Abstract
Background
Localizing the origin of outflow tract ventricular tachycardias (OTVT) is hindered by lack of accuracy of electrocardiographic (ECG) algorithms and infrequent spontaneous premature ventricular complexes (PVCs) during electrophysiological studies.
Objectives
To prospectively assess the performance of noninvasive electrocardiographic mapping (ECM) in the pre-/periprocedural localization of OTVT origin to guide ablation and to compare the accuracy of ECM with that of published ECG algorithms.
Methods
Patients with symptomatic OTVT/PVCs undergoing clinically indicated ablation were recruited. The OTVT/PVC origin was mapped preprocedurally by using ECM, and 3 published ECG algorithms were applied to the 12-lead ECG by 3 blinded electrophysiologists. Ablation was guided by using ECM. The OTVT/PVC origin was defined as the site where ablation caused arrhythmia suppression. Acute success was defined as abolition of ectopy after ablation. Medium-term success was defined as the abolition of symptoms and reduction of PVC to less than 1000 per day documented on Holter monitoring within 6 months.
Results
In 24 patients (mean age 50 ± 18 years) recruited ECM successfully identified OTVT/PVC origin in 23/24 (96%) (right ventricular outflow tract, 18; left ventricular outflow tract, 6), sublocalizing correctly in 100% of this cohort. Acute ablation success was achieved in 100% of the cases with medium-term success in 22 of 24 patients. PVC burden reduced from 21,837 ± 23,241 to 1143 ± 4039 (P < .0001). ECG algorithms identified the correct chamber of origin in 50%–88% of the patients and sublocalized within the right ventricular outflow tract (septum vs free-wall) in 37%–58%.
Conclusions
ECM can accurately identify OTVT/PVC origin in the left and the right ventricle pre- and periprocedurally to guide catheter ablation with an accuracy superior to that of published ECG algorithms.
Abbreviations: CT, computed tomographic; EF, ejection fraction; ECG, electrocardiographic; ECM, electrocardiographic mapping; EPS, electrophysiological study; LV, left ventricular/ventricle; LVOT, left ventricular outflow tract; OTVT, outflow tract ventricular tachycardia; PVC, premature ventricular complex; PVS, programmed ventricular stimulation; RV, right ventricular/ventricle; RVOT, right ventricular outflow tract; VT, ventricular tachycardia
Keywords: Ventricular tachycardia, Premature ventricular complex, Outflow tract tachycardia
Introduction
Outflow tract ventricular tachycardia (OTVT) or frequent premature ventricular complexes (PVCs) often occur in the absence of structural heart disease and account for 10% of all ventricular tachycardia (VT).1, 2 The majority of these originate from the right ventricular outflow tract (RVOT) and the remainder from the left ventricular outflow tract (LVOT), including the cusps of the aortic valve.3 Catheter ablation within these complex anatomical structures can be effective in eliminating symptoms and reversing PVC-induced cardiomyopathy, but there remain significant limitations to current mapping techniques, including poor spatial resolution of pace mapping, inaccurate localization of VT origin based on electrocardiographic (ECG) algorithms, and, especially limiting, lack of spontaneous ectopy rendering activation mapping ineffective.4, 5, 6
Body surface electrocardiographic mapping (ECM) is a noninvasive technique providing global electroanatomical maps of both ventricular chambers by projecting 252 body surface electrode signals onto the epicardial geometry of the heart derived from a noncontrast thoracic computed tomographic (CT) scan.7 This technology has the advantage of being able to display the onset and electrical propagation of a single PVC, recorded preprocedurally, onto the biventricular geometry to guide ablation.
The objectives of this prospective study are 2-fold: first, to compare the accuracy of noninvasive ECM with that of validated ECG algorithms in localizing OTVT origin preprocedurally, and second, to assess the periprocedural capabilities of ECM as a novel mapping tool to guide ablation of OTVT.
Methods
Study population
Patients undergoing clinically indicated ablation for symptomatic PVCs or asymptomatic but frequent (≥10,000/24 h) PVCs with an inferior axis on the 12-lead ECG were prospectively recruited into the study. In addition, patients with asymptomatic and/or infrequent PVCs in the presence of idiopathic globally impaired left ventricular (LV) function were included. All patients gave written informed consent before the procedure. Local research ethics committee approval was granted for the study.
Preprocedural mapping using ECM
Antiarrhythmic drugs were discontinued at least 5 half-lives before the procedure. The ECM system has been described previously.7, 8, 9 Briefly, it consists of a 252-electrode vest applied to the patient’s torso, acquiring electrical data at 2 kHz. After vest application, patients undergo noncontrast thoracic CT scans with an axial resolution of 3 mm. The 252 body surface electrodes and epicardial surface geometry are spatially labeled and defined from CT images. These body surface signals and geometric data are integrated to construct 3-dimensional electroanatomical maps composed of more than 1500 unipolar epicardial signals. These data are then applied to an algorithm to localize the origin of ectopy (Figure 1A). Following the CT scan, patients can ambulate with the vests, which, in our study, were applied from up to 5 hours before the procedure until the ablation was completed. ECM mapping was performed with the patient in a recumbent position.
