Abstract
Embryonic exposure to excess circulating fuels is proposed to underlie diabetic embryopathy. To isolate the effects of hyperglycemia from the many systemic anomalies of diabetes, we infused 4 mg/min glucose into the left uterine artery of non-diabetic pregnant rats on gestation days (GD) 7–9. Right-sided embryos and dams exhibited no glucose elevation. Embryos were assessed on GD13, comparing the left versus right uterine horns. Hyperglycemic exposure increased rates of embryopathy, resorptions, and worsened embryopathy severity. By contrast, saline infusion did not affect any of these parameters. To assess for possible embryopathy susceptibility bias between uterine horns, separate dams were given retinoic acid (25 mg/kg, a mildly embryopathic dose) systemically on GD7.5. The resultant embryopathy rates were equivalent between uterine horns. We conclude that hyperglycemia, even in the absence of systemic maternal diabetes, is sufficient to produce in vivo embryopathy during organogenesis.
Keywords: Embryopathy, Teratogenesis, Hyperglycemia, Diabetes, Fuel-Mediated Teratogenesis, Animal Model
1. Introduction
Diabetes mellitus exposes the embryo to a myriad of maternal insults including hyperglycemia, altered lipid levels, and inflammation. The effects of diabetes during pregnancy include devastating consequences to the developing embryo. Seven to twenty percent of women with pregestational diabetes experience adverse pregnancy outcomes, including spontaneous abortions, stillbirths, neonatal death and congenital malformations [1–3]. With improved care of diabetes before and during pregnancy and of infants born to diabetic mothers, perinatal mortality has declined. However congenital malformations have emerged as the leading cause of death in this population of infants[4,5]. Infants born to mothers with pregestational diabetes are eight times more likely to have major malformations than those born to mothers without diabetes [6]. Associated anomalies include brain and neural tube defects, caudal regression syndrome, skeletal dysplasia, congenital heart defects, gastrointestinal and genitourinary tract anomalies [5,7]. Multiple birth defects are also more common[7]. Significant progress has been made understanding the fetal-sided molecular mechanisms of diabetes-induced teratogenesis [8], which include oxidative stress [9,10], reduced Pax3 expression [11], and PKC activation [12].
The fuel mediated teratogenesis hypothesis states that maternal-diabetes induced embryopathy is caused by exposure of the embryo to excess circulating maternal energy fuels[13]. These exposures include excesses of glucose, ketones, fatty acids, triglycerides and variably glycerol [14,15]. Because improved glycemic control is associated with a lower incidence of pregnancy loss and congenital malformations [5,16–18], glucose is generally implicated as the major teratogen. In vitro, embryos cultured in defined media have supported this hypothesis for excesses of glucose [19,20] and/or ketones [21–23]. In vivo, glucose administration at hyperglycemia-inducing doses is sufficient to induce embryopathy [24]. Because systemic hyperglycemia induced by glucose administration produces a myriad of other acute systemic aberrations including altered circulating fuels, systemic oxidative stress, and inflammation [25–30], it is uncertain whether the consequences are related to localized effects of hyperglycemia on the embryo and/or downstream systemic maternal effects of hyperglycemia. It thus could be the case that localized hyperglycemia is insufficient to induce embryopathy in vivo, lacking required synergy with other perturbations of diabetes and systemic hyperglycemia [23].
The objective of this study was to determine whether hyperglycemia local to the developing embryo is sufficient to induce embryopathy in vivo, even in the absence of maternal diabetes. To meet this objective, we utilized a recently developed rat model that exposes select embryos to isolated hyperglycemia without maternal systemic hyperglycemia[31]. Our approach takes advantage of the bicornate nature of the rat uterus whereby each horn has a distinct blood supply. Glucose is infused directly into the left uterine artery circulation, producing hyperglycemia in the blood supply of that uterine horn alone, while the mother and contralateral uterine horn experience no significant elevation of blood glucose [31]. Here, we report use of this approach to test the fuel mediated teratogenesis hypothesis in vivo, finding that hyperglycemia in the left uterine artery during organogenesis is sufficient to induce embryopathy even in the absence of maternal diabetes.
2. Materials and Methods
2.1 Animals and Breeding
All procedures were performed within the regulations of the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and were approved by the University of Iowa Institutional Animal Care and Use Committee. Hsd:Sprague-Dawley SD rats (Harlan Laboratories, Inc., Indianapolis, IN) were housed in a temperature controlled, 12 hour light-dark cycled animal care facility with free access to water and conventional diet. Rats were bred and checked daily. Gestational day (GD) 0 was defined as the morning of a positive vaginal swab for the presence of spermatozoa.
2.2 Catheter placement
On GD1, a vascular catheter was inserted into the left femoral artery and secured in place just proximal to the left uterine blood supply as previously described [31]. Catheterization was performed at this point in gestation to allow stabilization of uterine blood flow prior to implantation, which typically occurs on GD 4 or 5. The catheter was kept patent utilizing an indwelling solution of 0.3 ml of 500 U/ml heparin in autoclaved glycerol. The distal end of the catheter was tunneled subcutaneously to the interscapular region for subsequent access.
2.3 Glucose infusion
On GD7, the distal end of the catheter was isolated from the interscapular region under brief isoflurane-oxygen anesthesia and connected to a single channel swivel (Instech Laboratories Inc., Plymouth Meeting, PA), affording the animal free movement about the cage. Glucose (20% dextrose in normal saline containing 5 U/ml heparin) was infused at 4 mg/minute (20µl/min) for 48 hours on GD7-9 (Figure 1A). Glucose infusion at this rate produces marked diabetic-level hyperglycemia in the uterine artery while there is no significant increase in maternal systemic or right uterine horn blood glucose [31]. Non-fasting, whole blood glucose concentrations were measured by tail nick sampling with a One Touch Ultra meter (LifeScan Inc., Milpitas, CA) at GD1, 7, 9 and 13. As a control for the effects of the catheter presence and infusion, some mothers underwent an identical protocol infusing normal saline containing 5 U/ml heparin rather than glucose on GD7-9.
