation assay (RIPA) buffer (PMSF: RIPA = 1:200). We then calculated the protein concentration using protein quantitative reagent kit-bicinchoninic acid (BCA) method. Equal amounts of proteins were separated through sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked in phosphate-buffered saline with tween 20 (PBST) containing 5% nonfat milk for 1 hour at room temperature before being blotted with the appropriate primary antibody overnight at 4°C (MST1, 1:1000, Abcam Company, Cambridge, UK; YAP, 1:1000, Abcam Company, Cambridge, UK; TEAD1, 1:1000, Abcam Company, Cambridge, UK; CDX2, 1:1000, ZSGB-BIO, Beijing, China). The membranes were washed with phosphate-buffered saline (PBS) and incubated with goat anti-rabbit horseradish peroxidase–conjugated secondary antibodies (Abcam Company, Cambridge, UK) for 1 hour at room temperature. The antibody–protein complexes were visualized by chemiluminescence and the specific protein bands were photographed by VILBER Fusion FX7 image system. Quantity One software was used for the analysis and quantification of WB image. β-actin was used as the internal control.
Statistical analysis
Statistical analysis was undertaken using SPSS 19.0. The one-way analysis of variance (ANOVA) was used for continuous data, and P < 0.05 was considered to indicate significant difference. The correlations were analyzed by Pearson's correlation coefficient, and P < 0.05 was considered to indicate significant difference.
RESULTS
The relative expression levels of Hippo pathway molecules
In this study, qRT-PCR showed that the mRNA expression levels of MST1 and LATS2 were significantly lower in CRC tissues than those in colorectal adenomas and adjacent nontumorous tissues (P < 0.01). The mRNA expression levels of MST1 and LATS2 showed an increasing tendency from CRC to colorectal adenomas and then to adjacent nontumorous tissues [Figure 2]. Conversely, the mRNA expression levels of YAP, TAZ, and TEAD1 obviously increased in CRC compared with colorectal adenomas and adjacent nontumorous tissues (P < 0.01) and showed a decreasing tendency [Figure 2]. On determining the expression levels of MST1, YAP, and TEAD1 by WB, we found that MST1 protein in colorectal adenomas and adjacent nontumorous tissues increased 0.3676 and 0.7039 times, respectively, compared with CRC [Figure 3]. Compared with CRC, YAP protein expression level decreased by 31.58% and 51.22% and TEAD1 protein expression level decreased by 20.87% and 40.11% in colorectal adenomas and adjacent nontumorous tissues, respectively, which were consistent with the results of qRT-PCR [Figures 2 and 3]. The results of Pearson's Chi-square test showed that the expression of MST1 was positively correlated with the expression of LATS2 (r = 0.96, P < 0.01), the expression of LATS2 correlated negatively with YAP and TAZ (r = −0.86, P < 0.01; r = −0.93, P < 0.01), and the expression of TEAD1 correlated positively with YAP and TAZ (r = 0.96, P < 0.01; r = 0.98, P < 0.01).
Figure 2.
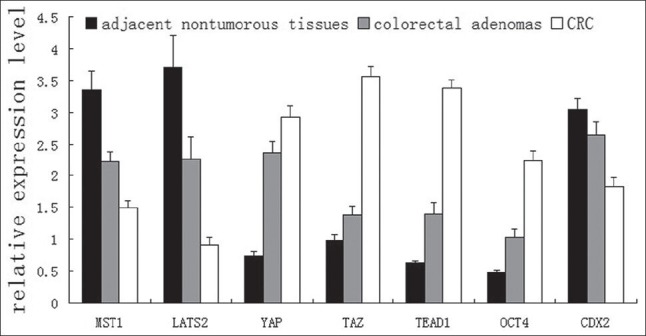
Relative expression levels of genes in adjacent nontumorous tissues, colorectal adenomas, and CRC detected by qRT-PCR
Figure 3.
