Abstract
Double Holliday junctions (dHJS) are important intermediates of homologous recombination. The separate junctions can each be cleaved by DNA structure-selective endonucleases known as Holliday junction resolvases. Alternatively, double Holliday junctions can be processed by a reaction known as “double Holliday junction dissolution.” This reaction requires the cooperative action of a so-called “dissolvasome” comprising a Holliday junction branch migration enzyme (Sgs1/BLM RecQ helicase) and a type IA topoisomerase (Top3/TopoIIIα) in complex with its OB (oligonucleotide/oligosaccharide binding) fold containing accessory factor (Rmi1). This review details our current knowledge of the dissolution process and the players involved in catalyzing this mechanistically complex means of completing homologous recombination reactions.
Double Holliday junctions (dHJs) form during the repair of double-strand breaks by homologous recombination. A sophisticated reaction involving a “dissolvasome” complex is a major route for dissipation of these dHJs.
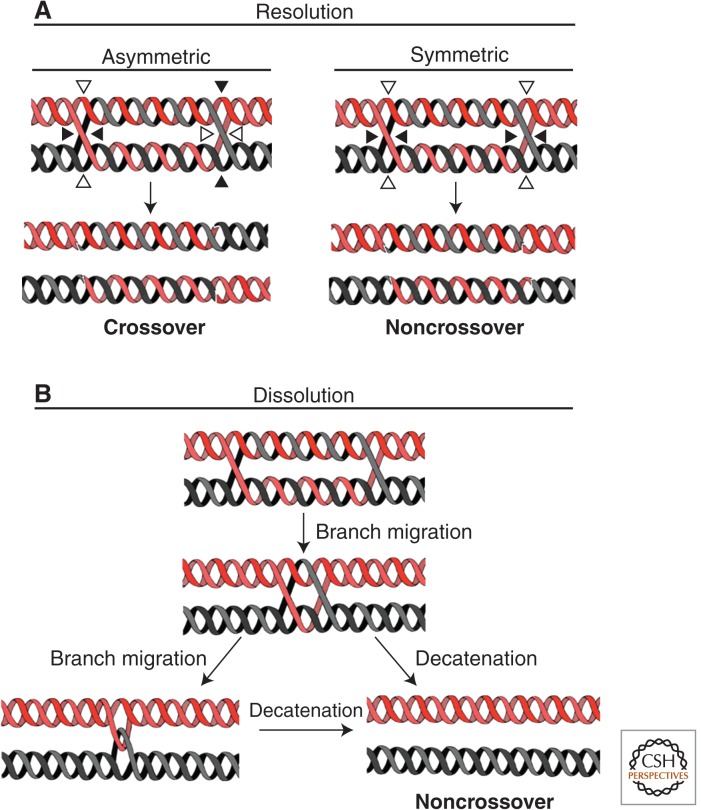
For decades, homologous recombination (HR) was defined as a mechanism for the production of new allelic combinations during meiosis because it can generate so-called crossing-over (see Mehta and Haber 2014). Crossovers are likely generated by the asymmetric cleavage of a key intermediate in HR, the dHJ, by the action of structure-selective endonucleases called “resolvases” (Fig. 1A) (see Wyatt and West 2014). In addition to its essential function during meiosis, HR has proven to be a crucial DNA repair pathway in mitotic cells. Precisely because it has the potential to generate crossing-over, the resolution of dHJ by resolvases affords a high risk of genomic instability in these circumstances. Indeed, when HR is engaged between two homologous chromosomes or two homeologous sequences, dHJ resolution could lead, respectively, to loss of heterozygosity or gross chromosomal rearrangements. Thus, an alternative mechanism allowing dHJ processing without crossing-over would appear essential when HR is used for DNA repair. Such a mechanism, termed dHJ dissolution, is thought to be a major route for dissipation of dHJs arising from HR repair (LaRocque et al. 2011; Krejci et al. 2012). During dHJ dissolution, the two HJs are branch migrated toward one another until they form a hemicatenated intermediate that can be decatenated by a topoisomerase (Fig. 1B). This sophisticated reaction is performed by the so-called “dissolvasome” complex composed of a specific RecQ helicase (BLM in humans/Sgs1 in budding yeast) and a type IA topoisomerase known as topoisomerase III (Fig. 2; for general reviews about RecQ helicases and topoisomerases, see Champoux 2001; Wang 2002; Bachrati and Hickson 2003; Viard and de la Tour 2007; Chu and Hickson 2009; Vindigni and Hickson 2009.
Figure 1.
Double Holliday junction processing pathways. (A) During HJ resolution, each HJ of a dHJ is cleaved by a structure-selective endonuclease (resolvase). Depending on the combination of cleavage orientations, which can be asymmetric or symmetric, this process can generate both crossover and noncrossover products. In contrast, during dissolution (B), each strand engaged in the dHJ is reassociated with its original complementary strand, preventing exchange of genetic material between the two homologous sequences (and hence generating exclusively noncrossover products). DHJ dissolution (B) is initiated by migration of the HJs toward one another. The fusion/collapse of the two HJs results in a hemicatenated intermediate. Decatenation of this intermediate regenerates the original DNA species present before the initiation of HR.
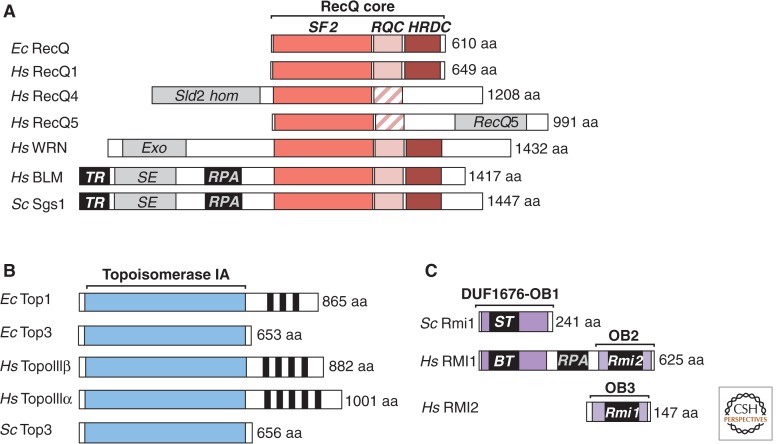
Figure 2.
Domain organization of RecQ helicases, topoisomerases IA, and RMI proteins. (A) Most of the RecQ helicase members share a superfamily 2 helicase domain (SF2), a RecQ conserved domain (RQC), and a helicase and RNase D carboxy-terminal domain (HRDC). Besides this “RecQ core” domain, some RecQ helicases contain amino-terminal and carboxy-terminal extensions that vary in size, sequence, and functionality (e.g., SLD2 homology domain in RECQ4, and a signature motif in the carboxy-terminal domain of RECQ5). The hatched boxes denote partially degenerate RQC domains. BLM/Sgs1 helicases share a common domain organization, including an amino-terminal extension that includes domains for interaction with both TopoIII/RMI1 (TR) and replication protein A (RPA), in addition to a region that has been proposed to be required for DNA strand exchange (SE) activity. (B) All type IA topoisomerases contain a conserved catalytic domain (topoisomerase IA). Some topoisomerase IA enzymes also exhibit a carboxy-terminal extension, frequently composed of zinc finger motifs (black boxes), which is believed to mediate protein–DNA and protein–protein interactions. The contribution of the carboxy-terminal extension to dissolution is unknown. The regions interacting with other components of the dissolvasome are unknown. (C) In RMI1 proteins, only the DUF1676 and the OB-fold domain 1 (OB1) are conserved from yeast to human. The OB1 associates with both BLM/Sgs1 and topoisomerase III (BT/ST). In addition, human RMI1 exhibits a carboxy-terminal extension, composed of a middle region, which mediates RPA binding, and a second OB fold (OB2), which is able to associate with RMI2. RMI2 is also an OB-fold protein (OB3) that stably associates with the dissolvasome in human cells. In total, therefore, the human RMI1/2 complex contains three OB folds.
