Abstract
Diabetes is characterized by ‘glucotoxic’ loss of pancreatic β-cell function and insulin content, but underlying mechanisms remain unclear. A mouse model of insulin-secretory deficiency induced by β-cell inexcitability (KATP gain-of-function) demonstrates development of diabetes and reiterates the features of human neonatal diabetes. In the diabetic state, β-cells lose their mature identity and dedifferentiate to neurogenin3-positive and insulin-negative cells. Lineage-tracing experiments show that dedifferentiated cells can subsequently re-differentiate to mature neurogenin3-negative, insulin-positive, β-cells after lowering of blood glucose by insulin therapy. We demonstrate here that β-cell dedifferentiation, rather than apoptosis, is the main mechanism of loss of insulin-positive cells, and re-differentiation accounts for restoration of insulin content and antidiabetic-drug responsivity in these animals. These results may help explain gradual decrease in β-cell mass in long-standing diabetes, and recovery of β-cell function and drug responsivity in type-2 diabetic patients following insulin therapy, and suggest an approach to rescuing ‘exhausted’ β-cells in diabetes.
Keywords: glucotoxicity; diabetes; insulin; mice; apoptosis; KATP; death; differentiation; dedifferentiation; mechanism; glucose; therapy; treatment; channel, neurogenin, progenitor, stem
INTRODUCTION
Type 2 diabetes is characterized by β-cell dysfunction, the mechanism of which is controversial (Ahlqvist et al., 2011; Butler et al., 2003; Hur et al., 2010; Nolan and Prentki, 2008; Prentki and Nolan, 2006; Puri and Hebrok, 2012; Robertson et al., 2004; Talchai et al., 2012b; Wajchenberg, 2007). When faced with persistent hyperglycemia, the normal pancreatic β-cell first responds with compensatory increase in insulin secretion and β-cell mass (Ahren, 2005; Bernal-Mizrachi et al., 2000; Heit et al., 2006; Jhala et al., 2003). However, chronic hyperglycemia gradually also leads to a paradoxical ‘glucotoxic’ loss of β-cell mass and insulin content that has typically been attributed to enhanced β-cell apoptosis (Butler et al., 2003; Lupi and Del Prato, 2008; Poitout and Robertson, 2008; Porat et al., 2011; Prentki and Nolan, 2006). Progressive deterioration in β-cell function and marked reduction of β-cell mass are classic findings in type-2 diabetic human islets, regardless of the therapy (Cnop et al., 2005; Del Prato et al., 2007; Sakuraba et al., 2002; UKPDS-group, 1998a, b), and both reduced glucose-stimulated insulin secretion (GSIS), and increased rate of β-cell apoptosis and decreased β-cell survival have been detected in islets from human diabetic pancreases (Butler et al., 2003; Tanaka et al., 2002; Weinberg et al., 2007). In general, however, the impairment of β-cell function in diabetic islets may be much greater than could be explained by the observed increase in the rate of apoptosis (Butler et al., 2003) and β-cell death may not be the main contributor to the marked loss of β-cell mass.
An alternative mechanism for diabetic loss of insulin content has recently received attention (Talchai et al., 2012b). The transcription factor FoxO1 is a major determinant of cell fate in enteroendocrine cells. In islets that lack FoxO1 in β-cells, Talchai et al demonstrated β-cell dedifferentiation to endocrine progenitor -like cells during stress-induced hyperglycemia. In addition to processes impinging on β-cell survival and hence on islet mass, β-cell dedifferentiation can also be observed in vitro (Weinberg et al., 2007). Dedifferentiation in common forms of β-cell failure has also been inferred from partial pancreatectomy studies (Jonas et al., 1999). Together, these studies raise the possibility that dedifferentiation and conversion into other endocrine cell types may be an under-recognized mechanism of β-cell failure in multiple forms of diabetes, and, moreover, that this process might conceivably be reversible.
Insulin secretory failure due to inexcitability is a major cause of monogenic neonatal diabetes (Flanagan et al., 2009; Gloyn et al., 2004) and a prominent contributor to human type 2 diabetes (Nielsen et al., 2003; Riedel et al., 2005; Villareal et al., 2009). Our studies reveal that a major mechanism of β-cell loss in diabetes resulting from secretory failure due to inexcitability (Remedi et al., 2009) is also dedifferentiation. Even more striking, additional experiments show that intensive insulin therapy, by reversing the hyperglycemia, leads to re-differentiation to mature β-cells. These results provide a potential explanation for gradual decrease in β-cell mass in long standing and poorly controlled human diabetes, as well as for recovery of β-cell function and sulfonylurea responsivity as can be observed in type-2 diabetic patients after intensive insulin therapy (Torella et al., 1991; Wajchenberg, 2007).
RESULTS
KATP-GOF mice develop diabetes with dramatic loss of insulin content
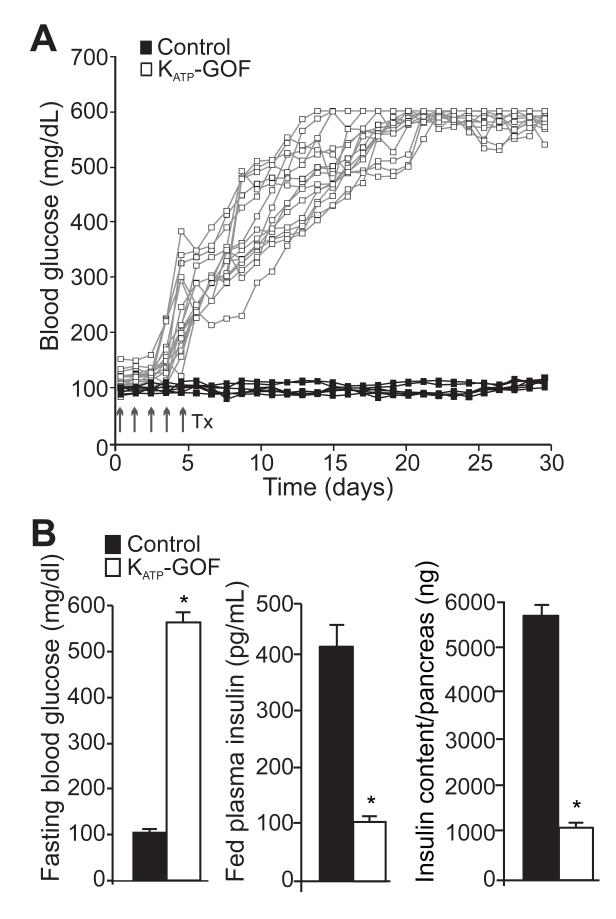
Following tamoxifen injection, two month-old Pdx1PBCreERTM Kir6.2[K185Q,ΔN30] (KATP-GOF) mice express the ATP-insensitive Kir6.2[K185Q,ΔN30] transgene, as well as an eGFP reporter. The animals develop severe diabetes within two weeks after tamoxifen induction (Figure 1A), as a result of the loss of glucose-dependent insulin secretion (Remedi et al., 2011; Remedi et al., 2009). Fed and fasting blood glucose rise to >500mg/dl in all KATP-GOF mice within ~20 days after tamoxifen induction of transgene expression, and remain high thereafter (Figure 1A,B). Insulin secretion is extremely low and insulin content is markedly decreased in KATP-GOF animals with respect to control mice (Figure 1B). These findings thus reiterate key features of human neonatal diabetes resulting from severe KATP-GOF mutations (Flanagan et al., 2009; Gloyn et al., 2004; Matthews et al., 1998; Nolan et al., 2011; Pearson et al., 2006; Shimomura et al., 2007), as well as the consequences of KATP-GOF that result from the Type 2 diabetes-associated polymorphism (E23K) in the Kir6.2 subunit of the KATP channel (Nielsen et al., 2003; Villareal et al., 2009).
Figure 1. KATP-GOF mice develop profound diabetes.
(A) Fed blood glucose (individual traces) from control (black squares) and KATP-GOF (white squares) mice after tamoxifen induction of transgene expression. (B) Fasting blood glucose, plasma insulin and total insulin content per pancreas in control (black) and KATP-GOF (white) mice 30 days after tamoxifen induction (n=10-12 mice per group, mean + SEM, * p<0.05, with respect to control).
