Abstract
Purpose
To analyze factors that predict the use of trimodality treatment (chemotherapy, surgery, and radiation therapy [RT]) and evaluate the impact that trimodality treatment use has on survival for patients with inflammatory breast cancer (IBC).
Methods
Using the National Cancer Data Base, patients who underwent surgical treatment of nonmetastatic IBC from 1998 to 2010 were identified. We collected demographic, tumor, and treatment data and analyzed treatment and survival trends over time. Logistic regression and Cox proportional hazard models were used to examine factors predicting treatment and survival.
Results
We identified 10,197 patients who fulfilled study criteria. The use of trimodality therapy fluctuated annually (58.4% to 73.4%). Patients who were older, diagnosed earlier in the study period, lived in regions of the country outside of the Midwest, had lower incomes or public insurance, and had a higher comorbid score were significantly less likely to receive trimodality therapy (all P < .05). Five- and 10-year survival rates were highest among patients receiving trimodality treatment (55.4% and 37.3%, respectively) compared with patients who received the combination of surgery plus chemotherapy, surgery plus RT, or surgery alone. After adjusting for potential confounding variables, use of trimodality therapy remained a significant independent predictor of survival.
Conclusion
Underutilization of trimodality therapy negatively impacted survival for patients with IBC. The use of trimodality therapy increased marginally with time, but there remain significant factors associated with differences in use of trimodality treatment. We have identified specific barriers to care that may be targeted to improve treatment delivery and potentially improve patient outcomes.
INTRODUCTION
Inflammatory breast cancer (IBC) is a rare but aggressive disease. Although IBC represents less than 5% of breast cancer diagnoses annually,1 current population-based studies suggest an increasing incidence.2 When compared with patients with similarly staged, noninflammatory, locally advanced breast cancer, patients with IBC have a worse prognosis, with 5-year overall survival rates of 34% to 47%.3–5
Current IBC treatment guidelines published by the National Comprehensive Cancer Network recommend anthracycline-based neoadjuvant chemotherapy followed by modified radical mastectomy and postmastectomy radiation therapy (RT) to the chest wall and draining lymphatics6 (trimodality treatment). These guidelines have remained largely unchanged, despite sophisticated advances in the treatment of noninflammatory breast cancer, and are based on highly specialized single-institution retrospective studies that demonstrate a survival advantage with the use of trimodality treatment.4,7–9 Studies evaluating contemporary treatment trends, adherence to the use of trimodality treatment, and subsequent impact on patient survival are lacking for patients with IBC.
It was our aim, therefore, to examine treatment trends and outcomes among women who underwent surgical treatment of IBC. To create a homogeneous group of patients who were healthy enough to undergo surgical treatment of IBC and were therefore more likely to be candidates for aggressive therapy, we focused on women who had surgery as part of their treatment. In this specific group of women, we evaluated the annual trends in the use of trimodality treatment, identified patient factors associated with nonuse of trimodality treatment, and studied the impact that partial, nontrimodality therapy had on overall patient survival in the contemporary era.
METHODS
Data Source and Study Cohort
Designed to serve as a comprehensive cancer care resource, The National Cancer Data Base (NCDB) is a collaborative effort of the American College of Surgeons, the American Cancer Society, and the Commission on Cancer. To assess local, regional, and national trends and standards, the database collects patient demographic, tumor, treatment, and outcome variables from the approximately 1,450 Commission on Cancer–approved hospitals across the United States. This is estimated to represent approximately 70% of all new cancer diagnoses in the country annually.10 Data reported to the NCDB are retrospective and de-identified, ensuring confidentiality.
Using the NCDB, patients who underwent surgical treatment of nonmetastatic IBC from 1998 to 2010 were identified. We included female patients who underwent surgical resection of any type (segmental mastectomy or mastectomy with or without axillary dissection) with any histologic breast cancer subtype and any nodal status who had a clinical diagnosis of T4d (stage IIIB or IIIC according to the American Joint Committee on Cancer fifth or sixth editions) breast cancer.11 Patients who did not receive surgical treatment or had metastatic disease were excluded. Patients were further excluded if they had a prior cancer diagnosis or were diagnosed by autopsy or death certificate.
Study Variables
We collected patient demographics (age, race, Charlson-Deyo comorbidity score, insurance status, geographic location, and income), tumor characteristics (grade and nodal status), and treatment administration (treatment facility type, operation performed, and use of trimodality therapy). Although estrogen receptor status and progesterone receptor status are site-specific data points accounted for in the NCDB, we excluded this variable from our analysis because of large amounts of missing data.