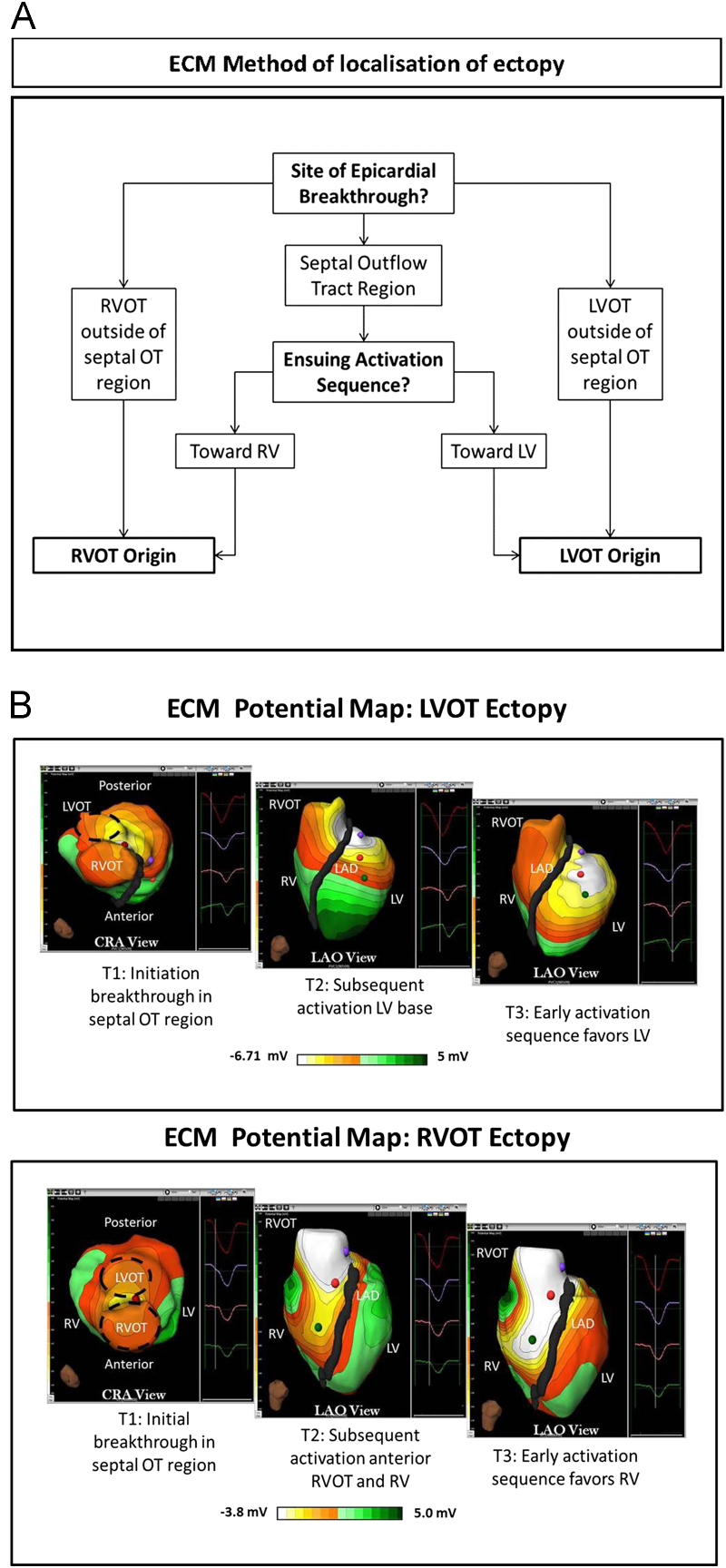
Figure 1.
A: ECM method of localization of ectopy origin. B: ECM potential maps of RVOT and LVOT ectopy. The images show the ECM potential PVC map from the cranial and LAO views at 3 time points: T1, initiation of epicardial breakthrough, and 2 later time points (T2 and T3). Top: After epicardial breakout in the septal groove, the ensuing activation spreads directly anteriorly toward the RV, suggesting RVOT origin. The successful ablation site here was in the mid-septal RVOT. Bottom: After epicardial breakout in the septal groove, the ensuing activation spreads posteriorly toward the LV, favoring the left ventricle. The successful ablation site here was in the anterolateral LVOT. CRA = cranial; ECM = electrocardiographic mapping; LAD = left anterior descending; LAO = left anterior oblique; LV = left ventricle; LVOT = left ventricular outflow tract; PVC = premature ventricular complex; OT = outflow tract; RV = right ventricle; RVOT = right ventricular outflow tract.
Three types of electroanatomical maps are generated by ECM: potential, activation, and voltage maps (Figure 1, Figure 2).
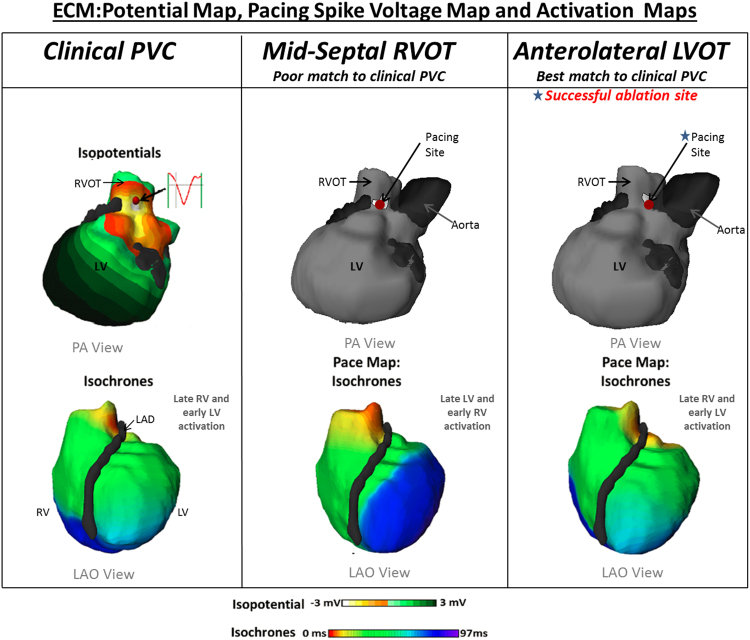
Figure 2.