Insulin content and insulin-positive β-cells are restored in KATP-GOF diabetic mice after chronic insulin therapy
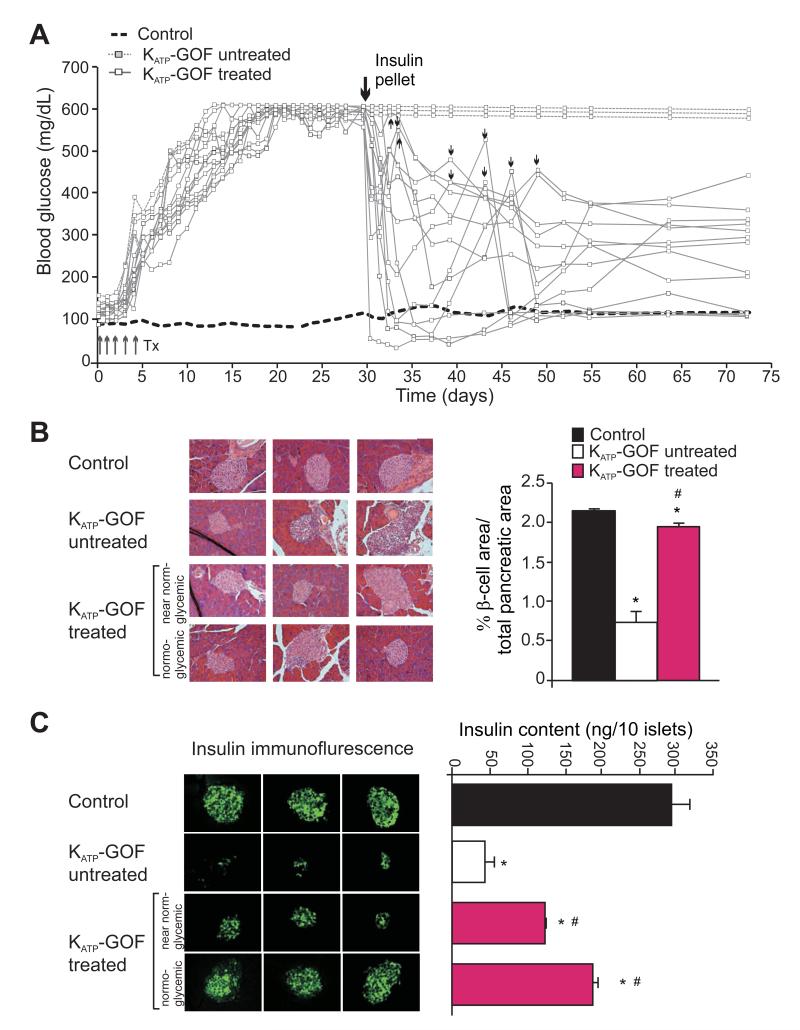
Following the induction of diabetes in KATP-GOF animals, reduction of plasma insulin level was accompanied by gradual loss of islet insulin content and insulin-positive β-cells (Figure 2B,C) (Remedi et al., 2009). We previously showed that this secondary loss could be avoided by maintenance of normoglycemia during and following disease induction, either by syngeneic islet transplantation, or by sulfonylurea treatment, if initiated at disease onset (Remedi et al., 2011; Remedi et al., 2009). However, once the disease has developed, sulfonylurea treatment is relatively ineffective, readily explained as a consequence of the marked loss of islet insulin content that rapidly develops (Remedi et al., 2009). Similar processes may also underlie gradual loss of drug responsivity in long-term or poorly controlled human diabetes, and raises the possibility that loss of insulin content might actually be restorable if glucose levels are normalized, and that drug-responsivity may then also be restored.
Figure 2. Insulin therapy restores endogenous insulin content in diabetic KATP-GOF mice.
(A) Fed blood glucose in control (average, black dashed line) and in KATP-GOF untreated (white, dashed line) and insulin-treated (white, solid line) mice after tamoxifen induction of transgene expression. Big arrow indicates first insulin pellet implantation, and small arrows a second insulin pellet implanted in individual mice as necessary (blood glucose >400mg/dl). (B) (left panels) High magnification sections of pancreases stained with hematoxylin-eosin from control and KATP-GOF untreated and insulin-treated mice. (right panels) Pancreatic β-cell area (from panel C below) in control (black) KATP-GOF untreated (white) and insulin-treated (pink) mice. (C) (left panels) Insulin immunofluorescence and (right panel) insulin content per islet in control (black) and KATP-GOF mice, untreated 30 days after tamoxifen injection (white), and insulin-treated (70 days after tamoxifen, 40 days after insulin pellet implantation, pink) (n=3-6 mice per group, mean + SEM). Insulin-treated KATP-GOF mice were divided in two groups at the time of sacrifice following the criteria of blood glucose levels: near normoglycemic and normoglycemic. Significant differences *p<0.05 with respect to control and #p<0.05 with respect to untreated KATP-GOF mice.
To examine this possibility directly, severely diabetic KATP-GOF mice (blood glucose >500mg/dl for ~3 weeks), were divided into two groups: (1) untreated; (2) chronically treated with insulin by implantation of slow-release insulin pellets, in an attempt to restore normoglycemia. Untreated KATP-GOF mice maintained persistently elevated blood glucose (Figure 2A). Insulin-treated KATP-GOF mice all demonstrated a marked reduction in blood glucose (Figure 2A), although the degree of normalization was variable; some mice essentially achieved normoglycemia with only one pellet implanted, whereas others required a second pellet to achieve a sustained lowering of blood glucose levels (Figure 2A).
In untreated diabetic KATP-GOF pancreases, immunohistochemistry reveals disrupted architecture with a marked loss of insulin content and insulin-positive β-cells in islets from KATP-GOF animals that were sacrificed at 30 days following tamoxifen induction (Figure 2B,C). However, insulin content and insulin-positive β-cells were fully restored in islets from the second set of diabetic KATP-GOF animals that were treated with insulin by implantation of slow-release pellets at 30 days following disease induction, and then sacrificed 40 days later (i.e. after 40 days of insulin treatment, Figure 2C). Notably, islets from insulin-treated mice, in which normalization of glycemic control was incomplete, showed a less pronounced recovery of insulin content than islets from animals in which glucose was almost fully normalized (Figure 2C). Moreover, total insulin content per pancreas, which was markedly reduced from 574±21 ng in control to 102±6 ng in untreated KATP-GOF mice (Figure 1B), was almost completely restored (to 495+14 ng) in insulin-treated KATP-GOF mice.
β-cell death may not explain the marked loss of insulin content and insulin-positive β-cells in KATP-GOF islets
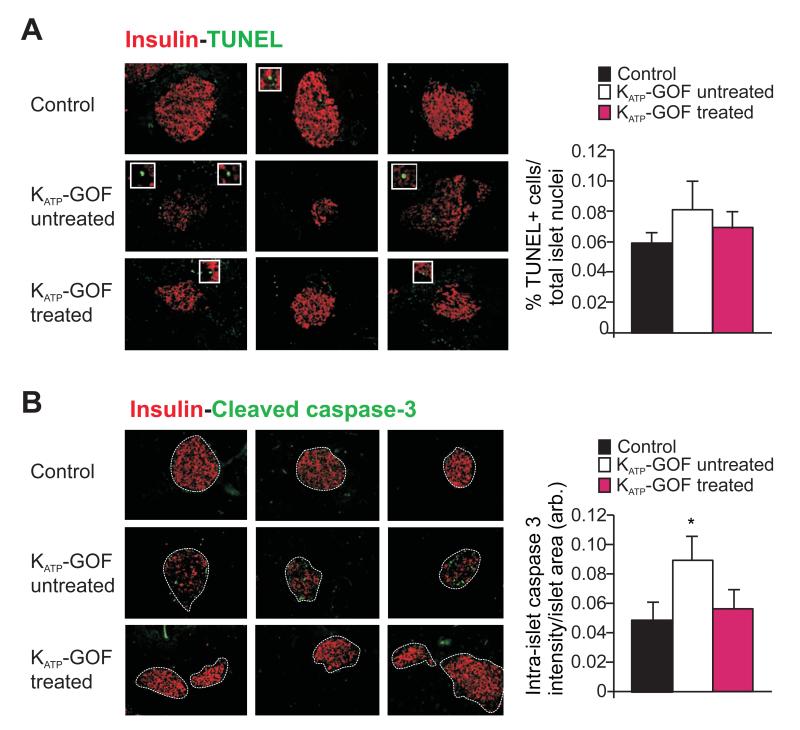
Increased apoptosis may contribute to β-cell loss in type 2 diabetes (Butler et al., 2003; Poitout and Robertson, 2008; Prentki and Nolan, 2006), and the marked reduction of insulin content in diabetic KATP-GOF islets (Figure 2B,C) raises the possibility that increased apoptosis might be responsible. Apoptosis was therefore assessed in pancreatic sections from control and KATP-GOF (untreated and insulin-treated) mice by TUNEL and cleaved caspase-3 assays. TUNEL staining showed only a slight (non-significant) increase in the number of apoptotic cells within diabetic islets from untreated KATP-GOF mice, compared with islets from control and insulin-treated mice (Figure 3A). Since TUNEL detects DNA-stand breaks in terminal cell stages, it is possible that the frequency of TUNEL-positive cells does not accurately reflect the level of apoptosis in these samples; therefore we additionally assessed the levels of cleaved caspase-3. Cleaved caspase-3 positivity showed a small but significant increase in untreated KATP-GOF islets, but was not different between control and insulin-treated islets (Figure 3B).