In this review, we first take a historical look at the experimental evidence that led some groups to formulate the proposal that a reaction akin to dissolution must exist, and which then led Wu and Hickson (2003) to confirm its existence by reconstitution of the dissolution reaction in vitro using purified proteins. Following that, we will review the individual and combined roles of the components of what we will term the dHJ dissolvasome. Although many mechanistic aspects of dHJ dissolution remain obscure, several biochemical studies have provided a general understanding of this conceptually simple, but mechanistically complex, reaction.
BACKGROUND TO THE DISCOVERY OF DHJ DISSOLUTION
Well before the discovery of the dHJ dissolution process, the two catalytic subunits of the dissolvasome, a specific RecQ helicase and a type IA topoisomerase, were individually known for their crucial role in preventing genomic instability generated during HR.
Background on RecQ Helicases
Although the first RecQ helicase family member to be identified was RecQ of Escherichia coli, this review will focus on the eukaryotic homologs, because they are better characterized in terms of their interactions with dHJs (for a detailed review on RecQ of E. coli, see Nakayama 2005). Nevertheless, it is clear that the malfunction of a RecQ helicase causes genome instability in all organisms (for review, see Chu and Hickson 2009; Bernstein et al. 2010; Larsen and Hickson 2013). In particular, a consistent observation in recQ helicase mutants is an increase in the frequency of crossing-over, which is generally detected by scoring the level of sister chromatid exchanges (SCEs) or loss of heterozygosity between homologs. For example, Saccharomyces cerevisiae sgs1 mutants show elevated levels of mitotic HR, illegitimate recombination (Gangloff et al. 1994; Watt et al. 1996; Yamagata et al. 1998; Onoda et al. 2000), and gross chromosomal rearrangements (Myung et al. 2001; Myung and Kolodner 2002). More specifically, Ira et al. (2003) showed that sgs1 mutants have an elevated frequency of crossing-over during HR, suggesting a role of Sgs1 in crossover suppression. Among the five human RecQ homologs, BLM is believed to be an Sgs1 ortholog. Sgs1 and BLM share similar sequences, domain architecture, and functionality (Fig. 2) (Kusano et al. 1999). BLM is defective in a rare autosomal recessive disorder called Bloom’s syndrome (BS) characterized by growth retardation, immune deficiency, reduced fertility, sensitivity to sunlight, and a high risk of developing various types of cancer (German 1993; Luo et al. 2000). Cells derived from BS patients show genomic instability, including the hallmark feature of an elevated frequency of crossover recombination—as scored by measuring SCE (Chaganti et al. 1974; German 1993).
Background on Type IA Topoisomerases
All topoisomerases are crucial for the proper transmission of the genetic information caused by their essential role during maintenance of cellular DNA supercoiling homeostasis and decatenation of sister chromatids in mitosis (for review, see Champoux 2001; Wang 2002; Viard and de la Tour 2007). It is worth noting that a subclass of topoisomerases, known as the type IA topoisomerase, plays little or no direct role in chromosome segregation or in regulating DNA supercoiling. Nevertheless, type IA topoisomerases are strongly conserved throughout evolution (Fig. 2) (Forterre and Gadelle 2009), and their essential nature has been emphasized by a plethora of genetic studies. Higher eukaryotic cells have two type IA topoisomerases, TopoIIIα and TopoIIIβ. Although TopoIIIα is involved in DHJ dissolution, the function of TopoIIIβ remains unclear and will not be discussed further here. Deletion of the gene encoding TopoIIIα leads to a lethal phenotype associated with severe developmental defects in mice, Drosophila melanogaster, and Arabidopsis thaliana (Li and Wang 1998; Plank et al. 2005; Hartung et al. 2007). In chicken DT40 cells, TopoIIIα depletion also causes cell death associated with dramatic genomic instability (Seki et al. 2006). In Schizosaccharomyces pombe, deletion of top3+ is also lethal and cell death is associated with chromosome missegregation (Goodwin et al. 1999; Maftahi et al. 1999; Oh et al. 2002). In contrast, S. cerevisiae top3Δ strains are viable, but are very slow growing, and show a pleiotropic phenotype, including hyperrecombination, sensitivity to DNA-damaging agents, defects in accurate chromosome segregation, and a failure to complete meiosis (Wallis et al. 1989; Gangloff et al. 1994; Chakraverty et al. 2001).
Genetic and Physical Interactions between RecQ Helicases and Topoisomerase III
A possible functional interaction between the Sgs1/BLM RecQ helicases and type IA topoisomerases was first suggested by studies showing that mutations in their genes genetically interact. For the most part, mutations in SGS1 and TOP3 are epistatic, and indeed deletion of SGS1 is a strong suppressor of the drastic growth and DNA repair defects in top3 mutants (Gangloff et al. 1994, 1999; Watt et al. 1996). A similar genetic interaction has also been found in S. pombe, where deletion of the SGS1 homolog, rqh1+, can rescue the lethality associated with top3Δ (Goodwin et al. 1999; Maftahi et al. 1999). In DT40 cells, the disruption of the RecQ helicase BLM suppresses genomic instability defects associated with TopIIIα depletion, without actually preventing cell death (Seki et al. 2006). In addition to this conserved genetic interaction, Sgs1/BLM RecQ helicases and type IA topoisomerases physically interact, as has been shown in S. cerevisiae between Top3 and Sgs1 (Bennett et al. 2000; Fricke et al. 2001), in S. pombe between Top3 and Rqh1 (Laursen et al. 2003; Ahmad and Stewart 2005), and in human cells between BLM and TopoIIIα (Johnson et al. 2000; Wu et al. 2000). More important, this physical interaction is crucial for Sgs1/BLM to maintain genomic stability in vivo, because the first 100 aa residues of Sgs1, which mediate the physical association with Top3, are required for the complementation of the sgs1 phenotypes (Gangloff et al. 1994; Bennett et al. 2000; Duno et al. 2000; Mullen et al. 2000; Fricke et al. 2001; Ui et al. 2001; Onodera et al. 2002; Ira et al. 2003; Weinstein and Rothstein 2008). Similarly, the putative TopoIIIα interaction domain of BLM is necessary for the suppression of the elevated SCE phenotype of BS cells (Wu et al. 2000; Hu et al. 2001).
Connections between RecQ Helicases/Type IA Topoisomerases and HR
The hyper-crossing-over phenotypes of cells lacking Sgs1/BLM and Top3/TopoIIIα suggest a role in HR. The search for other (non-sgs1) suppressors of top3Δ growth impairment in S. cerevisiae and S. pombe identified genes encoding proteins involved in the initial steps of HR, such as RAD51 and rad22+, respectively (Oakley et al. 2002; Shor et al. 2002; Laursen et al. 2003). Moreover, sgs1/top3 mutants accumulate unprocessed HR DNA intermediates following exposure to a DNA damaging agent (Liberi et al. 2005; Mankouri and Hickson 2006; Carotenuto and Liberi 2010). These data indicated that RecQ helicase/topoisomerase III act on DNA intermediates that build up during HR. The identification of those intermediates as being HJs was suggested by three additional facts. First, the viability of cells lacking BLM, Sgs1, and/or Top3 depends on functional DNA structure-selective endonucleases implicated as HJ resolvases such as Mus81-Mms4 (Kaliraman et al. 2001; Mullen et al. 2001; Andersen et al. 2011; Wechsler et al. 2011). Second, the synthetic lethality of sgs1 mus81 double mutants can be suppressed either by the overexpression of the bacterial HJ resolvase, RusA, or by the inactivation of the early steps of HR (Mullen et al. 2001; Fabre et al. 2002; Bastin-Shanower et al. 2003). Similar results were obtained in S. pombe (Boddy et al. 2000; Doe et al. 2002), and in D. melanogaster (Trowbridge et al. 2007; Andersen et al. 2011). Third, it was demonstrated that the HR intermediates accumulating in sgs1 and top3 mutants during a perturbed S-phase contain HJs (Bzymek et al. 2010; Ashton et al. 2011; Mankouri et al. 2011).