Patients were classified into the following four groups based on treatment received: surgery only; surgery plus chemotherapy; surgery plus RT; or surgery, RT, and chemotherapy (trimodality therapy). The use of trimodality therapy was defined as all three methods of treatment given in the first course of treatment recorded in the treatment plan and administered to patients before disease progression or recurrence. We evaluated the distribution of treatment across each year of the study. Chemotherapy and RT are reported as simply administered or not administered; because of the large amount of missing data, the sequence of administration was not evaluated, and data regarding the administration of specific chemotherapeutic agents are not available in the cancer registry. This study was determined to be exempt from review under Code of Federal Regulations 45 part 46.101(b) by the local institutional review board Human Subjects Committee.
Data Analysis
Univariable analysis was performed to evaluate the association between each variable and the use of trimodality therapy. To evaluate how the patient's demographic, tumor, and treatment variables were associated with the administration of trimodality therapy, we further grouped patients as those who received trimodality therapy versus those who did not. Data were compared between the groups using χ2 tests for categorical variables and t tests or nonparametric approaches (Wilcoxon rank sum or Kruskal-Wallis test) for continuous variables. Logistic regression analysis was used to assess the multivariable relationship between patient characteristics and the probability of receiving trimodality therapy.
Overall survival (OS) time was measured from the time of diagnosis until the time of death or last follow-up. We used Kaplan-Meier methods to estimate unadjusted OS rates for patients in each of the four treatment groups. Differences in OS were compared using the log-rank test. We then used Cox proportional hazards models to examine the association between patient characteristics and treatment variables on OS in multivariable analysis. We examined the proportional hazards assumption of the Cox model and did not detect any violations.
Because the Charlson-Deyo score was not collected until 2003, we performed the model-building procedures twice for each outcome (predictors of trimodality therapy and predictors of survival). First, we included the Charlson-Deyo score in the model selection procedure, which limited the analysis to patients who were diagnosed in and after 2003. We then excluded the Charlson-Deyo score from the second round model fitting, so that the patients who were diagnosed before 2003 could be included in the multivariable analysis, thus maximizing the follow-up time for patients in the study. Finding little difference in the covariates that predicted use of trimodality therapy or survival and recognizing the importance of adjusting for comorbid diseases in the surgical population, we report the results from the model that included Charlson-Deyo score.
Data were analyzed using SAS version 9.2 (SAS Institute, Cary, NC) and S-Plus version 8.04 (Statistical Sciences, Seattle, WA). For all statistical testing, we used a two-sided significance level of P = .05.
RESULTS
We identified 10,197 patients who fulfilled study criteria (Table 1). The incidence of patients who underwent surgery for nonmetastatic IBC remained stable across all years of the study. The majority of patients were white, insured, and with a low Charlson-Deyo score. Most tumors were poorly differentiated or undifferentiated (60.5%). Mastectomy (either unilateral or bilateral) was the most common operation (n = 9,524, 93.4%), with a minority of women undergoing segmental mastectomy or an unknown or unreported breast operation. Although all patients in the study underwent breast surgery for their IBC, 13.2% (n = 1,345) of patients did not have pathologic lymph node evaluation, and an additional 16.7% (n = 1,701) did not have pathologic lymph node status reported, resulting in nearly 30% of patients with an unknown lymph node status.
Table 1.
