Abstract
The past decade has seen an explosion in research focusing on innate immunity. Through a wide range of mechanisms including phagocytosis, intracellular killing, and activation of pro-inflammatory or antiviral cytokine production via pattern recognition receptors, the cells of the innate immune system initiate and support adaptive immunity. The effects of aging on innate immune responses remain incompletely understood, particularly in humans. Here, we review advances in the study of human immunosenescence in the diverse cells of the innate immune system, including neutrophils, monocytes, macrophages, NK and NKT cells, and dendritic cells—with a focus on consequences for the response to infection or vaccination in old age.
Introduction
Infectious diseases remain an important cause of morbidity and mortality in aged adults, who are more susceptible to severe infections, take longer to recover from infections and are frequently less responsive to vaccination. This is in part a consequence of immunosenescence, or the functional deterioration of the immune system with age. Both the adaptive and innate immune systems are influenced by immunosenescence, though the division between innate and adaptive immunity is somewhat artificial given the interplay between these two systems in the genesis of an immune response. In broad terms, innate immune responses constitute the earliest responses to pathogens, are less specific, and lack immunological memory. Innate immunity is mediated by a diverse group of cell types and mechanisms, including monocytes/macrophages, NK and NKT cells, dendritic cells (DC), neutrophils, eosinophils and basophils, and by the elaboration of proinflammatory cytokines, type I interferons (IFN) and other soluble factors. Studies on the aging of innate immunity in animal models are reviewed elsewhere in this issue; here, we will focus as far as possible on recent advances in the understanding of aging in the human innate immune system.
Monocytes and macrophages
Monocytes originate from a myeloid stem cell progenitor and differentiate to macrophages with specialised functions in a range of tissues including bone, brain, lung, liver, and skin. Recent studies indicate more complexity in monocyte lineages, and the potential for differentiation of monocyte populations into not only macrophages but also specific classes of inflammatory DC as well [1]. The majority of studies of the effects of aging on macrophage phagocytic function have been carried out in murine systems [2], and information on human macrophages is more limited, with early studies showing intact phagocytic function in aging [3] and other results indicating that the percentage of CD68-positive cells decreased in the bone marrow of adults, compared to children [4]. Critical to this question is whether Toll-like Receptor (TLR) function is altered in monocytes or macrophages in the context of aging. The TLRs are a family of invariant pattern recognition receptors specific for highly conserved portions of pathogens; 11 human TLRs have been identified to date, and known ligands include lipopeptides from bacteria, mycobacteria and fungi, LPS (TLR4), bacterial flagellin, and nucleic acids (double-stranded and single-stranded RNA and unmethylated CpG oligodeoxynucleotides). TLRs are expressed on a wide range of cells in the immune system, particularly antigen presenting cells, and TLR activation results in both proinflammatory cytokine responses via NF-κB-dependent pathways and the upregulation of type I IFN and IFN-dependent genes [5]. Thus, TLRs play a crucial role in linking innate to adaptive immune responses.
Early studies evaluating the effects of aging on LPS-mediated cytokine responses by human monocytes (some pre-dating the identification of TLR4 as a component of the LPS receptor) are in general conflicting, with some studies showing an increase in LPS-induced cytokine secretion, and others showing unchanged or decreased secretion [6–10]. These diverging results likely reflect heterogeneity in the (relatively small) numbers of participants evaluated in each study as well as differences in experimental protocols. For example, these studies relied upon different cell enrichment protocols, and variation in preparations of LPS might also have contributed to heterogeneity in results; in addition, some studies have limited enrollment using the highly restrictive SENIEUR protocol [11], while others have not. A recent study evaluated a wide range of TLR ligands in peripheral blood mononuclear cell (PBMC) samples from young and old individuals stimulated without adherence in round-bottom wells, with cytokine production in monocytes identified via flow cytometry and intracellular staining. In an analysis of 154 young and old individuals, an age-associated reduction in tumor necrosis factor (TNF)-α and IL-6 production in monocytes was observed following stimulation with agonists of the TLR1/2 heterodimer. This study also utilized mixed effect multivariable statistical analysis to account for covariates between young and old individuals that are a universal source of heterogeneity (e.g. gender, race, medication usage, co-morbid medical conditions) in studies of human aging (notably, the old cohort evaluated was a largely healthy group, with over 70% reporting no co-morbid illnesses) [12]. This functional defect in cytokine production was strongly correlated with a decrease in surface expression of TLR1, but not TLR2, on the surface of monocytes from old, compared to young individuals; in addition, an age-associated decrease in TLR4 surface expression was also observed [12]. An age-associated decrease in ssRNA-induced (TLR7) IL-6 production was also observed; TNF-α and IL-6 production following engagement of TLR2/6, TLR4, and TLR5 appeared grossly intact, though it remains possible that alterations in functions of these TLRs could be uncovered at different ligand concentrations. However, subsequent analyses of TLR-induced upregulation of costimulatory proteins on monocytes revealed a substantial age-associated defect in CD80 (B7-1) expression on monocytes that was statistically significant for all TLR ligands tested (engaging TLR1/2, TLR2/6, TLR4, TLR5, and TLR7/8). Furthermore, this defect in TLR-induced CD80 expression predicted the compromised generation of a protective antibody response to influenza immunization (which is known to have markedly decreased efficacy in old individuals) [13]. Taken together, these data provide evidence for age-associated defects in TLR-induced production of IL-6 and TNF-α, particularly in response to engagement of TLR1/2; by contrast, a generalized defect was observed for TLR-induced CD80 upregulation in monocytes from old individuals.
A recent study provided evidence for age-associated defects in TLR function in human macrophages in the context of infection with West Nile Virus (WNV), a mosquito-borne flavivirus recently introduced to North America with disproportionate morbidity (particularly from meningoencephalitis) and mortality in old individuals [14]. Primary human macrophages from old individuals were found to express lower levels of TLR3 than macrophages from young individuals at baseline; however, WNV infection in vitro resulted in the downregulation of TLR3 in macrophages from young individuals, and unchanged TLR3 gene expression but increased TLR3 protein levels in macrophages from old individuals. Such an increase in TLR3 protein expression could result in an exaggerated inflammatory response to viral infection that might provide an explanation for the worsened WNV disease seen in elderly individuals. In young individuals, the WNV-induced decrease in TLR3 was found to result from an interaction between the glycosylated WNV envelope protein and the DC-SIGN lectin on macrophages that attenuates STAT1 phosphorylation and subsequent signaling; by contrast, such decreased STAT1 signaling was deficient in macrophages from old individuals. Therefore, in response to WNV, macrophages from old individuals appear to have defective DC-SIGN-WNV signaling that results in an inability to downregulate TLR3; it is attractive to speculate that this could result in inappropriately sustained TLR3 engagement during viral infection.
Taken together, these findings provide evidence for defects in TLR function in human monocytes and macrophages from old individuals (Table 1); further studies under differing TLR stimulation conditions and in old cohorts with increased co-morbid medical conditions or disability may reveal additional age-associated phenotypes that contribute not only to adverse outcomes from infectious diseases but also to mortality in aged individuals as well [15]. Of particular interest will be the study of resident macrophages present in such tissues as lung (alveolar macrophages), liver (Kupfer cells), and brain (microglia), currently incomplete in human systems; for example, evidence is emerging for increased activation of microglia with normal aging, and in particular in association with neurodegenerative diseases [16]. In addition, whether specific infection states may influence TLR expression and function remains a possibility to be addressed in larger studies of old and young individuals [17].