ECM pacing spike voltage maps and activation maps. Left panel: Potential map of clinical PVC initiation (top) and activation map of biventricular global activation (below). Middle and right panels: Pacing spike voltage (top) and activation (bottom) maps created from mid-septal RVOT and anterolateral LVOT pacing, respectively. Despite the close anatomical proximity of the 2 pacing sites (red dot), the global activation demonstrates a clear difference, allowing localization of ectopy to the anterolateral LVOT. See accompanying supplementary videos. ECM = electrocardiographic mapping; LAO = left anterior oblique; LVOT = left ventricular outflow tract; PA = posteroanterior; PVC = premature ventricular complex; RVOT = right ventricular outflow tract.
The potential map is a dynamic map that displays global propagation of the depolarization wave front, detected by the earliest QS over the chosen cardiac interval (Figure 1B and Online Supplemental Video 1).
The activation map is a static map displaying the activation sequence across a chosen cardiac interval. The map assigns an activation time to each unipolar electrogram on the epicardial geometry (Figure 2). Activation calculations are computed from both the signal’s maximum negative change of voltage (−dV/dT) and the signal morphology of neighboring electrograms. The activation sequence is represented on the map by the color red as early and by purple as late, with reference to the earliest electrogram.
The voltage map displays the signal amplitude at each point on the epicardial geometry over the selected interval (white represents high amplitude and red represents low amplitude). As the system is unable to track the catheter location in real time, a voltage map of the pacing spike (pacing spike voltage map) can localize the pacing stimulus as a surrogate marker for catheter location (displayed as a red dot on the map in Figure 2).
All clinical VT/PVC morphologies were captured by the system and processed to display a potential and an activation map. The origin of the PVC was localized by the ECM system to 1 of 12 segments in the RVOT (high/low; posteroseptal/midseptal/anteroseptal/posterior free wall/anterior free wall/mid free wall of the RVOT) and 1 of 5 segments in the LVOT (noncoronary cusp/right coronary cusp/left coronary cusp/aortomitral continuity/septal LVOT). When clinical PVCs were captured preprocedurally, these maps were generated before the patient entered the electrophysiology laboratory. PVCs of the highest frequency were deemed to be of most clinical relevance.
Electrophysiological study
Venous access was obtained from the right femoral veins (8- and 6-F sheaths), and arterial access was obtained from the right femoral artery (8 F) for hemodynamic monitoring and retrograde access to the LVOT. A pentapolar catheter was inserted under fluoroscopic guidance via the femoral venous sheath into the right ventricular (RV) apex for pacing and to act as an intracardiac activation reference. A standard 4-mm nonirrigated tip ablation catheter (eg, Biosense Webster Inc, Diamond Bar, CA) was used. A maximum power delivery of up to 50 W with a maximum temperature limited to 60ºC was used for the RVOT and the LVOT. Left-sided mapping was performed via the retrograde aortic approach with systemic heparinization, targeting serial activated clotting time (ACT) values of greater than 300 seconds.
Spontaneous clinical PVCs captured periprocedurally were cross-referenced with PVCs captured preprocedurally on ECM to confirm a match. The 12-lead ECG of the clinical VT/PVC was displayed on a recording system (Bard Labsystem Pro, Bard Electrophysiology Division, Lowell, MA) as a template for pace mapping. If no spontaneous PVCs were present, programmed ventricular stimulation (PVS) was performed by pacing at twice the diastolic threshold and pulse width of 2 ms from the RVA and the RVOT at 2 drive cycle lengths with up to 3 extrastimuli with or without isopretenerol.
After the ECM identification of the PVC origin, the ablation catheter was fluoroscopically guided to perform pace mapping at various sites within the segment of interest containing the PVC origin as identified by the ECM system. At each site, pacing was performed to create ECM maps and the ablation site was identified if it fulfilled the following criteria:
-
1.
the resultant potential and activation maps corresponded with those during the clinical VT/PVC and
-
2.
the catheter location on the voltage map corresponded anatomically with the earliest site of electrical activation during VT/PVC (Figure 2).
At this location, the match of the pace map on the 12-lead ECG to the clinical VT/PVC and the prematurity of local activation as recorded by the distal bipole of the ablation catheter in relation to QRS onset during spontaneous VT/PVC also measured and documented.
After ablation at the site identified by ECM mapping, if spontaneous ectopy was still evident then operator discretion was used to continue mapping using ECM or conventional criteria.
The PVC origin was defined by the site at which ablation resulted in loss of spontaneous ectopy or if ablation resulted in noninducibility.
The successful site of ablation was stored as standard fluoroscopic right anterior oblique and left anterior oblique views. Acute procedural success was defined as the absence, or noninducibility, of the targeted VT/PVC during a 20-minute observation period with repeated PVS after delivery of the last ablation lesion.