Patient Demographic and Tumor and Treatment Characteristics
| Characteristic | No. of Patients (N = 10,197) | % |
|---|---|---|
| Patient factors | ||
| Age, years | ||
| < 40 | 1,055 | 10.3 |
| 40-49 | 2,397 | 23.5 |
| 50-59 | 3,162 | 31.0 |
| 60-69 | 1,968 | 19.3 |
| ≥ 70 | 1,615 | 15.4 |
| Race | ||
| White | 8,436 | 82.7 |
| Black | 1,371 | 13.5 |
| Other | 277 | 2.7 |
| Unknown | 113 | 1.1 |
| Charlson-Deyo Score | ||
| 0 | 5,271 | 51.7 |
| 1 | 808 | 7.9 |
| 2 | 159 | 1.6 |
| Unknown | 3,959 | 38.8 |
| Primary payer | ||
| Not insured | 462 | 4.5 |
| Private insurance | 5,862 | 57.5 |
| Public insurance | 3,608 | 35.4 |
| Unknown | 265 | 2.6 |
| Geographic location | ||
| Atlantic | 1,499 | 14.7 |
| Great Lakes | 1,790 | 17.6 |
| Midwest | 900 | 8.8 |
| Mountain | 478 | 4.7 |
| Northeast | 529 | 5.2 |
| Pacific | 1,404 | 13.8 |
| South | 669 | 6.6 |
| Southeast | 2,031 | 19.9 |
| West | 897 | 8.8 |
| Income | ||
| < $30,000 | 1,410 | 13.8 |
| $30,000-$34,900 | 1,787 | 17.5 |
| $35,000-$45,900 | 2,641 | 25.9 |
| > $46,000 | 3,872 | 38.0 |
| Unknown | 487 | 4.8 |
| Community | ||
| Urban/metro: population > 1,000,000 | 537 | 5.3 |
| Urban/metro: population 250,000-1,000,000 | 5,040 | 49.4 |
| Urban/metro: population < 250,000 | 1,896 | 18.6 |
| Urban: population > 20,000 adjacent to metro area | 1,080 | 10.6 |
| Urban: population > 20,000 not adjacent to metro area | 445 | 4.4 |
| Urban: population 2,500-19,999 adjacent to metro area | 176 | 1.7 |
| Urban: population 2,500-19,999 not adjacent to metro area | 543 | 5.3 |
| Rural: population < 2,500 adjacent to metro area | 301 | 3.0 |
| Rural: population < 2,500 not adjacent to metro area | 84 | 0.8 |
| Tumor factors | ||
| Grade | ||
| Well differentiated | 223 | 2.2 |
| Moderately differentiated | 2,189 | 21.5 |
| Poorly differentiated | 5,923 | 58.1 |
| Undifferentiated | 246 | 2.4 |
| Unknown | 1,616 | 15.9 |
| Nodal stage | ||
| 0 | 1,707 | 16.7 |
| 1 | 2,845 | 27.9 |
| 2 | 1,642 | 16.1 |
| 3 | 957 | 9.4 |
| Unknown | 3,046 | 29.9 |
| Treatment factors | ||
| Treatment facility type | ||
| Academic/research program | 3,240 | 31.8 |
| Community cancer program | 1,538 | 15.1 |
| Comprehensive community cancer program | 5,242 | 51.4 |
| Other | 177 | 1.7 |
| Operation performed | ||
| Segmental mastectomy | 536 | 5.3 |
| Unilateral mastectomy | 1,079 | 10.6 |
| Ipsilateral mastectomy + CPM | 224 | 2.2 |
| Unilateral MRM | 6,890 | 67.6 |
| Ipsilateral MRM + CPM | 1,131 | 11.1 |
| Unilateral RM | 162 | 1.6 |
| Ipsilateral RM + CPM | 38 | 0.4 |
| Other/Unknown | 137 | 1.3 |
| Treatment received | ||
| Surgery alone | 500 | 4.9 |
| Surgery + chemo | 2,728 | 26.8 |
| Surgery + RT | 158 | 1.6 |
| Surgery + RT + chemo | 6,811 | 66.8 |
Abbreviations: chemo, chemotherapy; CPM, contralateral prophylactic mastectomy; MRM, modified radical mastectomy; RM, radical mastectomy; RT, radiation therapy.
Chemotherapy with or without RT was administered to most patients (n = 9,539, 93.5%) and was most frequently reported as a multiagent regimen; of patients treated with chemotherapy, 90.7% (n = 8,654) were given more than one systemic agent. Compared with surgery alone, surgery plus chemotherapy without RT, or surgery plus RT without chemotherapy, trimodality therapy was the most common treatment (66.8%). The specific sequence of therapeutic administration was not reported for more than half of the patients in the study (n = 6,274).
The use of trimodality therapy fluctuated annually, between 58.4% and 73.4% (Fig 1). Over the course of the study, trimodality therapy administration increased steadily until it reached a maximum of 73.4% in 2004. In the years that followed, use varied; by 2010, only 65.9% of patients received trimodality treatment for IBC. Surgery with chemotherapy was the second most common treatment regimen (26.8%). Surgery with RT in the absence of chemotherapy was a rare treatment combination, accounting for less than 2% of the treatment plan in any given study year.
Fig 1.
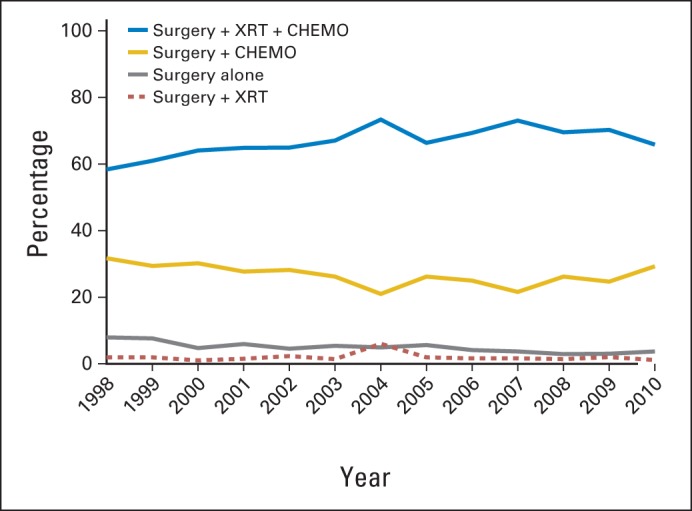
Distribution of treatment for each year of study. CHEMO, chemotherapy; XRT, radiation therapy.