Table 1.
Summary of alterations in NK cell, monocyte, and dendritic cell function associated with aging in humans.
| Effect | Refs | |
|---|---|---|
| NK cells | ↑ Numbers | [41–43] |
| ↓ Cytotoxicity | [42,46–49] | |
| Intact antibody-dependent cytotoxicity | [51,52] | |
| ↓ IL-2–dependent IFN-γ and IL-2– and IL-12–induced chemokine production (MIP-1α, RANTES, and IL-8) | [53,54] | |
| Monocytes | ↑, ↓ Or unchanged LPS-induced cytokine production | [6–10] |
| ↓ TLR1/2-induced IL-6 and TNF-α production | [12] | |
| ↓ TLR1 and TLR4 surface expression | [12] | |
| ↓ TLR-induced CD80 upregulation | [13] | |
| ↓ DC-SIGN signaling in macrophages (to West Nile virus) | [14] | |
| ↓ Phagocytosis | [3] | |
| ↓ Percentage CD68-positive macrophages in bone marrow | [4] | |
| Dendritic cells | ↑ Or unchanged number or percentage of mDCs | [94,96] |
| ↓ Or unchanged number or percentage of pDCs | [94–96] | |
| ↓ Langerhans cell density in skin | [99,100] | |
| Unchanged transendothelial migration, preserved ability of antigen-pulsed DCs to stimulate T-cell proliferation | [98,107,108] | |
| ↓ Pinocytosis or endocytosis and impaired chemokine-induced migration | [96] | |
| ↓ LPS-induced IL-12 production in mDCs | [94] | |
| ↓ IFN-α production in PBMCs | [95] | |
| ↑ TLR4- and TLR8-induced IL-6 and TNF-α production in MDDCs | [96] | |
| ↑ Self-DNA-induced production of IFN-α and IL-6 in MDDCs | [110] | |
| ↓ Akt-phosphorylation, ↑ p38 phosphorylation (MDDCs) | [96] |
NK, natural killer; IL, interleukin; IFN, interferon; MIP, macrophage inflammatory protein; RANTES, regulated upon activation, normal T-cell expressed, and secreted; LPS, lipopolysaccharide; TLR, Toll-like receptor; TNF, tumor necrosis factor; DC-SIGN, dendritic cell–specific ICAM-3 grabbing nonlectin; mDC, myeloid dendritic cell; pDC, plasmacytoid dendritic cell; PBMC, peripheral blood mononuclear cell; MDDC, monocyte derived dendritic cell.
Findings from several groups add an additional layer of complexity to the understanding of aging in cells of the monocyte/macrophage lineage: the observation of a heightened pro-inflammatory milieu in old individuals with higher levels of cytokines such as TNF-α, IL-6, and IL-1β, among others, and proinflammatory markers such as C-reactive protein and clotting factors— collectively a condition termed “inflamm-aging” [18,19]. Work from a number of groups has focused in particular on IL-6 [20]; notably, the concentration of IL-6 in human serum appears to progressively increase in concentration with age [21,22], though this has not been universally observed [23]. Increased levels of IL-6 are strongly associated with increased disability in elderly individuals [24–26], and with the geriatric syndrome of frailty [27] which has been validated as a predictor of adverse health outcomes including disability, falls, hospitalization, and mortality [28]. However, whether IL-6 plays a direct role in mediating these phenotypes remains unclear, and to date, analyses of IL-6 promoter single nucleotide polymorphisms associated with higher levels of IL-6 have not yielded clear results [29]. Such elevated proinflammatory responses might have additional clinical implications for conditions such as sepsis, which is associated with substantially elevated mortality in elderly individuals [30]
A variety of influences contribute to the apparent paradox of defects in TLR function and observations of increased proinflammatory cytokine levels in the context of aging. In some circumstances age-associated dysregulation of cytokine production could result in elevated basal cytokine levels that are refractory to further stimulation by TLR ligands or other pathogen-associated motifs. In addition, studies of serum levels of cytokines might also reflect production from a variety of tissue types; while monocytes/macrophages are a major source of IL-6, it is also produced by endothelial cells, adipocytes, muscle cells, stromal cells and other cell types which are susceptible to the aging process [31]. For example, it is notable that LPS-induced production of IL-1 and IL-8 (as measured by ELISA) was increased in old, compared to young adults in a whole blood assay but decreased in old, compared to young individuals when isolated PBMCs were evaluated [32]. In this regard, senescent fibroblasts and epithelial cells show a marked proinflammatory phenotype, secreting significant levels of IL-6 and IL-8 [33]. Finally, the behavior of cells that are the source for proinflammatory cytokines evaluated in the context of aging might also be influenced by the complex interplay of immunologic, hormonal, and neuroendocrine factors in vivo [34,35]. For example, proinflammatory cytokine production by monocytes/macrophages is modulated by both adipokines [36] and adrenal hormones [37], whose circulating levels are altered with age.
Therefore, the significant age-related increase in circulating levels of inflammatory cytokines, resulting in levels of TNF-α and IL-6 that are 2–3 fold higher in old compared to young humans, is likely to reflect the cumulative effect of modest cytokine output from cells of the monocyte lineage as well as aged stromal cells such as fibroblasts. Furthermore, the detrimental effect of this chronic inflamed state on health in old age is now being realized, with clear roles for inflamm-aging in cardiovascular disease, sarcopaenia and metabolic syndrome [38].
Natural Killer (NK) cells and NKT cells
NK cells are innate cytotoxic lymphocytes that play an important role in host defense against certain malignancies and viral infections. In response to IL-2, NK cells increase their cytolytic activity against target cells and become so-called lymphokine-activated killer cells (LAKs). In early studies using bulk populations of leukocytes, LAK activity from peripheral blood lymphocytes derived from both young and aged donors appeared to have comparable overall cytotoxic reactivity against the NK-sensitive tumor cell line K562. Similarly, non-MHC-restricted, constitutive (i.e. IL-2 independent) NK-mediated cytolytic activity also appeared preserved in the context of human aging, as assessed by both chromium release (which quantifies the lytic activity at the cell population level) and evaluation of the binding to and recognition of tumor cells and the magnitude of lethal hit delivery [39,40]. However, the absolute number of NK cells have been observed to increase with age, and is associated with an increase in the CD56dim population of NK cells with a mature phenotype) [41–45] and it now seems that this phenomenon can mask age-related defects in NK cell function. In fact, when analyzed on a per-cell basis by cloning or flow cytometry, age-associated defects in both NK cytotoxicity and LAK cell activity were observed [43,46–49]. This age-associated defect in NK cell cytotoxicity appears associated with impaired inositol triphosphate (IP3) generation [50]; notably, this alteration in inositol phospholipid metabolism was not observed for antibody-dependent cytotoxicity, which in early studies appeared unperturbed in NK cells from young and old individuals [51,52]. Thus, signal transduction pathways mediating spontaneous vs. antibody-dependent cytotoxicity in human NK cells appear to be differentially influenced by aging.
