Summary
Objective
To examine racial differences in tibiofemoral joint (TFJ) and patellofemoral joint (PFJ) radiographic osteoarthritis in African-American (AA) and Caucasian men and women.
Method
Multiple logistic regression was used to evaluate cross-sectional associations between race and tibiofemoral osteoarthritis (TF-OA) and the presence, severity and location of individual radiographic features of tibiofemoral joint osteoarthritis [TFJ-OA] (osteophytes, joint space narrowing [JSN], sclerosis and cysts) and patellofemoral joint osteoarthritis (PFJ-OA) (osteophytes, JSN and sclerosis), using data from the Johnston County Osteoarthritis Project. Proportional odds ratios (POR) assessed severity of TF-OA, TFJ and PFJ osteophytes, and JSN, adjusting for confounders. Generalized estimating equations accounted for auto-correlation of knees.
Results
Among 3187 participants (32.5% AAs; 62% women; mean age 62 years), 6300 TFJ and 1957 PFJ were included. Compared to Caucasians, AA men were more likely to have TF-OA (adjusted odds ratio [aOR] = 1.36; 95% CI, 1.00–1.86); tri-compartmental TFJ and PFJ osteophytes (aOR = 3.06; 95%CI = 1.96–4.78), and TFJ and PFJ sclerosis. AA women were more likely than Caucasian to have medial TFJ and tri-compartmental osteophytes (aOR = 2.13; 1.55–2.94), and lateral TFJ sclerosis. AAs had more severe TF-OA than Caucasians (adjusted cumulative odds ratio [aPOR] = 2.08; 95% CI, 1.19–3.64 for men; aPOR = 1.56; 95% CI, 1.06–2.29 for women) and were more likely to have lateral TFJ JSN.
Conclusions
Compared to Caucasians, AAs were more likely to have more severe TF-OA; tri-compartmental disease; and lateral JSN. Further research to clarify the discrepancy between radiographic features in OA among races appears warranted.
Keywords: Osteoarthritis, Knee, Radiographic features, Tibiofemoral joint, Patellofemoral joint
Introduction
Radiographic tibiofemoral osteoarthritis (TF-OA) has been reported to be more common in African-American (AA) women than in Caucasian women1–3. Anderson and Felson1 showed in their analysis of the first National Health and Nutrition Examination Survey (NHANES-I, 1971–75) that AA women were about twice as likely as Caucasian women to have radiographic TF-OA, while Sowers and colleagues2 reported that peri-menopausal AA women from Michigan were almost three times as likely to have radiographic TF-OA as Caucasian women. Recent analyses of the Third NHANES (1991–1994) showed that AAs were almost twice as likely as Caucasians to have TF-OA3. We recently reported that African-American men and women were both more likely to have radiographic knee OA and more severe TF-OA than Caucasian men and women4.
These studies1–4 examined racial differences in the presence of TF-OA using the Kellgren–Lawrence (K–L) score and did not evaluate individual radiographic features or the patellofemoral joint (PFJ). Although K–L grade remains the most used and accepted score for epidemiologic studies, it emphasizes the presence of osteophytes, makes no distinction between the medial or lateral compartment, and assesses other individual radiographic OA features (i.e., joint space narrowing [JSN], sclerosis and cysts) conjointly in the highest levels5–9. In addition, K–L grade also excludes the analysis of the PFJ.
We wondered whether the composite K–L classification might mask racial differences in the presence, severity, and importantly, the compartmental and intra-compartmental location, of specific radiographic features that might elucidate potential mechanisms, expose associations with features having possible different etiopathogenesis, and improve understanding of the observed racial differences in TF-OA. Determining if race is a risk factor for only some, or for all individual radiographic features in TF-and patellofemoral joint osteoarthritis (PFJ-OA) would have implication regarding understanding disparities in joint replacement and designing biomechanical and other interventions to prevent or treat OA.
The main purpose of our study was (1) to examine racial differences in radiographic features, their severity and location, among AA and Caucasian men and women, considering TF-OA (K–L grade), individual radiographic features of TF-OA (osteophytes, JSN, sclerosis and cysts) and PFJ OA (osteophytes, JSN, sclerosis), and (2) to see if these differences were independent of age, body mass index (BMI), history of knee injury, and educational attainment. This extends our previous descriptive observations in K–L TF-OA4 and is the first racially balanced, population-based study to evaluate racial differences in these outcomes in AA and Caucasian men and women, and the first study to include description of PFJ OA in AAs.