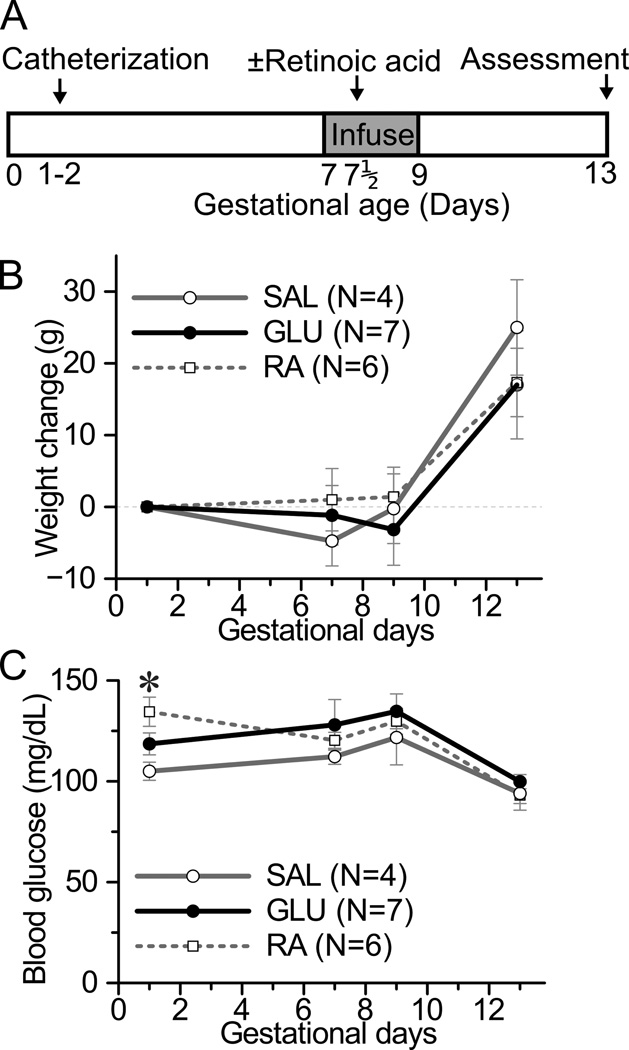
Figure 1. Experimental design and maternal characteristics by experimental group.
(A) Timeline: A catheter infusing into the left uterine artery was placed on gestational days 1–2. (B,C) There were three experimental groups of dams: glucose (GLU, black line, filled circle), saline (SAL, grey line, open circle), and retinoic acid (RA, dotted line, open square). GLU and SAL dams received glucose (4 mg/min) or normal saline, respectively, infused via the catheter on gestational days 7–9. Retinoic acid (25 mg/kg) was administered to RA dams intraperitoneally on gestational day 7.5. A portion of RA dams also received a catheter with or without saline infusion. Embryos were assessed on gestational day 13 (GLU and RA) or 14 (SAL). (B) Maternal weight change from baseline (gestational day 1) by experimental group. (C) Maternal systemic blood glucose by experimental group. The day 9 blood glucose was measured prior to discontinuing infusions. *p<0.05 for difference between RA and SAL mothers by ANOVA and Tukey's HSD post-doc analysis. Note that at day 1 the three groups had not yet received differing treatments. There were no other statistical differences between groups.
2.4 Retinoic acid
To assess for possible lateralizing embryopathy predilections, we utilized a mildly embryopathic dose of the teratogen retinoic acid (RA) administered systemically [32]. On GD 7.5, select dams received 25mg/kg of RA intraperitoneally. RA (Sigma Aldrich Inc., St Louis) was prepared immediately before administration in a light protected manner by dissolution in a minimal amount of dimethyl-sulfoxide followed by suspension in medium chain triglyceride or peanut oil to a concentration of 2 mg/ml. A portion of the retinoic acid treated dams also underwent left uterine artery catheter placement on GD1 with or without infusion of saline containing 5 U/ml heparin on GD7-9 to assure that catheter infusion, alongside a mild systemic teratogen, did not increase embryopathy on one side versus the other.
2.5 Outcome Measures
Embryos were accessed by terminal cesarean section on GD13 (glucose and RA dams) or GD14 (saline dams). Rats completely lacking any viable embryos were not considered. The physical location of the embryos was recorded, being from the left or the right uterine horn and numbered consecutively by proximity to the cervix. Embryos were collected, within the amniotic-sac when intact, and placed in 10% neutral buffered formalin. Embryos were carefully dissected free of extra-embryonic tissues using a binocular dissection microscope. Embryonic resorption was defined as the presence of a chorion without embryo or the presence of embryonic tissue lacking any identifiable morphology. Non-resorption embryos were assessed visually for apparent malformations using a classification rubric [33]. Final classifications were adjudicated by a single individual for consistency. Normal embryos exhibited natural body flexion, a long curved tail, intact spinal column with closed anterior and posterior neural pores, and normally placed and sized cardiac sac and limb buds. Minor embryonic malformation was defined as exhibiting one limited deviation from this pattern, such as a short tail, or small localized neural tube opening. Major embryonic malformation was defined as a significant deviation, for example that might impact embryonic, fetal, or neonatal viability, such as a large neural tube defect involving the brain, or absence of cardiac structures. Embryopathy was defined as the presence of any malformation or resorption. Embryopathy rate was defined as the fraction of embryos in a given horn exhibiting any degree of embryopathy. Embryopathy severity was quantified adapting a scoring system of 0–10 [34–36]: normal embryos received a score of zero, embryos assessed as having a minor and major malformation were scored 1 and 5 respectively, and embryos with multiple major malformations or embryos with resorptions were scored 10. The latter scoring reflects that a significant portion of embryos with multiple major malformation were likely non-viable or even partly resorbed.
2.6 Statistical Analyses
Statistical comparison of embryopathy rates or severity scores between uterine horns presents several challenges and opportunities. For one, the number of embryos varies across uterine horns, leading to an inherently unbalanced situation. For example, a horn containing a total of two embryos should yield less statistical confidence than a horn containing eight embryos. Another challenge is that the sample distribution of embryopathy rates and/or severity scores may be complex and not well modeled by parametric approaches. Additionally, there is a natural pairing between uterine horns by mother. The effective N of a given experiment should be the number of mothers, as each mother represents an independent replicate. However, the number of assessed embryos should not be neglected. We thus used a random permutation testing approach[37], accounting for these challenges and opportunities, to assess the significance of differences in embryopathy rates between matched left and right uterine horn pairs, across mothers. This approach has the advantages of (i) stemming naturally from the natural pairing between uterine horns, (ii) not requiring a specific sample distribution, and (iii) accounting for the inherently unbalanced pairing between uterine horns. The mean embryopathy rate difference between the left and right uterine horns, E̅Δ, across N mothers was calculated as
where Le,i is the number of embryopathic embryos in the ith mother's left uterine horn, Ln,i is the total number of embryo implantations in that horn, and Re,i and Rn,i are the corresponding right uterine horn values. Mean embryopathy rate differences under the null hypothesis, E̅Δ,H0, were estimated from the same group of mothers using permutation testing. This was accomplished by assigning, in silico, each mothers embryopathic embryos randomly among all her implantation sites across the right and left uterine horns without replacement, across all mothers but permuting only within each mother. This was performed for a series of independent permutations, thus constructing a distribution of E̅Δ,H0. Comparison of E̅Δ to this distribution in a two-tailed approach yielded the p-value. For comparison of embryopathy severity scores, an identical approach was used substituting scores for rates. Non-paired comparisons between groups were performed using ANOVA and Tukey's HSD test. All statistical analyses were performed using the open source statistical programming language R [38]; R code will be shared upon request. Means ± standard error of the means are reported.