In addition to their cytotoxic functions against infected and cancerous cells, NK cells also secrete immunoregulatory factors upon activation. It has been observed that IL-2-induced IFN-γ production, and IL-2 or IL-12-induced production of chemokines such as MIP-1α, RANTES, and IL-8 are decreased in NK cells from aged individuals [53,54]. Thus, while the diminished lytic efficiency of individual NK cells that occurs during aging may be compensated by an increase in NK cell number, impaired production of cytokines and chemokines is likely to compromise NK-cell driven adaptive immune responses in the elderly (Table 1); consistent with this hypothesis, infection risk and mortality in elderly individuals appear to be strongly correlated with NK cell activity [55]. Ongoing studies indicate that alterations in zinc homeostasis may contribute to age-associated NK defects in cytotoxicity, which appear to improve with zinc supplementation [56,57].
NKT cells constitute a unique and heterogeneous T-cell population that shares some functional (cytotoxicity) and phenotypic (expression of NK-associated receptors such as CD161) characteristics with NK cells. Classical NKT cells in humans are CD1d-restricted, responsive to α-galactosyl-ceramide (α-GalCer), and are characterised by the expression of an invariant TCRα chain encoded by Vα24/JαQ gene segments, and T cell receptor (TCR) Vβ11 (such cells are often termed invariant, or iNKT cells) [58]. NKT cells can respond very rapidly to antigenic challenge by production of cytokines (especially IL-4 or IFN-γ) and therefore influence adaptive immune responses. A decreased percentage of CD3+Vα24+ cells has been reported in peripheral blood from elderly donors that is accompanied by at least a trend toward impairment in α-GalCer-induced proliferation, depending upon the study evaluated [59–61]. In addition, recent in vitro studies using cell cultures enriched for iNKT cells indicate that iNKT cells from old individuals shift from a TH1 toward a TH2 cytokine profile (as manifested by decreased ratios of IFN-γ/IL-4 and IFN-γ/IL-10) compared to iNKT cells from young individuals [60].
In addition to the iNKT cells discussed above, NKT-like cells constitute a subset of (mainly CD8+) T lymphocytes expressing NK-associated receptors such as CD16, CD56, CD57, CD161, CD94 and NKG2A [62]. It is known that such NKT-like cells constitute a significant proportion of CD3+ T cells from elderly individuals, healthy donors or centenarians [63]. Recent studies of CD8 T cells expressing CD56 indicate that such T cells are largely CD28null, and accumulate both in T cells induced to undergo senescence and in cross-sectional analyses of young vs. old individuals. Notably, CD56 cross-linking without TCR ligation on these T cells resulted in NF-κB activation and pro-inflammatory cytokine production, suggesting a potential mechanism for TCR-independent activation of senescent T cells from aging individuals [64]. In addition, CD28null T cells from old individuals also acquire expression of Killer Ig-like Receptors (KIRs) usually found on NK cells [65], and recent studies have begun to elucidate epigenetic mechanisms associated with KIR expression in aged human T cells [66]. Because KIRs are a genetically polymorphic family with both inhibitory and stimulatory members, the contribution of KIR expression on T cells to immunosenescence remains incompletely understood.
Neutrophils, Eosinophils and Basophils
Granulocytes are involved in the earliest responses to microbial and parasitic infections and their bactericidal armory includes the generation of reactive oxygen and nitrogen species and the release of a range of degradative enzymes and antimicrobial peptides. The appropriate initiation and resolution of their inflammatory responses is crucial to the clearance of infections and the prevention of non-specific tissue damage leading to chronic inflammatory disease and frailty. That the function of these cells might be compromised by aging is indicated not only by the increased morbidity and mortality due to bacterial infections in the elderly [67,68], but also by the wealth of clinical data showing that age is an independent risk factor for the development of chronic inflammatory diseases which include a pathogenic role for neutrophils, for example rheumatoid arthritis [69,70].
At present, limited data exist on age-associated changes in eosinophil and basophil function in humans. Analysis of age-related changes in peripheral blood eosinophil function in 30 young and old human subjects with asthma showed decreased degranulation (as measured by IL-5-induced degranulation of eosinophil-derived neurotoxin) in old individuals (55 to 88 years of age) [71]. A trend toward decreased production of superoxide anions was also observed in eosinophils from old individuals, but eosinophil adhesion, chemotaxis, and number in the sputum were unchanged. Basophil degranulation was initially reported to be impaired in an aged cohort [72], while a later study found higher reactivity from basophils from old subjects for both the maximum proportion of anti-IgE-induced histamine release as well as sensitivity to a standard concentration of anti-IgE [73]. With the recent demonstration of roles for basophils in CD4 TH2 cell differentiation [74] and in enhancing humoral immune memory responses [75], studies in aging humans are needed to examine these newly discovered functions.
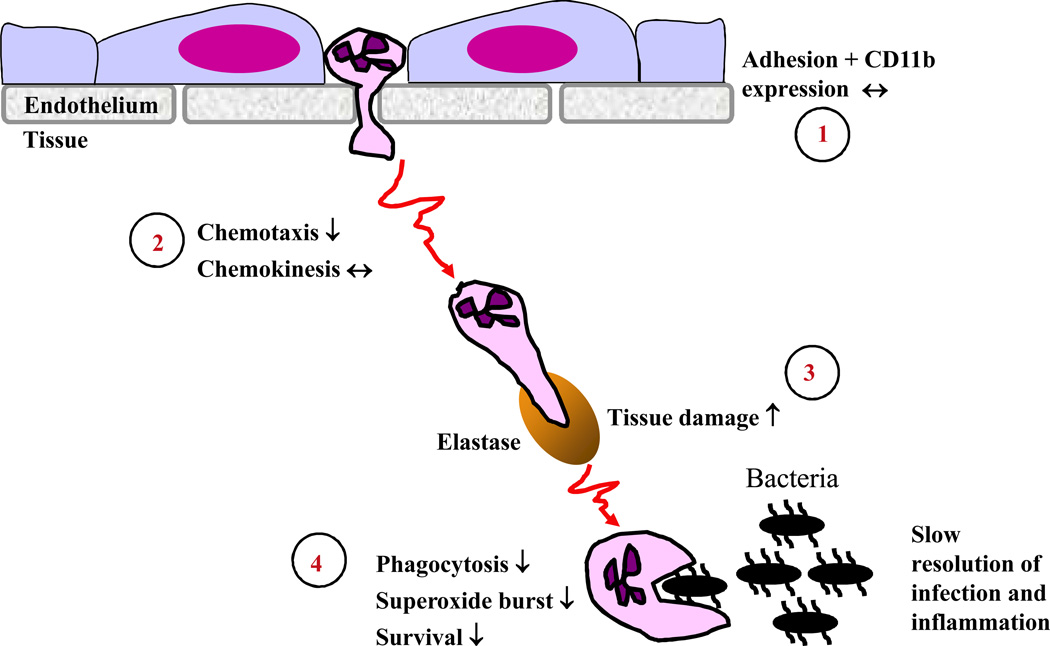
In contrast, our understanding of the effect of age upon neutrophil function is more comprehensive, reflecting the dominant role of these cells in innate bacterial immunity. This literature has been reviewed thoroughly by several authors [76–80] and will only be outlined here in order to facilitate the discussion of the potential impact of these changes on innate immunity and pathology. Research spanning two decades has shown that most aspects of neutrophil function are decreased in aged humans including chemotaxis, phagocytosis of microbes and generation of superoxide in response to stimulation by soluble host and bacterial factors (GM-CSF, LPS, fMLP) or opsonised bacteria (Figure 1)[76,79]. In addition, neutrophils are the shortest lived blood cell with a half-life of 8–12 hours and their lifespan is extended at sites of inflammation by survival signals provided by pro-inflammatory cytokines (GM-CSF, type 1 IFN) and bacterial products (fMLP, LPS). This mechanism is important in order to maximise the anti-bacterial potential of neutrophils during an infection. This ability to respond to survival signals, specifically GM-CSF [81], is also reduced with age, with implications not only for the ability of old adults to clear infections but also for the resolution of inflammation itself. Neutrophils die by apoptosis, but if the number of apoptotic neutrophils at the inflamed site exceeds the capacity of macrophages to clear the dead cells, they could progress to secondary necrosis leading to persistence of inflammation [82].