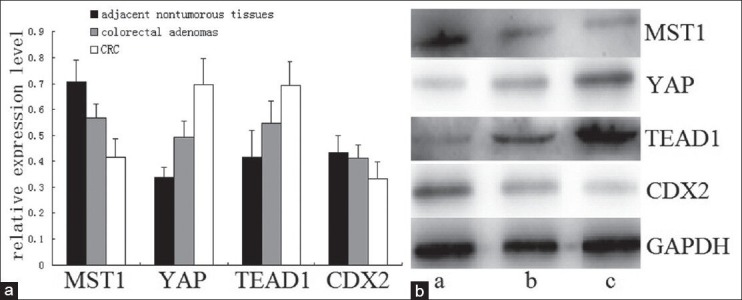
Expression of MST1, YAP, TEAD1, and CDX2 proteins in adjacent nontumorous tissues, colorectal adenomas, and CRC detected by western blot. (a) Relative expression levels of MST1, YAP, TEAD1, and CDX2 proteins. (b) Bands of MST1, YAP, TEAD1, and CDX2 proteins (a, adjacent nontumorous tissues; b, colorectal adenomas; c, CRC)
The expression levels of CDX2 and OCT4
As shown in Figures 2 and 3, the results of qRT-PCR and WB indicated that the expression of CDX2 was significantly lower in CRC than that in colorectal adenomas and adjacent nontumorous tissues (all P < 0.01). However, there was no significant difference between the latter two groups (qRT-PCR, P = 0.113; WB, P = 0.151). In Figure 2, it is observed that the expression of OCT4 gradually increased from adjacent nontumorous tissues to colorectal adenomas and then to CRC (P < 0.01). Furthermore, Pearson's Chi-square test showed the expression levels of YAP and TEAD1 were negatively correlated with the expression of CDX2 (r = −0.977, P < 0.01; r = −0.933, P < 0.01) and positively correlated with OCT4 (r = −0.971, P < 0.01; r = −0.942, P < 0.01). Meanwhile, there was a significant negative correlation between OCT4 and CDX2 (r = −0.98, P < 0.01).
Association of Hippo pathway components with the clinicopathologic characteristics of CRC patients
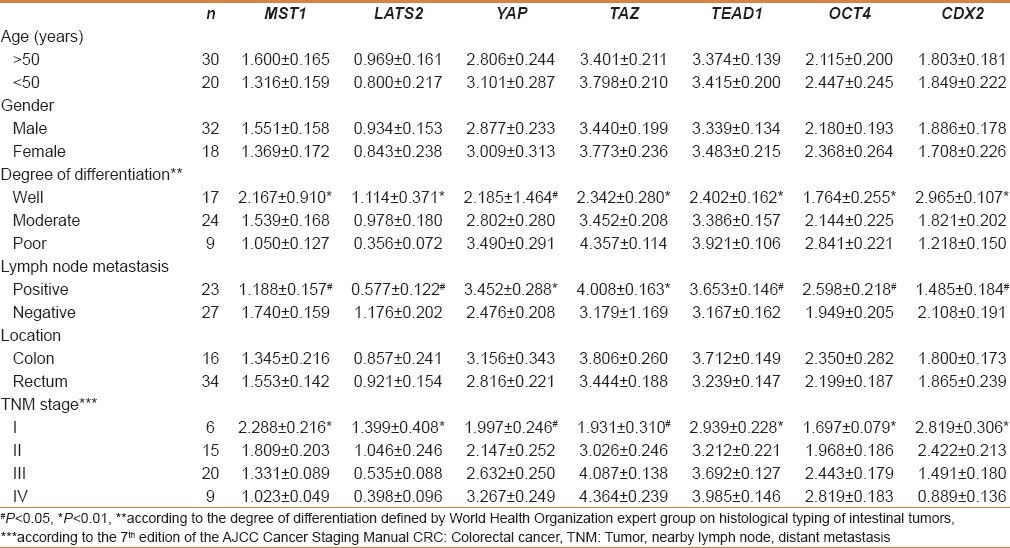
In Table 2, it is found the expression levels of MST1, LATS2, and CDX2 in patients with lymph node metastasis were significantly lower than those in patients without lymph node metastasis (P < 0.05). On the contrary, the expression levels of YAP, TAZ, TEAD1, and OCT4 in patients with lymph node metastasis were significantly higher than those in patients without lymph node metastasis (YAP and TAZ, P < 0.01; TEAD1 and OCT4, P < 0.05). The results of qRT-PCR showed the mRNA expression levels of MST1, LATS2, and CDX2 gradually decreased from good to poor differentiation (P < 0.01) and the expression levels of YAP, TAZ, TEAD1, and OCT4 gradually increased from poor to good differentiation (YAP, P < 0.05; others, P < 0.01). As shown in Table 2, the expression of Hippo pathway components was significantly associated with TNM stage in CRC. The mRNA expression levels of MST1, LATS2, and CDX2 gradually decreased from TNM I to IV stage (P < 0.01) and the mRNA expression levels of YAP, TAZ, TEAD1, and OCT4 gradually increased from TNM I to IV stage (YAP and TAZ, P < 0.05; TEAD and OCT4, P < 0.01). In additon, there was no significant correlation with age, gender, and location of cancer [Table 2].