Figure 3. Small increase in apoptosis in KATP-GOF diabetic mice.
(A,B) (left panels) Representative pancreatic sections (left) and (right panel) quantification of apoptosis from control (black) and KATP-GOF untreated (white) and insulin-treated (pink) mice immunostained for insulin (red) and apoptosis (green) using TUNEL (A) and cleaved caspase-3 (B). Data represent n=4 mice per group, 5 pancreatic sections per mouse. (* p<0.05 with respect to control).
Hyperglycemia drives dedifferentiation of KATP-GOF pancreatic β-cells to an insulin-negative, neurogenin3 positive phenotype
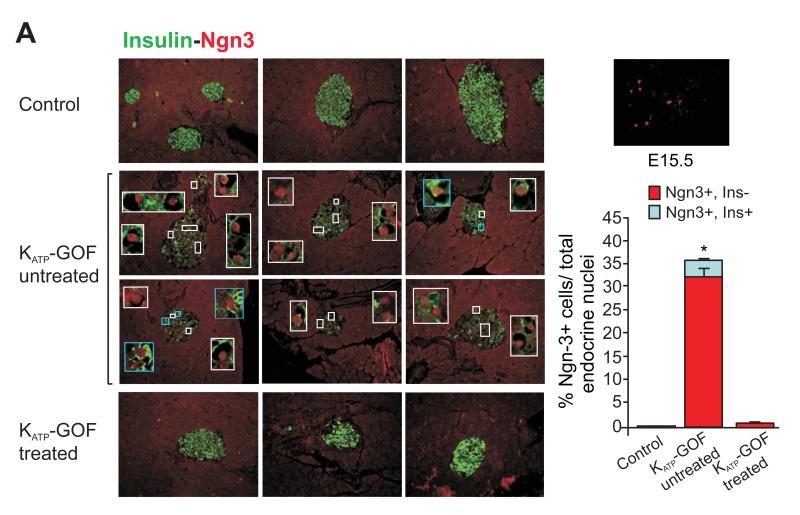
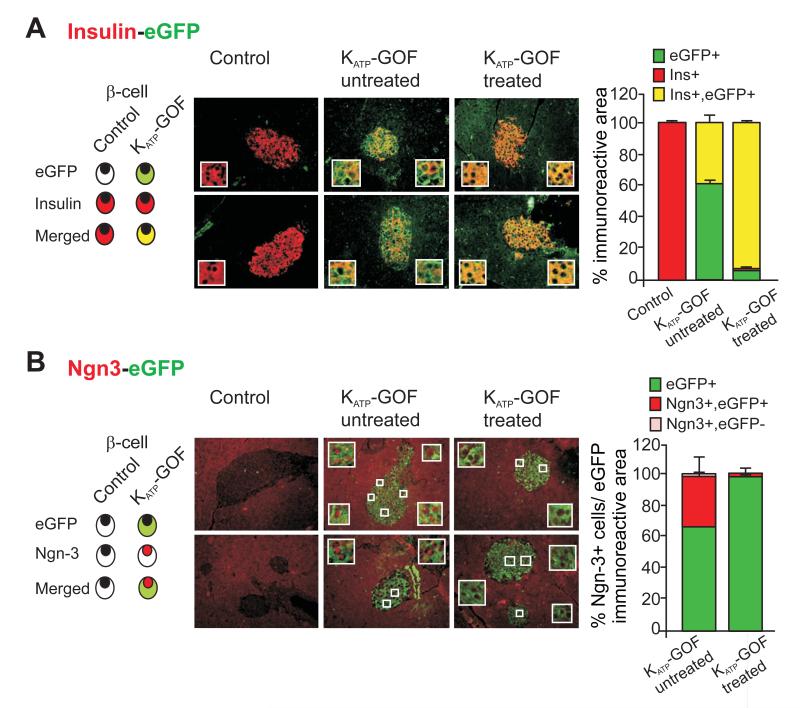
Given the only slight increase in apoptosis in even severely diabetic KATP-GOF islets, the recent findings of Talchai et al. (Talchai et al., 2012b) raise the possibility that dedifferentiation might be an alternative mechanism underlying the dramatic loss of insulin-positive β-cells in these mice. Pancreatic sections from untreated and insulin-treated KATP-GOF mice were immunostained for neurogenin3 (Ngn3), a marker of islet progenitor cells (Gu et al., 2002; Xu et al., 2008). There was essentially no Ngn3 positivity in control islets, but we observed a high number of dedifferentiated, Ngn3 positive, cells in diabetic KATP-GOF mouse islets using both Santa Cruz and BCBC Ngn3 antibodies (Figure 4 and S3A). Importantly, almost all Ngn3-positive cells were insulin-negative (Figure 4). These results indicate that in the diabetic condition, a significant number of mature insulin-containing β-cells are replaced by insulin-negative and Ngn3-positive cells, which might actually have arisen by dedifferentiation from mature β-cells. In order to lineage trace the loss of insulin-positive cells as well as the origin of Ngn3-positive cells, pancreatic sections were double immunostained for insulin or Ngn3 and eGFP, which is co-expressed with the KATP-GOF mutant transgene following tamoxifen-induction (Remedi et al., 2009), and therefore indicates cells that are, or were, mature insulin-producing β-cells. Double immunostaining for insulin and eGFP shows that almost all cells in the islet core are eGFP-positive in untreated diabetic KATP-GOF islets, but only ~40% express insulin (Figure 5A). Moreover, in these severely diabetic KATP-GOF islets, essentially all Ngn3 positive cells also express eGFP (Figure 5B), indicating that the Ngn3 positive cells were also originally mature β-cells and providing compelling evidence for dedifferentiation as the primary mechanism for the loss of β-cell phenotype. A question arises as to what happens to the insulin, since these islets are electrically inexcitable and lack glucose-dependent insulin secretion. Clearly the cells are depleted of insulin, but this is presumably either through intracellular degradation or depletion through basal secretion, once the cells turn off insulin production (i.e. as they dedifferentiate). To examine potential plasticity and pluripotency of β-cell dedifferentiation, we also looked for expression of other stem cell markers in diabetic KATP-GOF islets. Quantitative real time PCR shows an increase in mRNA expression levels of islet progenitor markers Ngn3, Nanog and L-Myc (Figure S2D), and a corresponding decrease in the levels of β-cell specific markers Pdx1, Nkx6.1 and MafA (Figure S2C) in diabetic islets, consistent with β-cell dedifferentiation to endocrine progenitor cells in the diabetic state. Immunohistologically, we detected Oct4, Nanog, and Nestin expression in control E15.5 fetal tissue, although we were unable to detect these markers in adult KATP-GOF diabetic islets (Figure S2B), and thus we cannot confirm that the elevated message levels are reflected in elevated protein levels.
Figure 4. β-cell dedifferentiation in severely diabetic KATP-GOF mice and dramatic β-cell re-differentiation in islets from KATP-GOF insulin-treated mice.
(left panels) Representative pancreatic sections from control and KATP-GOF untreated and insulin-treated mice double immunostained for insulin (green) and Ngn3 (Santa Cruz antibody, red). White bordered insets show Ngn3 positive and insulin-negative cells within islets. Blue bordered insets show occasional Ngn3 positive and insulin-positive cells. (top right) Ngn3 positivity in E15.5 fetal tissue. (bottom right) Percentage of Ngn3 positive cells, either insulin negative (red) or insulin positive (light blue from each condition (mean + s.e.m). Data represent n=5-8 mice per group, 5 pancreatic sections per mouse. *Significant differences p<0.05 with respect to control and KATP-GOF insulin-treated mice.
Figure 5. Dedifferentiated and re-differentiated islet cells are originated from former adult β-cells.
(A,B) (left panels) Representative images of double immunostaining for eGFP (indicating transgene expression in pancreatic β-cells) and insulin (A) or Ngn3 (B) on pancreatic sections from control and both untreated and insulin-treated KATP-GOF mice. Insets show representative cells. (right panels) Percentage immunopositive area of the islet insulin and eGFP (A), or for Ngn3 and eGFP (mean ± s.e.m) (B). Data represent n=5 mice per group, 5 pancreatic sections per mouse.