These genetic studies indicate that the RecQ-topoisomerase III complex actually plays a role in completion of HR, representing an alternative to the classical HJ resolvase pathway for the dissipation of dHJ in a way that prevents crossing-overs. Several groups proposed that two HJs could theoretically be dissipated without resolvases by a two-step mechanism involving the convergent branch migration of dHJ, followed by the release of hemicatenanes generated after the collapse of the two junctions (Fig. 1B) (Nasmyth 1982; Wang et al. 1990; Duguet 1997; Wu and Hickson 2001; Ira et al. 2003). Using an oligo-based dHJ substrate (OligoDHJ; Fig. 3), Wu and Hickson (2003) demonstrated that the BLM/TopoIIIα complex catalyzes such a reaction in vitro, shedding light on most of the observations accumulated during years of genetic analysis on those enzymes.
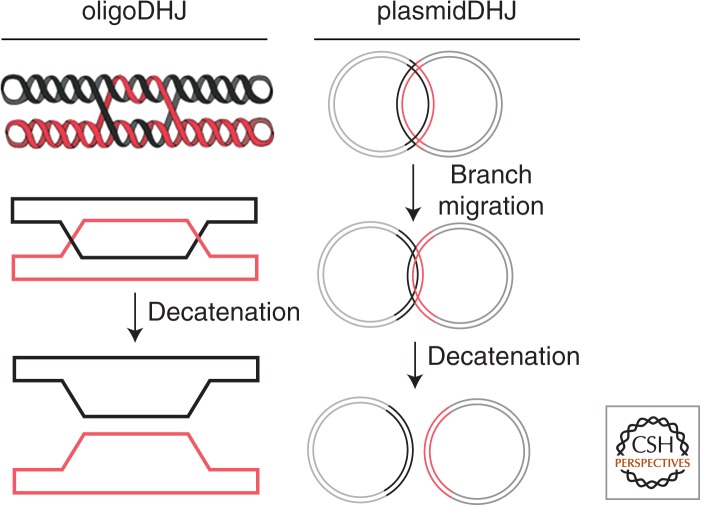
Figure 3.
DHJ substrates. Several substrates have been used to study HJ dissolution in vitro. The oligoDHJ substrate (left) is generated by the annealing and ligation of two oligonucleotides, leading to the formation of two HJ separated by 14 bp of quasi-homology. The processing of this substrate by the dissolvasome requires little or no branch migration allowing the investigation of the decatenation step. Following dissolution, the two strands forming the oligoDHJ substrate are physically separated and remain circular. The plasmidDHJ (right) comprises two circular dsDNA molecules associated though a region of homology (depicted in black and red). This substrate permits the analysis of the entire dissolution reaction, which in this case will lead to the complete separation of the two plasmids.
MECHANISTIC INSIGHT INTO THE DISSOLUTION OF A DOUBLE HJ (dHJ)
Two substrates are commonly used to study dissolution in vitro (Fig. 3). One is composed of two ligated oligonucleotides, which we will refer to as the oligoDHJ. This molecule can be viewed as the product of convergent branch migration at or near the point where decatenation can occur, allowing analysis of the decatenation step (Fu et al. 1994; Wu and Hickson 2003). Although this substrate is quite easy to prepare (Bachrati and Hickson 2009), it does not contain HJs that can migrate freely and, therefore, does not permit the biochemical analysis of the convergent branch migration step of dissolution. By contrast, the second, much larger substrate (termed plasmidDHJ) does contain two migratable HJs but is technically much more challenging to prepare (Plank and Hsieh 2006; Chen et al. 2013). The lack of a facile method for the preparation of a substrate containing two fully branch migratable HJs represents one important difficulty with conducting biochemical analysis of the dHJ dissolution reaction in vitro.
Importance of Protein Complex Formation for Dissolution
The genetic data reviewed above suggested that the physical interaction between the helicase and the topoisomerase component of the dissolvasome would be crucial for the dissolution reaction in vivo. Consistent with this, highly efficient dissolution is observed in vitro only when BLM or Sgs1 are incubated in the presence of the topoisomerase III enzyme from the same species. Moreover, the BLM/Sgs1 component cannot be replaced by another RecQ helicase (Wu and Hickson 2003; Wu et al. 2005; Plank et al. 2006; Raynard et al. 2006; Cejka et al. 2010). However, a BLM mutant lacking the first 212 residues shown to be responsible for interaction with TopoIIIα is as efficient as the full-length BLM in catalyzing dissolution of the oligoDHJ substrate (Wu et al. 2005). Together, these data indicate that BLM/TopoIIIα physical interaction is not absolutely required for dissolution in vitro. Consistent with this, the reaction can occur, albeit quite inefficiently, when BLM/Sgs1 is utilized together with a noncognate type IA topoisomerase (Wu et al. 2006). Hence, beside their ability to interact together, BLM/Sgs1 and type IA topoisomerases must exhibit specific properties that allow them to catalyze the different steps leading to HJ dissolution.
The Decatenation Step
The hemicatenated DNA structure generated by the fusion/collapse of two HJs is the key intermediate in dHJ dissolution. Although the actual structure of two fused HJs is not known, it is likely to correspond to a combination of one or more single-stranded catenanes (Fig. 1B). Among all topoisomerases, only type IA topoisomerases can perform the decatenation reaction required for the topological separation of the two duplexes (Wu and Hickson 2003; Plank et al. 2006; Wu et al. 2006; Cejka et al. 2010). This specificity has been attributed to the unique ability of type IA topoisomerases to catalyze efficient single stranded decatenation (Brown and Cozzarelli 1981; Harmon et al. 1999; Yang et al. 2010; Cejka et al. 2012). The specific requirement of a type IA topoisomerase during the last step of HJ dissolution may also reside in their affinity for complex DNA structures. Indeed, both yTop3 and hTopoIIIα exhibit remarkable affinity for HJ-containing substrates that are largely double stranded in character (Fig. 3) (Wu et al. 2006; Chen and Brill 2007). This is surprising because catalysis by the type IA topoisomerase requires the presence of single-stranded DNA (ssDNA) in the substrate (Srivenugopal et al. 1984; Kirkegaard and Wang 1985; Kim and Wang 1992; Chen and Brill 2007). Type IA topoisomerases may be viewed, therefore, as DNA structure-selective topoisomerases, making them ideally suited for the processing of complex DNA structures.
Function of the RecQ Helicase during Decatenation
Despite the above discussion, the type IA topoisomerase cannot perform efficient decatenation of a dHJ alone, because this requires the presence of the RecQ helicase. Moreover, the helicase likely plays an active role during decatenation as evidenced by the requirement of its ATPase activity for the reaction (Wu and Hickson 2003; Raynard et al. 2006; Bussen et al. 2007; Cejka et al. 2010; Yang et al. 2010; Chen et al. 2014). It is likely that the RecQ unwinding activity remodels the substrate into a conformation that can be efficiently processed by the type IA topoisomerase—for instance, by providing accessible ssDNA. Indeed, Sgs1/BLM helicases are known to stimulate both the relaxation and decatenation activities of Top3/TopoIIIα (Wu and Hickson 2002; Yang et al. 2010; Cejka et al. 2012). However, none of the other RecQ helicases tested thus far can assist the type IA topoisomerase during DHJ decatenation (Wu and Hickson 2003; Wu et al. 2005; Plank et al. 2006; Cejka et al. 2010). This observation suggests that the Sgs1/BLM subfamily might possess functional domain(s) that are missing from or altered in other RecQ helicases and that are crucial during dissolution. For instance, the unique HRDC domain of BLM is absolutely required for dissolution (Bernstein and Keck 2005; Wu et al. 2005). This domain displays specific DNA binding properties allowing BLM to specifically bind to and unwind a HJ (Janscak et al. 2003; Wu et al. 2005). More important, amino-terminal extensions of several RecQ helicases are known to carry additional activities providing a functional specialization (Fig. 2) (Huang et al. 1998; Matsuno et al. 2006). Although the extreme amino-terminal region of BLM/Sgs1 where TopoIIIα/Top3 binds is dispensable for efficient dissolution of the oligoDHJ substrate (Wu et al. 2005), a function for the extended amino-terminal region during catalysis of convergent branch migration should not be excluded.