Univariable analysis demonstrated that the use of trimodality therapy was associated with younger age, type of treating facility, geographic location, rural/urban setting, year of diagnosis, race, insurance status, education, income, and the presence of comorbid conditions. Table 2 lists the multivariable analysis results of factors that were significantly associated with the use of trimodality therapy. When including the Charlson-Deyo score of comorbid conditions in the model, trimodality therapy was administered more frequently to patients who were younger; who were diagnosed later in the study period; who were treated in the Great Lakes, Midwest, and Northeast versus other regions of the country; and who had private insurance or a lower comorbid score (P < .05). These same factors were also significant when the Charlson-Deyo score was not included in the analysis. The type of treating facility did not significantly impact the use of trimodality therapy after adjusting for other potential confounding variables in the multivariable analysis, despite it being significant in univariable analysis. Tumor grade and nodal status did not impact therapy in either model.
Table 2.
Predictors of the Use of Trimodality Therapy: Multivariable Analysis Results
| Factor | Odds Ratio | 95% CI |
|---|---|---|
| Patient factors | ||
| Age, years* | ||
| < 40 | 1.00 | |
| 40-49 | 1.13 | 0.90 to 1.41 |
| 50-59 | 1.08 | 0.87 to 1.34 |
| 60-69 | 0.87 | 0.69 to 1.10 |
| ≥ 70 | 0.47 | 0.37 to 0.61 |
| Charlson-Deyo score* | ||
| 0 | 1.00 | |
| 1 | 0.81 | 0.69 to 0.96 |
| 2 | 0.63 | 0.44 to 0.88 |
| Primary payer* | ||
| Public insurance | 1.00 | |
| Private insurance | 1.19 | 1.03 to 1.37 |
| Not insured | 0.99 | 0.74 to 1.32 |
| Community* | ||
| Urban/metro: population > 1,000,000 | 1.00 | |
| Urban/metro: population 250,000-1,000,000 | 1.18 | 1.01 to 1.38 |
| Urban/metro: population < 250,000 | 1.71 | 1.39 to 2.12 |
| Urban: population > 20,000 adjacent to metro area | 1.02 | 0.76 to 1.36 |
| Urban: population > 20,000 not adjacent to metro area | 1.39 | 0.88 to 2.19 |
| Urban: population 2,500-19,999 adjacent to metro area | 1.51 | 1.14 to 2.00 |
| Urban: population 2,500-19,999 not adjacent to metro area | 1.27 | 0.89 to 1.79 |
| Rural: population < 2,500 adjacent to metro area | 1.29 | 0.72 to 2.32 |
| Rural: population < 2,500 not adjacent to metro area | 0.92 | 0.51 to 1.67 |
| Geographic location* | ||
| Atlantic | 1.00 | |
| Great Lakes | 1.33 | 1.08 to 1.63 |
| Midwest | 1.39 | 1.07 to 1.81 |
| Mountain | 0.85 | 0.62 to 1.17 |
| Northeast | 1.26 | 0.91 to 1.73 |
| Pacific | 1.01 | 0.81 to 1.26 |
| South | 0.80 | 0.61 to 1.06 |
| Southeast | 0.91 | 0.75 to 1.11 |
| West | 0.58 | 0.46 to 0.74 |
| Income* | ||
| > $46,000 | 1.00 | |
| $35,000-$45,900 | 0.95 | 0.82 to 1.11 |
| $30,000-$34,900 | 0.76 | 0.64 to 0.91 |
| < $30,000 | 0.77 | 0.64 to 0.94 |
| Treatment factors | ||
| Year of diagnosis* | ||
| 2003 | 1.00 | |
| 2004 | 1.46 | 1.15 to 1.87 |
| 2005 | 0.91 | 0.73 to 1.14 |
| 2006 | 1.09 | 0.86 to 1.37 |
| 2007 | 1.32 | 1.04 to 1.66 |
| 2008 | 1.13 | 0.90 to 1.41 |
| 2009 | 1.23 | 0.98 to 1.54 |
| 2010 | 0.99 | 0.79 to 1.26 |
P < .05.
The unadjusted median OS for all patients in the study cohort was 61 months. Median OS was significantly higher among patients receiving trimodality therapy (72 months) compared with surgery alone (26 months; P < .05, Fig 2). Not surprisingly, 5- and 10-year survival rates were highest among patients in the trimodality group (55.4% and 37.3%, respectively) compared with any other treatment group. Five-and 10-year survival rates were similar for patients who received the combination of surgery plus chemotherapy (42.9% and 28.5%, respectively) or surgery plus RT (40.7% and 23.5%, respectively). Long-term survival rates were lowest among patients treated with surgery alone; the 10-year survival rate was 16.5% for this group of patients.