Re-differentiation to insulin-producing β-cells following chronic lowering of blood glucose
As shown above, Ngn3 positive cells were not observed in islets from control islets, nor in insulin-treated KATP-GOF islets from animals after reduction of blood glucose levels (Figure 5B). Instead, following lowering of blood glucose in treated animals, eGFP and insulin are again expressed throughout the core (Figure 5). This suggests that substantial reduction of systemic hyperglycemia, in this case by insulin therapy, actually permits re-differentiation of the Ngn3 positive cells to mature, insulin-containing β-cells. Tamoxifen can remain in the body for an extended period, and may continue to label significant numbers of cells for a few weeks after treatment (Reinert et al., 2012), but the finding that almost all cells in the islet core stain positive for the eGFP reporter, 70 days after initial tamoxifen induction (Figure 5) strongly argues that these were the same cells that were originally induced to express eGFP as mature β-cells. Importantly, mRNA expression levels of the β-cell markers Pdx1, Nkx6.1 and MafA are restored to normal levels in insulin-treated islets (Figure S2C), with a concomitant decrease in the progenitor markers Ngn3, Oct4, Nanog and L-Myc (Figure S2D), consistent with dedifferentiated cells re-differentiating to mature β-cells.
To investigate the alternative possibility that an abnormally high β-cell proliferation might underlie restoration of the insulin-expressing cells, we performed immunostaining for the cell cycle marker Ki67. The fraction of Ki67 positive β-cells per islet was not different between control, diabetic or treated conditions (Figure S1), providing evidence of similarly low rates of proliferation in each, and suggesting that the re-appearance of mature β-cells is not likely to be the result of proliferation of residual, pre-existing, β-cells.
Endogenous Insulin secretion in response to sulfonylureas, but not to glucose, is restored in re-differentiated islets from insulin-treated KATP-GOF mice
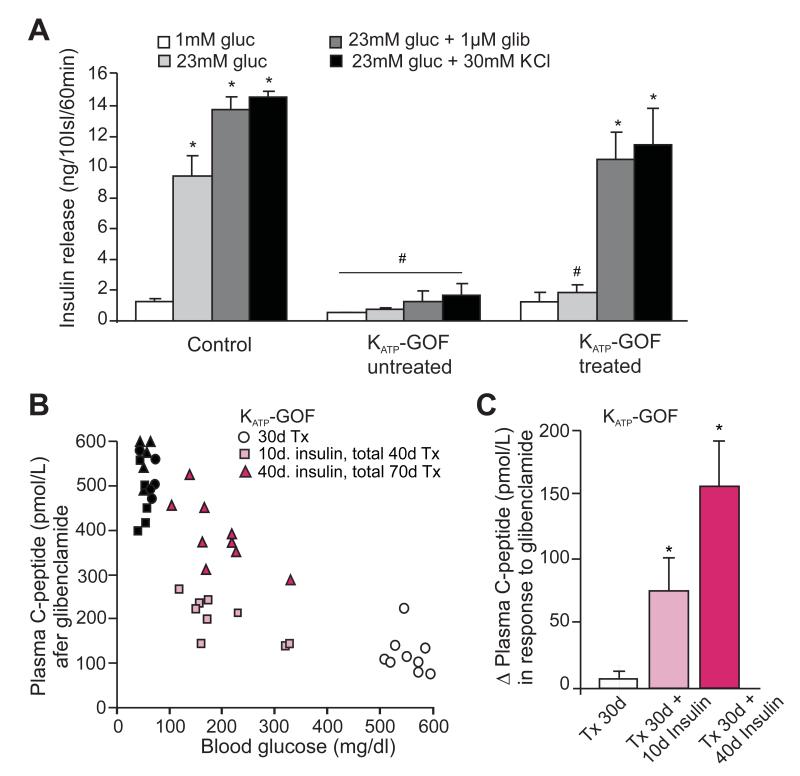
The above results imply that the restoration of islet insulin content, following normalization of glycemia, results from re-differentiation of pre-existing KATP-GOF β-cells that had dedifferentiated to insulin-negative, Ngn3-positive, cells. If this is correct, then these cells, in addition to still expressing eGFP, should also still be expressing the KATP-GOF transgene, and hence should remain electrically inexcitable, and non-responsive to glucose, but responsive to sulfonylureas. Insulin secretion in low (1 mM) glucose or in response to the sulfonylurea glibenclamide, was not different between control and insulin-treated KATP-GOF islets (Figure 6A) but, in excellent agreement with the above prediction, robust glucose-dependent secretion was present in control islets, but not in insulin-treated and control islets (Figure 6A). Together with the immunohistochemical findings, these results provide strong evidence that dedifferentiated and then re-differentiated cells were originally KATP-GOF mutant β-cells.
Figure 6. Glibenclamide-dependent C-peptide release is restored only in KATP-GOF insulin-treated mice.
(A) Insulin secretion from islets isolated from control and KATP-GOF untreated and insulin-treated mice (70 days after tamoxifen, ~40 days on insulin therapy). Islets were incubated in low (1mM) and high (23mM) glucose, and in the presence of the sulfonylurea glibenclamide (1μM) or the depolarizing agent KCl (30mM). Significant differences #p<0.05 with respect to control under the same condition and *p<0.05 with respect to 1mM glucose within the same group. (B) Plasma C-peptide (individual values) 30 min. after glibenclamide stimulation in control (30 days after Tx: black circles; 40 days after Tx: black squares, and 70 days after Tx: black triangles), KATP-GOF untreated 30 days after tamoxifen injection (white, 30 days after Tx, 30d) and KATP-GOF treated with insulin for 10 days (10d insulin, total 40 days after Tx, light pink) and 40 days (40d insulin, total 70 days after Tx, dark pink), mice. (C) Delta plasma C-peptide in response to glibenclamide in KATP-GOF untreated (white) and KATP-GOF insulin-treated (10 and 40 days, light and dark pink, respectively), mice (n=5-9 mice per group, experiments made in triplicates, mean + SEM). Significant differences *p<0.05 with respect to KATP-GOF untreated mice.
Sulfonylurea-dependent insulin secretion is reinstated in KATP-GOF mice after insulin therapy
Restoration of sulfonylurea-sensitive insulin secretion in insulin-treated KATP-GOF islets would also suggest that in vivo sulfonylurea-sensitive secretion should also be restored. Artefactually high insulin levels are measured in the blood of insulin-treated animals (3251 ± 487 ng/ml) which obviates assessment of the levels of endogenous insulin itself. We therefore measured C-peptide levels in mice following injection with the sulfonylurea, glibenclamide. Control mice all showed high (400-600 pmol/l) plasma C-peptide levels (Figure 6B). Thirty days after tamoxifen injection, untreated diabetic KATP-GOF showed very low levels of C-peptide (30d Tx, Figure 6B), consistent with the marked increase in the number of dedifferentiated, Ngn3 positive, cells (Figure 4 and S3A) and decrease in insulin content within their islets (Figure 2B,C). However, C-peptide levels were considerably higher in the same KATP-GOF mice after a subsequent 10 days with insulin treatment (10d insulin, total 40 days Tx, Figure 6B, light pink squares) and even higher after 40 days of insulin treatment (40d insulin, total 70 days Tx, Figure 6B, dark pink squares). In a sub-set of KATP-GOF animals, C-peptide levels were measured both before and 30 minutes after glibenclamide injection (Figure 6C). In untreated diabetic KATP-GOF animals, there was no significant response to the drug, but there was increasingly marked glibenclamide response with time after treatment. These results nicely correlate with the increase in insulin content and number of insulin-positive β-cells that was observed in insulin-treated islets. Importantly, and as predicted, both KATP-GOF untreated or insulin-treated (10d or 40d) mice demonstrate low C-peptide levels in fed conditions, and only mice that have been treated with insulin show restoration of C-peptide release in response to sulfonylurea stimulation (Figure 6C, C-peptide release after glibenclamide over basal). Together, these results demonstrate that restoration of β-cell insulin-positivity and islet insulin content in diabetic mice by insulin therapy indeed leads to reestablishment of antidiabetic drug-responsivity.