Convergent Branch Migration Step
If the type IA topoisomerase surely provides the crucial catalytic activity during decatenation, the RecQ helicase is certainly the central catalytic component of the dissolvasome during convergent branch migration. Indeed, many RecQ helicases, including BLM and Sgs1, exhibit efficient branch migration activities on a single HJ (Constantinou et al. 2000; Karow et al. 2000; Bugreev et al. 2008; Cejka and Kowalczykowski 2010). The mechanism of this branch migration activity is unknown, but it might result from the association of the unwinding and annealing activities of RecQ helicases (Cheok et al. 2005). More important, besides the fact that many RecQ helicases can catalyze single HJ branch migration, thus far only the Sgs1/BLM subtype has a proven role during dHJ dissolution. This specificity highlights once again the potential existence of atypical properties shared only by Sgs1 and BLM. For example, the substrate specificity provided by the poorly conserved HRDC domain of the BLM/Sgs1 helicases could play a crucial function during convergent branch migration (Wu et al. 2005). An alternative model, yet to be demonstrated, proposes that the RecQ core domain simply functions in the translocation of the protein along ssDNA, whereas it is the amino-terminal domain that catalyzes branch migration via a strand exchange activity (Chen and Brill 2010).
Function of the Type IA Topoisomerase during Convergent Branch Migration
In the context of a topologically closed dHJ (exemplified by the plasmidDHJ substrate), each strand of a duplex is associated with its complementary strand in the other duplex through base pairing and topological linking (Fig. 3). Therefore, the initial step of dHJ dissolution cannot be catalyzed by a helicase alone; instead, it requires the combined action of a helicase and a topoisomerase to permit the two HJs to converge. According to the twin supercoiling domain model, branch migration catalyzed by a helicase will lead to the redistribution of topological links, with an accumulation of positive supercoils between the two junctions and negative supercoils behind them (Liu and Wang 1987; Plank et al. 2006). These torsional stresses must be dissipated by the action of a topoisomerase. Indeed, in vitro, Sgs1 together with wheat germ TopoIB, which exhibits an efficient relaxation activity of both positive and negative supercoils, can catalyze convergent branch migration of the plasmidDHJ to generate products corresponding to a nearly or completely fused HJ (Cejka et al. 2010). However, the final decatenation step still requires a type IA topoisomerase. This observation indicates that, at least in vitro, Sgs1 does not require the presence of its Top3 partner to catalyze convergent branch migration, and that the dissipation of the topological stress generated during convergent branch migration can occur through an “uncoupled” mechanism. Having said that, as a component of the same complex, the type IA topoisomerase subunit of the dissolvasome likely supports this function in vivo. Consistent with this, when assayed on the plasmidDHJ substrate, the association of Sgs1 or D. melanogaster BLM with its cognate type IA topoisomerase formed a proficient branch migrating machine (Plank et al. 2006; Cejka et al. 2010). The precise mechanism by which Top3 assists the efficient convergent branch migration catalyzed by Sgs1 in a topologically closed substrate remains unclear, mainly because of the well-established inefficiency of type IA topoisomerases to relax either negative or positive supercoils. However, one must consider that the affinity of type IA topoisomerases for a four-way junction, as well as its association with a cognate RecQ helicase, would allow its positioning in the vicinity of the HJ and provide the ssDNA necessary for relaxation of torsional stress (Cejka et al. 2012). One could also speculate that the type IA topoisomerase directly manipulates the topology of the HJ itself, allowing the branch migration to occur without generating torsional stress (Duguet 1997; Plank and Hsieh 2009).
In the previous paragraphs, we have attempted to describe the individual contribution of the two catalytic subunits of the dissolvasome during the convergent branch migration and decatenation steps of dissolution. However, the dissolution reaction appears to result not only from the simple addition of two individual activities, but also from a potential intricate cooperation of these activities, emphasizing the complex nature of dissolution reaction. This view is further strengthened by the presence and the function of the third component of the minimal dissolvasome core complex, RMI1.
RMI1
Rmi1, an Integral Component of the Core Dissolvasome
In S. cerevisae, two independent approaches led to the identification of Rmi1 as a candidate protein functioning alongside Sgs1 and Top3 in the maintenance of genomic stability (Bellaoui et al. 2003; Tong et al. 2004). rmi1 mutants phenocopy all investigated Δtop3-associated phenotypes including slow growth, sensitivity to DNA damaging agents, an increased rate of mitotic recombination, meiotic defects, gross chromosomal rearrangements, and an accumulation of HR intermediates (Chang et al. 2005; Mullen et al. 2005; Ashton et al. 2011). Moreover, Δrmi1 is synthetically lethal with mutations in genes encoding putative HJ resolvases, and this synthetic lethality can be suppressed by inactivation of early steps of HR. Finally, rmi1 is epistatic to top3, and the slow growth phenotype of Δrmi1 strains can be suppressed by mutations in the SGS1 gene (Chang et al. 2005; Mullen et al. 2005). Rmi1 is a conserved protein, being present in nearly all eukaryotes although no ortholog has been found in D. melanogaster (Yin et al. 2005; Chen et al. 2012). As with TopoIIIα, Rmi1 homologs are essential in S. pombe, A. thaliana, and mice (Chang et al. 2005; Hartung et al. 2008; Chen et al. 2011; Guiraldelli et al. 2013). Finally, in HeLa cells, depletion of RMI1 by siRNA is associated with an increased level of SCE (Yin et al. 2005).
The confirmation that Rmi1 is an integral and conserved component of the dissolvasome came with the finding that the human homolog of yeast Rmi1, RMI1 (originally designated BLAP75, a BLM-associated protein of 75 kDa) forms a stable complex with both BLM and TopoIIIα in vivo and in vitro (Meetei et al. 2003; Yin et al. 2005; Raynard et al. 2006). Similarly, Rmi1 was also found in a complex with Sgs1 and Top3 in yeast (Chang et al. 2005; Mullen et al. 2005; Chen and Brill 2007). Although Rmi1/RMI1 seems to interact independently with Sgs1/BLM and Top3/TopoIIIα, it forms a stable heterodimer with the type IA topoisomerase. In contrast, it appears that at least a proportion of the RecQ helicase is found outside of the apparently obligate Rmi1/Top3 complex in cells (Mullen et al. 2005; Chen and Brill 2007).
Role of RMI1 during HJ Dissolution
RMI1 strongly stimulates the dissolution reaction catalyzed by BLM and TopoIIIα, and this stimulation requires Rmi1interaction with BLM and TopoIIIα (Raynard et al. 2006, 2008; Wu et al. 2006; Cejka et al. 2010). Importantly, the stimulatory effect of RMI1 on dissolution is so impressive that the reaction catalyzed by TopoIIIα and BLM alone appears extremely inefficient by comparison. RMI1 is, therefore, considered to be a key component of the efficient dissolvasome.
Human RMI1 is composed of two OB-fold domains linked together by an undefined domain (Fig. 2). However, only the amino-terminal domain of hRMI1 is conserved in yeast. This conserved OB-fold domain is required for TopoIIIα and BLM binding and is sufficient for efficient dissolution in vitro (Raynard et al. 2008). As a consequence, the conserved dissolvasome core complex can be considered as being composed of TopoIIIα, BLM, together with the conserved amino-terminal domain of RMI1.