Figure 1. Proposed consequences of the age-related loss of human neutrophil function.
1) Neutrophil adhesion to vascular endothelium and initial extravasation into tissue is unaffected by aging [89]. 2) Chemotaxis appears to be compromised by aging whilst chemokinesis remains intact [112], reducing the efficiency of migration to the infected site. 3) Migration through tissue involves secretion of proteases such as neutrophil elastase from azurophilic granules. Reduced chemotactic activity would increase exposure of tissue to such proteases, increasing collateral damage to healthy tissue. 4) At the site of infection itself neutrophil uptake of microbes and subsequent killing involving generation of superoxide are both reduced with age [76,77,112], due in part to reduced responsiveness to priming agents such as GM-CSF [81]. GM-CSF also extends neutrophil lifespan at sites of infection and thus reduced responsiveness to this cytokine increases apoptosis. Each of these factors will contribute to the reduced ability of aged adults to clear bacterial infections and to resolve inflammation promptly.
One conclusion from an analysis of the existing literature is that many of the defects in neutrophil function might arise from changes to the cell membrane and/or to proximal events in receptor signaling. For example, neutrophil priming and activation in response to a range of ligands is decreased with age, including fMLP, GM-CSF, G-CSF and LPS [76], as is the response to ligation of the neutrophil receptor TREM-1 [83]. These receptors have quite distinct modes of signaling and their modulation during aging suggests a common underlying mechanism. Some studies [84] have reported increased neutrophil membrane fluidity, related to a decrease in cholesterol content, in peritoneal neutrophils from aged rats and this is associated with a 40% decrease in superoxide generation in response to the bacterial peptide fMLP. However, the superoxide response to the phorbol ester PMA, which activates downstream signaling events via direct activation of protein kinase C (PKC), was not affected, suggestive of a defect proximal to the receptor. Lipid rafts are regions of reduced fluidity within the phospholipid bilayer and are enriched for cholesterol and phospholipids with long, saturated fatty acid side chains. Lipid rafts are important for the regulation of cell signaling as they provide a means to segregate receptors and their proximal signaling components within the membrane, with receptor activity modulated by the inclusion or exclusion of signaling elements in the lipid raft associated signalosome [85]. Alterations to membrane fluidity would thus impact upon lipid raft formation and integrity with consequences for a range of membrane receptors. Although similar studies of the lipid composition of neutrophils from old humans have not been reported, there is evidence that lipid raft function might be compromised in human neutrophils with age. Indeed the negative regulator of GM-CSF receptor signaling SHP-1 is excluded from lipid rafts within 1 minute of stimulation of young neutrophils with GM-CSF, but remains associated with lipid rafts in neutrophils from old donors [86], thus maintaining inhibition of receptor signaling. At the same time, agonist receptors such as TREM-1 and TLR4 show reduced recruitment to lipid rafts in neutrophils of aging donors, compromising their signaling function [83,87]. Taken together these data might explain the reduction in downstream signaling events in old neutrophils seen following ligation of receptors such as TREM-1 and the receptor for GM-CSF including decreased activation of the JAK-STAT, ERK1/2, PLCγ-PKC and PI3 kinase-AKT pathways [78,83,88] mediating superoxide generation, chemotaxis and anti-apoptotic processes [78].
Whether the decline in the various aspects of neutrophil function has differential consequences for immunity in old age, and thus identifies potential targets for intervention, is an important but largely unanswered question. The process of extravasation from the circulation appears unaffected by aging, with adhesion to endothelial cells, expression of adhesion molecules and recruitment to inflamed skin all unaltered in neutrophils from aged donors [89,90]. However, the movement of neutrophils towards a site of inflammation is affected by two components of migration, namely chemokinesis (speed of movement) and chemotaxis (directional movement). To date only a single study has assessed the effect of age on both elements of neutrophil migration and the data indicate that chemokinesis is intact but chemotaxis is reduced with age [112]. The efficiency of migration to the infected site could thus be affected as a result of reduced chemotactic ability and both cross-sectional and longitudinal studies have shown that this is associated with increased morbidity and mortality in aged patients with infections following trauma [91,92]. There is thus no evidence that neutrophil recruitment to inflamed sites is reduced with age [89], rather that there is a failure to resolve the inflammation. We propose that this could result from several aspects of neutrophil senescence. For instance, as neutrophils migrate towards a site of infection, their movement through tissues is effected by the release of proteases such as elastase. Once in the tissue, the age related decrease in chemotactic ability [92, 112] could result in increased collateral damage to healthy tissues due to reduced directional movement and persistence towards the site of infection (Figure 1); reduced phagocytic function, bactericidal activity (superoxide generation) and inability to extend neutrophil lifespan via inflammatory survival factors, will all compromise the efficiency of removal of microbes and thus extend the time to resolve the inflammation. Interestingly, studies of neutrophil function in centenarians revealed that phagocytic ability was increased whilst superoxide generation in response to the phorbol ester PMA was reduced compared to middle aged subjects [45]. These data suggest that maintenance of neutrophil phagocytic capacity is important for longevity and can overcome the age-related decline in superoxide generating ability. Whether any of the more recently discovered mechanisms that are induced to actively resolve inflammation, such as production of Resolvins, are also reduced with age remains to be determined and will represent an important area of research for the future.