3. Results
Three groups of mothers were studied: mothers that received left uterine artery hyperglycemic infusion on GD7-9 (“GLU”), mothers that received left uterine artery saline infusion on GD7-9 (“SAL”), and mothers that received systemic retinoic acid on GD7.5 (“RA”) (Figure 1A). Weight change during pregnancy did not differ among the three groups (Figure 1B). Likewise, maternal systemic blood glucose measured at the start or conclusion of the infusion period did not differ among the groups (Figure 1C). Maternal insulin levels measured at the conclusion of the infusion did not differ statistically between groups, though there was a trend towards higher levels in the GLU dams (Supplemental Figure 1). The number of implantations per horn did not statistically differ between uterine horns regardless of the presence or absence of a catheter at 6.7±0.6 or 7.4±0.6 respectively (N=16–18 horns, p=n.s.).
3.1 Embryopathy Rate
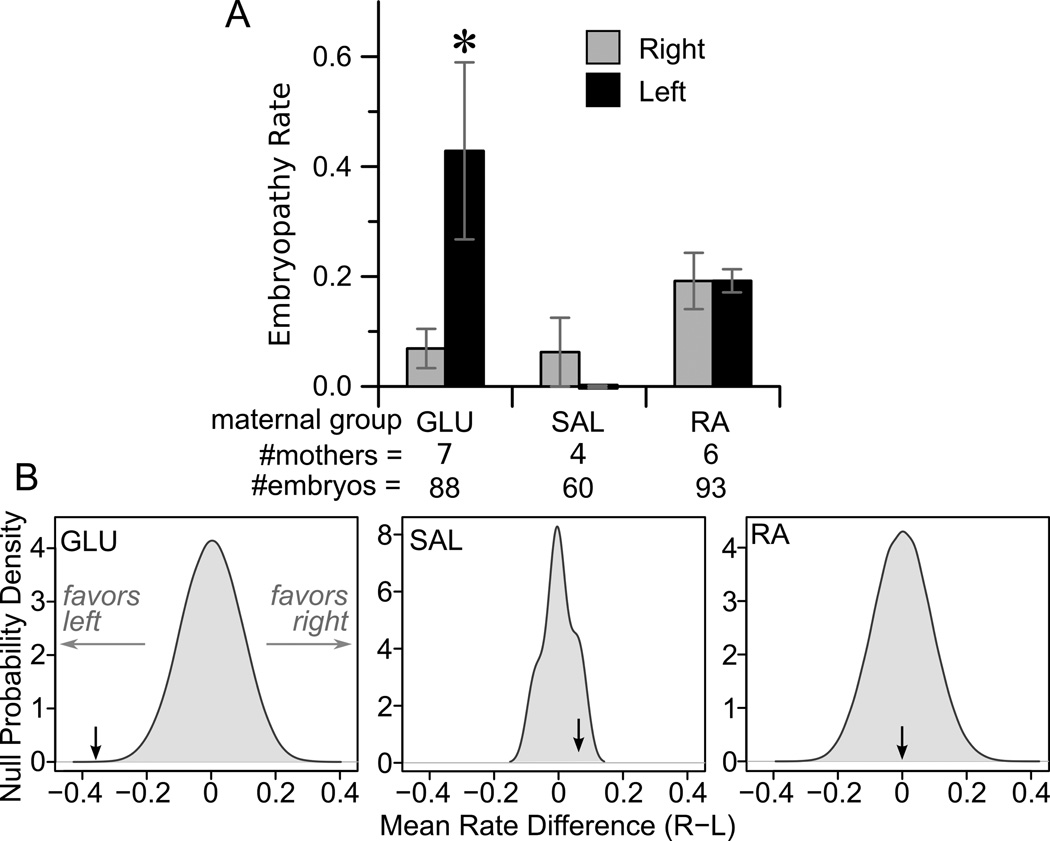
The primary outcomes of interest were whether differences existed in embryopathy rates between the left and right uterine horns for each of the three groups of mothers studied. A low embryopathy rate was observed in the right uterine horn of GLU dams (0.07), similar to embryopathy rates previously observed in control rat pregnancies when examined in mid-gestation [39], whereas the rate in the left (i.e. hyperglycemia exposed) uterine horn exceeded 0.4 (Figure 2A). To assess for statistical significance of this difference, we used random permutation testing, allowing determination of the null probability densities for the mean difference in embryopathy rates between uterine horns (Figure 2B). For glucose infusion, the actual observed mean difference in rate between horns was skewed significantly left of the null distribution (p<0.001). By contrast, for dams treated with SAL or RA the embryopathy rate was not different between horns (Figure 2A). Likewise, the mean difference between uterine horns fell in the middle of the null distribution (Figure 2B), yielding non-significance at p=0.47 (SAL) and 0.99 (RA).
Figure 2. Comparison of embryopathy rates between uterine horns.
(A) Mean embryopathy rates in the left (black bars) and right (grey) uterine horns. *P<0.001 (B) Null distributions for the difference between right and left uterine horn embryopathy rates, determined by random permutation testing, are shown in grey for the three experimental groups. The actual observed embryopathy rate difference is shown as a dark arrow.
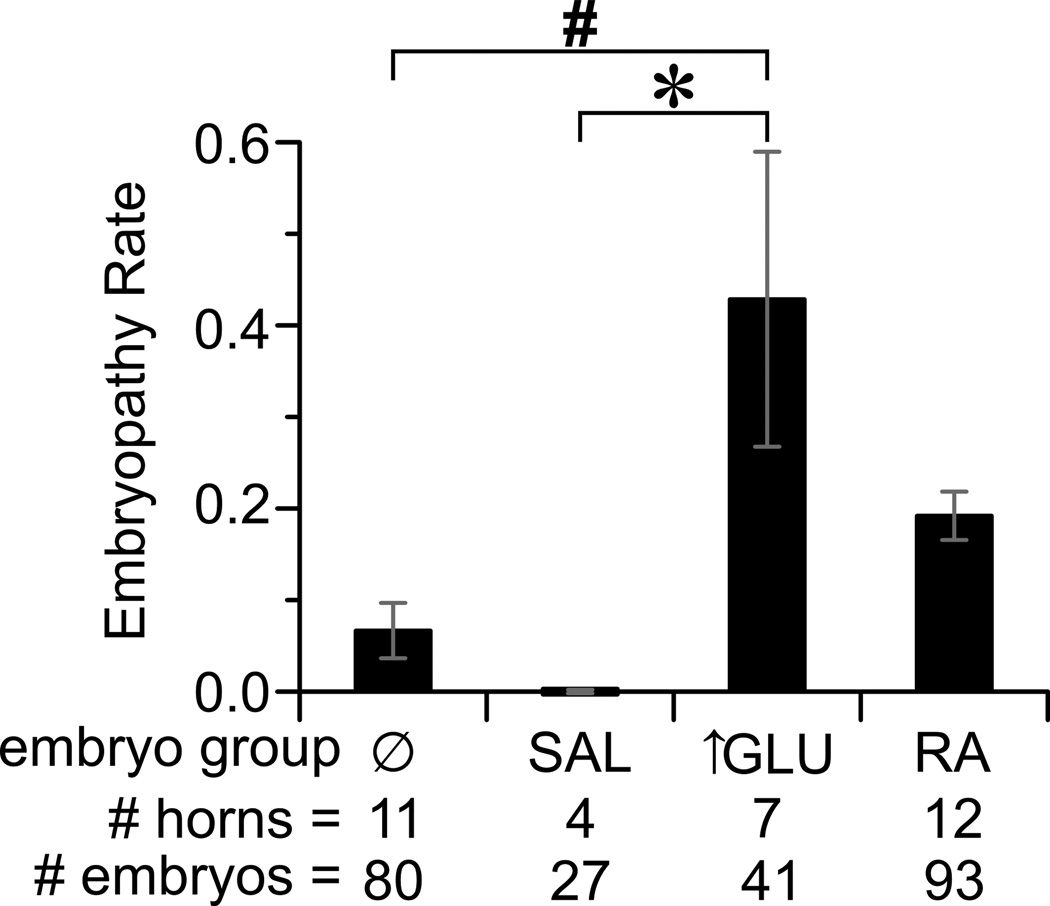
We performed a secondary analysis, grouping uterine horns by exposure. The exposures occurred in 4 groups: none, saline, hyperglycemia, and RA (Figure 3). Grouping in this manner demonstrated that the lowest embryopathy rate occurred among embryos in uterine horns with no exposure; those exposed to saline had similarly low rates. The highest rates of embryopathy occurred in hyperglycemia exposed horns and were significantly higher than that in saline- and non-exposed horns. Embryos in RA exposed horns had intermediate embryopathy rates. Among embryos in the glucose infused horns, embryopathy was more likely in those embryos closest to the uterine artery infusion (i.e. the embryos in the inferior aspect of the uterine horn) (Supplemental Figure S2). This inferior skew in embryopathy likelihood was not observed in any of the other uterine horn exposures. These results not only demonstrated the embryopathic nature of hyperglycemic infusion, but also indicated that the infusion and catheter were not inherently embryopathic since the embryopathy rate in the saline infused horns was extremely low.