ECG algorithms to identify VT origin
We hypothesized that ECM would be superior to validated ECG algorithms at localizing VT origin. The V2 transition ratio (algorithm B) published by Betensky et al10 localizes ectopy origin to either the LV or the RV. Zhang et al11 (algorithm A) and Ito et al12 (algorithm C) published criteria that allow further sublocalization within the RV (septum vs free-wall). To assess ECG accuracy at PVC localization, 3 electrophysiologists blinded to the ECM data and ablation outcomes applied these 3 algorithms retrospectively to the same 12-lead ECGs of clinical VT/PVC. The intra- and interobserver variability was also tested. All ECGs were digitalized, and durations and amplitudes were measured at a magnification of 200×. One month after the first round of analysis, the same set of ECGs were reanalyzed by the same electrophysiologists. The performance of ECM and 3 ECG algorithms was determined to identify the site of successful ablation.
Follow-up
Antiarrhythmic medications were not resumed in all patients after ablation. β-Blockers were continued, if required, for other clinical indications. All patients underwent echocardiography before ablation, and those with impaired LV function or a postablation PVC burden of 10,000/24 h underwent a repeat scan postablation. Patients were reviewed in the arrhythmia clinic 3–6 months after the procedure, and each patient underwent a clinical interview to assess symptomatology, 12-lead ECG, and 24-hour Holter monitoring. Arrhythmia recurrence was defined as symptomatic recurrence with documented clinical arrhythmia or PVC burden exceeding 1000 in 24 hours on Holter monitoring. Symptoms were classified into 4 categories; complete absence, significantly improved, unchanged, or worsened.
Statistical analysis
Continuous variables are expressed as mean ± SD. Categorical variables were compared by using the Wilcoxon signed-rank test. The free marginal multi-rater kappa was used to assess the chance-adjusted measure of agreement between electrophysiologists analyzing the ECGs using 3 different algorithms. The kappa coefficient was also used for intra-rater agreement testing. Statistical analysis was performed by using SPSS statistical software (release 21.0, SPSS, Chicago, IL). A P value of ≤.05 was considered statistically significant. A kappa value of ≥0.7 indicated adequate agreement above chance.
Results
Effectiveness of ECM at localizing VT/PVC origin
Twenty-four clinical VT/PVC morphologies (23 with left bundle branch block) were targeted in 24 patients (8 men; mean age 50 ± 18 years). In 21 of 24 patients with spontaneous PVC/VT preprocedurally, the PVC origins could be located on the ECM geometry before electrophysiological study (EPS). In the remaining 3 patients, ECM located the PVC origin periprocedurally. In all but one patient, PVCs occurred spontaneously or with the initiation of isoproterenol infusion. In only 1 patient was PVS useful for PVC induction.
The first ablation lesion was delivered at all 24 sites identified by pre-/periprocedural ECM, which resulted in acute procedural success in 23 of 24 patients. The local bipolar electrogram at these sites during clinical ectopy preceded VT/PVC QRS onset by 29 ± 9 ms (range 10–45 ms). At 22 of 24 sites, a 12/12 pace match was achieved. The best pace map at the remaining 2 sites was 11/12 and 10/12.
The mean number of ablation lesions, including consolidation lesions, was 7.5 ± 7 before PVC quiescence.
Of interest was patient 11 (see case study 1 in the Online Data Supplement), who demonstrated not only the limitation of conventional mapping but where all 3 ECG algorithms, applied by all assessors, located the site of origin in the incorrect chamber.
ECM failed to identify the appropriate site of ablation in a single case (patient 19), where the origin of a left bundle branch block PVC with QRS transition at lead V3/V4 was localized by the ECM to the RVOT. However, at this site, “His” bundle capture was noted with an 11/12 ECG pace-map match and local activation which preceded QRS onset by 40 ms. Ablation was commenced at low-power settings and was stopped early owing to the occurrence of a single nonconducted sinus beat. Pacing in the surrounding RVOT region did not reproduce the clinical PVC ECM activation map, which led to exploration of the LVOT. ECM maps created in the LVOT identified a perfect match at the region between the right coronary cusp and the noncoronary cusp with an early local electrogram (−45 ms) and a 12/12 pace-map match. Fluoroscopically this site was anatomically adjacent to the RVOT site explored earlier.
Acute procedural success was achieved in all patients after ablation at 18 RV and 6 LV sites: 3 posteroseptal RVOT, 8 anteroseptal RVOT, 4 mid-septal RVOT, 2 posterolateral RVOT, 4 left coronary cusp, 1 noncoronary cusp/right coronary cusp junction, and 2 aortomitral continuity sites. The mean power delivered was 37 ± 13 W.
Six patients had previously undergone EPS; 3 having had unsuccessful ablation. One patient with recurrent symptoms had undergone 4 attempts at ablation, but on each previous occasion, ablation could not be completed owing to lack of spontaneous clinical ectopy.
ECG algorithms vs ECM at localizing VT/PVC origin
ECM predicted the correct ventricular chamber (left vs right) in 96% of the cases (Table 1). In 23 of 24 cases in which ECM predicted the correct ventricular chamber, the correct region within that chamber was sublocalized in 100% of the cases.
Table 1.