Dendritic Cells
Studies regarding the effects of aging on the frequency and properties of human DC have yielded varied conclusions. Several distinct subsets of DC have been identified including myeloid DC (mDC) and plasmacytoid DC (pDC). pDCs appear to be especially important for type I IFN production and antiviral responses, while mDCs express a broader array of TLRs and facilitate adaptive T cell responses, in part through TLR-induced IL-12 production in the induction of TH1 responses [93]. In one study, the number of mDCs was reported to increase with age, and an increased proportion of mDCs from old participants was found to have a more mature phenotype, as evidenced by increased expression of CD86 and CD83 [94]; no change in pDC numbers was observed in this study, though an age-associate decrease in pDCs was observed by other investigators [95]. Other studies reported no change in the percentage of mDCs and pDCs in young versus old individuals, although absolute numbers were not calculated [96]. No significant changes have been reported in the derivation of DCs (which generally have a phenotype resembling mDCs) from monocytes using GM-CSF and IL-4 [96–98]. The reasons for these differences remain unclear, but certainly heterogeneity in the aged cohorts evaluated might be an important consideration. Such heterogeneity may result not only from genetic factors, but also from differences in functional status and comorbid medical conditions that were largely not reported in these studies. Some information is available regarding tissue-specific DC populations; for example, decreases in human Langerhans cell (LC) densities have been observed in the epidermis of the skin [99,100]. Some evidence for age-associated morphologic alterations in LC density have been reported, such as a decrease in dendritic-branching processes; in comparisons of old vs. young individuals with chronic periodontitis, decreased densities of intraepithelial LCs were observed, with an age-associated shift to a more mature phenotype, as evidenced by CD83 and DC-LAMP immunostaining [100–102]. Advances in this area have been slowed by availability of material (i.e. requirement for biopsy) for human studies and by the technical challenges of quantitation and characterization of LCs from skin biopsy specimens. Such limitations have also restricted study of the effect of aging on follicular dendritic cells (FDC), whose immune complex trapping function in germinal centres is crucial to the production of plasma cells secreting high affinity antibodies. However, studies in mice indicate that this is an area that should be pursued in humans, as FDC from old mice have reduced levels of receptors for complement and antibody and show reduced trafficking to B cell follicles [103]. These combined defects are likely to contribute to reduced germinal centre formation and humoral immunity in aged mice and might well contribute to the compromised vaccination response in terms of antibody titre, affinity and repertoire, seen in aged humans [104–106].
The extent of immunosenescence in human dendritic cells remains an area of ongoing study. In general, antigen presenting functions in human DCs, as assessed by transendothelial DC migration and the ability of antigen-pulsed DCs to stimulate T cell proliferation, appear largely preserved in the context of aging [98,107,108]. However, it remains possible that age-associated impairment in DC may be uncovered in the setting of specific infectious agents, as evidenced by decreased IFN-γ ELISPOT in response to RSV-infected (but not influenza virus-infected) monocyte-derived DCs from old individuals [109]. Previous studies of TLR-induced cytokine production in humans have reported an age-associated defect in LPS-induced IL-12 production in mDCs (assessed via intracellular cytokine staining) [94], and decreased IFN-α protein production (likely with pDCs an important source) was reported following stimulation of PBMCs with Herpes Simplex virus [95].
In contrast, recent studies have demonstrated increased production of TNF-α and IL-6 in response to LPS and ssRNA, and increased production of IL-6 and IFN-α in response to self-DNA stimulation of monocyte-derived DC from old, compared to young participants [96,110]. However, phagocytic function and migration of such monocyte-derived DCs toward CCL19 or SCF-1 in a transwell assay were decreased in old individuals, suggesting defects in antigen presentation function. Decreased PI-3 kinase activity, as manifested by decreased AKT phosphorylation, was also reported in monocyte-derived DCs from old individuals, and was associated with increased levels of phosphorylation of the mitogen activated protein kinases p38 and ERK. PI-3 kinase signaling has been reported to be both a negative and positive regulator of TLR-mediated cytokine responses [111], and whether the observed hyper-responsiveness to TLR stimulation with concomitant impaired phagocytic and migration functions of these DCs derived from old individuals are both consequences of the multifunctional role of the PI3-K pathway in DCs remains to be determined. Age-associated alterations in human DCs are summarized in Table 1.
These intriguing findings call for additional studies of a wider range of TLRs in human DC populations. It remains possible that such laboratory generated, monocyte-derived DCs could be a model for a class of inflammatory mDCs that could arise from monocyte precursors in vivo. If so, these monocyte-derived cells could represent a potential source for the elevated proinflammatory cytokine levels associated with aspects of the aged innate immune system. However, studies of primary DCs will also be informative to provide additional information on mDC and particularly pDC populations that have not been derived from treatment ex vivo with GM-CSF and IL-4, since the possibility exists that growth factor stimulation could mask age-associated alterations in DC function.
Concluding remarks
Several aspects of the innate immune response are affected by normal human aging, resulting in a reduced ability to provide the immediate response to bacterial and viral pathogens and also to integrate with and influence the adaptive immune response. The mechanisms underlying these changes are now beginning to be characterised and include alterations in the activity of a variety of innate immune cell receptors and their downstream signaling pathways as well as changes to the numbers of certain cells within the circulation. That this loss of innate cell function has consequences for immunity and longevity is also clear, with reduced neutrophil and NK cell activity predictive of increased mortality in old adults [55,92] and dysregulation of TLR function affecting vaccine responsiveness and hyper-responsiveness to viral infection in aged individuals [13,14]. Indeed, such biomarkers could be used to define an Innate Immune Risk Phenotype, to complement and expand the Immune Risk Phenotype already established for elements of the adaptive immune response [106].
Acknowledgements
Work in the authors’ laboratories was supported in part by an Arthritis Research Campaign (ARC) programme grant (to J.M.L.), by the National Institutes of Health (N01-AI-50031, AI 070343), by the Center of Excellence in Aging at Yale University, funded by the John A. Hartford Foundation, and by the Claude D. Pepper Older Americans Independence Center at Yale University (P30 AG021342). E.F. is an Investigator of the Howard Hughes Medical Institute.