Beside its ability to physically associate with BLM and TopoIIIα, RMI1 does not seem to exhibit any obvious biochemical property that would readily explain its contribution to dissolution. Because RMI1 is an OB fold containing protein (Fig. 2), it is tempting to speculate that it is a DNA binding protein (Murzin 1993). RMI1 does exhibit some affinity for DNA that can only be observed with high protein concentrations or with cross-linking treatment (Mullen et al. 2005; Wu et al. 2006; Chen and Brill 2007; Xu et al. 2008; Wang et al. 2010). Thus, it is unlikely that its role in dissolution depends entirely on its apparently unimpressive DNA binding properties. Moreover, the putative DNA binding domain of RMI1 has been shown to correspond to the nonconserved carboxy-terminal OB-fold domain, dispensable for dissolution stimulation of the human BLM/TopoIIIα decatenation activity (Fig. 2) (Raynard et al. 2008). The role of the Rmi1 subunit during dissolution is mainly attributed to its direct influence on TopoIIIα/Top3 biochemical properties. Indeed, Rmi1/RMI1 proteins have been shown to significantly enhance Top3/TopoIIIα binding to different substrates including the dHJ and ssDNA (Wu et al. 2006; Chen and Brill 2007) and to influence several activities of topoisomerase III enzymes (Chen and Brill 2007; Yang et al. 2010; Cejka et al. 2012). Importantly, in each case, the effect of Rmi1/RMI1 on Top3/TopoIIIα activity is synergistically enhanced in the presence of Sgs1/BLM, indicating cooperation between RMI1 and BLM in the stimulation of the topoisomerase (Wu et al. 2006; Yang et al. 2010, 2012; Cejka et al. 2012).
Analysis of the effect of RMI1 on the dissolution of the plasmidDHJ substrate indicated that Rmi1 functions primarily in the decatenation step of dissolution, playing little or no role during convergent branch migration (Cejka et al. 2010). However, because the TopoIIIα/RMI1 subcomplex strongly stimulates BLM unwinding activity of a four-way junction, an involvement of TopoIIIα/RMI1 during the branch migration process is possible (Bussen et al. 2007; Raynard et al. 2008). This stimulation requires only the conserved amino-terminal portion of RMI1 (Fig. 2), suggesting that the stimulation of the RecQ helicase unwinding activity by the Topoisomerase/Rmi1 subcomplex might be a conserved feature during evolution (Raynard et al. 2008). The synergistic influence exerted by Sgs1/BLM and Top3/TopoIIIα on each other in the presence of Rmi1/RMI1 emphasizes the highly sophisticated reaction required for efficient HJ dissolution. In this context, the Rmi1 subunit might act as both the architect and the choreographer of the dissolvasome, being responsible for the tight association and coordination between the two catalytical subunits. Indeed, recent biochemical and structural data strongly suggest that Rmi1 regulates the decatenation function of Top3 thoroughly altering the dynamics of the opening and closing of the topoisomerase gate (Cejka et al. 2012; Bocquet et al. 2014).
An Extended Human Dissolvasome Complex
Efficient dissolution of the more complex plasmidDHJ substrate also seems to require the presence of the ssDNA binding protein RPA (Plank et al. 2006; Cejka et al. 2010). This stimulatory effect is attributed to the binding to and stimulation of BLM/Sgs1 helicase activity by RPA, suggesting that RPA could be part of an extended dissolvasome complex (Brosh et al. 2000; Meetei et al. 2003; Doherty et al. 2005; Hegnauer et al. 2012). This proposal is supported by recent findings showing that the stimulatory effect of hRPA on dHJ dissolution requires both its ssDNA binding activity and a direct physical interaction with RMI1 (Xue et al. 2013). Importantly, however, the hRPA interacting domain of RMI1 resides in the nonconserved central region of hRMI1, suggesting that the physical integration of RPA within the dissolvasome might not be conserved in yeast (Xue et al. 2013). Consistently, yRPA and E. coli single-strand binding protein (SSB) stimulate dissolution by yeast Sgs1/Top3/Rmi1 complex to a similar degree (Cejka et al. 2010). hRPA is not the only protein found to physically associate with the carboxy-terminal extension found in higher eukaryotic RMI1. Indeed, this nonconserved region is responsible for the association of the dissolvasome complex with FANCM and RMI2 (Deans and West 2009; Yang et al. 2010; Hoadley et al. 2012; Manthei and Keck 2013). Among them, only RMI2 is thought to be important for the functionality of the complex in vivo (Fig. 2) (Singh et al. 2008; Xu et al. 2008; Hoadley et al. 2010). However, this additional OB fold containing protein has a minor effect on the efficiency of dissolution in vitro (Singh et al. 2008; Xu et al. 2008).
CONCLUDING REMARKS
Ten years after its discovery, the precise mechanism by which the dissolvasome catalyzes dissolution still remains enigmatic. Indeed, despite its apparent simplicity, dissolution clearly requires some remarkably complicated biochemical cooperation between a type IA topoisomerase, a RecQ helicase, and an OB-fold protein. Many aspects of the process remain elusive and will deserve attention in the future. For instance, the reasons underlying the apparent specialization of one subgroup of RecQ helicases defined by Sgs1/BLM in dissolution and the mechanical contribution of Type IA topoisomerase during convergent branch migration are still unknown. In addition, several structural elements might be illuminating to understand the architecture of the complex. It is noteworthy that the dissolvasome complex is thought to dissipate other DNA structures such as late replication intermediates or catenated DNA (Wu et al. 1999; Mankouri and Hickson 2007; Suski and Marians 2008; Cejka et al. 2012; Manthei and Keck 2013). Because the mechanism underlying those processes and dissolution might be similar, it would also be informative to carry on mechanistic studies of those reactions.
ACKNOWLEDGMENTS
We thank Drs. Hocine Mankouri and Kata Sarlos for helpful comments on the manuscript. Work in the authors’ laboratory is supported by the Nordea Foundation, The Villum Kann Rasmussen Fund, The European Research Council, The Danish Medical Research Council, and The Danish Cancer Society.
Footnotes
Editors: Stephen Kowalczykowski, Neil Hunter, and Wolf-Dietrich Heyer
Additional Perspectives on DNA Recombination available at www.cshperspectives.org
REFERENCES
*Reference is also in this subject collection.