Figure 3. Comparison of embryopathy rates by differing exposures.
Embryopathy rates by exposure: none (Ø), saline (SAL), hyperglycemia (↑GLU), or retinoic acid (RA). * P<0.05, # P<0.01 for the indicated effect by 1-way ANOVA and Tukey's HSD post-doc analysis.
We also sought to control for the effect of the catheter and infusion in the presence of a teratogenic milieu. To accomplish this, a subset of the RA dams received a catheter with saline infusion, a catheter without infusion, or no catheter/infusion. Embryopathy rates were identical between these three groups (Table 1), indicating that the catheter and/or infusion per se were not embryopathic, even in the presence of a systemic teratogen.
TABLE 1.
Embryopathy rates in uterine horns of RA-treated dams
| group |
N |
embryopathy rate |
|||
|---|---|---|---|---|---|
| RA | catheter | saline-infusion | horns | embryos | mean±sem |
| + | + | + | 3 | 24 | 0.20±0.04 |
| + | + | − | 2 | 15 | 0.19±0.03 |
| + | − | − | 7 | 54 | 0.19±0.04 |
p=0.99 for difference between groups by one-way anova.
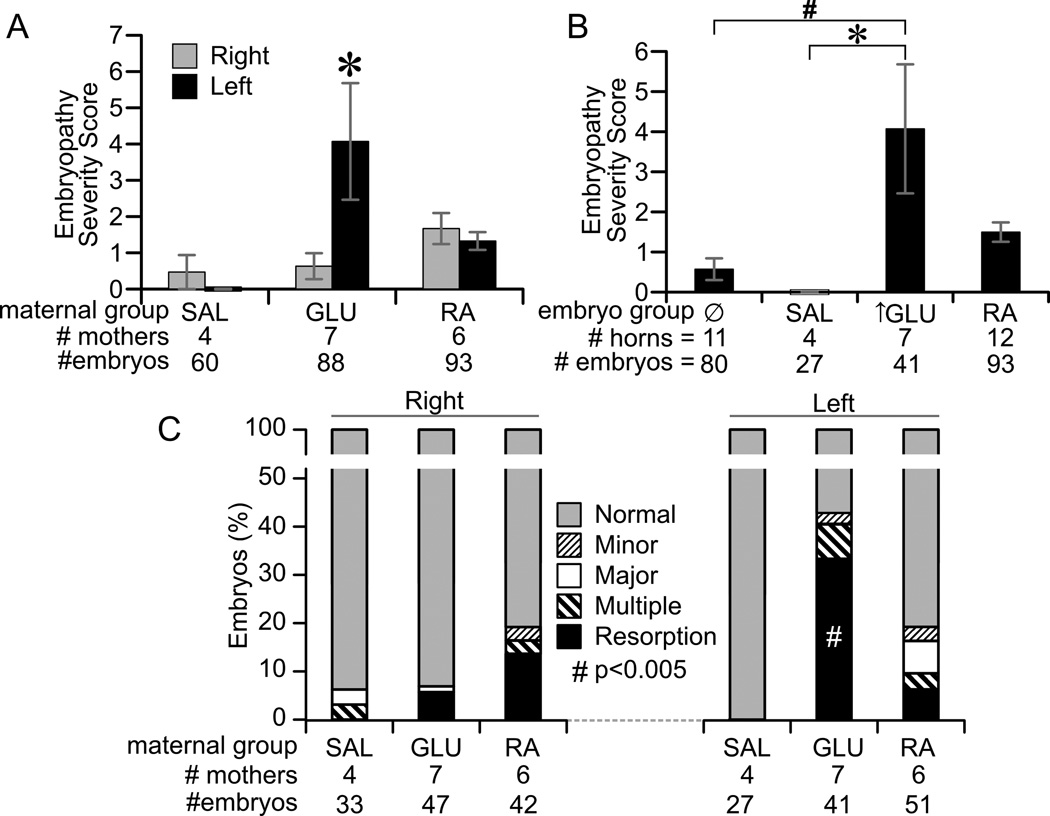
3.2. Embryopathy Severity
A range of embryopathy was observed from minor malformations to embryonic resorption (Figure 4). We scored this severity using an existing metric ranging 0 – 10. The mean severity score was significantly greater in hyperglycemia infused uterine horns than the contralateral non-exposed horns (Figure 5A). By contrast, there was no statistical difference between left and right horns for saline and RA exposed dams (Figure 5A). Mean severity scores were also assessed among the four groups of uterine horn exposures. Hyperglycemic infusion significantly increased the severity score as compared to saline- and non-exposed uterine horns (Figure 5B). The mean percent of outcomes per horn classified as normal, minor malformation, major malformation, multiple malformations, and resorptions is shown in Figure 5C. Resorptions represented the major fraction of embryopathy in hyperglycemia exposed uterine horns. We thus assessed whether the mean rate of resorptions was different between left and right horns in each group, using random permutation testing. This analysis showed that resorption rates were significantly higher in the left versus right uterine horns for GLU dams (Figure 5C).