Accuracy of ECM with successful ablation site
| Patient | Successful ablation site | ECM location | ECG pace-map match | Endocardial earliest local activation timing (pre-QRS) |
|---|---|---|---|---|
| 1 | RVOT | RVOT | 12 | 36 |
| 2 | RVOT | RVOT | 12 | 14 |
| 3 | AMC | AMC | 12 | 38 |
| 4 | RVOT | RVOT | 12 | 28 |
| 5 | RVOT | RVOT | 12 | NA |
| 6 | AMC | AMC | 12 | 10 |
| 7 | RVOT | RVOT | 12 | 40 |
| 8 | RVOT | RVOT | 12 | 28 |
| 9 | RVOT | RVOT | 12 | 36 |
| 10 | LCC | LCC | 12 | 28 |
| 11 | LCC | LCC | 11 | 34 |
| 12 | RVOT | RVOT | 12 | 22 |
| 13 | RVOT | RVOT | 10 | 22 |
| 14 | RVOT | RVOT | 12 | 36 |
| 15 | RVOT | RVOT | 12 | 40 |
| 16 | LVOT | LCC | 12 | 30 |
| 17 | RVOT | RVOT | 12 | 35 |
| 18 | RVOT | RVOT | 12 | 28 |
| 19 | RVOT/NCC | RVOT near His | 12 | 45 |
| 20 | RVOT | RVOT | 12 | 40 |
| 21 | RVOT | RVOT | 12 | 20 |
| 22 | RVOT | RVOT | 12 | 28 |
| 23 | RVOT | RVOT | 12 | 26 |
| 24 | RVOT | RVOT | 12 | 30 |
| Correct diagnosis | 23/24 | |||
| Diagnostic accuracy | 96% |
AMC = aortomitral continuity; ECM = electrocardiographic mapping; LCC = left coronary cusp; LVOT = left ventricular outflow tract; NCC = noncoronary cusp; RCC = right coronary cusp; RVOT = right ventricular outflow tract.
The diagnostic accuracy of ECGs analyzed retrospectively by 3 independent electrophysiologists for LVOT vs RVOT varied from 79% to 88% using algorithm A, from 67% to 71% using algorithm B, and from 50% to 63% using algorithm C. The correct sublocalization of ectopy origin within the RVOT septum vs RVOT free wall using algorithms A and C varied from 37% to 58% and from 37% to 53%, respectively (Table 2).
Table 2.
(A) ECG localization of ectopy origin between the RV and the LV using algorithms A–C and (B) sublocalization in the RVOT (septum vs free-wall) using algorithms A and C, compared with successful ablation site, as tested by 3 independent assessors
| Accuracy of ECG Algorithms in localising within the LVOT vs RVOT |
|||
|---|---|---|---|
| Algorithm A | Algorithm B | Algorithm C | |
| Assessor 1 | 79% | 67% | 50% |
| Assessor 2 | 88% | 67% | 63% |
| Assessor 3 | 83% | 71% | 58% |
| Accuracy of ECG Algorithms in sub-localising within the RVOT: septum vs free-wall |
||
|---|---|---|
| Algorithm A | Algorithm C | |
| Assessor 1 | 42% | 37% |
| Assessor 2 | 58% | 53% |
| Assessor 3 | 37% | 42% |
ECG = electrocardiographic; LV = left ventricle; RV = right ventricle; RVOT = right ventricular outflow tract.
The inter-rater agreement for ECG analysis was measured for localization to the RVOT septum, RVOT free wall, or LV using ECG algorithms A and C and for RV vs LV for algorithm B. Free marginal kappa values of 0.15 (overall agreement of 43%) and 0.54 (overall agreement of 69%) were obtained for ECG algorithms A and C, respectively, confirming poor inter-assessor agreement. An acceptable kappa value of 0.72 was obtained for algorithm B with 86% overall agreement.
Intraobserver agreement testing performed within 3 months of the initial ECG analyses reproduced similar results (Table 3).
Table 3.
Intraobserver agreement was tested by using the kappa coefficient for all 3 ECG algorithms on all 24 ECGs of clinical PVC by 2 independent assessors
| Intra-observer ECG agreement |
|||
|---|---|---|---|
| Algorithm A | Algorithm B | Algorithm C | |
| Assessor 2 | 0.85 | 1 | 0.80 |
| Assessor 3 | 0.87 | 0.62 | 1 |
ECG = electrocardiographic; PVC = premature ventricular complex.
Clinical outcomes
All patients experienced significant improvement in symptoms in the immediate postoperative period. Twenty-one patients were completely symptom-free or experienced symptomatic improvement with significant reduction in OTVT/PVC burden over a mean follow-up duration of 11 ± 4 months (from 21,837 ± 23,241 to 1143 ± 4039; Z = −4.286; P < .0001).
Three patients reported recurrence in symptoms or worsening of symptoms during extended follow-up. Two of these 3 patients had repeated Holter ECGs, which documented a significant reduction in ectopy. In patient 9, clinical ectopy was reduced from 500 to less than 40 per day. In patient 21, symptoms recurred 3 weeks. Holter monitoring showed a sustained PVC reduction from 14,000 to 4700. Patient 8 had recurrence of frequent PVCs, which were from 3 distinctly different origins and were successfully ablated during repeat ablation.
The mean LV ejection fraction (EF) for the group was 57% ± 11%. Four patients were found to have moderate to severe impairment of LV systolic function (EF −37.8% ± 10.5%; PVC burden 22,045 ± 15,057 in 24 hours). Repeat scans after ablation in this cohort did not show significant improvement in the LV EF (39% ± 22%; PVC burden 5444 ± 9842). One of these patients has been discussed previously (patient 8).
Prior failed procedures
Six patients had previously attended for 9 attempted ablations collectively, which were either unsuccessful or aborted owing to lack of spontaneously occurring clinical PVCs.