Fig 2.
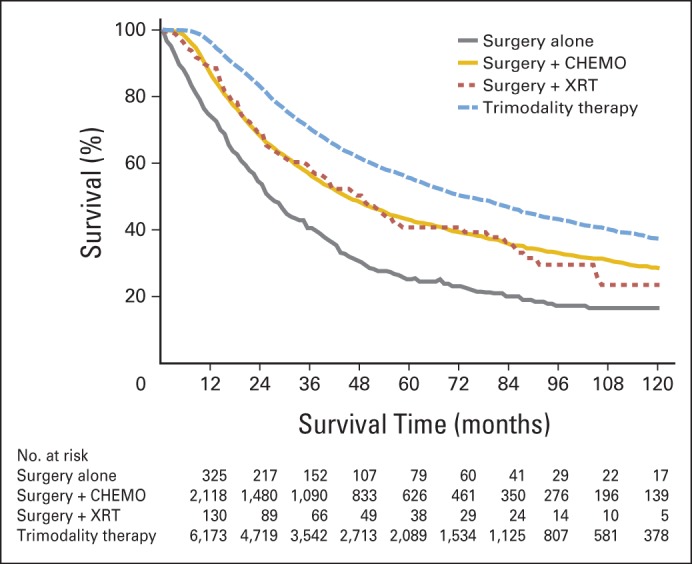
Ten-year unadjusted overall survival based on treatment administered. CHEMO, chemotherapy; XRT, radiation therapy.
Survival rates were predicted not only by type of treatment, but also by age, race, Charlson-Deyo comorbidity score, insurance status, tumor grade, and nodal status (P < .05; Table 3). White patients with fewer comorbidities, younger patients, patients who were privately insured, those who had well or moderately differentiated tumors, and those without evidence of nodal metastases had significantly improved survival. We further found that survival was significantly better among patients treated at academic-based programs when compared with patients treated at community cancer centers (hazard ratio, 1.37; P < .001) or comprehensive community cancer programs (hazard ratio, 1.17; P = .006).
Table 3.
Predictors of Overall Survival: Multivariable Analysis Results
| Factor | Hazard Ratio | 95% CI |
|---|---|---|
| Patient factors | ||
| Age, years* | ||
| < 40 | 1.00 | |
| 40-49 | 0.85 | 0.70 to 1.03 |
| 50-59 | 0.92 | 0.77 to 1.11 |
| 60-69 | 0.86 | 0.70 to 1.05 |
| ≥ 70 | 1.18 | 0.95 to 1.46 |
| Race* | ||
| Black | 1.00 | |
| White | 0.73 | 0.63 to 0.84 |
| Other | 0.58 | 0.40 to 0.83 |
| Charlson-Deyo score* | ||
| 0 | 1.00 | |
| 1 | 1.13 | 0.98 to 1.31 |
| 2 | 1.35 | 1.02 to 1.77 |
| Primary payer* | ||
| Public insurance | 1.00 | |
| Private insurance | 0.82 | 0.72 to 0.93 |
| Not insured | 0.96 | 0.73 to 1.26 |
| Tumor factors | ||
| Grade* | ||
| Well differentiated | 1.00 | |
| Moderately differentiated | 0.93 | 0.65 to 1.33 |
| Poorly differentiated | 1.78 | 1.25 to 2.51 |
| Undifferentiated | 1.74 | 1.07 to 2.80 |
| Nodal stage* | ||
| 0 | 1.00 | |
| 1 | 1.50 | 1.28 to 1.77 |
| 2 | 2.15 | 1.84 to 2.50 |
| 3 | 2.92 | 2.50 to 3.43 |
| Treatment factors | ||
| Treatment facility type* | ||
| Academic/research program | 1.00 | |
| Community cancer program | 1.37 | 1.18 to 1.59 |
| Comprehensive community cancer program | 1.17 | 1.04 to 1.31 |
| Other specified types of cancer programs | 1.04 | 0.57 to 1.89 |
| Treatment received* | ||
| Surgery + RT + chemo | 1.00 | |
| Surgery + chemo | 1.64 | 1.46 to 1.84 |
| Surgery + RT | 1.47 | 0.96 to 2.24 |
| Surgery alone | 2.28 | 1.80 to 2.89 |
Abbreviations: chemo, chemotherapy; RT, radiation therapy.
P < .05.