- Andersen SL, Kuo HK, Savukoski D, Brodsky MH, Sekelsky J 2011. Three structure-selective endonucleases are essential in the absence of BLM helicase in Drosophila. PLoS Genet 7: e1002315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton TM, Mankouri HW, Heidenblut A, McHugh PJ, Hickson ID 2011. Pathways for Holliday junction processing during homologous recombination in Saccharomyces cerevisiae. Mol Cell Biol 31: 1921–1933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Hickson ID 2003. RecQ helicases: Suppressors of tumorigenesis and premature aging. Biochem J 374: 577–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachrati CZ, Hickson ID 2009. Dissolution of double Holliday junctions by the concerted action of BLM and topoisomerase IIIα. Methods Mol Biol 582: 91–102 [DOI] [PubMed] [Google Scholar]
- Bastin-Shanower SA, Fricke WM, Mullen JR, Brill SJ 2003. The mechanism of Mus81-Mms4 cleavage site selection distinguishes it from the homologous endonuclease Rad1-Rad10. Mol Cell Biol 23: 3487–3496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellaoui M, Chang M, Ou J, Xu H, Boone C, Brown GW 2003. Elg1 forms an alternative RFC complex important for DNA replication and genome integrity. EMBO J 22: 4304–4313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett RJ, Noirot-Gros MF, Wang JC 2000. Interaction between yeast Sgs1 helicase and DNA topoisomerase III. J Biol Chem 275: 26898–26905 [DOI] [PubMed] [Google Scholar]
- Bernstein DA, Keck JL 2005. Conferring substrate specificity to DNA helicases: Role of the RecQ HRDC domain. Structure 13: 1173–1182 [DOI] [PubMed] [Google Scholar]
- Bernstein KA, Gangloff S, Rothstein R 2010. The RecQ DNA helicases in DNA repair. Annu Rev Genet 44: 393– 417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocquet N, Bizard AH, Abdulrahman W, Larsen NB, Faty M, Cavadini S, Bunker RD, Kowalczykowski SC, Cejka P, Hickson ID, et al. 2014. Structural and mechanistic insight into Holliday-junction dissolution by Topoisomerase IIIα and RMI1. Nat Struct Mol Biol 2121: 261–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy MN, Lopez-Girona A, Shanahan P, Interthal H, Heyer WD, Russell P 2000. Damage tolerance protein Mus81 associates with the FHA1 domain of checkpoint kinase Cds1. Mol Cell Biol 20: 8758–8766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosh RM Jr., Li JL, Kenny MK, Karow JK, Cooper MP, Kureekattil RP, Hickson ID, Bohr VA 2000. Replication protein A physically interacts with the Bloom’s syndrome protein and stimulates its helicase activity. J Biol Chem 275: 23500–23508 [DOI] [PubMed] [Google Scholar]
- Brown PO, Cozzarelli NR 1981. Catenation and knotting of duplex DNA by type 1 topoisomerases: A mechanistic parallel with type 2 topoisomerases. Proc Natl Acad Sci 78: 843–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugreev DV, Brosh RM Jr., Mazin AV 2008. RECQ1 possesses DNA branch migration activity. J Biol Chem 283: 20231–20242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussen W, Raynard S, Busygina V, Singh AK, Sung P 2007. Holliday junction processing activity of the BLM-Topo IIIα-BLAP75 complex. J Biol Chem 282: 31484–31492 [DOI] [PubMed] [Google Scholar]
- Bzymek M, Thayer NH, Oh SD, Kleckner N, Hunter N 2010. Double Holliday junctions are intermediates of DNA break repair. Nature 464: 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carotenuto W, Liberi G 2010. Mitotic inter-homologue junctions accumulate at damaged DNA replication forks in recQ mutants. DNA Repair 9: 661–669 [DOI] [PubMed] [Google Scholar]
- Cejka P, Kowalczykowski SC 2010. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds Holliday junctions. J Biol Chem 285: 8290–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Plank JL, Bachrati CZ, Hickson ID, Kowalczykowski SC 2010. Rmi1 stimulates decatenation of double Holliday junctions during dissolution by Sgs1-Top3. Nat Struct Mol Biol 17: 1377–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Plank JL, Dombrowski CC, Kowalczykowski SC 2012. Decatenation of DNA by the S. cerevisiae Sgs1-Top3-Rmi1 and RPA complex: A mechanism for disentangling chromosomes. Mol Cell 47: 886–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti RS, Schonberg S, German J 1974. A manyfold increase in sister chromatid exchanges in Bloom’s syndrome lymphocytes. Proc Natl Acad Sci 71: 4508–4512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraverty RK, Kearsey JM, Oakley TJ, Grenon M, de La Torre Ruiz MA, Lowndes NF, Hickson ID 2001. Topoisomerase III acts upstream of Rad53p in the S-phase DNA damage checkpoint. Mol Cell Biol 21: 7150–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux JJ 2001. DNA topoisomerases: Structure, function, and mechanism. Annu Rev Biochem 70: 369–413 [DOI] [PubMed] [Google Scholar]
- Chang M, Bellaoui M, Zhang C, Desai R, Morozov P, Delgado-Cruzata L, Rothstein R, Freyer GA, Boone C, Brown GW 2005. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J 24: 2024–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Brill SJ 2007. Binding and activation of DNA topoisomerase III by the Rmi1 subunit. J Biol Chem 282: 28971–28979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CF, Brill SJ 2010. An essential DNA strand-exchange activity is conserved in the divergent N-termini of BLM orthologs. EMBO J 29: 1713–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, You MJ, Jiang Y, Wang W, Li L 2011. RMI1 attenuates tumor development and is essential for early embryonic survival. Mol Carcinog 50: 80–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Wu CH, Plank JL, Hsieh TS 2012. Essential functions of C terminus of Drosophila Topoisomerase IIIα in double Holliday junction dissolution. J Biol Chem 287: 19346–19353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Plank JL, Willcox S, Griffith JD, Hsieh TS 2013. Improved methods for creating migratable Holliday junction substrates. Nucleic Acids Res 41: e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Plank JL, Willcox S, Griffith JD, Hsieh TS 2014. Top3α is required during the convergent migration step of double Holliday junction dissolution. PloS ONE 9: e83582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheok CF, Wu L, Garcia PL, Janscak P, Hickson ID 2005. The Bloom’s syndrome helicase promotes the annealing of complementary single-stranded DNA. Nucleic Acids Res 33: 3932–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu WK, Hickson ID 2009. RecQ helicases: Multifunctional genome caretakers. Nat Rev Cancer 9: 644–654 [DOI] [PubMed] [Google Scholar]
- Constantinou A, Tarsounas M, Karow JK, Brosh RM, Bohr VA, Hickson ID, West SC 2000. Werner’s syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep 1: 80–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans AJ, West SC 2009. FANCM connects the genome instability disorders Bloom’s syndrome and Fanconi anemia. Mol Cell 36: 943–953 [DOI] [PubMed] [Google Scholar]
- Doe CL, Ahn JS, Dixon J, Whitby MC 2002. Mus81-Eme1 and Rqh1 involvement in processing stalled and collapsed replication forks. J Biol Chem 277: 32753–32759 [DOI] [PubMed] [Google Scholar]
- Doherty KM, Sommers JA, Gray MD, Lee JW, von Kobbe C, Thoma NH, Kureekattil RP, Kenny MK, Brosh RM Jr 2005. Physical and functional mapping of the replication protein interaction domain of the Werner and Bloom syndrome helicases. J Biol Chem 280: 29494–29505 [DOI] [PubMed] [Google Scholar]
- Duguet M 1997. When helicase and topoisomerase meet! J Cell Sci 110: 1345–1350 [DOI] [PubMed] [Google Scholar]
- Duno M, Thomsen B, Westergaard O, Krejci L, Bendixen C 2000. Genetic analysis of the Saccharomyces cerevisiae Sgs1 helicase defines an essential function for the Sgs1-Top3 complex in the absence of SRS2 or TOP1. Mol Gen Genet 264: 89–97 [DOI] [PubMed] [Google Scholar]
- Fabre F, Chan A, Heyer WD, Gangloff S 2002. Alternate pathways involving Sgs1/Top3, Mus81/ Mms4, and Srs2 prevent formation of toxic recombination intermediates from single-stranded gaps created by DNA replication. Proc Natl Acad Sci 99: 16887–16892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forterre P, Gadelle D 2009. Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res 37: 679–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricke WM, Kaliraman V, Brill SJ 2001. Mapping the DNA topoisomerase III binding domain of the Sgs1 DNA helicase. J Biol Chem 276: 8848–8855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu TJ, Tse-Dinh YC, Seeman NC 1994. Holliday junction crossover topology. J Mol Biol 236: 91–105 [DOI] [PubMed] [Google Scholar]