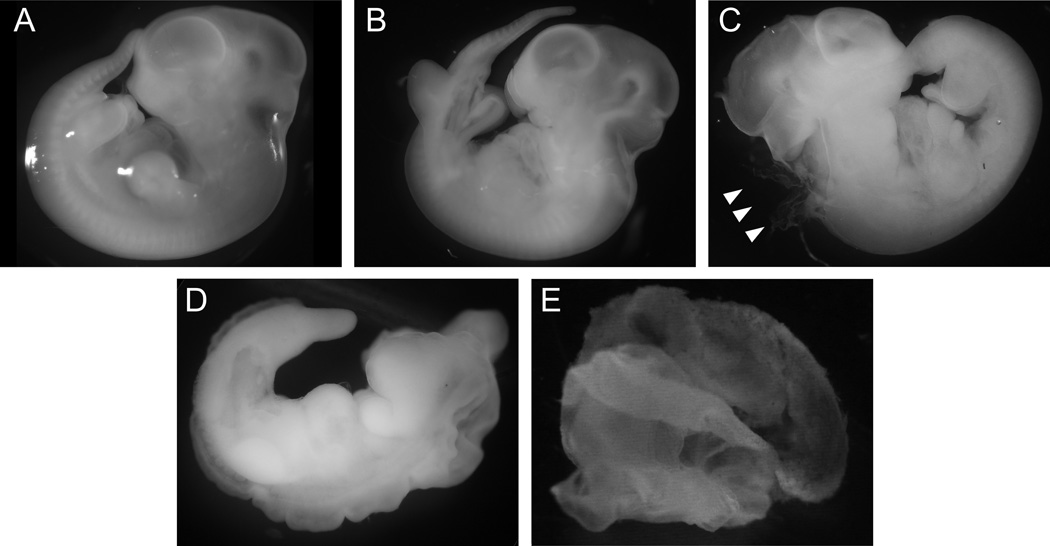
Figure 4. Example micrographs across a range of embryopathy severity outcomes at gestational day 13.
(A) Normal embryo. (B) Minor embryonic malformation, specifically disrupted caudal formation. (C) Major embryonic malformation, specifically a hindbrain neuropore defect (arrows). (D) Multiple apparent embryonic malformations. (E) Resorption, specifically empty amnion.
Figure 5. Embryopathy severity.
(A) Comparison of mean embryopathy severity scores between left (black) and right (grey) uterine horns. *p<0.001 for difference between horns by random permutation testing. (B) Comparison of mean embryopathy severity scores between embryos grouped by exposure. * p<0.05 for indicated effect by ANOVA. (C) Distribution of embryopathy classes among embryos from left and right uterine horns. Embryos were classified as being normal (grey), or having a minor malformation (small cross-hatch), major malformation (white), multiple malformations (large cross hatch), or undergoing resorption (black). # p<0.005 for difference between left and right uterine horns by random permutation testing on either resorptions.
Morphological embryo outcomes ranged from minor variations such as shortened tails to severe malformations including neural tube defects, sacral agenesis, or cardiac hypoplasia. We classified the types of malformations into 6 broad and arbitrary categories and determined the mean rates for the four uterine exposure groups (Table 2). In general, the rates of many malformation classes were increased in the hyperglycemia and/or RA groups. However, the total number of events per class was small and no differences between groups were statistically significant.
TABLE 2.
Mean rates of apparent malformations by exposure group.
| group | # | NTD | craniofacial | caudal | cor/thor | abd/GI | limb |
|---|---|---|---|---|---|---|---|
| con | 11 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 |
| SAL | 4 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| glucose | 7 | 0.00 ± 0.00 | 0.07 ± 0.05 | 0.07 ± 0.05 | 0.07 ± 0.05 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| RA | 12 | 0.05 ± 0.02 | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.00 ± 0.00 |
Mean rates observed of specific malformation classes are shown, determined from the number of indicated uterine horns.
Abbreviations: NTD – neural tube defect; caudal – caudal regression; cor/thor – cardiac or thoracic malformation; abd/GI – abdominal or gastrointestinal malformation. There were no significant differences, by 1-way anova, between groups for each malformation class.
4. Discussion
The fuel mediated teratogenesis hypothesis[13] has been difficult to refine in vivo due to the multifaceted nature of maternal diabetes. Maternal diabetes exposes embryos to a wide swath of perturbations that extend beyond hyperglycemia alone. To address this, we developed an approach[31] to selectively expose embryos in vivo to hyperglycemia in non-diabetic mothers. Using this approach we found that hyperglycemia, localized only to the uterus and its blood supply, is sufficient to markedly increase embryopathy prevalence and severity, even in the absence of other factors of maternal diabetes. Consistent with prior in vivo rodent studies [40], we found that susceptibility to diabetic embryopathy is high on gestational days 7–9, that embryonic resorptions outnumber simple malformations, and that a wide range of malformations are induced. Likewise, our results are reflective of pregestational diabetes in humans, which is associated with increased risk of pregnancy loss and a wide range of birth defects including brain and neural tube defects, caudal regression syndrome, and congenital heart defects [5], and high risk of multiple defects [7]. Our findings recapitulate prior findings in rodents and humans, and thus show that localized hyperglycemia without systemic diabetes is sufficient to induce the full spectrum of diabetic embryopathy. Thus, this work lends strong support to the fuel mediated teratogenesis hypothesis specifically as regards glucose. The major advance of our work is to isolate the specific effects of hyperglycemia, while minimizing and accounting for secondary maternal phenomenon, in an in vivo system.
To date, tests of the fuel mediated teratogenesis hypothesis have been conducted via three general approaches: epidemiologic, in vitro, and in vivo study. Epidemiologic studies have consistently identified a major role for hyperglycemia in mediating diabetes-related embryopathy. Namely, better glycemic control early in pregnancy leads to a marked reduction in the risk of birth defects[41]. However, these observations do not yield definitive mechanistic insight, for many aspects of diabetes, ketones levels for example, improve as glucose levels are lowered. In vitro studies on cultured embryos have advanced the field greatly and demonstrate teratogenic effects of glucose [19,42] and/or ketones [21,22]. However, the in vivo circumstance is more complex, involving bi-directional interactions between the embryonic, extra-embryonic, and maternal tissues; in vitro embryo studies do not always replicate in vivo toxicologies [8,43–46].
In vivo, rodents have been used as a model for they develop embryopathy similar to humans when diabetes is present during organogenesis. There are two conventional in vivo approaches to testing the fuel mediated teratogenesis hypothesis: (i) induction of diabetes in the pregnant rodent by various means such as streptozotocin [47], (ii) systemic administration of glucose [24,48]. However, neither of these approaches allows analysis of the isolated local effects of individual fuels on diabetic embryopathy because (i) systemic diabetes induces multifaceted irregularities in the maternal circulation, including abnormal energy fuels, inflammation, and oxidative stress, as noted in the introduction, and (ii) systemic induction of hyperglycemia by glucose administration produces a myriad of rapid (within hours) systemic aberrations including increased plasma triglycerides [25], oxidative stress and systemic inflammation [28–30], altered insulinemia [49], altered gene expression in tissues [50], and altered lipid and amino acid metabolism [26,27]. One elegant in vivo study tested whether hyperglycemia is necessary to induce diabetes-related embryopathy by treating diabetic pregnant rodents with phlorizin to selectively ameliorate the hyperglycemia but not the insulinopenic state [24]. This reduced the rates of embryopathy, indicating that hyperglycemia is necessary for diabetes-related teratogenesis. The results of this prior study are complimentary to our present study demonstrating that hyperglycemia is sufficient to induce embryopathy.