Table 2.
Association of hippo pathway components with the clinicopathologic characteristics of CRC patients
DISCUSSION
MST1 and LATS2 are upstream components in the Hippo pathway according to Dong's elucidation of Hippo signal pathway in mammals.[9] MST1, the ortholog of Drosophila protein kinase hpo, is a STE20-like kinase containing Ser/Thr protein kinase domain in the N-terminal and SARAH domain in the C-terminal. The activated MST1 phosphorylates and activates its direct substrate, LATS2. The phosphorylation of LATS2 is enhanced by SAV1 (defined as the adaptor protein, also called hWW45 in human beings), which combines with MST1 and LATS2 through the SARAH domain of MST1 and PPXY motifs of LATS2, respectively.[10] Zhou[6,11] confirmed that MST1 is required for tumorigenesis suppression by knockdown of MST1 gene, and downregulation of MST1 mRNA expression in adult mouse liver leads to the onset of hepatocyte proliferation, massive overgrowth, and multifocal hepatocyte carcinoma eventually. Xu[12] demonstrated that MST1 performs tumor suppressor function by transfecting recombinant eukaryotic expression vector which contains human wild-type MST1 gene to human non-small-cell lung cancer (A549 cells). In this study, MST1 and LATS2 are suppressed in colorectal adenomas and CRC, and the expression levels of MST1 and LATS2 show a decreasing trend from adjacent nontumorous tissues to CRC. All these suggest that the inactivation of MST1 and LATS2 might result in progression from colorectal adenomas to CRC because of the absence of proliferation and tumorigenesis suppressing function. In addition, the expression of MST1 shows a positive correlation with LATS2 in CRC, which indicates that the downregulation of MST1 might be followed by the suppression of LATS2. However, the mechanism of suppression has not been found. Previous researches[6,13] suggest that the downregulation is associated with promoter hypermethylation and gene deletion.