One patient with a previously abandoned study due to noninducibility did not have any spontaneous PVCs during the preprocedural mapping period. Single beats of the clinical arrhythmia could be consistently induced at the end of each drive train during PVS. These PVCs were sufficient for the ECM to localize the arrhythmia origin to guide successful ablation (see case study 2 in the Online Data Supplement).
All 6 patients were successfully treated by using ECM. PVC burden on Holter monitoring for this cohort was reduced from 14,672 ± 16,969 to 10 ± 16 per 24 hours (P < .05) during a mean follow-up duration of 13 ± 3 months.
Discussion
Pre- and periprocedural mapping using ECM successfully identifies the OTVT/PVC origin to guide ablation, even in patients with minimal ectopy at the time of ablation.
Limitations of conventional intraprocedural localization of ectopy origin
During the EPS, the origin of ectopy is localized by pace mapping in regions of interest on the basis of analysis of the 12-lead ECG during OTVT/PVC, with the aim of achieving an exact 12-lead match with spontaneous OTVT/PVC.13 However, pace mapping can be imprecise with exact matches achieved from pacing sites more than 2 cm away from the origin of PVC.14, 15 Activation mapping using unipolar and bipolar electrograms can also be used to corroborate the pace-map findings in patients with frequent OTVT/PVCs. However, the mean area of myocardium activated within the first 10 ms during RVOT VT can range from 1.3 to 6.4 cm2. This, along with an interobserver variability of up to 5 ms or more in the manual assignment of activation time, is a significant limitation of activation mapping.14, 15
In addition, sequential activation mapping, with or without 3-dimensional electroanatomical navigation systems, is heavily dependent on sufficient spontaneous or induced clinical ectopy. As seen in 1 patient in our series, attempts to localize and ablate VT origin were abandoned during 4 previous procedures owing to minimal spontaneous ectopy despite pacing maneuvers and pharmacological adjuvants. The limitations of both activation and pace mapping were clearly illustrated in patient 11 (see case study 1 in the Online Data Supplement).
These disadvantages can be overcome by the use of an invasive noncontact mapping Ensite array that allows for mapping of single beats of ectopy. However, it requires systemic heparinization, even in the RV, increasing the risk of bleeding complications. Furthermore, only a single chamber can be mapped at a time. ECM overcomes these 2 limitations and even allows the patient to remain ambulatory for several hours before the EPS to allow spontaneous arrhythmia to be recorded without prolonging invasive procedural time.
The ability to predict left-sided arrhythmia from right-sided arrhythmia could better guide informed consent with regard to procedural time and risk, although complete certainty is still not possible with current noninvasive systems.
Limitations of 12-lead ECG localization of ectopy origin
The ability to predict left-sided arrhythmia from right-sided arrhythmia is of great importance as it guides informed consent with regard to procedural time and risk. The 12-lead ECG of OTVT/PVC is conventionally used preprocedurally for this purpose to plan access (ie, RV vs LV) and ablation strategy.
ECG criteria have been extensively published, describing characteristics of ectopic foci originating from the RVOT, the LVOT, and the aortomitral continuity, anterior interventricular vein, and the aortic sinus cusps.10, 11, 12, 16, 17
The V2 transition ratio (algorithm B) correctly predicted LVOT vs RVOT origin in 91% of the cases in their study.10 In our cohort, the algorithm was correct in 67%–71% of the patients when applied by 3 independent electrophysiologists with an acceptable inter-rater kappa agreement value of 0.72.
Algorithms A and C not only differentiate LVOT from RVOT origin but also sublocalize within the RVOT, with published sensitivity, specificity, and positive predictive values ranging from 70% to 90%. However, the application of the protocols in our cohort yielded the correct location (RV free-wall vs RV septum vs LV) in only 46%–63% of the cases, with poor interobserver agreement kappa values of 0.15 and 0.54.
As the algorithms become more specific at sublocalization, there are an increasing number of steps and measurements required to reach a diagnosis, thus increasing the chances of error and variability in results. We observed that the reduction in the reliability of each algorithm was proportional to the number of steps needed to reach a diagnosis. Algorithm B, involving 3 steps and localizing between the RVOT and the LVOT, yielded an acceptable result, with 86% overall agreement between the 3 assessors. However, algorithm A, involving 5 steps and sublocalizing to 8 regions (LV, near His, RV: anteroseptal, posteroseptal, mid-septal, anterolateral, posterolateral, and mid-lateral), yielded only 43% overall agreement between assessors.
These algorithms rely on measurements of parameters, including amplitude of the S wave in lead V6, ratio of PVC QRS duration to preceding sinus rhythm QRS duration, R/S-wave amplitude ratio and R-wave duration index, which can be prone to errors from a wandering baseline, lead noise, and absence of a suitable sinus rhythm beat preceding the PVC QRS, all of which are encountered in day-to-day clinical practice. In addition, interpretation and measurement of such parameters are inconsistent when performed on a 12-lead ECG at 25-mm speed in the absence of electronic calipers.
One striking example of ambiguity in such measurements is the presence or absence of an S wave in lead V6 >0.1 mV, which is the first step in algorithm C. We demonstrated a consistent disagreement between the assessors across several ECGs, with a poor inter-rater kappa agreement of 0.4 for this parameter alone. Two ECG examples are shown in Online Supplemental Figure 3. Similar variations were noted from measurements made for other ratios and indices mentioned above.