Our novel approach circumvents the above noted limitations of other in vivo and in vitro studies by inducing hyperglycemia in an anatomically localized fashion and avoiding systemic maternal hyperglycemia thus minimizing any systemic perturbations. Furthermore, any unrecognized systemic perturbations induced by the hyperglycemic infusion will affect embryos of both uterine horns equally. In this manner, the embryos in the right uterine horn are an ideal control in that they will be exposed to the same developmental environment, except for the local effects of hyperglycemia in the left uterine horn. Thus, this approach allows isolation of the localized effects of hyperglycemia on the developing embryo in vivo.
Using this approach also provides opportunity to examine the effects of hyperglycemia at various stages of development. We selected hyperglycemic exposure during GD7-9, which is a key time for organogenesis including neurulation, heart and somite formation in the rat. We then assessed embryonic outcomes on gestational day 13 or 14, paralleling multiple prior studies which assessed embryonic outcomes of experimental diabetic pregnancy during gestational days 10–14 [9,10,12,24,33,42]. However, there is an experimental trade off in studying outcomes early versus late in gestation. Specifically, some mild malformations may be too minor to be visually evident early in gestation but would be apparent at later time points. Our study was not designed to ascertain malformations independent of embryopathy. This is because (i) of uncertainty in determining the primacy of apparent major malformations versus partial resorption in embryos and (ii) the number of isolated malformations were low, such that there was insufficient power to make conclusions. Among non-severely affected embryos, hyperglycemic exposure as compared to controls may have increased the risk of isolated malformation (odd ratio 1.8, 95% confidence interval, 0.2–20.1). Had we scored the pregnancy outcomes at term, it would not be surprising to observe even higher rates of total adverse outcomes including pregnancy loss.
Our dextrose infusion rate was chosen to simulate poorly controlled diabetic glucose levels. Uterine blood flow at this gestational age is on the order of 0.5 mL/min/horn [51]. The infusion of 4 mg/min glucose thus should produce an estimated uterine artery glucose increment of roughly 800 mg/dL. Importantly, the infusion rate (20 µl/min) will produce only a minimal local dilution of blood by the infusate (roughly 4%) and thus would not be expected to cause embryopathy of its own accord. Consistent with this, there was no increase in embryopathy observed with saline infusion at 20 µl/min. It is likely that less severe hyperglycemic exposure would produce less embryonic resorption, but it remains to be determined whether this would be manifest as a greater fraction of embryos with isolated malformations.
Although it is labor intensive, our novel approach has a number of strengths. The embryos in the non-exposed right uterine horn represent an ideal control for the hyperglycemia exposed embryos in the left uterine horn. This is because the right and left embryos have similar genetic make-up and experience the same in utero environment with the exception that the left sided embryos receive first pass exposure to the infusate. Otherwise, the embryos for a given mother are exposed to the same developmental environment including any systemic modulating factors, such as maternal diet or administration of synergistic teratogens like retinoic acid. It is possible that the glucose infusion may have induced systemic changes in the maternal milieu, such as increased insulin levels. Importantly, in our model, the embryos in both uterine horns will experience such systemic perturbations. Thus, hyperglycemia remains the one factor differing between the two horns, allowing isolation of its local effects on embryonic development. There is another advantage of our model for non-inbred models such as the Sprague-Dawley rat, in that embryos from the left and right uterine horns of a given mother will be more genetically similar than offspring compared between mothers. We took advantage of this natural pairing by using a paired statistical approach which accounted for the inherent unbalanced nature of embryo numbers between horns and mothers. Other advantages for this model include the ability to precisely control the timing of hyperglycemic exposure, thus allowing study of its effects at narrow stages of embryogenesis. Likewise, the dose of infused glucose can be precisely controlled and varied. This advantage is partly offset by the difficulty in knowing the exact degree of hyperglycemia to which the embryos are exposed in real time, as there is currently no experimental approach to accurately measure uterine artery blood glucose. The glucose infusion rate utilized in this study is expected to have produced considerable hyperglycemia as might occur during very poorly controlled diabetes. This degree of hyperglycemia might induce embryopathy in part via an osmotic effect, which could be assessed in future studies by infusion of a non-fully metabolized but osmotically active carbohydrate such as L-glucose. We initially envisioned placing a symmetrically identical catheter for the right uterine artery as a control. However, the vascular anatomy on the right does not perfectly mirror that on the left, and thus an identical catheter control for the right was not possible. Because of anatomic differences between the two uterine horns, it is conceivable that hyperglycemic infusion into the right uterine horn might produce a different embryopathy rate than that observed during hyperglycemic infusion into the left uterine artery. As a control we assessed for teratogenic effects of the catheter and saline infusion on the left side, showing that catheter and infusion are not inherently embryopathic at baseline or in the presence of a teratogenic potential.
This model could be applied to other suspected teratogens that produce complex systemic effects in order to determine whether the agent is directly teratogenic. An experimental requirement for this approach is that the agent under study is cleared rapidly from the maternal circulation such that it does not build up to an appreciable degree systemically in the mother or contralateral uterine horn.
In summary, these results show that exposure to hyperglycemia during key portions of development is highly embryopathic and provide in vivo support for the fuel mediated teratogenesis hypothesis. Our findings thus reinforce the need for strict avoidance of hyperglycemia during organogenesis in women with pregestational diabetes.
Supplementary Material
Highlights.
Glucose was infused into the left uterine artery on gestational days 7–9.
Mothers, and thus the right uterine horn blood supply, had normal glucoses.
Embryopathy was significantly elevated only among the left uterine embryos.
Thus, systemic hyperglycemia is not necessary for diabetic embryopathy.
This new approach separates local from systemic embryopathic actions.
Acknowledgments
The authors thank Drs. Frank Morriss and Jeffrey Murray for comments, and Steven Bullard, Tyler Fisher, and Dr. Jianrong Yao (all of University of Iowa) for technical assistance.