References
- 1.Auffray C, et al. Blood Monocytes: Development, Heterogeneity, and Relationship with Dendritic Cells. Annu Rev Immunol. 2009 doi: 10.1146/annurev.immunol.021908.132557. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian C, et al. MacrophAging: a cellular and molecular review. Immunobiology. 2005;210:121–126. doi: 10.1016/j.imbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Fietta A, et al. Influence of aging on some specific and nonspecific mechanisms of the host defense system in 146 healthy subjects. Gerontology. 1994;40:237–245. doi: 10.1159/000213591. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa T, et al. Age-related changes of human bone marrow: a histometric estimation of proliferative cells, apoptotic cells, T cells, B cells and macrophages. Mech Ageing Dev. 2000;117:57–68. doi: 10.1016/s0047-6374(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 5.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Clark JA, Peterson TC. Cytokine production and aging: overproduction of IL-8 in elderly males in response to lipopolysaccharide. Mech Ageing Dev. 1994;77:127–139. doi: 10.1016/0047-6374(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 7.Delpedro AD, et al. Signal transduction in LPS-activated aged and young monocytes. J Interferon Cytokine Res. 1998;18:429–437. doi: 10.1089/jir.1998.18.429. [DOI] [PubMed] [Google Scholar]
- 8.Mariani E, et al. RANTES and MIP-1alpha production by T lymphocytes, monocytes and NK cells from nonagenarian subjects. Exp Gerontol. 2002;37:219–226. doi: 10.1016/s0531-5565(01)00187-5. [DOI] [PubMed] [Google Scholar]
- 9.Gon Y, et al. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 10.van Duin D, Shaw AC. Toll-like receptors in older adults. J Am Geriatr Soc. 2007;55:1438–1444. doi: 10.1111/j.1532-5415.2007.01300.x. [DOI] [PubMed] [Google Scholar]
- 11.Ligthart GJ, et al. Admission criteria for immunogerontological studies in man: the SENIEUR protocol. Mech Ageing Dev. 1984;28:47–55. doi: 10.1016/0047-6374(84)90152-0. [DOI] [PubMed] [Google Scholar]
- 12.van Duin D, et al. Age-associated defect in human TLR-1/2 function. J Immunol. 2007;178:970–975. doi: 10.4049/jimmunol.178.2.970. [DOI] [PubMed] [Google Scholar]
- 13.van Duin D, et al. Prevaccine determination of the expression of costimulatory b7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 14.Kong KF, et al. Dysregulation of TLR3 impairs the innate immune response to West Nile virus in the elderly. J Virol. 2008;82:7613–7623. doi: 10.1128/JVI.00618-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van den Biggelaar AHJ, et al. Impaired innate immunity predicts frailty in old age. The Leiden 85-plus study. Exp Gerontol. 2004;39:1407. doi: 10.1016/j.exger.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Dilger RN, Johnson RW. Aging, microglial cell priming, and the discordant central inflammatory response to signals from the peripheral immune system. J Leukoc Biol. 2008;84:932–939. doi: 10.1189/jlb.0208108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Comin F, et al. Ageing and Toll-like receptor expression by innate immune cells in chronic human schistosomiasis. Clin Exp Immunol. 2007;149:274–284. doi: 10.1111/j.1365-2249.2007.03403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fagiolo U, et al. Increased cytokine production in mononuclear cells of healthy elderly people. Eur J Immunol. 1993;23:2375–2378. doi: 10.1002/eji.1830230950. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi C, et al. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 20.Ershler WB, Keller ET. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 21.Forsey RJ, et al. Plasma cytokine profiles in elderly humans. Mech Ageing Dev. 2003;124:487–493. doi: 10.1016/s0047-6374(03)00025-3. [DOI] [PubMed] [Google Scholar]
- 22.Giuliani N, et al. Serum interleukin-6, soluble interleukin-6 receptor and soluble gp130 exhibit different patterns of age- and menopause-related changes. Exp Gerontol. 2001;36:547–557. doi: 10.1016/s0531-5565(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 23.Beharka AA, et al. Interleukin-6 production does not increase with age. J Gerontol A Biol Sci Med Sci. 2001;56:B81–B88. doi: 10.1093/gerona/56.2.b81. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri M, et al. Chronic inflammation and the effect of IGF-I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab. 2003;284:E481–E487. doi: 10.1152/ajpendo.00319.2002. [DOI] [PubMed] [Google Scholar]
- 25.Cohen HJ, et al. Coagulation and activation of inflammatory pathways in the development of functional decline and mortality in the elderly. Am J Med. 2003;114:180–187. doi: 10.1016/s0002-9343(02)01484-5. [DOI] [PubMed] [Google Scholar]
- 26.Ferrucci L, et al. Serum IL-6 level and the development of disability in older persons. J Am Geriatr Soc. 1999;47:639–646. doi: 10.1111/j.1532-5415.1999.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 27.Leng SX, et al. Inflammation and frailty in older women. J Am Geriatr Soc. 2007;55:864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 28.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Di Bona D, et al. Effect of interleukin-6 polymorphisms on human longevity: a systematic review and meta-analysis. Ageing Res Rev. 2009;8:36–42. doi: 10.1016/j.arr.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Opal SM, et al. The immunopathogenesis of sepsis in elderly patients. Clin Infect Dis. 2005;41(Suppl 7):S504–S512. doi: 10.1086/432007. [DOI] [PubMed] [Google Scholar]
- 31.Maggio M, et al. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol A Biol Sci Med Sci. 2006;61:575–584. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gabriel P, et al. Overproduction of monokines by leukocytes after stimulation with lipopolysaccharide in the elderly. Exp Gerontol. 2002;37:235–247. doi: 10.1016/s0531-5565(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 33.Coppe JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stout RD, Suttles J. Immunosenescence and macrophage functional plasticity: dysregulation of macrophage function by age-associated microenvironmental changes. Immunol Rev. 2005;205:60–71. doi: 10.1111/j.0105-2896.2005.00260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neuroimmune Biology. Elsevier; 2004. [Google Scholar]
- 36.Lago R, et al. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Padgett DA, Loria RM. Endocrine regulation of murine macrophage function: effects of dehydroepiandrosterone, androstenediol, and androstenetriol. J Neuroimmunol. 1998;84:61–68. doi: 10.1016/s0165-5728(97)00244-0. [DOI] [PubMed] [Google Scholar]
- 38.Vasto S, et al. Inflammatory networks in ageing, age-related diseases and longevity. Mech Ageing Dev. 2007;128:83–91. doi: 10.1016/j.mad.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 39.Yamashita N, et al. Effects of aging on the in vitro response of human lymphocytes to interleukin-2. Jpn J Med. 1984;23:211–215. doi: 10.2169/internalmedicine1962.23.211. [DOI] [PubMed] [Google Scholar]
- 40.Krishnaraj R. Immunosenescence of human NK cells: effects on tumor target recognition, lethal hit and interferon sensitivity. Immunol Lett. 1992;34:79–84. doi: 10.1016/0165-2478(92)90030-r. [DOI] [PubMed] [Google Scholar]