DISCUSSION
We found that patients with nonmetastatic IBC who received trimodality treatment had the best survival rates when compared with patients receiving less comprehensive treatment regimens. By uniquely focusing our study on patients who underwent surgical treatment of their nonmetastatic IBC, we have isolated a cohort of patients with access to medical care who were clinically well enough to undergo aggressive treatment, eliminating patients who presented with an advanced disease state; those who experienced progression while on chemotherapy rendering them nonsurgical candidates; and those in such medically frail condition so as to preclude full multimodality treatment. Despite this, we found statistically significant differences in treatment administration and associated outcomes based on patients' demographic status.
Current recommendations for the multidisciplinary care of IBC are largely based on observational studies or extrapolated from patients with noninflammatory, locally advanced breast cancer,12 resulting in consensus-based rather than evidence-based treatment recommendations. Clinicians are further challenged by the fact that IBC is a clinical diagnosis representing a broad spectrum of disease presentation. It was not surprising, therefore, that we identified variable treatment administration patterns based on year of treatment administration and geographic location, recognizing that treatment patterns may fluctuate with current expert opinion, community awareness, or geographic trends. We were, however, surprised to find that 5.3% of women in the study were treated with substandard segmental mastectomy rather than total mastectomy, a surgical option associated with poor cosmetic outcomes and unacceptably high positive margin and local recurrence rates.13 We also found it notable that nearly one third of women did not have pathologic lymph node status reported, despite the knowledge that IBC is associated with axillary lymph node involvement in 55% to 85% of cases and is a predictor of patient survival.1,14 It is unclear from the data what specific factors drove the surgical decision-making process for these women.
In one of the largest single-institution studies evaluating contemporary chemotherapy outcomes for patients with IBC, Cristofanilli et al7 found that the addition of a taxane to traditional anthracycline-based chemotherapy (fluorouracil, doxorubicin, and cyclophosphamide) resulted in improvements in progression-free survival and OS for patients with estrogen receptor–negative tumors. A separate study demonstrated that patients with IBC treated with anthracycline-based chemotherapy plus a taxane had higher rates of pathologic complete response.15 In our study, 93.6% of women were treated with some form of systemic chemotherapy; 90% of those were treated with a multiagent chemotherapy regimen, although the specific agents given were not available. It is therefore unclear what impact, if any, the use of taxanes had on survival in the current study, although we recognize that changing treatment trends may have an impact on patient outcomes over time.
Contrary to the high percentage of patients receiving chemotherapy in our study, an alarming number of women did not receive postoperative RT. More than a quarter of the patients (26.8%) received the combination of chemotherapy and surgery, without RT. This resulted in a significant detrimental impact on OS. By focusing our study on the surgical population, we have created a cohort of women who had access to medical care; despite this, patients with lower incomes or public insurance were less likely to receive complete trimodality treatment than those with higher incomes or private insurance. No prior studies have examined the compliance rates of postmastectomy RT specifically for patients with IBC, although our data are consistent with population-based studies evaluating the compliance of postoperative RT for patients undergoing breast-conserving surgery for noninflammatory breast cancer.16 In a recent study, Pan et al17 found that women with young children were less likely to receive guideline-recommended RT after surgical treatment of noninflammatory breast cancer. Although it is quite likely that the reasons for noncompliance of treatment are multifactorial, clinicians must consider the individual impact of each patient's social setting, recognizing that unique financial challenges including travel, childcare, and days away from work may influence a woman's ability to fully adhere to her cancer treatment plan. Given that recurrence rates in IBC are relatively high and take place within a shorter time period than in other forms of breast cancer,18 adherence to RT may play a greater role in locoregional control. The ongoing nationwide trends in underutilization of RT warrant further investigation into the specific patient and physician factors that preclude comprehensive cancer care.
When analyzing annual changes in the use of trimodality treatment, we found no definitive trend. Although the range of trimodality treatment increased in the early study years, from 58.4% in 1999 to 73.4% in 2004, the upward trend in use did not continue from 2004 to 2010; instead, it remained relatively stable, suggesting there remain significant limitations in therapy administration for women with treatable IBC. Some of those limitations are likely to be patient-driven, given our finding that younger patients with a lower comorbid score were more likely to receive trimodality treatment compared with patients who were older or with more comorbid diagnoses. This also may be the result of treatment delivery bias, with younger, healthier patients being the target of more aggressive, guideline-compliant therapy. Other significant social factors, including patient insurance status, geographic location, and income, also predicted use of trimodality therapy. These important findings highlight ongoing nationwide disparities in cancer care.