Dedifferentiated β-cells do not transdifferentiate to glucagon-producing α-cells
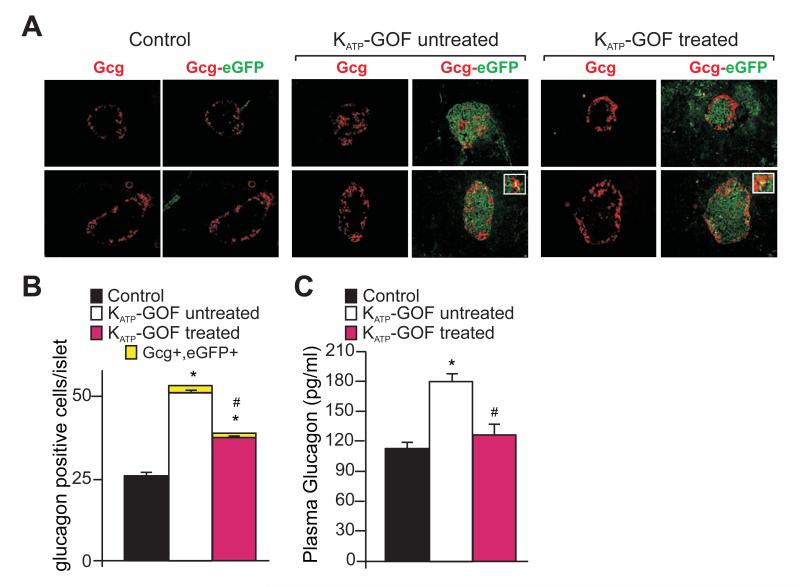
As reported previously (Remedi et al., 2009), severely diabetic KATP-GOF mice demonstrate a significant increase in glucagon immunoreactive area, as well as elevated plasma glucagon levels, but all of these are substantially reversed after insulin therapy (Figure 7). By lineage tracing analysis we can examine the possibility of trans-differentiation of eGFP-positive β-cells to glucagon-producing α-cells via Ngn3 positive progenitors. Pancreatic sections from control and KATP-GOF mice were double stained for glucagon (Gcg) and eGFP (which will indicate KATP-GOF former β-cells) (Figure 7A). Ngn3The number of glucagon positive cells increases in the diabetic state, but only a very small number of cells co-stain for both Gcg and eGFP in untreated or treated islets (Figure 7A,B), indicating that the increase in the number of α-cells in these islets is not substantially attributable to conversion from β-cells.
Figure 7. Increased α-cell number and plasma glucagon in KATP-GOF mice.
(A) Representative images of double immunostaining for eGFP (indicating transgene expression in pancreatic β-cells) and glucagon on pancreatic sections from control and both untreated and insulin-treated KATP-GOF mice. White bordered insets show co-staining for both glucagon and eGFP. Quantification of glucagon positive cells (B) and plasma glucagon (C) from control (black) and KATP-GOF untreated (white) and insulin-treated (pink) mice (mean + SEM). Data represent n=5 mice per group, 5 pancreatic sections per mouse. Significant differences *p<0.05 with respect to control and #p<0.05 with respect to untreated KATP-GOF mice.
Rip-KATP-GOF mice also develop severe diabetes and show similar dedifferentiation of β-cells, and re-differentiation after insulin therapy
Pdx1 is also expressed in ductal and even acinar cells, raising the formal possibility that newly formed β-cells might derive from non-β-cells that express Pdx1 during tamoxifen treatment. We therefore performed similar experiments to those above on animals in which the KATP-GOF transgene (Kir6.2 [K185Q,ΔN30]) was expressed under the control of the rat insulin promoter in Rip-Cre expressing mice (Rip-KATP-GOF). Rip-KATP-GOF mice show hyperglycemia immediately after birth, with development of severe diabetes over time (Remedi et al., 2009), similar to Pdx KATP-GOF mice. Severely diabetic, 75 day-old Rip-KATP-GOF mice were implanted with slow release insulin pellets and blood glucose was followed over a period of 40 days (Figure S3A). Islet insulin content, which was markedly reduced compared to age-matched littermates, was also dramatically restored following insulin treatment (Figure S3B,C). There was again a striking increase in Ngn3 positive/insulin negative cells in untreated Rip-KATP-GOF mice, that disappeared following treatment (Figure S3D,E). Again, Ngn3 positive cells and insulin positive cells both co-stained with eGFP positive cells, again providing compelling evidence that dedifferentiated as well as re-differentiated cells originated from former β-cells (Figure S3E). Glucagon immunostaining also demonstrated a significant increase in α-cell population in diabetic Rip-KATP-GOF mice (Figure S3F).
DISCUSSION
This study shows that (1) loss of insulin content during the progression of diabetes that results from β-cell secretory failure, occurs primarily through glucotoxic β-cell dedifferentiation, rather than apoptosis; (2) restoration of normoglycemia results in re-differentiation to insulin-positive β-cells.
Controlling blood glucose is enough to reverse glucotoxicity in diabetes
It is generally well-established that hyperglycemia negatively impacts β-cell secretory capacity, but the underlying mechanisms and treatability are not completely understood. A progressive deterioration in β-cell function is a common finding in patients with both Type 2 diabetes (Matthews et al., 1998; Nolan et al., 2011; Rhodes, 2005; Sakuraba et al., 2002; UKPDS-group, 1998a, b) and KATP-dependent monogenic diabetes (Pearson et al., 2006). We previously demonstrated that chronic hyperglycemia gradually leads to a dramatic loss of insulin-positive cells and insulin content in our KATP-GOF mouse model of neonatal diabetes (Remedi et al., 2009), correlating with the findings obtained in other rodent models of diabetes (Girard et al., 2009; Jonas et al., 2009; Laybutt et al., 2003; Porat et al., 2011; Talchai et al., 2012b). In this paper we demonstrate that chronic insulin treatment not only normalizes blood glucose in diabetic KATP-GOF mice, but also dramatically restores insulin content and insulin-positive β-cells. The secondary consequences of diabetes and their reversal were strikingly reflected in vivo by loss, and reversal, of insulin and C-peptide release in response to injected glibenclamide, and in isolated islets by restored glibenclamide- and KCl-dependent insulin secretion. These results suggest that relieving β-cells from systemic hyperglycemia may actually restore insulin content and thereby improve β-cell drug responsiveness.
There has been much interest in the notion that β-cells become ‘exhausted’ due to the excess demands of secretion induced by hyperglycemia in the diabetic state, and that β-cell ‘rest’ via exogenous insulin treatment permits restoration of β-cell function in type-2 diabetic patients (Alvarsson et al., 2008; Greenwood et al., 1976; Qvigstad et al., 2004; Torella et al., 1991; Weng et al., 2008). The present results show that insulin therapy, by correcting the systemic diabetes, leads to restoration of endogenous islet insulin content and antidiabetic drug responsivity, in islets that are intrinsically inexcitable, and are therefore chronically low in [Ca2+]i, and do not secrete insulin in response to glucose (Benninger et al., 2011; Remedi et al., 2009). Thus it must be somewhere upstream of excitability and secretion that is being affected. As we showed previously (Benninger et al., 2011), glucose-dependent metabolism, as assessed by NAD(P)H autofluorescence, is actually elevated in diabetic KATP-GOF islets, potentially a consequence of chronic in vivo hyperglycemia. Thus we suggest that it is ‘rest’ from hyperstimulation of metabolism, rather than ‘rest’ from hyperexcitability and secretion that permits β-cell recovery of insulin-positive cells and insulin content (Nichols and Remedi, 2012). The underlying mechanism of this improvement might also be key to the beneficial effects of insulin therapy and tight glycemic control in type-2 diabetic patients (Alvarsson et al., 2003; Ilkova et al., 1997; UKPDS-group, 1998a, b; Wajchenberg, 2007; Weng et al., 2008).
Apoptosis versus dedifferentiation as a response to hyperglycemia
In this paper we demonstrate that hyperglycemia-induced glucotoxicity, with marked loss of islet insulin content and insulin-positive β-cells, is accompanied by a small increase of apoptosis (Figure 3), similar to the levels of apoptosis detected in human diabetic islets (Butler et al., 2007; Rahier et al., 2008). However, the number of cells exhibiting positive TUNEL staining or cleaved caspase-3 is markedly less than the number of insulin-negative cells (Figure 3), suggesting that β-cell dedifferentiation, rather than apoptosis, may be the major mechanism underlying the loss of insulin-positivity. Ngn3 expression is essentially undetectable in islets from control mice, reportedly present at very low levels in adult mouse pancreases (Wang et al., 2009), but it is clearly elevated in insulin-negative cells of untreated KATP-GOF islets (Figure 4, S2A); and at least at the messenger level, Ngn3 expression as well as of other progenitor markers, Nanog and L-Myc is elevated in diabetic islets form KATP-GOF diabetic mice (Figure S2D). These results are very comparable to the finding of marked increase in the number of dedifferentiated cells within stressed FoxO1-deficient and insulin-resistant GIRKO and db/db models of type-2 diabetes (Talchai et al., 2012b). As Talchai et al. suggest, such dedifferentiation, rather than a truly degenerative state, may provide an advantage to the islet and, as we show below, a potential route to the ultimate reversibility of islet demise. The underlying mechanism for the dedifferentiation is still not clear, but might be linked to downregulation of FoxO1 following hyperglycemia-induced oxidative stress (Benninger et al., 2011; Kitamura et al., 2005; Talchai et al., 2012a). The phenomenon may provide an explanation for slow decline of β-cell mass in human diabetes (Rahier et al., 2008), although no changes in mRNA expression levels of Ngn3 or other stem cells markers, were detected in a recent study (Guo et al., 2013) of human type-2 diabetic islets (with the exception of Oct4 which increased significantly). The dramatic decrease in mRNA levels of the β-cell transcription factors Pdx1, MafA and Nkx6.1 in our diabetic KATP-GOF mice (Figure S2C) correlates well with the marked reduction in these transcription factors in both human and mouse type-2 diabetic islets (Guo et al., 2013; Talchai et al., 2012b), reflecting loss of β-cell identity in glucotoxic conditions in vivo.