Further inaccuracies in ECG algorithms are introduced by individual variability in the spatial relationship between the heart, thoracic wall, and overall body habitus across patients. This is taken into account by the ECM system owing to its use of true cardiac anatomy from segmented CT geometry, making it highly accurate at localization even in the presence of underlying structural cardiac abnormalities. An additional strength of the system is that the high-density ECM vest is registered to the patient’s individually segmented CT geometry, which removes the potential for ECG lead-placement errors. The ECM vest can be applied at a time and location remote to the ablation procedure and catheter laboratory in which maneuvers to induce ectopy, including aerobic exercises on the treadmill, can be performed for preprocedural mapping. To avoid any potential shifts in geometry, ECM maps after exercise were created with the patient in the recumbent position.
By overcoming the aforementioned limitations, ECM accurately identified OTVT/PVC origin in the LV and correctly sublocalized in each chamber in all patients.
Study limitations
A limitation of this study is the small patient group, which does not cover the full spectrum of possible idiopathic OTVT locations; however, as a proof of concept, it demonstrates the feasibility and accuracy of ECM at pre- and periprocedural localization of the OTVT/PVC origin even in patients with minimal ectopy burden. In 3 of 24 patients, the clinical PVC did not occur spontaneously during the preprocedural mapping period, which precluded preprocedural identification of the PVC origin. However, it is possible that an extended period of vest wearing or the use of isopretenerol infusion could increase the likelihood of recording the clinical PVC.
It is also important to acknowledge that the cardiac vectors are projected onto the epicardial shell, although this did not appear to affect the high ablation success rate.
Inability to track the ablation catheter or perform distance measurements on the geometry limits testing of the system’s resolution (within millimeters) of fine localization of arrhythmia origin and successful ablation site for comparison.
The need for CT scanning and associated radiation exposure using ECM (mean 148 mGy·cm) should be weighed against the benefits of accurate localization of ectopy origin and potential reduction in procedural and fluoroscopic time, which warrant further studies.
Conventional catheter ablation of PVCs with unambiguous localization from the ECG and frequent intraprocedural ectopics is typically straightforward and associated with excellent clinical outcomes. However, the use of the ECM system may be particularly beneficial in guiding more challenging cases, such as patients with infrequent PVC burden and multiple PVC morphologies.
Conclusions
Noninvasive ECM can accurately identify VT origin in both LVOT and RVOT pre- and periprocedurally to guide catheter ablation with an accuracy superior to that of established ECG algorithms.
Footnotes
This work was supported by the National Institute for Health Research Biomedical Research Centre and the ElectroCardioMaths Programme of the Imperial British Heart Foundation Centre of Research Excellence. Dr Jamil-Copley is funded by grant PG/10/37/28347 from the British Heart Foundation.
Mr Bokan is a paid employee of Cardioinsight Technologies. As a paid employee of Cardioinsight Technologies, he is also a stockholder of Cardioinsight Technologies.
Appendix
Supplementary data
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.hrthm.2014.01.013.
Appendix
Supplementary data
Spontaneous clinical PVC
This video displays the biventricular activation wavefront of the spontaneous clinical PVC. The PVC origin from within the septal region is clear and can be correlated to the PA view (top panel) in Figure 2 of the main manuscript. The movie displays the ensuing activation as the white area propagating from the OT region down along the upper third of the LAD before spreading to the LV. This suggests a PVC origin from the septal LVOT.
Septal LVOT pacemapping
This video displays the biventricular activation wavefront when we pacemap from the LVOT at the septal origin of the clinical PVC as suggested by ECM. Activation is similar to the spontaneous clinical PVC and travels along the LAD and then towards the LV confirming that this is the best ablation site as the activation corresponds to the activation seen in Video 1.
Septal RVOT pacemapping
This displays the biventricular activation wavefront when we pacemap from the septal RVOT close to the septal origin of the spontaneous clinical PVC. Activation clearly propagates towards the RV unlike the PVC activation wavefront demonstrated in Video 1. This confirms that this anatomical location is not the origin of the PVC.