We acknowledge that this analysis is limited to a subset of relatively healthy women who underwent surgery for a rare disease and were treated at highly specialized institutions, thus potentially limiting the generalizability of our findings. We further recognize that using a large, national database of retrospective data is subject to patient selection and institution reporting bias. Indeed, our 5-year survival rate of 55.4% is higher than reported in the literature3,4 and likely reflects the biases inherent in this population of women with localized disease who underwent surgery for IBC when compared with the general population of women diagnosed with IBC. Similarly, our findings are limited by the completeness of data reported in the NCDB. Studies have demonstrated that there are substantial variations in the reporting of data within the NCDB across cancer sites and geographic location when compared with national cancer data sets such as the American Cancer Society Cancer Facts and Figures; the Surveillance, Epidemiology, and End Results Database; or the United States Cancer Statistics report.19 We found in this specific data set, for example, that a substantial number of patients did not have documentation of lymph node status or hormone receptor status, a limitation inherent to the use of pre-existing clinical data. Additionally, the NCDB does not capture HER2 mutation status, thus eliminating the ability to adjust the survival data against this important prognostic indicator.
These limitations are offset, however, by the ability to analyze a large number of patients with a rare disease. Using data collected in the NCDB, we analyzed outcomes from more than 10,000 patients with IBC and demonstrated that within this population, underutilization of trimodality therapy had a direct impact on overall patient survival. To create a homogeneous cohort, we performed an analysis specifically of patients who were candidates for surgery and who, therefore, would have been more likely to be candidates for aggressive, thorough, multimodality oncologic care. Despite this, we found underutilization of trimodality therapy and a particular underutilization of RT across all years of study with no improvements seen since 2004. Importantly, we were able to identify significant patient factors associated with differences in use of trimodality treatment. By identifying that younger, healthier patients with higher incomes, with private insurance, and living in certain geographic areas of the country are more likely to receive complete trimodality treatment of IBC, we have highlighted ongoing disparities in breast cancer care. These findings can be used to help develop comprehensive support programs designed to target potential barriers to care. Social programs focusing on patient outreach and education, community transportation options for elderly patients, on-site child care for mothers with young children, local IBC support groups, and the addition of regional RT centers designed to make access to RT more readily available would likely serve to lessen disparate treatment delivery and subsequently improve patient outcomes for women with this highly aggressive, deadly form of breast cancer.
Glossary Terms
- American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM staging:
a cancer staging system that describes the extent of cancer in a patient's body. “T” describes the size of the tumor and whether it has invaded nearby tissue; “N” describes regional lymph nodes that are involved; “M” describes distant metastasis (spread of cancer from one body part to another). The TNM Classification of Malignant Tumours was developed and maintained by the UICC to achieve consensus on one globally recognized standard for classifying the extent of spread of cancer. The TNM classification was also used by the AJCC. In 1987, the UICC and AJCC staging systems were unified into a single staging system. Prognosis of a patient is defined by TNM classification.
- estrogen receptor (ER):
ligand-activated nuclear proteins, belonging to the class of nuclear receptors, present in many breast cancer cells that are important in the progression of hormone-dependent cancers. After binding, the receptor-ligand complex activates gene transcription. There are two types of estrogen receptors (ERα and ERβ). ERα is one of the most important proteins controlling breast cancer function. ERβ is present in much lower levels in breast cancer, and its function is uncertain. Estrogen receptor status guides therapeutic decisions in breast cancer.
- HER2/neu (human epidermal growth factor receptor 2):
also called ErbB2. HER2/neu belongs to the epidermal growth factor receptor (EGFR) family and is overexpressed in several solid tumors. Like EGFR, it is a tyrosine kinase receptor whose activation leads to proliferative signals within the cells. On activation, the human epidermal growth factor family of receptors are known to form homodimers and heterodimers, each with a distinct signaling activity. Because HER2 is the preferred dimerization partner when heterodimers are formed, it is important for signaling through ligands specific for any members of the family. It is typically overexpressed in several epithelial tumors.
- inflammatory breast cancer:
a clinical diagnosis characterized by rapid enlargement of the breast, generalized induration in the presence or absence of a distinct breast mass, edema of the skin of the breast, erythema that must involve more than one third of the breast, and biopsy-proven carcinoma.
- logistic regression analysis:
a multivariable regression model in which the log of the odds of a time-fixed outcome event (eg, 30-day mortality) or other binary outcome is related to a linear equation.
- overall survival:
the duration between random assignment and death.
- Surveillance, Epidemiology, and End Results (SEER):
a national cancer registry that collects information from all incident malignancies in multiple geographic areas of the United States.
- taxanes:
a class of chemotherapy that leads to the disruption of microtubule function and thus stops cell division. Paclitaxel and docetaxel are examples of taxanes.