Re-differentiation as a response to normalization of glycemia
We demonstrate here a dramatic recovery of insulin-positive β-cells and islet insulin content, and hence sulfonylurea responsivity, after insulin therapy in KATP-GOF mice (Figures 2,5,6). The level of this recovery is proportional to the level of rescue of glycemia, pointing to hyperglycemia as the controlling feature. The recovery of insulin content (Figure 2C) is paralleled by loss of Ngn3 staining (Figures 4,5), with maintenance of eGFP staining in the recovered islet cells (Figure 5), indicating that recovery involves re-differentiation of the same former β-cells within the islet, and with no evidence for trans-differentiation of dedifferentiated β-cells to α-cells (Figure 7). This conclusion is supported by the finding that, while multiple markers of cell dedifferentiation are up-regulated, and specific β-cell markers are reduced, in diabetic islets, all marker expression levels are restored to normal after intensive insulin therapy (Figure 4, S2). Essentially the same pattern of dedifferentiation and re-differentiation after insulin therapy is demonstrated in diabetes driven by Rip-Cre induced expression of the same KATP GOF transgene (Figure S3), providing further evidence that resurgent β-cells are derived from the previously dedifferentiated β-cells, and excluding the possibility that these cells might be derived from ductal or acinar Pdx1-positive cells in the Pdx-Cre driven model. More generally, these findings provide potential explanation for reversibility of β-cell dysfunction and restoration of drug responsivity that is seen in human type 2 diabetics following β-cell ‘rest’ with intensive insulin therapy (Alvarsson et al., 2008; Ilkova et al., 1997; Torella et al., 1991; UKPDS-group, 1998b; Wajchenberg, 2007; Weng et al., 2008). They also raise the exciting possibility that the gradual decrease in antidiabetic drug responsivity that is frequently observed in diabetic patients may similarly be reversed by intensive normalization of blood glucose levels.
CONCLUSIONS
Our findings reveal a mechanism underlying loss of islet insulin content in response to hyperglycemia in a mouse model of KATP-dependent diabetes. These results might explain decrease in β-cell mass in multiple forms of long-standing or poorly-controlled diabetes. They suggest that targeting glucotoxic β-cell dysfunction and strictly normalizing blood glucose and systemic diabetes could induce cell re-differentiation to mature β-cells and hence restoration of drug responsivity, providing a novel approach to rescuing ‘exhausted’ β-cells in diabetes.
EXPERIMENTAL PROCEDURES
Chronic insulin therapy in a mouse model of Neonatal diabetes
All experiments were performed in compliance with the institutional guidelines and approved by the Washington University Animal Studies Committee. Rat-insulin promoter (Rip) and tamoxifen inducible Pdx1PBCreERTM β-cell specific KATP gain-of-function (Kir6.2 [K185Q,ΔN30]) mutant mice were previously generated. In the case of inducible mice, transgene expression was induced by 5 consecutive injections of tamoxifen as previously described (Remedi et al., 2009). Littermate controls were used in all experiments. KATP-GOF transgenic mice with severe diabetes (blood glucose >500mg/dl) for several weeks were anesthetized and implanted with time released insulin pellets (release 0.1U/day/implant for 60 days, Linbit™, Linshin Canada Inc.) and blood glucose was followed over-time.
Blood glucose, plasma insulin, glucagon and C-peptide levels
Tail blood was assayed for glucose content using Glucometer Elite-Xl (Bayer Corp, Elkhart-IN.). The limit of detection was 600mg/dl, and glucose at/above this level is recorded as 600mg/dl, but considered to be a lower limit of the true value. Plasma insulin was measured using Singulex Erenna method (Washington University Immunoassay Core) and glucagon by using radioimmunoassay (RIA, Millipore). C-peptide was measured using ELISA (Alpco Diagnostics, Washington University Immunoassay Core).
Pancreatic islet isolation
Mice were anesthetized with Isofluorane (0.2ml) and killed by cervical dislocation, the bile duct was cannulated and perfused with Hank’s solution (Sigma) containing collagenase (Collagenase Type XI, Sigma). Pancreases were removed and digested at 37°C, hand shaken and washed in cold Hank’s solution. Islets were isolated by hand under a dissecting microscope and maintained overnight in CMRL-1066 (5.6mM glucose) culture medium (GIBCO) supplemented with fetal calf serum (10%), penicillin (100U/ml), and streptomycin (100μg/ml) (Remedi et al., 2009).
Insulin secretion and content
Following overnight incubation in low glucose CMRL-1066 medium, islets (10 per well in 12 well plates) were pre-incubated in glucose-free CMRL-1066 plus 3 mM glucose (only for insulin secretion), then incubated for 60 min at 37°C in CMRL-1066 plus different [glucose], 1 μM glibenclamide, or 30 mM KCl, as indicated. After the incubation period, the medium was removed and assayed for insulin release. Experiments were repeated in triplicate. For islet insulin content, groups of 5 islets were disrupted using ethanol-HCl extraction and sonicated on ice for estimation of insulin content. Whole pancreas was removed and disrupted in homogenization buffer using a polytron homogenizer. Samples were centrifuged and supernatant was used for measurement of total insulin content per pancreas. Insulin secretion and content were measured using a Rat Insulin radioimmunoassay according to manufacturer’s procedure (RIA, Millipore, St. Charles, MO) (Remedi et al., 2009).
Immunohistochemical and morphometric analysis
Pancreases from control, untreated KATP-GOF (day 30 after tamoxifen induction) and insulin treated KATP-GOF mice (day 70 after tamoxifen induction, ~40 days after insulin pellet implantation) were fixed in 10% formalin, and paraffin-embedded for sectioning. Four to eight mice from each genotype were sampled on 5μm thick sections, 150μm apart and used for immunohistochemical and morphometric analysis. For morphometric analysis, at least 5 pancreatic sections from 3-8 mice from each genotype were covered systematically by accumulating images from non-overlapping fields on an inverted fluorescence Zeiss microscope or on a Zeiss LSM 510 laser scanning confocal microscope. Hematoxylin-Eosin (HE) staining was carried out as described previously (Remedi and Nichols, 2008). Antigen retrieval was performed for nuclear transcription factor detection (Nacalai USA) (Talchai et al., 2012b). For insulin-positivity determination, islet insulin immunoreative cross-sectional area and total pancreatic area were measured and calculated using Meta Morph imaging software (Universal Imaging Corp) and expressed as percentage of β-cell area relative to the total pancreatic area. Apoptosis was determined on pancreatic paraffin sections by using Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL, ApopTag Plus Fluorescein In situ Apoptosis, Chemicon International, Inc) and cleaved caspase-3 staining techniques. Cleaved caspase-3 primary antibody (Cell Signaling) was detected by using secondary antibody conjugated with AlexaTM 488 (green, Molecular Probes, Eugene-OR). For apoptosis quantification, number of TUNEL positive cells per total islet nuclei (counterstained with DAPI); or intra-islet caspase-3 intensity per total islet area were determined. Quantification of Ngn3 was performed in pancreatic sections double stained for insulin and Ngn3, by counting the number of Ngn3 positive cells, insulin-negative or – positive in z stack section images acquired in a Zeiss LSM 510 laser confocal microscope in a merged red and green image. The presence of eGFP (indicating KATP-GOF transgene expression on pancreatic β-cells) was assessed in pancreatic sections using eGFP primary antibody. Quantification of lineage tracing experiments in KATP-GOF mice was performed by determining eGFP, insulin and glucagon immunoreactive areas, and the ratio of overlapping area/total immunoreactive area. Ngn3 positive cells were counted and expressed as a percentage of Ngn3 positive cells per total eGFP immunoreactive area. To estimate α-cell population, the total number of glucagon positive cells was counted from several pancreatic sections immunostained for glucagon. For proliferation analysis, Ki67 positive β-cell cells were counted in pancreatic sections co-stained for insulin, and expressed as a percentage of Ki67 positive cells per total β-cell area. Primary antibodies used: insulin (rabbit monoclonal, Cell Signaling or guinea pig polyclonal, Abcam), neurogenin3 (goat polyclonal for the N-terminus of Ngn3, Santa Cruz, Figures 4, 5) and Beta Cell Biology Consortium (mouse BCBC F25A1B3, Figure S2A), eGFP (rabbit polyclonal, Molecular Probes, Invitrogen), glucagon (guinea pig polyclonal, Millipore), Ki67 (rabbit polyclonal, Abcam), Nanog (rabbit polyclonal, Abcam), Oct4 (rabbit polyclonal, Stemgent), Nestin (mouse monoclonal, Abcam). Detection was performed by using secondary antibody conjugated with AlexaTM 488 (green) or AlexaTM 594 (red) fluorescent dyes (Molecular Probes, Eugene-OR) (Remedi et al., 2009).
