Abstract
Autophagy is upregulated during ischemia–reperfusion (IR)-induced and cisplatin-induced acute kidney injury (AKI). Proximal tubule-specific Atg7 knockout mice exhibited increased renal injury compared with wild-type mice following cisplatin- and IR-induced AKI. Inhibition of autophagy by chloroquine aggravated AKI, whereas upregulation of autophagy by rapamycin recovered lost renal function and histology, further indicating a protective role of autophagy in AKI. These findings reported by Jiang et al. will provide stimulus to further examine the role and mechanism of the enhancement of autophagy in AKI.
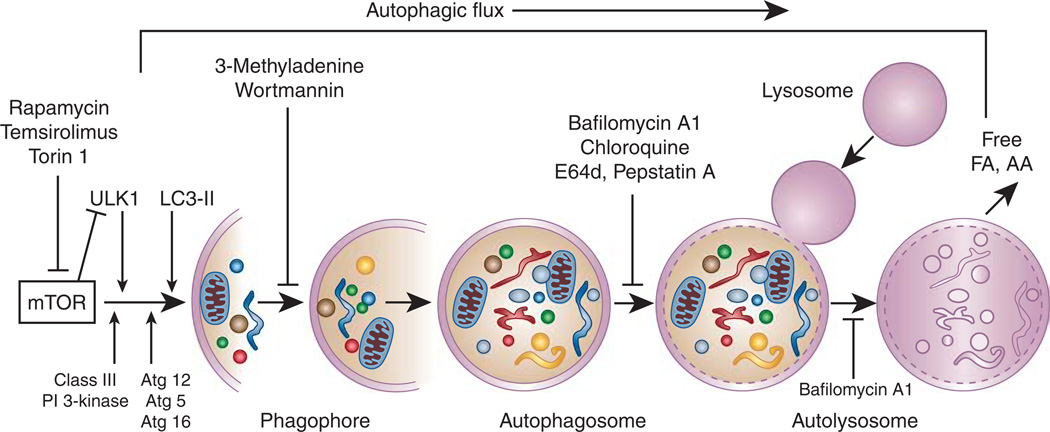
Autophagy is a catabolic process of degradation of long-lived proteins, cellular macromolecules, and intracellular organelles through the lysosomal hydrolases. In this process, a double-membrane structure known as an autophagosome sequesters and delivers cytoplasmic contents to the lysosome for degradation. Free amino acids and fatty acids generated on degradation of cellular components are recycled to synthesize new proteins and bioenergetic supplies of the cell. Thus, under normal physiological conditions, basal autophagy plays a homeostatic role that maintains cellular homeostasis and quality control. Autophagy is induced in response to stress conditions including cell starvation, growth factor deprivation, hypoxia, and oxidant injury. Under stress conditions, autophagy induction is generally considered to play an adaptive role that ensures cell survival. The molecular machinery involved in autophagosome formation is composed of evolutionarily conserved Atg-related proteins originally identified in yeast.1 The formation of an autophagosome is initiated by several autophagic protein complexes, including the unc-51-like kinase 1 or 2 (ULK1 or ULK2) complex, the class III phosphatidylinositol 3-kinase complex, Atg12–Atg5–Atg16 conjugation, and lipidation of microtubule-associated protein 1 light chain 3 (LC3) with phosphatidylethanolamine to form LC3-II1 (Figure 1). The elongation and expansion steps in autophagosome formation involve two ubiquitin-like proteins, Atg12 and Atg8/LC3 (Figure 1). The conjugation of Atg12 to Atg5 is catalyzed by Atg7 and Atg10 (E1- and E2-like enzymes, respectively) to form covalently linked Atg12–Atg5. Following formation of the Atg12–Atg5 conjugate, Atg16L non-covalently associates with this conjugate to produce the Atg12–Atg5–Atg16 multimeric complex.1 At present, there is great interest in studying autophagy under various pathological conditions, including renal diseases, partly because of the recognition that autophagy is an evolutionarily conserved process and its activation may play a pro-survival role. Atg knockout mice were originally considered to be used in evaluating the specific role of autophagy in mammalian cells; however, most of the Atg knockout mice showed embryonic or neonatal lethality.2 Tissue-specific Cre-lox-based conditional Atg knockout mice are now being generated to directly evaluate the specific role of autophagy in disease conditions.
Figure 1. The autophagy pathway and autophagic flux.