Supplementary Material
Supplementary Material
References
- 1.Aliot E., Stevenson G., Amendral-Garrote J. EHRA/HRS Expert consensus on catheter ablation of ventricular arrhythmias. Europace. 2009;11:771–817. doi: 10.1093/europace/eup098. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa M., Takahashi N., Nobe S., Ichinose M., Ooie T., Yufu F., Shigematsu S., Hara M., Yonemochi H., Saikawa T. Gender differences in various types of idiopathic ventricular tachycardia. J Cardiovasc Electrophysiol. 2002;13:633–638. doi: 10.1046/j.1540-8167.2002.00633.x. [DOI] [PubMed] [Google Scholar]
- 3.Lerman B.B., Stein K.M., Markowitz S.M., Mittal S., Iwai S. In: Cardiac Electrophysiology: from Cell to Bedside. 4th ed. Zipes D.P., Jaife J., editors. WB Saunders; Philadelphia, PA: 2004. Ventricular tachycardia in patients with structurally normal hearts; p. 668. [Google Scholar]
- 4.Joshi S., Wilber D. Ablation of idiopathic right ventricular outflow tract tachycardia: current perspectives. J Cardiovasc Electrophysiol. 2005;16:s52–s58. doi: 10.1111/j.1540-8167.2005.50163.x. [DOI] [PubMed] [Google Scholar]
- 5.Mountantonakis S.E., Frankel D.S., Gerstenfeld E.P. Reversal of outflow tract ventricular premature depolarization-induced cardiomyopathy with ablation: effect of residual arrhythmia burden and preexisting cardiomyopathy on outcome. Heart Rhythm. 2011;8:1608–1614. doi: 10.1016/j.hrthm.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 6.Asirvatham S.J. Correlative anatomy for the invasive electrophysiologist: outflow tract and supravalvar arrhythmia. J Cardiovasc Electrophysiol. 2009;20:955–968. doi: 10.1111/j.1540-8167.2009.01472.x. [DOI] [PubMed] [Google Scholar]
- 7.Ramanathan C., Jia P., Ghanem R., Ryu K., Rudy Y. Activation and repolarization of the normal heart under complete physiological conditions. Proc Natl Acad Sci U S A. 2006;103:6309–6314. doi: 10.1073/pnas.0601533103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanem R.N., Ramanathan C., Ryu K., Markowitz A., Rudy Y. Noninvasive electrocardiographic imaging (ECGI): comparison to intraoperative mapping in patients. Heart Rhythm. 2005;2:339–354. doi: 10.1016/j.hrthm.2004.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cuculich P.S., Zhang J., Wang Y., Desouza K.A., Vijayakumar R., Woodard P.K., Rudy Y. The electrophysiological cardiac ventricular substrate in patients after myocardial infarction: noninvasive characterization with electrocardiographic imaging. J Am Coll Cardiol. 2011;58:1893–1902. doi: 10.1016/j.jacc.2011.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Betensky B.P., Park R.E., Marchlinski F.E., Hutchinson M., Garcia F.C., Dixit S., Callans D.J., Cooper J.M., Bala R., Lin D., Riley M.P., Gerstenfeld E.P. The V2 transition ratio: a new electrocardiographic criterion for distinguishing left from right ventricular outflow tract tachycardia origin. J Am Coll Cardiol. 2011;57:2255–2262. doi: 10.1016/j.jacc.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Zhang F., Chen M., Yang B., Ju W., Chen H., Yu J., Lau C.P., Cao K., Tse H.F. Electrocardiographic algorithm to identify the optimal target ablation site for idiopathic right ventricular outflow tract ventricular premature contraction. Europace. 2009;11:1214–1220. doi: 10.1093/europace/eup231. [DOI] [PubMed] [Google Scholar]
- 12.Ito S., Tada H., Naito S., Kurosaki K., Ueda M., Hoshizaki H., Miyamori I., Oshima S., Taniguchi K., Nogami A. Development and validation of an ECG algorithm for identifying the optimal ablation site for idiopathic ventricular outflow tract tachycardia. J Cardiovasc Electrophysiol. 2003;14:1280–1286. doi: 10.1046/j.1540-8167.2003.03211.x. [DOI] [PubMed] [Google Scholar]
- 13.Bala R., Marchlinski F. Electrocardiographic recognition and ablation of outflow tract ventricular tachycardia. Heart Rhythm. 2007;4:366–370. doi: 10.1016/j.hrthm.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 14.Azegami K., Wilber D.J., Aarruda M., Lin A.C., Denman R.A. Spatial resolution of pace-mapping and activation mapping in patients with idiopathic right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2002;39:1808–1812. [Google Scholar]
- 15.Bogun F., Taj M., Ting M., Kim H.M., Reich S., Good E., Jongnarangsin K., Chugh A., Pelosi F., Oral H., Morady F. Spatial resolution of pace-mapping of idiopathic ventricular tachycardia/ectopy originating in the right ventricular outflow tract. Heart Rhythm. 2008;5:39–44. doi: 10.1016/j.hrthm.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T., McElderry T., Doppalapudi H., Okada T., Murakami Y., Yoshida Y., Yoshida N., Inden Y., Murohara T., Plumb V., Kay N. Idiopathic ventricular arrhythmias originating from the left ventricular summit. Circ Arrhythm Electrophysiol. 2010;3:616–623. doi: 10.1161/CIRCEP.110.939744. [DOI] [PubMed] [Google Scholar]
- 17.Ouyang F., Fotuhi P., Yen Ho S., Hebe J., Volkmer M., Goya M., Burns M., Antz M., Ernst S., Cappato R., Kuck K.-H. Repetitive monomorphic ventricular tachycardia originating from the aortic sinus cusp: electrocardiographic characterisation from the guiding catheter ablation. J Am Coll Cardiol. 2002;39:500–508. doi: 10.1016/s0735-1097(01)01767-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spontaneous clinical PVC
This video displays the biventricular activation wavefront of the spontaneous clinical PVC. The PVC origin from within the septal region is clear and can be correlated to the PA view (top panel) in Figure 2 of the main manuscript. The movie displays the ensuing activation as the white area propagating from the OT region down along the upper third of the LAD before spreading to the LV. This suggests a PVC origin from the septal LVOT.
Septal LVOT pacemapping
This video displays the biventricular activation wavefront when we pacemap from the LVOT at the septal origin of the clinical PVC as suggested by ECM. Activation is similar to the spontaneous clinical PVC and travels along the LAD and then towards the LV confirming that this is the best ablation site as the activation corresponds to the activation seen in Video 1.
Septal RVOT pacemapping
This displays the biventricular activation wavefront when we pacemap from the septal RVOT close to the septal origin of the spontaneous clinical PVC. Activation clearly propagates towards the RV unlike the PVC activation wavefront demonstrated in Video 1. This confirms that this anatomical location is not the origin of the PVC.
Supplementary Material
Supplementary Material