Quantitative RT-PCR analysis
Islets were isolated 30 days after tamoxifen injection and 70 days after tamoxifen (40 days after insulin pellet implantation) for KATP-GOF-untreated and insulin-treated mice respectively, and immediately processed for RNA isolation. Cellular RNA was isolated using RNeasy Mini Kit (Qiagen) and DNA was removed using DNase1 RNase free solution (Qiagen). cDNA was prepared from RNA using the High Capacity cDNA Reverse Transcription kit (Applied Biosystems), and quantitative RT-PCR was performed using the One-Step system and Taq-Man primers (Applied Byosystems). The experimental data were normalized using two reference genes: β-actin (Act) for high abundant genes, and β-actin and TATA binding protein (TBP) for low abundant genes. Messenger RNA changes were calculated by the comparative ΔCt method.
Statistics
Data are presented as mean ± SEM. Differences between two groups were tested using t-test and among several groups were tested using analysis of variance (ANOVA) and post-hoc Duncan’s test. * and # indicate significant differences among groups with p <0.05, non-significant differences are not indicated.
Supplementary Material
Highlights.
Loss of pancreatic β-cell insulin content in long-standing glucotoxic diabetes
β-cell dedifferentiation to neurogenin3-positive/insulin-negative cells in diabetes
Re-differentiation to insulin-positive β-cells after normalization of blood glucose
ACKNOWLEDGEMENTS
MSR and CGN designed the study. ZW, NY and MSR carried out the experiments. MSR and CGN wrote the paper. This work was supported by NIH grants (R01 DK098584 to MSR, R01 DK69445 to CGN, and Diabetes Research Training Center Grant 5P60 DK020579). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank Theresa M. Harter (Department of Cell Biology and Physiology, Washington University School of Medicine, Saint Louis, MO) for assistance with mouse breeding, maintenance and genotyping. We also thank Mr. Jonathan Friedman and Miss Mariana Alisio for technical assistance. We are extremely grateful to Maureen Gannon (Department of Molecular Physiology and Biophysics, Vanderbilt University, Nashville, Tennessee) and to Pedro Herrera (University of Geneva) for providing us with the tamoxifen inducible Pdx1PBCreERTM and Rip-Cre mice, respectively. The authors have no conflict-of-interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahlqvist E, Ahluwalia TS, Groop L. Genetics of type 2 diabetes. Clin Chem. 2011;57:241–254. doi: 10.1373/clinchem.2010.157016. [DOI] [PubMed] [Google Scholar]
- Ahren B. Type 2 diabetes, insulin secretion and beta-cell mass. Curr Mol Med. 2005;5:275–286. doi: 10.2174/1566524053766004. [DOI] [PubMed] [Google Scholar]
- Alvarsson M, Sundkvist G, Lager I, Berntorp K, Fernqvist-Forbes E, Steen L, Orn T, Holberg MA, Kirksaether N, Grill V. Effects of insulin vs. glibenclamide in recently diagnosed patients with type 2 diabetes: a 4-year follow-up. Diabetes Obes Metab. 2008;10:421–429. doi: 10.1111/j.1463-1326.2007.00719.x. [DOI] [PubMed] [Google Scholar]
- Alvarsson M, Sundkvist G, Lager I, Henricsson M, Berntorp K, Fernqvist-Forbes E, Steen L, Westermark G, Westermark P, Orn T, et al. Beneficial effects of insulin versus sulphonylurea on insulin secretion and metabolic control in recently diagnosed type 2 diabetic patients. Diabetes Care. 2003;26:2231–2237. doi: 10.2337/diacare.26.8.2231. [DOI] [PubMed] [Google Scholar]
- Benninger RK, Remedi MS, Head WS, Ustione A, Piston DW, Nichols CG. Defects in beta cell Ca(2)+ signalling, glucose metabolism and insulin secretion in a murine model of K(ATP) channel-induced neonatal diabetes mellitus. Diabetologia. 2011;54:1087–1097. doi: 10.1007/s00125-010-2039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernal-Mizrachi E, Wice B, Inoue H, Permutt MA. Activation of serum response factor in the depolarization induction of Egr-1 transcription in pancreatic islet beta-cells. J Biol Chem. 2000;275:25681–25689. doi: 10.1074/jbc.M003424200. [DOI] [PubMed] [Google Scholar]
- Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003 doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- Butler PC, Meier JJ, Butler AE, Bhushan A. The replication of beta cells in normal physiology, in disease and for therapy. Nat Clin Pract Endocrinol Metab. 2007;3:758–768. doi: 10.1038/ncpendmet0647. [DOI] [PubMed] [Google Scholar]
- Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- Del Prato S, Bianchi C, Marchetti P. beta-cell function and anti-diabetic pharmacotherapy. Diabetes Metab Res Rev. 2007;23:518–527. doi: 10.1002/dmrr.770. [DOI] [PubMed] [Google Scholar]
- Flanagan SE, Clauin S, Bellanne-Chantelot C, de Lonlay P, Harries LW, Gloyn AL, Ellard S. Update of mutations in the genes encoding the pancreatic beta-cell K(ATP) channel subunits Kir6.2 (KCNJ11) and sulfonylurea receptor 1 (ABCC8) in diabetes mellitus and hyperinsulinism. Hum Mutat. 2009;30:170–180. doi: 10.1002/humu.20838. [DOI] [PubMed] [Google Scholar]
- Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, Abdulkader F, Clark A, Ball V, Zubcevic L, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JMCL, Molnes J, et al. Activating Mutations in the Gene Encoding the ATP-Sensitive Potassium-Channel Subunit Kir6.2 and Permanent Neonatal Diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Greenwood RH, Mahler RF, Hales CN. Improvement in insulin secretion in diabetes after diazoxide. Lancet. 1976;1:444–447. doi: 10.1016/s0140-6736(76)91473-2. [DOI] [PubMed] [Google Scholar]
- Gu G, Dubauskaite J, Melton DA. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- Guo S, Dai C, Guo M, Taylor B, Harmon JS, Sander M, Robertson RP, Powers AC, Stein R. Inactivation of specific beta cell transcription factors in type 2 diabetes. J Clin Invest. 2013 doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature. 2006;443:345–349. doi: 10.1038/nature05097. [DOI] [PubMed] [Google Scholar]
- Hur KY, Jung HS, Lee MS. Role of autophagy in beta-cell function and mass. Diabetes Obes Metab. 2010;12(Suppl 2):20–26. doi: 10.1111/j.1463-1326.2010.01278.x. [DOI] [PubMed] [Google Scholar]
- Ilkova H, Glaser B, Tunckale A, Bagriacik N, Cerasi E. Induction of long-term glycemic control in newly diagnosed type 2 diabetic patients by transient intensive insulin treatment. Diabetes Care. 1997;20:1353–1356. doi: 10.2337/diacare.20.9.1353. [DOI] [PubMed] [Google Scholar]
- Jhala US, Canettieri G, Screaton RA, Kulkarni RN, Krajewski S, Reed J, Walker J, Lin X, White M, Montminy M. cAMP promotes pancreatic beta-cell survival via CREB-mediated induction of IRS2. Genes Dev. 2003;17:1575–1580. doi: 10.1101/gad.1097103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas JC, Bensellam M, Duprez J, Elouil H, Guiot Y, Pascal SM. Glucose regulation of islet stress responses and beta-cell failure in type 2 diabetes. Diabetes Obes Metab. 2009;11(Suppl 4):65–81. doi: 10.1111/j.1463-1326.2009.01112.x. [DOI] [PubMed] [Google Scholar]
- Jonas JC, Sharma A, Hasenkamp W, Ilkova H, Patane G, Laybutt R, Bonner-Weir S, Weir GC. Chronic hyperglycemia triggers loss of pancreatic beta cell differentiation in an animal model of diabetes. J Biol Chem. 1999;274:14112–14121. doi: 10.1074/jbc.274.20.14112. [DOI] [PubMed] [Google Scholar]
- Kitamura YI, Kitamura T, Kruse JP, Raum JC, Stein R, Gu W, Accili D. FoxO1 protects against pancreatic beta cell failure through NeuroD and MafA induction. Cell Metab. 2005;2:153–163. doi: 10.1016/j.cmet.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Laybutt DR, Glandt M, Xu G, Ahn YB, Trivedi N, Bonner-Weir S, Weir GC. Critical reduction in beta-cell mass results in two distinct outcomes over time. Adaptation with impaired glucose tolerance or decompensated diabetes. J Biol Chem. 2003;278:2997–3005. doi: 10.1074/jbc.M210581200. [DOI] [PubMed] [Google Scholar]