- 41.Thompson JS, et al. The immune status of healthy centenarians. J Am Geriatr Soc. 1984;32:274–281. doi: 10.1111/j.1532-5415.1984.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 42.Borrego F, et al. NK phenotypic markers and IL2 response in NK cells from elderly people. Exp Gerontol. 1999;34:253–265. doi: 10.1016/s0531-5565(98)00076-x. [DOI] [PubMed] [Google Scholar]
- 43.Vitale M, et al. The impairment of natural killer function in the healthy aged is due to a postbinding deficient mechanism. Cell Immunol. 1992;145:1–10. doi: 10.1016/0008-8749(92)90307-b. [DOI] [PubMed] [Google Scholar]
- 44.Sansoni P, et al. Lymphocyte subsets and natural killer cell activity in healthy old people and centenarians. Blood. 1993;82:2767–2773. [PubMed] [Google Scholar]
- 45.Miyaji C, et al. Functional alteration of granulocytes, NK cells, and natural killer T cells in centenarians. Hum Immunol. 2000;61:908–916. doi: 10.1016/s0198-8859(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 46.Facchini A, et al. Increased number of circulating Leu 11+ (CD 16) large granular lymphocytes and decreased NK activity during human ageing. Clin Exp Immunol. 1987;68:340–347. [PMC free article] [PubMed] [Google Scholar]
- 47.Mariani E, et al. Age-associated changes in CD8+ and CD16+ cell reactivity: clonal analysis. Clin Exp Immunol. 1990;81:479–484. doi: 10.1111/j.1365-2249.1990.tb05359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ogata K, et al. Natural killer cells in the late decades of human life. Clin Immunol Immunopathol. 1997;84:269–275. doi: 10.1006/clin.1997.4401. [DOI] [PubMed] [Google Scholar]
- 49.Kutza J, Murasko DM. Age-associated decline in IL-2 and IL-12 induction of LAK cell activity of human PBMC samples. Mech Ageing Dev. 1996;90:209–222. doi: 10.1016/0047-6374(96)01772-1. [DOI] [PubMed] [Google Scholar]
- 50.Mariani E, et al. Age-dependent decreases of NK cell phosphoinositide turnover during spontaneous but not Fc-mediated cytolytic activity. Int Immunol. 1998;10:981–989. doi: 10.1093/intimm/10.7.981. [DOI] [PubMed] [Google Scholar]
- 51.Edwards DL, Avis FP. Antibody-dependent cellular cytotoxicity effector cell capability among normal individuals. J Immunol. 1979;123:1887–1893. [PubMed] [Google Scholar]
- 52.Fernandes G, Gupta S. Natural killing and antibody-dependent cytotoxicity by lymphocyte subpopulations in young and aging humans. J Clin Immunol. 1981;1:141–148. doi: 10.1007/BF00922755. [DOI] [PubMed] [Google Scholar]
- 53.Krishnaraj R, Bhooma T. Cytokine sensitivity of human NK cells during immunosenescence. 2. IL2-induced interferon gamma secretion. Immunol Lett. 1996;50:59–63. doi: 10.1016/0165-2478(96)02519-9. [DOI] [PubMed] [Google Scholar]
- 54.Mariani E, et al. Chemokine production by natural killer cells from nonagenarians. Eur J Immunol. 2002;32:1524–1529. doi: 10.1002/1521-4141(200206)32:6<1524::AID-IMMU1524>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 55.Ogata K, et al. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin Exp Immunol. 2001;124:392–397. doi: 10.1046/j.1365-2249.2001.01571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mariani E, et al. Effect of zinc supplementation on plasma IL-6 and MCP-1 production and NK cell function in healthy elderly: interactive influence of +647 MT1a and −174 IL-6 polymorphic alleles. Exp Gerontol. 2008;43:462–471. doi: 10.1016/j.exger.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 57.Mocchegiani E, et al. Metallothioneins/PARP-1/IL-6 interplay on natural killer cell activity in elderly: parallelism with nonagenarians and old infected humans. Effect of zinc supply. Mech Ageing Dev. 2003;124:459–468. doi: 10.1016/s0047-6374(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 58.Bendelac A, et al. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 59.DelaRosa O, et al. Valpha24+ NKT cells are decreased in elderly humans. Exp Gerontol. 2002;37:213–217. doi: 10.1016/s0531-5565(01)00186-3. [DOI] [PubMed] [Google Scholar]
- 60.Jing Y, et al. Aging is associated with a rapid decline in frequency, alterations in subset composition, and enhanced Th2 response in CD1d-restricted NKT cells from human peripheral blood. Exp Gerontol. 2007;42:719–732. doi: 10.1016/j.exger.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 61.Peralbo E, et al. Decreased frequency and proliferative response of invariant Valpha24Vbeta11 natural killer T (iNKT) cells in healthy elderly. Biogerontology. 2006;7:483–492. doi: 10.1007/s10522-006-9063-5. [DOI] [PubMed] [Google Scholar]
- 62.Tarazona R, et al. Increased expression of NK cell markers on T lymphocytes in aging and chronic activation of the immune system reflects the accumulation of effector/senescent T cells. Mech Ageing Dev. 2000;121:77–88. doi: 10.1016/s0047-6374(00)00199-8. [DOI] [PubMed] [Google Scholar]
- 63.Peralbo E, et al. Invariant NKT and NKT-like lymphocytes: two different T cell subsets that are differentially affected by ageing. Exp Gerontol. 2007;42:703–708. doi: 10.1016/j.exger.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 64.Lemster BH, et al. Induction of CD56 and TCR-independent activation of T cells with aging. J Immunol. 2008;180:1979–1990. doi: 10.4049/jimmunol.180.3.1979. [DOI] [PubMed] [Google Scholar]
- 65.Abedin S, et al. Diversity of NKR expression in aging T cells and in T cells of the aged: the new frontier into the exploration of protective immunity in the elderly. Exp Gerontol. 2005;40:537–548. doi: 10.1016/j.exger.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 66.Li G, et al. Epigenetic mechanisms of age-dependent KIR2DL4 expression in T cells. J Leukoc Biol. 2008;84:824–834. doi: 10.1189/jlb.0807583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Laupland KB, et al. Population-based study of the epidemiology of and the risk factors for invasive Staphylococcus aureus infections. J Infect Dis. 2003;187:1452–1459. doi: 10.1086/374621. [DOI] [PubMed] [Google Scholar]
- 68.Schneider EL. Infectious diseases in the elderly. Ann Intern Med. 1983;98:395–400. doi: 10.7326/0003-4819-98-3-395. [DOI] [PubMed] [Google Scholar]
- 69.Edwards SW, Hallett MB. Seeing the wood for the trees: the forgotten role of neutrophils in rheumatoid arthritis. Immunol Today. 1997;18:320–324. doi: 10.1016/s0167-5699(97)01087-6. [DOI] [PubMed] [Google Scholar]
- 70.Raza K, et al. Early rheumatoid arthritis is characterized by a distinct and transient synovial fluid cytokine profile of T cell and stromal cell origin. Arthritis Res Ther. 2005;7:R784–R795. doi: 10.1186/ar1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mathur SK, et al. Age-related changes in eosinophil function in human subjects. Chest. 2008;133:412–419. doi: 10.1378/chest.07-2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwarzenbach HR, et al. Skin reactivity, basophil degranulation and IgE levels in ageing. Clin Allergy. 1982;12:465–473. doi: 10.1111/j.1365-2222.1982.tb01645.x. [DOI] [PubMed] [Google Scholar]
- 73.Marone G, et al. Human basophil releasability. I. Age-related changes in basophil releasability. J Allergy Clin Immunol. 1986;77:377–383. doi: 10.1016/s0091-6749(86)80121-x. [DOI] [PubMed] [Google Scholar]
- 74.Sokol CL, et al. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Denzel A, et al. Basophils enhance immunological memory responses. Nat Immunol. 2008;9:733–742. doi: 10.1038/ni.1621. [DOI] [PubMed] [Google Scholar]