- Gangloff S, McDonald JP, Bendixen C, Arthur L, Rothstein R 1994. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: A potential eukaryotic reverse gyrase. Mol Cell Biol 14: 8391–8398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangloff S, de Massy B, Arthur L, Rothstein R, Fabre F 1999. The essential role of yeast topoisomerase III in meiosis depends on recombination. EMBO J 18: 1701–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J 1993. Bloom syndrome: A Mendelian prototype of somatic mutational disease. Medicine 72: 393–406 [PubMed] [Google Scholar]
- Goodwin A, Wang SW, Toda T, Norbury C, Hickson ID 1999. Topoisomerase III is essential for accurate nuclear division in Schizosaccharomyces pombe. Nucleic Acids Res 27: 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiraldelli MF, Eyster C, Pezza RJ 2013. Genome instability and embryonic developmental defects in RMI1 deficient mice. DNA Repair 12: 835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon FG, DiGate RJ, Kowalczykowski SC 1999. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: A conserved mechanism for control of DNA recombination. Mol Cell 3: 611–620 [DOI] [PubMed] [Google Scholar]
- Hartung F, Suer S, Puchta H 2007. Two closely related RecQ helicases have antagonistic roles in homologous recombination and DNA repair in Arabidopsis thaliana. Proc Natl Acad Sci 104: 18836–18841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung F, Suer S, Knoll A, Wurz-Wildersinn R, Puchta H 2008. Topoisomerase 3α and RMI1 suppress somatic crossovers and are essential for resolution of meiotic recombination intermediates in Arabidopsis thaliana. PLoS Genet 4: e1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegnauer AM, Hustedt N, Shimada K, Pike BL, Vogel M, Amsler P, Rubin SM, van Leeuwen F, Guenole A, van Attikum H, et al. 2012. An N-terminal acidic region of Sgs1 interacts with Rpa70 and recruits Rad53 kinase to stalled forks. EMBO J 31: 3768–3783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley KA, Xu D, Xue Y, Satyshur KA, Wang W, Keck JL 2010. Structure and cellular roles of the RMI core complex from the Bloom syndrome dissolvasome. Structure 18: 1149–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoadley KA, Xue Y, Ling C, Takata M, Wang W, Keck JL 2012. Defining the molecular interface that connects the Fanconi anemia protein FANCM to the Bloom syndrome dissolvasome. Proc Natl Acad Sci 109: 4437–4442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Beresten SF, van Brabant AJ, Ye TZ, Pandolfi PP, Johnson FB, Guarente L, Ellis NA 2001. Evidence for BLM and Topoisomerase IIIα interaction in genomic stability. Hum Mol Genet 10: 1287–1298 [DOI] [PubMed] [Google Scholar]
- Huang S, Li B, Gray MD, Oshima J, Mian IS, Campisi J 1998. The premature ageing syndrome protein, WRN, is a 3′ → 5′ exonuclease. Nat Genet 20: 114–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janscak P, Garcia PL, Hamburger F, Makuta Y, Shiraishi K, Imai Y, Ikeda H, Bickle TA 2003. Characterization and mutational analysis of the RecQ core of the bloom syndrome protein. J Mol Biol 330: 29–42 [DOI] [PubMed] [Google Scholar]
- Kaliraman V, Mullen JR, Fricke WM, Bastin-Shanower SA, Brill SJ 2001. Functional overlap between Sgs1-Top3 and the Mms4-Mus81 endonuclease. Genes Dev 15: 2730–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karow JK, Constantinou A, Li JL, West SC, Hickson ID 2000. The Bloom’s syndrome gene product promotes branch migration of Holliday junctions. Proc Natl Acad Sci 97: 6504–6508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim RA, Wang JC 1992. Identification of the yeast TOP3 gene product as a single strand-specific DNA topoisomerase. J Biol Chem 267: 17178–17185 [PubMed] [Google Scholar]
- Kirkegaard K, Wang JC 1985. Bacterial DNA topoisomerase I can relax positively supercoiled DNA containing a single-stranded loop. J Mol Biol 185: 625–637 [DOI] [PubMed] [Google Scholar]
- Krejci L, Altmannova V, Spirek M, Zhao X 2012. Homologous recombination and its regulation. Nucleic Acids Res 40: 5795–5818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusano K, Berres ME, Engels WR 1999. Evolution of the RECQ family of helicases: A Drosophila homolog, Dmblm, is similar to the human Bloom syndrome gene. Genetics 151: 1027–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRocque JR, Stark JM, Oh J, Bojilova E, Yusa K, Horie K, Takeda J, Jasin M 2011. Interhomolog recombination and loss of heterozygosity in wild-type and Bloom syndrome helicase (BLM)-deficient mammalian cells. Proc Natl Acad Sci 108: 11971–11976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen NB, Hickson ID 2013. RecQ helicases: Conserved guardians of genomic integrity. Adv Exp Med Biol 767: 161–184 [DOI] [PubMed] [Google Scholar]
- Laursen LV, Bjergbaek L, Murray JM, Andersen AH 2003. RecQ helicases and topoisomerase III in cancer and aging. Biogerontology 4: 275–287 [DOI] [PubMed] [Google Scholar]
- Li W, Wang JC 1998. Mammalian DNA topoisomerase IIIα is essential in early embryogenesis. Proc Natl Acad Sci 95: 1010–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberi G, Maffioletti G, Lucca C, Chiolo I, Baryshnikova A, Cotta-Ramusino C, Lopes M, Pellicioli A, Haber JE, Foiani M 2005. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev 19: 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LF, Wang JC 1987. Supercoiling of the DNA template during transcription. Proc Natl Acad Sci 84: 7024–7027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo G, Santoro IM, McDaniel LD, Nishijima I, Mills M, Youssoufian H, Vogel H, Schultz RA, Bradley A 2000. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat Genet 26: 424–429 [DOI] [PubMed] [Google Scholar]
- Maftahi M, Han CS, Langston LD, Hope JC, Zigouras N, Freyer GA 1999. The top3+ gene is essential in Schizosaccharomyces pombe and the lethality associated with its loss is caused by Rad12 helicase activity. Nucleic Acids Res 27: 4715–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri HW, Hickson ID 2006. Top3 processes recombination intermediates and modulates checkpoint activity after DNA damage. Mol Biol Cell 17: 4473–4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankouri HW, Hickson ID 2007. The RecQ helicase-topoisomerase III-Rmi1 complex: A DNA structure-specific “dissolvasome?” Trends Biochem Sci 32: 538–546 [DOI] [PubMed] [Google Scholar]
- Mankouri HW, Ashton TM, Hickson ID 2011. Holliday junction-containing DNA structures persist in cells lacking Sgs1 or Top3 following exposure to DNA damage. Proc Natl Acad Sci 108: 4944–4949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthei KA, Keck JL 2013. The BLM dissolvasome in DNA replication and repair. Cell Mol Life Sci 70: 4067–4084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno K, Kumano M, Kubota Y, Hashimoto Y, Takisawa H 2006. The N-terminal noncatalytic region of Xenopus RecQ4 is required for chromatin binding of DNA polymerase α in the initiation of DNA replication. Mol Cell Biol 26: 4843–4852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meetei AR, Sechi S, Wallisch M, Yang D, Young MK, Joenje H, Hoatlin ME, Wang W 2003. A multiprotein nuclear complex connects Fanconi anemia and Bloom syndrome. Mol Cell Biol 23: 3417–3426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Mehta A, Haber JE 2014. Sources of DNA double-strand breaks and models for recombinational DNA repair. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Brill SJ 2000. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics 154: 1101–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Kaliraman V, Ibrahim SS, Brill SJ 2001. Requirement for three novel protein complexes in the absence of the Sgs1 DNA helicase in Saccharomyces cerevisiae. Genetics 157: 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen JR, Nallaseth FS, Lan YQ, Slagle CE, Brill SJ 2005. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol Cell Biol 25: 4476–4487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murzin AG 1993. OB(oligonucleotide/oligosaccharide binding)-fold: Common structural and functional solution for non-homologous sequences. EMBO J 12: 861–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myung K, Datta A, Chen C, Kolodner RD 2001. SGS1, the Saccharomyces cerevisiae homologue of BLM and WRN, suppresses genome instability and homeologous recombination. Nat Genet 27: 113–116 [DOI] [PubMed] [Google Scholar]
- Myung K, Kolodner RD 2002. Suppression of genome instability by redundant S-phase checkpoint pathways in Saccharomyces cerevisiae. Proc Natl Acad Sci 99: 4500–4507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama H 2005. Escherichia coli RecQ helicase: A player in thymineless death. Mutat Res 577: 228–236 [DOI] [PubMed] [Google Scholar]