YAP and its paralog TAZ are the core components and downstream regulators of Hippo pathway and play a role in gene induction as transcriptional co-activators after they combine with TEAD1 leading to cell proliferation, organ size, epithelial-mesenchymal transition, and tumorigenesis.[9,14] YAP, the ortholog of Drosophila protein kinase Yki, is first cloned because of binding to the SH3. YAP and TAZ are phosphorylated by LATS2 after they combine with each other through WW domain and PPXY motifs and the phosphorylation sites are S127 and S89, respectively. Once combined with 14-3-3 protein, the phosphorylated YAP and TAZ proteins are fixed in the cytoplasm. The expression of YAP is upregulated in many tumors, such as gastric, esophageal, pulmonary, and hepatic carcinomas. Overexpression of YAP results in liver overgrowth and then progresses to HCC. YAP is an independent prognostic marker for overall survival and disease-free survival times of HCC patients and is associated with tumor differentiation.[15] This phenomenon is also found in intestinal cancer. Camargo et al., observed in their study that YAP could restrict the differentiation of stem cell in the intestine and expand multipotent undifferentiated progenitor cells.[16] Zhou et al., study confirms that overexpression of YAP promotes colonic tumorigenesis through inducing the ablation of kinases MST1 and MST2.[17] TAZ plays a similar function in cell proliferation and tumorigenesis. Previous studies have shown that the proliferative and oncogenic potential of TAZ is suppressed when TAZ gene is knocked down in non-small-cell lung carcinoma using siRNA transfection.[18] The qRT-PCR and WB results suggest that the expression levels of YAP and TAZ are both upregulated and show an uptrend from adjacent nontumorous tissues to CRC. Pearson's Chi-square test showed the mRNA expression level of LATS2 is negatively correlated with the mRNA expression levels of YAP and TAZ, which indicates YAP or TAZ might be overexpressed secondary to the suppression of MST1 and LATS2 in CRC and colorectal adenomas. Our results are consistent with Zhou et al., and previous researches. Therefore, the overexpression of YAP or TAZ, downstream regulators of Hippo pathway, might lead to excessive cell proliferation, polarity absence, adenoma formation, and CRC progression eventually. On analyzing the correlation between YAP/TAZ and the clinicopathologic characteristics of CRC patients, we found that the expression levels of YAP and TAZ were positively correlated with tumor differentiation, lymph node metastasis, and TNM stage. Also, there was no significant correlation with age, gender, and location of tumor, which is similar to the study results of Yuen et al.[19] So, the expression of YAP and TAZ might predict the prognosis for CRC patients.
As a co-transcriptional activator, TEAD1 is required for gene promoters in the nucleus. Previous researches show that TEAD1 gene knockdown restricts the proliferation and tumorigenesis potential of YAP and TAZ.[14,20] We found in our study that TEAD1 is overexpressed in CRC and colorectal adenomas, and is positively correlated with the expression of YAP and TAZ. This means TEAD1 might be co-overexpressed with YAP or TAZ to promote the expression of backward genes.
However, the question is which genes are promoted by YAP/TAZ-TEAD1 co-overexpression? CDX2 and OCT4 are overexpressed in ES cells and are the markers of pluripotency. CDX2 is initially co-expressed with and suppressed by OCT4 in the ES cells, and the suppression of OCT4 leads to the upregulation of CDX2.[21] In human beings, CDX2 is strictly expressed in the intestinal epithelial cells, which plays a critical role in directing intestinal development, differentiation and maintenance of the intestinal phenotype.[22] Previous research[23] shows that the expression of CDX2 is reduced in CRC. Mallo et al., reduced the tumorigenicity, resistance to apoptosis, and migration potential of HT29 cells after upregulating CDX2 expression by transfection with CDX2 cDNA. Therefore, Kim suggests that CDX2 is a tumor suppressor gene.[24] In addition, recent studies report that reduced CDX2 expression is associated with poor overall survival in patients with CRC and could be a prognostic indicator.[25] Another study[26] reports that TEAD/TEF family transcription factor could induce the expression of CDX2 in ES cells. So, we wonder if YAP/TAZ-TEAD1 complex would upregulate or downregulate CDX2 expression in patients with CRC. We found in our study that the mRNA and protein expression levels of CDX2 in CRC are significantly lower than those in colorectal adenomas and adjacent nontumorous tissues. Therefore, the expression of CDX2 might be reduced in CRC. Consequently, we detected the expression levels of OCT4 (the antagonist of CDX2) by qRT-PCR. OCT4 is required for maintaining the proliferation and pluripotency of ES cell, and OCT4 knockdown by RNA interference leads to triggering differentiation of ES cell at the morphologic and molecular level.[27] Overexpression of OCT4 in tumor stem cells has been found in many tumors, such as pulmonary, cervical, pancreatic, and colorectal carcinomas. Our results show the mRNA expression levels of OCT4 are significantly higher in CRC than those in colorectal adenomas and adjacent nontumorous tissues. Meanwhile, there is a significantly negative correlation between CDX2 and OCT4. This study indicates that the suppression of CDX2 might be associated with the overexpression of OCT4, which inhibits the expression of CDX2 in colorectal tumor stem cell, although TEAD1 might induce CDX2 expression. Hence, there is another hypothesis that YAP/TAZ-TEAD1 complex might reduce CDX2 expression due to the tumor microenvironment in colorectal stem cells instead of embryonic microenvironment. Besides, the expression of CDX2 is associated with tumor differentiation, lymph node metastasis, and TNM stage, and there is no significant correlation with age, gender, and location of tumor. Therefore, CDX2 might be treated as a prognostic factor for patients with CRC.