Footnotes
Supported in part by the National Cancer Institute Grant No. R01 CA079466 (Y.S.), the Grant Morgan Welch Inflammatory Breast Cancer Research Program and the State of Texas Rare and Aggressive Breast Cancer Research Program Grant (N.T.U.) and by Biostatistics Shared Resources at MD Anderson Grant No. CA16672 (H.Y.L and Y.S.).
Presented at the 67th Annual Cancer Symposium of the Society of Surgical Oncology, Phoenix, AZ, March 12-15, 2014.
Terms in blue are defined in the glossary, found at the end of this article and online at www.jco.org.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) and/or an author's immediate family member(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: None Research Funding: Simona F. Shaitelman, Elekta Expert Testimony: None Patents, Royalties, and Licenses: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: All authors
Financial support: Yu Shen
Collection and assembly of data: Heather Y. Lin, Yu Shen
Data analysis and interpretation: Natasha M. Rueth, Heather Y. Lin, Simona F. Shaitelman, Yu Shen, Gildy Babiera
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Anderson WF, Schairer C, Chen BE, et al. Epidemiology of inflammatory breast cancer (IBC) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hance KW, Anderson WF, Devesa SS, et al. Trends in inflammatory breast carcinoma incidence and survival: The Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–975. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cristofanilli M, Valero V, Buzdar AU, et al. Inflammatory breast cancer (IBC) and patterns of recurrence: Understanding the biology of a unique disease. Cancer. 2007;110:1436–1444. doi: 10.1002/cncr.22927. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Ueno NT, Valero V, et al. Differences in survival among women with stage III inflammatory and noninflammatory locally advanced breast cancer appear early: A large population-based study. Cancer. 2011;117:1819–1826. doi: 10.1002/cncr.25682. [DOI] [PubMed] [Google Scholar]
- 5.Masuda H, Brewer TM, Liu DD, et al. Long-term treatment efficacy in primary inflammatory breast cancer by hormonal receptor- and HER2-defined subtypes. Ann Oncol. 2014;25:384–391. doi: 10.1093/annonc/mdt525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program SEER Database: Incidence—SEER Regs Public-Use 2007. www.seer.cancer.gov.
- 7.Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer: The M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415–419. doi: 10.3816/cbc.2004.n.004. [DOI] [PubMed] [Google Scholar]
- 8.Fleming RY, Asmar L, Buzdar AU, et al. Effectiveness of mastectomy by response to induction chemotherapy for control in inflammatory breast carcinoma. Ann Surg Oncol. 1997;4:452–461. doi: 10.1007/BF02303668. [DOI] [PubMed] [Google Scholar]
- 9.Ueno NT, Buzdar AU, Singletary SE, et al. Combined-modality treatment of inflammatory breast carcinoma: Twenty years of experience at M. D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40:321–329. doi: 10.1007/s002800050664. [DOI] [PubMed] [Google Scholar]
- 10.Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: An introduction to available quality assessment tools. J Surg Oncol. 2009;99:488–490. doi: 10.1002/jso.21173. [DOI] [PubMed] [Google Scholar]
- 11.Edge S, Byrd DR, Compton CC, et al., editors. Chicago, IL: American Joint Committee on Cancer; 2010. Cancer Staging Handbook from the AJCC Cancer Staging Manual. www.cancerstaging.org. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 13.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: Consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamauchi H, Woodward WA, Valero V, et al. Inflammatory breast cancer: What we know and what we need to learn. Oncologist. 2012;17:891–899. doi: 10.1634/theoncologist.2012-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hennessy BT, Gonzalez-Angulo AM, Hortobagyi GN, et al. Disease-free and overall survival after pathologic complete disease remission of cytologically proven inflammatory breast carcinoma axillary lymph node metastases after primary systemic chemotherapy. Cancer. 2006;106:1000–1006. doi: 10.1002/cncr.21726. [DOI] [PubMed] [Google Scholar]
- 16.Hershman DL, Buono D, McBride RB, et al. Surgeon characteristics and receipt of adjuvant radiotherapy in women with breast cancer. J Natl Cancer Inst. 2008;100:199–206. doi: 10.1093/jnci/djm320. [DOI] [PubMed] [Google Scholar]
- 17.Pan IW, Smith BD, Shih YC. Factors contributing to underuse of radiation among younger women with breast cancer. J Natl Cancer Inst. 2014;106:djt340. doi: 10.1093/jnci/djt340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woodward WA, Buchholz TA. The role of locoregional therapy in inflammatory breast cancer. Semin Oncol. 2008;35:78–86. doi: 10.1053/j.seminoncol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Lerro CC, Robbins AS, Phillips JL, et al. Comparison of cases captured in the national cancer data base with those in population-based central cancer registries. Ann Surg Oncol. 2013;20:1759–1765. doi: 10.1245/s10434-013-2901-1. [DOI] [PubMed] [Google Scholar]