- Nasmyth KA 1982. Molecular genetics of yeast mating type. Annu Rev Genet 16: 439–500 [DOI] [PubMed] [Google Scholar]
- Oakley TJ, Goodwin A, Chakraverty RK, Hickson ID 2002. Inactivation of homologous recombination suppresses defects in topoisomerase III-deficient mutants. DNA Repair 1: 463–482 [DOI] [PubMed] [Google Scholar]
- Oh M, Choi IS, Park SD 2002. Topoisomerase III is required for accurate DNA replication and chromosome segregation in Schizosaccharomyces pombe. Nucleic Acids Res 30: 4022–4031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda F, Seki M, Miyajima A, Enomoto T 2000. Elevation of sister chromatid exchange in Saccharomyces cerevisiae sgs1 disruptants and the relevance of the disruptants as a system to evaluate mutations in Bloom’s syndrome gene. Mutat Res 459: 203–209 [DOI] [PubMed] [Google Scholar]
- Onodera R, Seki M, Ui A, Satoh Y, Miyajima A, Onoda F, Enomoto T 2002. Functional and physical interaction between Sgs1 and Top3 and Sgs1-independent function of Top3 in DNA recombination repair. Genes Genetic Syst 77: 11–21 [DOI] [PubMed] [Google Scholar]
- Plank JL, Hsieh TS 2006. A novel, topologically constrained DNA molecule containing a double Holliday junction: Design, synthesis, and initial biochemical characterization. J Biol Chem 281: 17510–17516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank J, Hsieh TS 2009. Helicase-appended topoisomerases: New insight into the mechanism of directional strand transfer. J Biol Chem 284: 30737–30741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plank JL, Chu SH, Pohlhaus JR, Wilson-Sali T, Hsieh TS 2005. Drosophila melanogaster topoisomerase IIIα preferentially relaxes a positively or negatively supercoiled bubble substrate and is essential during development. J Biol Chem 280: 3564–3573 [DOI] [PubMed] [Google Scholar]
- Plank JL, Wu J, Hsieh TS 2006. Topoisomerase IIIα and Bloom’s helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc Natl Acad Sci 103: 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynard S, Bussen W, Sung P 2006. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIα, and BLAP75. J Biol Chem 281: 13861–13864 [DOI] [PubMed] [Google Scholar]
- Raynard S, Zhao W, Bussen W, Lu L, Ding YY, Busygina V, Meetei AR, Sung P 2008. Functional role of BLAP75 in BLM-topoisomerase IIIα-dependent Holliday junction processing. J Biol Chem 283: 15701–15708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Nakagawa T, Seki T, Kato G, Tada S, Takahashi Y, Yoshimura A, Kobayashi T, Aoki A, Otsuki M, et al. 2006. Bloom helicase and DNA topoisomerase IIIα are involved in the dissolution of sister chromatids. Mol Cell Biol 26: 6299–6307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shor E, Gangloff S, Wagner M, Weinstein J, Price G, Rothstein R 2002. Mutations in homologous recombination genes rescue top3 slow growth in Saccharomyces cerevisiae. Genetics 162: 647–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TR, Ali AM, Busygina V, Raynard S, Fan Q, Du CH, Andreassen PR, Sung P, Meetei AR 2008. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev 22: 2856–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivenugopal KS, Lockshon D, Morris DR 1984. Escherichia coli DNA topoisomerase III: Purification and characterization of a new type I enzyme. Biochemistry 23: 1899–1906 [DOI] [PubMed] [Google Scholar]
- Suski C, Marians KJ 2008. Resolution of converging replication forks by RecQ and topoisomerase III. Mol Cell 30: 779–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong AH, Lesage G, Bader GD, Ding H, Xu H, Xin X, Young J, Berriz GF, Brost RL, Chang M, et al. 2004. Global mapping of the yeast genetic interaction network. Science 303: 808–813 [DOI] [PubMed] [Google Scholar]
- Trowbridge K, McKim K, Brill SJ, Sekelsky J 2007. Synthetic lethality of Drosophila in the absence of the MUS81 endonuclease and the DmBlm helicase is associated with elevated apoptosis. Genetics 176: 1993–2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ui A, Satoh Y, Onoda F, Miyajima A, Seki M, Enomoto T 2001. The N-terminal region of Sgs1, which interacts with Top3, is required for complementation of MMS sensitivity and suppression of hyper-recombination in sgs1 disruptants. Mol Genet Genomics 265: 837–850 [DOI] [PubMed] [Google Scholar]
- Viard T, de la Tour CB 2007. Type IA topoisomerases: A simple puzzle? Biochimie 89: 456–467 [DOI] [PubMed] [Google Scholar]
- Vindigni A, Hickson ID 2009. RecQ helicases: Multiple structures for multiple functions? HFSP J 3: 153–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R 1989. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58: 409–419 [DOI] [PubMed] [Google Scholar]
- Wang JC 2002. Cellular roles of DNA topoisomerases: A molecular perspective. Nat Rev Mol Cell Biol 3: 430–440 [DOI] [PubMed] [Google Scholar]
- Wang JC, Caron PR, Kim RA 1990. The role of DNA topoisomerases in recombination and genome stability: A double-edged sword? Cell 62: 403–406 [DOI] [PubMed] [Google Scholar]
- Wang F, Yang Y, Singh TR, Busygina V, Guo R, Wan K, Wang W, Sung P, Meetei AR, Lei M 2010. Crystal structures of RMI1 and RMI2, two OB-fold regulatory subunits of the BLM complex. Structure 18: 1159–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt PM, Hickson ID, Borts RH, Louis EJ 1996. SGS1, a homologue of the Bloom’s and Werner’s syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics 144: 935–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler T, Newman S, West SC 2011. Aberrant chromosome morphology in human cells defective for Holliday junction resolution. Nature 471: 642–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J, Rothstein R 2008. The genetic consequences of ablating helicase activity and the Top3 interaction domain of Sgs1. DNA Repair 7: 558–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID 2001. RecQ helicases and topoisomerases: Components of a conserved complex for the regulation of genetic recombination. Cell Mol Life Sci 58: 894–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID 2002. The Bloom’s syndrome helicase stimulates the activity of human topoisomerase IIIα. Nucleic Acids Res 30: 4823–4829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID 2003. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874 [DOI] [PubMed] [Google Scholar]
- Wu L, Karow JK, Hickson ID 1999. Genetic recombination: Helicases and topoisomerases link up. Curr Biol 9: R518–520 [DOI] [PubMed] [Google Scholar]
- Wu L, Davies SL, North PS, Goulaouic H, Riou JF, Turley H, Gatter KC, Hickson ID 2000. The Bloom’s syndrome gene product interacts with topoisomerase III. J Biol Chem 275: 9636–9644 [DOI] [PubMed] [Google Scholar]
- Wu L, Chan KL, Ralf C, Bernstein DA, Garcia PL, Bohr VA, Vindigni A, Janscak P, Keck JL, Hickson ID 2005. The HRDC domain of BLM is required for the dissolution of double Holliday junctions. EMBO J 24: 2679–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Bachrati CZ, Ou J, Xu C, Yin J, Chang M, Wang W, Li L, Brown GW, Hickson ID 2006. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc Natl Acad Sci 103: 4068–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Wyatt HDM, West SC 2014. Holliday junction resolvases. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Guo R, Sobeck A, Bachrati CZ, Yang J, Enomoto T, Brown GW, Hoatlin ME, Hickson ID, Wang W 2008. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev 22: 2843–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue X, Raynard S, Busygina V, Singh AK, Sung P 2013. Role of replication protein A in double Holliday junction dissolution mediated by the BLM-Topo IIIα-RMI1-RMI2 protein complex. J Biol Chem 288: 14221–14227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagata K, Kato J, Shimamoto A, Goto M, Furuichi Y, Ikeda H 1998. Bloom’s and Werner’s syndrome genes suppress hyperrecombination in yeast sgs1 mutant: Implication for genomic instability in human diseases. Proc Natl Acad Sci 95: 8733–8738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bachrati CZ, Ou J, Hickson ID, Brown GW 2010. Human topoisomerase IIIα is a single-stranded DNA decatenase that is stimulated by BLM and RMI1. J Biol Chem 285: 21426–21436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Bachrati CZ, Hickson ID, Brown GW 2012. BLM and RMI1 alleviate RPA inhibition of TopoIIIα decatenase activity. PloS ONE 7: e41208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, Li L, Wang W 2005. BLAP75, an essential component of Bloom’s syndrome protein complexes that maintain genome integrity. EMBO J 24: 1465–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]