CONCLUSIONS
The results obtained show that the Hippo pathway is suppressed and the downstream cascade kinases are overexpressed in both colorectal adenomas and CRC, which indicates that the suppression of Hippo pathway might be one mechanism in the pathogenesis from colorectal adenomas to CRC. The Hippo pathway is closely related to the tumor differentiation, lymph node metastasis, and TNM stage, which suggests that this signal pathway might serve as a prognostic indicator for patients with CRC. In accordance with these results, the target genes of Hippo pathway might be CDX2 and OCT4.
ACKNOWLEDGEMENT
This study was supported by Central Laboratory, Affiliated Hospital of the Medical College Qingdao University, and all the authors appreciate Dr. Yang's efforts for this study.
Footnotes
Source of Support: This study was supported by Central Laboratory, Affiliated Hospital of the Medical College Qingdao University
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2013 doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 4.Prasetyanti PR, Zimberlin CD, Bots M, Vermeulen L, De Sousa EM, Medema JP. Regulation of stem cell self-renewal and differentiation by Wnt and Notch are conserved throughout the adenoma-carcinoma sequence in the colon. Mol Cancer. 2013;12:126. doi: 10.1186/1476-4598-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010;24:862–74. doi: 10.1101/gad.1909210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi Y, Miyoshi Y, Takahata C, Irahara N, Taguchi T, Tamaki Y, et al. Down-regulation of LATS1 and LATS2 mRNA expression by promoter hypermethylation and its association with biologically aggressive phenotype in human breast cancers. Clin Cancer Res. 2005;11:1380–5. doi: 10.1158/1078-0432.CCR-04-1773. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki H, Kawano O, Endo K, Suzuki E, Yukiue H, Kobayashi Y, et al. Human MOB1 expression in non-small-cell lung cancer. Clin Lung Cancer. 2007;8:273–6. doi: 10.3816/CLC.2007.n.006. [DOI] [PubMed] [Google Scholar]
- 8.Kowalik MA, Saliba C, Pibiri M, Perra A, Ledda-Columbano GM, Sarotto I, et al. Yes-associated protein regulation of adaptive liver enlargement and hepatocellular carcinoma development in mice. Hepatology. 2011;53:2086–96. doi: 10.1002/hep.24289. [DOI] [PubMed] [Google Scholar]
- 9.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford SA, et al. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan EH, Nousiainen M, Chalamalasetty RB, Schafer A, Nigg EA, Sillje HH. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene. 2005;24:2076–86. doi: 10.1038/sj.onc.1208445. [DOI] [PubMed] [Google Scholar]
- 11.Zhou D, Conrad C, Xia F, Park JS, Payer B, Yin Y, et al. Mst1 and Mst2 maintain hepatocyte quiescence and suppress hepatocellular carcinoma development through inactivation of the Yap1 oncogene. Cancer Cell. 2009;16:425–38. doi: 10.1016/j.ccr.2009.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu CM, Liu WW, Liu CJ, Wen C, Lu HF, Wan FS. Mst1 overexpression inhibited the growth of human non-small cell lung cancer in vitro and in vivo. Cancer Gene Ther. 2013;20:453–60. doi: 10.1038/cgt.2013.40. [DOI] [PubMed] [Google Scholar]
- 13.Seidel C, Schagdarsurengin U, Blumke K, Wurl P, Pfeifer GP, Hauptmann S, et al. Frequent hypermethylation of MST1 and MST2 in soft tissue sarcoma. Mol Carcinog. 2007;46:865–71. doi: 10.1002/mc.20317. [DOI] [PubMed] [Google Scholar]