- 76.Fortin CF, et al. Aging and neutrophils: there is still much to do. Rejuvenation Res. 2008;11:873–882. doi: 10.1089/rej.2008.0750. [DOI] [PubMed] [Google Scholar]
- 77.Lord JM, et al. Neutrophil ageing and immunesenescence. Mech Ageing Dev. 2001;122:1521–1535. doi: 10.1016/s0047-6374(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 78.Tortorella C, et al. Age-related impairment of GM-CSF-induced signalling in neutrophils: role of SHP-1 and SOCS proteins. Ageing Res Rev. 2007;6:81–93. doi: 10.1016/j.arr.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 79.Schroder AK, Rink L. Neutrophil immunity of the elderly. Mech Ageing Dev. 2003;124:419–425. doi: 10.1016/s0047-6374(03)00017-4. [DOI] [PubMed] [Google Scholar]
- 80.Lord JM, et al. Synergistic effects of ageing and stress on neutrophil function. In: Fulop T, et al., editors. Handbook of Immunosenescence. Springer; 2009. pp. 475–498. [Google Scholar]
- 81.Fortin CF, et al. GM-CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology. 2007;8:173–187. doi: 10.1007/s10522-006-9067-1. [DOI] [PubMed] [Google Scholar]
- 82.Vandivier RW, et al. Burying the dead: the impact of failed apoptotic cell removal (efferocytosis) on chronic inflammatory lung disease. Chest. 2006;129:1673–1682. doi: 10.1378/chest.129.6.1673. [DOI] [PubMed] [Google Scholar]
- 83.Fortin CF, et al. Effects of aging on triggering receptor expressed on myeloid cells (TREM)-1-induced PMN functions. FEBS Lett. 2007;581:1173–1178. doi: 10.1016/j.febslet.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 84.Alvarez E, et al. Age-related changes in membrane lipid composition, fluidity and respiratory burst in rat peritoneal neutrophils. Clin Exp Immunol. 2001;124:95–102. doi: 10.1046/j.1365-2249.2001.01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 86.Fortin CF, et al. Impairment of SHP-1 down-regulation in the lipid rafts of human neutrophils under GM-CSF stimulation contributes to their age-related, altered functions. J Leukoc Biol. 2006;79:1061–1072. doi: 10.1189/jlb.0805481. [DOI] [PubMed] [Google Scholar]
- 87.Fulop T, et al. Signal transduction and functional changes in neutrophils with aging. Aging Cell. 2004;3:217–226. doi: 10.1111/j.1474-9728.2004.00110.x. [DOI] [PubMed] [Google Scholar]
- 88.Tortorella C, et al. Role of phosphoinositide 3-kinase and extracellular signal-regulated kinase pathways in granulocyte macrophage-colony-stimulating factor failure to delay fas-induced neutrophil apoptosis in elderly humans. J Gerontol A Biol Sci Med Sci. 2006;61:1111–1118. doi: 10.1093/gerona/61.11.1111. [DOI] [PubMed] [Google Scholar]
- 89.Biasi D, et al. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20:673–681. doi: 10.1007/BF01488803. [DOI] [PubMed] [Google Scholar]
- 90.Butcher SK, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J Leukoc Biol. 2001;70:881–886. [PubMed] [Google Scholar]
- 91.Egger G, et al. Blood polymorphonuclear leukocyte migration as a predictive marker for infections in severe trauma: comparison with various inflammation parameters. Intensive Care Med. 2004;30:331–334. doi: 10.1007/s00134-003-2111-6. [DOI] [PubMed] [Google Scholar]
- 92.Niwa Y, et al. Neutrophil chemotaxis, phagocytosis and parameters of reactive oxygen species in human aging: cross-sectional and longitudinal studies. Life Sci. 1989;44:1655–1664. doi: 10.1016/0024-3205(89)90482-7. [DOI] [PubMed] [Google Scholar]
- 93.Kadowaki N. The divergence and interplay between pDC and mDC in humans. Front Biosci. 2009;14:808–817. doi: 10.2741/3279. [DOI] [PubMed] [Google Scholar]
- 94.Della Bella S, et al. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin Immunol. 2007;122:220–228. doi: 10.1016/j.clim.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 95.Shodell M, Siegal FP. Circulating, interferon-producing plasmacytoid dendritic cells decline during human ageing. Scand J Immunol. 2002;56:518–521. doi: 10.1046/j.1365-3083.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- 96.Agrawal A, et al. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3-kinase-signaling pathway. J Immunol. 2007;178:6912–6922. doi: 10.4049/jimmunol.178.11.6912. [DOI] [PubMed] [Google Scholar]
- 97.Lung TL, et al. Unimpaired dendritic cells can be derived from monocytes in old age and can mobilize residual function in senescent T cells. Vaccine. 2000;18:1606–1612. doi: 10.1016/s0264-410x(99)00494-6. [DOI] [PubMed] [Google Scholar]
- 98.Steger MM, et al. Morphologically and functionally intact dendritic cells can be derived from the peripheral blood of aged individuals. Clin Exp Immunol. 1996;105:544–550. doi: 10.1046/j.1365-2249.1996.d01-790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bhushan M, et al. Tumour necrosis factor-alpha-induced migration of human Langerhans cells: the influence of ageing. Br J Dermatol. 2002;146:32–40. doi: 10.1046/j.1365-2133.2002.04549.x. [DOI] [PubMed] [Google Scholar]
- 100.Bodineau A, et al. Do Langerhans cells behave similarly in elderly and younger patients with chronic periodontitis? Arch Oral Biol. 2007;52:189–194. doi: 10.1016/j.archoralbio.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 101.Bodineau A, et al. Increase of gingival matured dendritic cells number in elderly patients with chronic periodontitis. Arch Oral Biol. 2009;54:12–16. doi: 10.1016/j.archoralbio.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 102.Zavala WD, Cavicchia JC. Deterioration of the Langerhans cell network of the human gingival epithelium with aging. Arch Oral Biol. 2006;51:1150–1155. doi: 10.1016/j.archoralbio.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 103.Szakal AK, et al. Molecular interactions of FDCs with B cells in aging. Semin Immunol. 2002;14:267–274. doi: 10.1016/s1044-5323(02)00059-3. [DOI] [PubMed] [Google Scholar]
- 104.Dunn-Walters DK, et al. Effects of age on antibody affinity maturation. Biochem Soc Trans. 2003;31:447–448. doi: 10.1042/bst0310447. [DOI] [PubMed] [Google Scholar]
- 105.Frasca D, et al. Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180:5283–5290. doi: 10.4049/jimmunol.180.8.5283. [DOI] [PubMed] [Google Scholar]
- 106.Wikby A, et al. Changes in CD8 and CD4 lymphocyte subsets, T cell proliferation responses and non-survival in the very old: the Swedish longitudinal OCTO-immune study. Mech Ageing Dev. 1998;102:187–198. doi: 10.1016/s0047-6374(97)00151-6. [DOI] [PubMed] [Google Scholar]
- 107.Castle SC, et al. Antigen presenting cell function is enhanced in healthy elderly. Mech Ageing Dev. 1999;107:137–145. doi: 10.1016/s0047-6374(98)00141-9. [DOI] [PubMed] [Google Scholar]
- 108.Pietschmann P, et al. Surface markers and transendothelial migration of dendritic cells from elderly subjects. Exp Gerontol. 2000;35:213–224. doi: 10.1016/s0531-5565(99)00089-3. [DOI] [PubMed] [Google Scholar]
- 109.Looney RJ, et al. Effect of aging on cytokine production in response to respiratory syncytial virus infection. J Infect Dis. 2002;185:682–685. doi: 10.1086/339008. [DOI] [PubMed] [Google Scholar]
- 110.Agrawal A, et al. Increased reactivity of dendritic cells from aged subjects to self-antigen, the human DNA. J Immunol. 2009;182:1138–1145. doi: 10.4049/jimmunol.182.2.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–363. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 112.Wenisch C, et al. Effect of age on human neutrophil function. J Leukoc Biol. 2000;67:40–45. doi: 10.1002/jlb.67.1.40. [DOI] [PubMed] [Google Scholar]