Autophagosome assembly in the autophagy pathway follows a series of steps. The process of autophagy begins in the cytoplasm by formation of a double-membrane structure, phagophore, or isolation membrane that has sequestered or engulfed the targeted portion of the cytoplasm, containing misfolded proteins, damaged macromolecules, and organelles. The ‘core’ autophagic machinery uses Atg proteins for the formation of the phagophore, and its subsequent elongation to mature autophagosome formation proceeds through a series of steps mediated by several functional protein complexes: (1) The ULK1/2 kinase complex is required for the induction of autophagy and participates at the site of autophagosome formation. The ULK1/2 (Atg1) complex is composed of ULK1/2, Atg13, FIP200, and Atg101. ULK1/2 is negatively regulated by mTORC1. (2) A class III phosphatidylinositol 3-kinase complex is required for nucleation of the phagophore membrane. Beclin1 (Atg6) and Atg14 are part of this complex. (3) The elongation and expansion steps in autophagosome formation involve two conjugation systems that require ubiquitin-like proteins, Atg12 and Atg8/LC3-II. Transmembrane Atg9 facilitates this step. The first conjugation system is facilitated by Atg7 and Atg10, which conjugate Atg12 to an internal lysine of Atg5. The Atg5–Atg12 conjugate subsequently associates non-covalently with Atg16 to form an Atg12–Atg5–Atg16 multimeric complex. The second conjugation step is the formation of LC3-II (Atg8-PE) by conjugation of LC3 with phosphatidylethanolamine (PE). The two conjugation systems are recruited to the phagophore membrane for phagophore expansion to complete the formation of the autophagosome. Once the autophagosome is formed, most of the Atg proteins are dissociated, which allows fusion with the lysosome to form the autolysosome. LC3-II remains present in both the membranes of the autophagosome. The sequestered contents and the inner membrane of the autolysosome are degraded by the lysosomal hydrolases. Autophagic flux is an indicator that the autophagy process has been completed. AA, amino acids; FA, fatty acids; PI, phosphatidylinositol.
Jiang et al.3 (this issue) report that proximal tubule-specific Cre-lox-based Atg7 knockout mice exhibited increased renal injury compared with wild-type mice in response to cisplatin-induced and ischemia–reperfusion (IR)-induced acute kidney injury (AKI). The proximal tubule-specific Atg7 knockout mice were normal at birth and did not show any overt phenotype in kidneys. Atg7 deletion was unable to produce autophagosomes in proximal tubules, since the Atg12–Atg5–Atg16 multimeric complex required for autophagosome biosynthesis is not formed in the absence of Atg7. Inhibition of autophagy by chloroquine administration worsened AKI, whereas upregulation of autophagy by rapamycin treatment ameliorated cisplatin nephrotoxicity. These findings are in close agreement with the recently published reports on the pro-survival role of autophagy in renal IR injury4,5 and cisplatin nephrotoxicity6 in tubule-specific Atg5 knockout mice. One of the interesting findings from Atg7- or Atg5-deficient kidneys is the rapid accumulation of p62 and formation of p62-positive inclusion bodies. p62, also known as sequestosome 1 (SQSTM1) or A170, is one of the key autophagy substrates. The expression levels of p62 can be used to assess autophagic flux in some contexts. In an efficient flux of the autophagic pathway, p62 interacts with LC3 through its LC3-interacting domain that enables incorporation of p62 and p62-bound polyubiquitinated protein aggregates into the autophagosome, where p62–LC3 complex and bound aggregates are subsequently degraded by the lysosome. However, during inactivation of autophagy such as in Atg7- or Atg5-deficient kidneys or during impaired autophagic flux, p62 cannot be degraded by the autophagy-lysosomal pathway and is accumulated. Also, p62 and associated aggregates are often found in cytoplasmic inclusion bodies that are composed of misfolded proteins and damaged organelles. A recent study has shown an inhibitory effect of accumulated p62 and associated polyubiquitinated protein aggregates on the ubiquitin–proteasome system.7 Therefore, further studies are needed to establish whether p62 aggregates compromise the impact of the ubiquitin–proteasome system in Atgdeficient kidneys. Another remarkable finding of Jiang et al.3 is that Atg5 deletion in proximal tubules results in accumulation of oxidative stress markers and increased proximal tubule apoptosis. However, the underlying mechanism in the increased production of oxidants and apoptosis due to Atg5 or Atg7 deletion needs to be examined further. Autophagy is vital for the kidney, and accumulating evidence has shown upregulation of autophagy in in vitro and in vivo experimental models of both AKI8–10 and chronic kidney disease. Many of these studies used pharmacological inhibitors of autophagy to delineate whether autophagy induction plays a pro-survival or a pro-death role. These studies provided initial information on the upregulation and role of autophagy in AKI. However, to directly determine the role of autophagy in AKI or chronic kidney disease, tissue-specific autophagy-deficient mice or tissue-specific overexpression of autophagy protein components that enhance autophagy in mice are required. Jiang and colleagues,3 by using proximal tubule-specific Atg7-deficient mice, validated previous studies that showed a prosurvival role of autophagy in AKI.
Further investigation by Jiang et al.3 demonstrated that administration of the lysosomotropic agent chloroquine impairs autophagic flux in cisplatin nephrotoxicity as shown by the accumulation of LC3-II and p62. At present, the efficiency of the autophagy-lysosomal degradation pathway is not known in AKI. Even a detailed time-course study on the activation of autophagy during the course of ischemic, reperfusion, or regeneration periods is not yet completely established. Since efficient flux is critical for cell survival, more careful studies are required for measurement of autophagic flux during IR and cisplatin injury. Efficient autophagic flux is dependent on the integrity of the lysosomes and their appropriate fusion with the autophagosomes. It may be necessary to critically assess lysosomal dysfunction in the pathogenesis of AKI. Thus, more studies are needed to understand alterations in the lysosomal membrane permeabilization similarly to what we now know about the mitochondrial membrane permeabilization in AKI. The process of the fusion of the lysosome with the autophagosome is also important for efficient lysosomal function in the clearance of the autophagosome cargo. Thus, the integrity of the lysosomal membranes and their fusion with the autophagosomes are important determinants in efficient autophagic flux during AKI.