- 14.Zhao B, Ye X, Yu J, Li L, Li W, Li S, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–71. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu MZ, Yao TJ, Lee NP, Ng IO, Chan YT, Zender L, et al. Yes-associated protein is an independent prognostic marker in hepatocellular carcinoma. Cancer. 2009;115:4576–85. doi: 10.1002/cncr.24495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Camargo FD, Gokhale S, Johnnidis JB, Fu D, Bell GW, Jaenisch R, et al. YAP1 increases organ size and expands undifferentiated progenitor cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 17.Zhou D, Zhang Y, Wu H, Barry E, Yin Y, Lawrence E, et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein overabundance. Proc Natl Acad Sci USA. 2011;108:E1312–20. doi: 10.1073/pnas.1110428108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, Hao Y, Liu N, Raptis L, Tsao MS, Yang X. TAZ is a novel oncogene in non-small cell lung cancer. Oncogene. 2011;30:2181–6. doi: 10.1038/onc.2010.606. [DOI] [PubMed] [Google Scholar]
- 19.Yuen HF, McCrudden CM, Huang YH, Tham JM, Zhang X, Zeng Q, et al. TAZ expression as a prognostic indicator in colorectal cancer. PloS One. 2013;8:e54211. doi: 10.1371/journal.pone.0054211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y, Xin Y, Ye F, Wang W, Lu Q, Kaplan HJ, et al. Taz-tead1 links cell-cell contact to zeb1 expression, proliferation, and dedifferentiation in retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2010;51:3372–8. doi: 10.1167/iovs.09-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niwa H, Toyooka Y, Shimosato D, Strumpf D, Takahashi K, Yagi R, et al. Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation. Cell. 2005;123:917–29. doi: 10.1016/j.cell.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Silberg DG, Swain GP, Suh ER, Traber PG. Cdx1 and cdx2 expression during intestinal development. Gastroenterology. 2000;119:961–71. doi: 10.1053/gast.2000.18142. [DOI] [PubMed] [Google Scholar]
- 23.Mallo GV, Soubeyran P, Lissitzky JC, Andre F, Farnarier C, Marvaldi J, et al. Expression of the Cdx1 and Cdx2 homeotic genes leads to reduced malignancy in colon cancer-derived cells. J Biol Chem. 1998;273:14030–6. doi: 10.1074/jbc.273.22.14030. [DOI] [PubMed] [Google Scholar]
- 24.Kim SP, Park JW, Lee SH, Lim JH, Jang BC, Jang IH, et al. Homeodomain protein CDX2 regulates COX-2 expression in colorectal cancer. Biochem Biophys Res Commun. 2004;315:93–9. doi: 10.1016/j.bbrc.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 25.Hong KD, Lee D, Lee Y, Lee SI, Moon HY. Reduced CDX2 expression predicts poor overall survival in patients with colorectal cancer. Am Surg. 2013;79:353–60. [PubMed] [Google Scholar]
- 26.Nishioka N, Inoue K, Adachi K, Kiyonari H, Ota M, Ralston A, et al. The Hippo signaling pathway components Lats and Yap pattern Tead4 activity to distinguish mouse trophectoderm from inner cell mass. Dev Cell. 2009;16:398–410. doi: 10.1016/j.devcel.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Hay DC, Sutherland L, Clark J, Burdon T. Oct-4 knockdown induces similar patterns of endoderm and trophoblast differentiation markers in human and mouse embryonic stem cells. Stem Cells. 2004;22:225–35. doi: 10.1634/stemcells.22-2-225. [DOI] [PubMed] [Google Scholar]