- Lupi R, Del Prato S. Beta-cell apoptosis in type 2 diabetes: quantitative and functional consequences. Diabetes Metab. 2008;34(Suppl 2):S56–64. doi: 10.1016/S1262-3636(08)73396-2. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. UK Prospective Diabetes Study (UKPDS) Group. Diabetic Medicine. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Nichols CG, Remedi MS. The diabetic β-cell: hyperstimulated vs. hyperexcited. Diabetes, Obesity and Metabolism. 2012;14 doi: 10.1111/j.1463-1326.2012.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen EM, Hansen L, Carstensen B, Echwald SM, Drivsholm T, Glumer C, Thorsteinsson B, Borch-Johnsen K, Hansen T, Pedersen O. The E23K variant of Kir6.2 associates with impaired post-OGTT serum insulin response and increased risk of type 2 diabetes. Diabetes. 2003;52:573–577. doi: 10.2337/diabetes.52.2.573. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Damm P, Prentki M. Type 2 diabetes across generations: from pathophysiology to prevention and management. Lancet. 2011;378:169–181. doi: 10.1016/S0140-6736(11)60614-4. [DOI] [PubMed] [Google Scholar]
- Nolan CJ, Prentki M. The islet beta-cell: fuel responsive and vulnerable. Trends Endocrinol Metab. 2008;19:285–291. doi: 10.1016/j.tem.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Pearson ER, Flechtner I, Njolstad PR, Malecki MT, Flanagan SE, Larkin B, Ashcroft FM, Klimes I, Codner E, Iotova V, et al. Switching from insulin to oral sulfonylureas in patients with diabetes due to Kir6.2 mutations. N Engl J Med. 2006;355:467–477. doi: 10.1056/NEJMoa061759. [DOI] [PubMed] [Google Scholar]
- Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porat S, Weinberg-Corem N, Tornovsky-Babaey S, Schyr-Ben-Haroush R, Hija A, Stolovich-Rain M, Dadon D, Granot Z, Ben-Hur V, White P, et al. Control of pancreatic beta cell regeneration by glucose metabolism. Cell Metab. 2011;13:440–449. doi: 10.1016/j.cmet.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri S, Hebrok M. Diabetic beta Cells: To Be or Not To Be? Cell. 2012;150:1103–1104. doi: 10.1016/j.cell.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qvigstad E, Kollind M, Grill V. Nine weeks of bedtime diazoxide is well tolerated and improves beta-cell function in subjects with Type 2 diabetes. Diabet Med. 2004;21:73–76. doi: 10.1046/j.1464-5491.2003.01091.x. [DOI] [PubMed] [Google Scholar]
- Rahier J, Guiot Y, Goebbels RM, Sempoux C, Henquin JC. Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes Metab. 2008;10(Suppl 4):32–42. doi: 10.1111/j.1463-1326.2008.00969.x. [DOI] [PubMed] [Google Scholar]
- Reinert RB, Kantz J, Misfeldt AA, Poffenberger G, Gannon M, Brissova M, Powers AC. Tamoxifen-Induced Cre-loxP Recombination Is Prolonged in Pancreatic Islets of Adult Mice. PLoS One. 2012;7:e33529. doi: 10.1371/journal.pone.0033529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Agapova SE, Vyas AK, Hruz PW, Nichols CG. Acute Sulfonylurea Therapy at Disease Onset Can Cause Permanent Remission of KATP-Induced Diabetes. Diabetes. 2011 doi: 10.2337/db11-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Kurata HT, Scott A, Wunderlich FT, Rother E, Kleinridders A, Tong A, Bruning JC, Koster JC, Nichols CG. Secondary consequences of beta cell inexcitability: identification and prevention in a murine model of K(ATP)-induced neonatal diabetes mellitus. Cell Metab. 2009;9:140–151. doi: 10.1016/j.cmet.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. PLoS Med. 2008;5:e206. doi: 10.1371/journal.pmed.0050206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- Riedel MJ, Steckley DC, Light PE. Current status of the E23K Kir6.2 polymorphism: implications for type-2 diabetes. Hum Genet. 2005;116:133–145. doi: 10.1007/s00439-004-1216-5. [DOI] [PubMed] [Google Scholar]
- Robertson RP, Harmon J, Tran PO, Poitout V. Beta-cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes. 2004;53(Suppl 1):S119–124. doi: 10.2337/diabetes.53.2007.s119. [DOI] [PubMed] [Google Scholar]
- Sakuraba H, Mizukami H, Yagihashi N, Wada R, Hanyu C, Yagihashi S. Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia. 2002;45:85–96. doi: 10.1007/s125-002-8248-z. [DOI] [PubMed] [Google Scholar]
- Shimomura K, Horster F, de Wet H, Flanagan SE, Ellard S, Hattersley AT, Wolf NI, Ashcroft F, Ebinger F. A novel mutation causing DEND syndrome: a treatable channelopathy of pancreas and brain. Neurology. 2007;69:1342–1349. doi: 10.1212/01.wnl.0000268488.51776.53. [DOI] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Kitamura T, DePinho RA, Accili D. Generation of functional insulin-producing cells in the gut by Foxo1 ablation. Nat Genet. 2012a;44:406–412. S401. doi: 10.1038/ng.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic beta Cell Dedifferentiation as a Mechanism of Diabetic beta Cell Failure. Cell. 2012b;150:1223–1234. doi: 10.1016/j.cell.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y, Tran PO, Harmon J, Robertson RP. A role for glutathione peroxidase in protecting pancreatic beta cells against oxidative stress in a model of glucose toxicity. Proc Natl Acad Sci U S A. 2002;99:12363–12368. doi: 10.1073/pnas.192445199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torella R, Salvatore T, Cozzolino D, Giunta R, Quatraro A, Giugliano D. Restoration of sensitivity to sulfonylurea after strict glycaemic control with insulin in non-obese type 2 diabetic subjects. Diabete et Metabolisme. 1991;17:443–447. [PubMed] [Google Scholar]
- UKPDS-group. UK Prospective Diabetes Study Group Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998a;352:854–865. [PubMed] [Google Scholar]
- UKPDS-group. UK Prospective Diabetes Study Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998b;352:837–853. [PubMed] [Google Scholar]
- Villareal DT, Koster JC, Robertson H, Akrouh A, Miyake K, Bell GI, Patterson BW, Nichols CG, Polonsky KS. Kir6.2 variant E23K increases ATP-sensitive K+ channel activity and is associated with impaired insulin release and enhanced insulin sensitivity in adults with normal glucose tolerance. Diabetes. 2009;58:1869–1878. doi: 10.2337/db09-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajchenberg BL. Endocr Rev. 2007;28:187–218. doi: 10.1210/10.1210/er.2006-0038. [DOI] [PubMed] [Google Scholar]
- Wang S, Jensen JN, Seymour PA, Hsu W, Dor Y, Sander M, Magnuson MA, Serup P, Gu G. Sustained Neurog3 expression in hormone-expressing islet cells is required for endocrine maturation and function. Proc Natl Acad Sci U S A. 2009;106:9715–9720. doi: 10.1073/pnas.0904247106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg N, Ouziel-Yahalom L, Knoller S, Efrat S, Dor Y. Lineage tracing evidence for in vitro dedifferentiation but rare proliferation of mouse pancreatic beta-cells. Diabetes. 2007;56:1299–1304. doi: 10.2337/db06-1654. [DOI] [PubMed] [Google Scholar]
- Weng J, Li Y, Xu W, Shi L, Zhang Q, Zhu D, Hu Y, Zhou Z, Yan X, Tian H, et al. Effect of intensive insulin therapy on beta-cell function and glycaemic control in patients with newly diagnosed type 2 diabetes: a multicentre randomised parallel-group trial. Lancet. 2008;371:1753–1760. doi: 10.1016/S0140-6736(08)60762-X. [DOI] [PubMed] [Google Scholar]
- Xu X, D’Hoker J, Stange G, Bonne S, De Leu N, Xiao X, Van de Casteele M, Mellitzer G, Ling Z, Pipeleers D, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.