Funding
This research was funded by grants R01 DK081548 (to A.W.N.), NIH T32 HL077344 (to M.L.B.), the Fraternal Order of Eagles Diabetes Research Center (to A.W.N.), American Diabetes Association Amaranth Fund 1-08-RA-142 (to A.W.N.), and the Children’s Miracle Network (to M.L.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Jensen DM, Damm P, Moelsted-Pedersen L, Ovesen P, Westergaard JG, Moeller M, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27:2819–2823. doi: 10.2337/diacare.27.12.2819. [DOI] [PubMed] [Google Scholar]
- 2.Bell R, Glinianaia SV, Tennant PWG, Bilous RW, Rankin J. Peri-conception hyperglycaemia and nephropathy are associated with risk of congenital anomaly in women with pre-existing diabetes: a population-based cohort study. Diabetologia. 2012 doi: 10.1007/s00125-012-2455-y. [DOI] [PubMed] [Google Scholar]
- 3.Al-Agha R, Firth RG, Byrne M, Murray S, Daly S, Foley M, et al. Outcome of pregnancy in type 1 diabetes mellitus (T1DMP): results from combined diabetes-obstetrical clinics in Dublin in three university teaching hospitals (1995–2006) Ir J Med Sci. 2012;181:105–109. doi: 10.1007/s11845-011-0781-6. [DOI] [PubMed] [Google Scholar]
- 4.Weintrob N, Karp M, Hod M. Short- and long-range complications in offspring of diabetic mothers. J Diabetes Complicat. 1996;10:294–301. doi: 10.1016/1056-8727(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 5.Reece EA, Homko CJ. Prepregnancy care and the prevention of fetal malformations in the pregnancy complicated by diabetes. Clin Obstet Gynecol. 2007;50:990–997. doi: 10.1097/GRF.0b013e31815a634b. [DOI] [PubMed] [Google Scholar]
- 6.Becerra JE, Khoury MJ, Cordero JF, Erickson JD. Diabetes mellitus during pregnancy and the risks for specific birth defects: a population-based case-control study. Pediatrics. 1990;85:1–9. [PubMed] [Google Scholar]
- 7.Correa A, Gilboa SM, Besser LM, Botto LD, Moore CA, Hobbs CA, et al. Diabetes mellitus and birth defects. Am J Obstet Gynecol. 2008;199:237.e1–237.e9. doi: 10.1016/j.ajog.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zabihi S, Loeken MR. Understanding diabetic teratogenesis: where are we now and where are we going? Birth Defects Res Part A Clin Mol Teratol. 2010;88:779–790. doi: 10.1002/bdra.20704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wentzel P, Ejdesjö A, Eriksson UJ. Maternal diabetes in vivo and high glucose in vitro diminish GAPDH activity in rat embryos. Diabetes. 2003;52:1222–1228. doi: 10.2337/diabetes.52.5.1222. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Weng H, Xu C, Reece EA, Yang P. Oxidative stress-induced JNK1/2 activation triggers proapoptotic signaling and apoptosis that leads to diabetic embryopathy. Diabetes. 2012;61:2084–2092. doi: 10.2337/db11-1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Viana M, Thirumangalathu S, Loeken MR. AMP-activated protein kinase mediates effects of oxidative stress on embryo gene expression in a mouse model of diabetic embryopathy. Diabetologia. 2012;55:245–254. doi: 10.1007/s00125-011-2326-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Zhiyong, Wu Y-K, Reece EA. Demonstration of the essential role of protein kinase C isoforms in hyperglycemia-induced embryonic malformations. Reprod Sci. 2008;15:349–356. doi: 10.1177/1933719108316986. [DOI] [PubMed] [Google Scholar]
- 13.Freinkel N. Banting Lecture 1980. Of pregnancy and progeny. Diabetes. 1980;29:1023–1035. doi: 10.2337/diab.29.12.1023. [DOI] [PubMed] [Google Scholar]
- 14.Jovanovic L, Metzger BE, Knopp RH, conley MR, Park E, Lee YJ, et al. The Diabetes in Early Pregnancy Study: beta-hydroxybutyrate levels in type 1 diabetic pregnancy compared with normal pregnancy. NICHD-Diabetes in Early Pregnancy Study Group (DIEP). National Institute of Child Health and Development. Diabetes Care. 1998;21:1978–1984. doi: 10.2337/diacare.21.11.1978. [DOI] [PubMed] [Google Scholar]
- 15.Montelongo A, Lasunción MA, Pallardo LF, Herrera E. Longitudinal study of plasma lipoproteins and hormones during pregnancy in normal and diabetic women. Diabetes. 1992;41:1651–1659. doi: 10.2337/diab.41.12.1651. [DOI] [PubMed] [Google Scholar]
- 16.Galindo A, Burguillo AG, Azriel S, de la Fuente P. Outcome of fetuses in women with pregestational diabetes mellitus. J Perinat Med. 2006;34:323–331. doi: 10.1515/JPM.2006.062. [DOI] [PubMed] [Google Scholar]
- 17.Pearson DWM, Kernaghan D, Lee R, Penney GC. The relationship between pre-pregnancy care and early pregnancy loss, major congenital anomaly or perinatal death in type I diabetes mellitus. BJOG. 2007;114:104–107. doi: 10.1111/j.1471-0528.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 18.Evers IM, de Valk HW, Visser GHA. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ. 2004;328:915. doi: 10.1136/bmj.38043.583160.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fraser RB, Waite SL, Wood KA, Martin KL. Impact of hyperglycemia on early embryo development and embryopathy: in vitro experiments using a mouse model. Hum Reprod. 2007;22:3059–3068. doi: 10.1093/humrep/dem318. [DOI] [PubMed] [Google Scholar]
- 20.Cockroft DL, Coppola PT. Teratogenic effects of excess glucose on head-fold rat embryos in culture. Teratology. 1977;16:141–146. doi: 10.1002/tera.1420160205. [DOI] [PubMed] [Google Scholar]
- 21.Sadler TW, Hunter ES, Wynn RE, Phillips LS. Evidence for multifactorial origin of diabetes-induced embryopathies. Diabetes. 1989;38:70–74. doi: 10.2337/diab.38.1.70. [DOI] [PubMed] [Google Scholar]
- 22.Freinkel N, Cockroft DL, Lewis NJ, Gorman L, Akazawa S, Phillips LS, et al. The 1986 McCollum award lecture. Fuel-mediated teratogenesis during early organogenesis: the effects of increased concentrations of glucose, ketones, or somatomedin inhibitor during rat embryo culture. Am J Clin Nutr. 1986;44:986–995. doi: 10.1093/ajcn/44.6.986. [DOI] [PubMed] [Google Scholar]
- 23.Buchanan TA, Denno KM, Sipos GF, Sadler TW. Diabetic teratogenesis. In vitro evidence for a multifactorial etiology with little contribution from glucose per se. Diabetes. 1994;43:656–660. doi: 10.2337/diab.43.5.656. [DOI] [PubMed] [Google Scholar]
- 24.Fine EL, Horal M, Chang TI, Fortin G, Loeken MR. Evidence that elevated glucose causes altered gene expression, apoptosis, and neural tube defects in a mouse model of diabetic pregnancy. Diabetes. 1999;48:2454–2462. doi: 10.2337/diabetes.48.12.2454. [DOI] [PubMed] [Google Scholar]