At present, the mechanism of induction of autophagy in AKI is not clearly understood. AKI may result from various pathological conditions, including oxidative stress, endoplasmic reticulum stress, mitochondrial and other intracellular organelle damage, DNA damage, and inflammation, all of which can activate and affect autophagy. Thus diverse signaling pathways involved in each of these conditions may be involved in activation of autophagy during AKI. Jiang et al.3 show that in response to cisplatin-induced AKI, proximal tubule-specific Atg7 knockout mice exhibited increased activation of p53 and c-Jun N-terminal kinase. Further studies will be required to understand the effect of Atg7 deletion or autophagy deficiency on these pathways. Jiang et al.3 also show that administration of rapamycin provides protection from cisplatin-induced AKI. However, care should be used in interpreting the results obtained with rapamycin. Rapamycin has been used commonly as an inducer of autophagy by virtue of its inhibition of mTORC1 kinase. Inhibition of mTORC1 kinase or inactivation of mTORC1 leads to activation of the first autophagy protein, ULK1/2 (yeast Atg1), which upregulates autophagy,11 and this induction of autophagy was shown in kidney cells on inhibition of mTOR kinase by rapamycin. However, the nutrient-sensing molecule mTOR is also an important regulator of cell growth and proliferation. Inhibition of mTOR may upregulate autophagy, and it may affect other vital processes in the cell. Therefore, rapamycin’s effect on autophagy induction and cell survival should be carefully evaluated. Previous studies have shown that mTOR is upregulated in AKI, and inhibition of mTOR by rapamycin is associated with a delay in recovery from AKI.12 Also, recent studies have shown that rapamycin is a poor inhibitor of mTOR and may not be very effective in inducing autophagy. Therefore, as an alternative to rapamycin, a genetic approach that specifically augments autophagy may offer a better tool to evaluate the role of autophagy in AKI. Current studies have examined the role of autophagy by inhibiting it through pharmacological inhibitors or by down-regulating it with genetic approaches. It will be useful to investigate the role of autophagy in AKI by enhancing it using genetic approaches.
The work presented by Jiang and colleagues3 provides direct evidence for a renoprotective role of autophagy in AKI. The findings highlight the importance of delineating the mechanisms involved in the upregulation and cytoprotective role of autophagy. Further studies will help in identifying autophagy or a specific event in the autophagic pathway as a therapeutic tool to prevent or reduce AKI.
Footnotes
DISCLOSURE
The author declared no competing interests.
REFERENCES
- 1.Mizushima N, Yoshimori T, Ohsumi Y. The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol. 2011;27:107–132. doi: 10.1146/annurev-cellbio-092910-154005. [DOI] [PubMed] [Google Scholar]
- 2.Mizushima N, Levine B. Autophagy in mammalian development and differentiation. Nat Cell Biol. 2010;12:823–830. doi: 10.1038/ncb0910-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu S, Hartleben B, Kretz O, et al. Autophagy plays a critical role in kidney tubule maintenance, aging and ischemia-reperfusion injury. Autophagy. 2012;8:826–837. doi: 10.4161/auto.19419. [DOI] [PubMed] [Google Scholar]
- 5.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. J Am Soc Nephrol. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takahashi A, Kimura T, Takabatake Y, et al. Autophagy guards against cisplatin-induced acute kidney injury. Am J Pathol. 2012;180:517–525. doi: 10.1016/j.ajpath.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Korolchuk VI, Menzies FM, Rubinsztein DC. Mechanisms of cross-talk between the ubiquitin-proteasome and autophagy-lysosome systems. FEBS Lett. 2010;584:1393–1398. doi: 10.1016/j.febslet.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 8.Kaushal GP, Kaushal V, Herzog C, et al. Autophagy delays apoptosis in renal tubular epithelial cells in cisplatin cytotoxicity. Autophagy. 2008;4:710–712. doi: 10.4161/auto.6309. [DOI] [PubMed] [Google Scholar]
- 9.Yang C, Kaushal V, Shah SV, et al. Autophagy is associated with apoptosis in cisplatin injury to renal tubular epithelial cells. Am J Physiol Renal Physiol. 2008;294:F777–F787. doi: 10.1152/ajprenal.00590.2007. [DOI] [PubMed] [Google Scholar]
- 10.Jiang M, Liu K, Luo J, et al. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. Am J Pathol. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamada Y, Yoshino K, Kondo C, et al. Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol Cell Biol. 2010;30:1049–1058. doi: 10.1128/MCB.01344-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. J Am Soc Nephrol. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]