- 25.Hirano T, Mamo JC, Furukawa S, Nagano S, Takahashi T. Effect of acute hyperglycemia on plasma triglyceride concentration and triglyceride secretion rate in non-fasted rats. Diabetes Res Clin Pract. 1990;9:231–238. doi: 10.1016/0168-8227(90)90050-4. [DOI] [PubMed] [Google Scholar]
- 26.Flakoll PJ, Hill JO, Abumrad NN. Acute hyperglycemia enhances proteolysis in normal man. Am J Physiol. 1993;265:E715–E721. doi: 10.1152/ajpendo.1993.265.5.E715. [DOI] [PubMed] [Google Scholar]
- 27.Winhofer Y, Krssák M, Jankovic D, Anderwald C-H, Reiter G, Hofer A, et al. Short-term hyperinsulinemia and hyperglycemia increase myocardial lipid content in normal subjects. Diabetes. 2012;61:1210–1216. doi: 10.2337/db11-1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marfella R, Quagliaro L, Nappo F, Ceriello A, Giugliano D. Acute hyperglycemia induces an oxidative stress in healthy subjects. Journal of Clinical Investigation. 2001;108:635–636. doi: 10.1172/JCI13727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, et al. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 30.Ling P-R, Mueller C, Smith RJ, Bistrian BR. Hyperglycemia induced by glucose infusion causes hepatic oxidative stress and systemic inflammation, but not STAT3 or MAP kinase activation in liver in rats. Metab Clin Exp. 2003;52:868–874. doi: 10.1016/s0026-0495(03)00057-x. [DOI] [PubMed] [Google Scholar]
- 31.Yao J, Wang C, Walsh SA, Hu S, Sawatzke AB, Dang D, et al. Localized fetomaternal hyperglycemia: spatial and kinetic definition by positron emission tomography. PLoS ONE. 2010;5:e12027. doi: 10.1371/journal.pone.0012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kistler A. Teratogenesis of retinoic acid in rats: susceptible stages and suppression of retinoic acid-induced limb malformations by cycloheximide. Teratology. 1981;23:25–31. doi: 10.1002/tera.1420230106. [DOI] [PubMed] [Google Scholar]
- 33.Chi MM, Pingsterhaus J, Carayannopoulos M, Moley KH. Decreased glucose transporter expression triggers BAX-dependent apoptosis in the murine blastocyst. J Biol Chem. 2000;275:40252–40257. doi: 10.1074/jbc.M005508200. [DOI] [PubMed] [Google Scholar]
- 34.Wentzel P, Thunberg L, Eriksson UJ. Teratogenic effect of diabetic serum is prevented by supplementation of superoxide dismutase and N-acetylcysteine in rat embryo culture. Diabetologia. 1997;40:7–14. doi: 10.1007/s001250050636. [DOI] [PubMed] [Google Scholar]
- 35.Ejdesjö A, Wentzel P, Eriksson UJ. Genetic and environmental influence on diabetic rat embryopathy. Am J Physiol Endocrinol Metab. 2011;300:E454–E467. doi: 10.1152/ajpendo.00543.2010. [DOI] [PubMed] [Google Scholar]
- 36.Eriksson UJ, Wentzel P, Minhas HS, Thornalley PJ. Teratogenicity of 3-deoxyglucosone and diabetic embryopathy. Diabetes. 1998;47:1960–1966. doi: 10.2337/diabetes.47.12.1960. [DOI] [PubMed] [Google Scholar]
- 37.Konietschke F, Pauly M. Bootstrapping and permuting paired t-test type statistics. Stat Comput n.d. :1–14. [Google Scholar]
- 38.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Google Scholar]
- 39.Wentzel P, Jansson L, Eriksson UJ. Diabetes in pregnancy: uterine blood flow and embryonic development in the rat. Pediatr Res. 1995;38:598–606. doi: 10.1203/00006450-199510000-00021. [DOI] [PubMed] [Google Scholar]
- 40.Eriksson RS, Thunberg L, Eriksson UJ. Effects of interrupted insulin treatment on fetal outcome of pregnant diabetic rats. Diabetes. 1989;38:764–772. doi: 10.2337/diab.38.6.764. [DOI] [PubMed] [Google Scholar]
- 41.Miller E, Hare JW, Cloherty JP, Dunn PJ, Gleason RE, Soeldner JS, et al. Elevated maternal hemoglobin A1c in early pregnancy and major congenital anomalies in infants of diabetic mothers. N Engl J Med. 1981;304:1331–1334. doi: 10.1056/NEJM198105283042204. [DOI] [PubMed] [Google Scholar]
- 42.Gareskog M, Cederberg J, Eriksson UJ, Wentzel P. Maternal diabetes in vivo and high glucose concentration in vitro increases apoptosis in rat embryos. Reprod Toxicol. 2007;23:63–74. doi: 10.1016/j.reprotox.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Buchanan T. Diabetes Mellitus: A Fundamental and Clinical Text. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. Effects of maternal diabetes mellitus on intrauterine development; pp. 1251–1265. [Google Scholar]
- 44.Robinson JF, Verhoef A, Pennings JLA, Pronk TE, Piersma AH. A comparison of gene expression responses in rat whole embryo culture and in vivo: time-dependent retinoic acid-induced teratogenic response. Toxicol Sci. 2012;126:242–254. doi: 10.1093/toxsci/kfr342. [DOI] [PubMed] [Google Scholar]
- 45.Robinson JF, Verhoef A, Piersma AH. Transcriptomic analysis of neurulation and early organogenesis in rat embryos: an in vivo and ex vivo comparison. Toxicol Sci. 2012;126:255–266. doi: 10.1093/toxsci/kfr343. [DOI] [PubMed] [Google Scholar]
- 46.Beaudoin AR, Fisher DL. An in vivo/in vitro evaluation of teratogenic action. Teratology. 1981;23:57–61. doi: 10.1002/tera.1420230108. [DOI] [PubMed] [Google Scholar]
- 47.Jawerbaum A, White V. Animal models in diabetes and pregnancy. Endocr Rev. 2010;31:680–701. doi: 10.1210/er.2009-0038. [DOI] [PubMed] [Google Scholar]
- 48.Leung MBW, Choy K-W, Copp AJ, Pang C-P, Shum ASW. Hyperglycaemia potentiates the teratogenicity of retinoic acid in diabetic pregnancy in mice. Diabetologia. 2004;47:515–522. doi: 10.1007/s00125-004-1350-6. [DOI] [PubMed] [Google Scholar]
- 49.Toschi E, Camastra S, Sironi AM, Masoni A, Gastaldelli A, Mari A, et al. Effect of acute hyperglycemia on insulin secretion in humans. Diabetes. 2002;51(Suppl 1):S130–S133. doi: 10.2337/diabetes.51.2007.s130. [DOI] [PubMed] [Google Scholar]
- 50.Tsintzas K, Norton L, Chokkalingam K, Nizamani N, Cooper S, Stephens F, et al. Independent and combined effects of acute physiological hyperglycaemia and hyperinsulinaemia on metabolic gene expression in human skeletal muscle. Clin Sci. 2013;124:675–684. doi: 10.1042/CS20120481. [DOI] [PubMed] [Google Scholar]
- 51.Buelke-Sam J, Holson JF, Nelson CJ. Blood flow during pregnancy in the rat: II. Dynamics of and litter variability in uterine flow. Teratology. 1982;26:279–288. doi: 10.1002/tera.1420260310. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.