Methods
STUDY POPULATION
The sample was composed of 3187 AAs and Caucasians participants enrolled into the Johnston County Osteoarthritis Project, an ongoing population-based prospective study of OA of the knee and hip in a rural North Carolina County. Project design, protocol and subject recruitment were described in detail elsewhere4,10. In brief, civilian, non-institutionalized African-American and Caucasian individuals 45 years of age and older were recruited by probability sampling of six townships in Johnston County between May 1991 and December 1997. All participants had two interviewer-administered home interviews, a limited clinical and functional examination, and X-rays of the knees; women over the age of 50 years and all men also underwent hip radiography. Height was measured without shoes and weight with a balance beam scale. The study was approved by the Institutional Review Boards of the Schools of Medicine and Public Health at the University of North Carolina at Chapel Hill and the Centers for Disease Control and Prevention. Written informed consent was obtained from all participants before the initiation of the interviews.
RADIOGRAPHIC EVALUATION
Anterior–posterior TFJ knee radiography with weight-bearing was obtained by standardized protocol. The protocol included a single radiograph of both knees fully extended, with joint spaces radiographically open, and no rotation. A horizontal X-ray beam was centered between the right and left patellas at the distance of 40 inches. Settings of 5 mA/s and 70 kVp were used, depending upon the knee thickness. Sunrise PFJ knee radiography view was added to the protocol after data collection had begun, and therefore was performed on only a subset of participants (31%) examined after this procedure was incorporated. There were no differences in age, gender, race, BMI, education level, or self-reported previous knee injury between those who underwent PFJ radiography and those who did not. The PFJ protocol included a single radiograph of both patellas flexed 45° with no femoral rotation, with the participant in a sitting position. A perpendicular X-ray beam was centered between the femoral condyles and the patella articular surfaces. The distance between tube and film was 40 inches. Settings of 5 mA/s and 60 kVp were used.
All radiographs were read by a single board certified bone and joint radiologist (JBR) with expertise in musculoskeletal imaging. The radiologist was blinded to each patient’s clinical history, physical examination and laboratory results. Intra-(JBR) and inter-rater reliability between the study radiologist (JBR) and a second radiologist in a subset of 163 knee and hip examinations resulted in a Kappa statistics equal to 0.910.
K–L grade11 and individual radiographic features were assessed using standard knee and PFJ atlases12. Among 3187 participants, a total of 6300 TFJ and 1957 PFJ knees were included in the analyses, after 74 knees were eliminated due to underlying conditions such as rheumatoid arthritis, amputation, total knee replacement, old knee fractures, surgically fused knee or knees from participants who could not stand on their own.
DEFINITIONS OF VARIABLES FOR ANALYSIS
Demographics and clinical variables
Participants were queried about their race, age, gender, education level and history of previous knee injury. Self-identified race (AA vs Caucasian) was the main exposure variable of interest. Potential confounders included age and BMI (weight in kg/height in m2) each used as continuous variables. Gender, previous knee injury (positive response to the question: ‘Have you ever injured your right, left knee?’), and educational level (<12 years, ≥12 years) were treated as binary.
Outcomes: TFJ OA and radiographic features (Table I)
Table I.
Categorization of TFJ and PFJ OA
| Presence of OA* | Severity of OA† | |
|---|---|---|
| TF-OA (K/L grade) | No: 0 = no OA; 1 = questionable OA | 2, 3, 4 |
| Yes: 2 = mild OA; 3 = moderate OA; 4 = severe OA | ||
| TFJ and PFJ osteophytes | No: 0 = absent | 1, 2, 3 |
| Yes: 1 = mild; 2 = moderate; 3 = severe | ||
| TFJ and PFJ JSN | No: 0 = normal | 1, 2, 3 |
| Yes: 1 = 33% abnormal; 2 = up to 66% abnormal; 3 = up to 100% abnormal | ||
| TFJ and PFJ sclerosis | No: 0 = absent | N/A |
| Yes: 1 = present | ||
| TFJ cysts | No: 0 = absent | N/A |
Odds ratios to assess the magnitude of association between race and radiographic features.
Proportional odds ratios for multiple categories to assess higher levels of severity.
TFJ OA was scored for each knee as K–L radiographic grade with the definition of TF-OA requiring a grade of at least 211. TFJ osteophytes and JSN were scored semi-quantitatively (0–3) for medial and lateral compartments13. Osteophyte location was evaluated at the medial and lateral compartments individually and together (medial and lateral); within medial and lateral compartments, osteophyte location was further classified as tibial and/or femoral. The rationale behind the last comparison was to evaluate in more detail our preliminary observations that AAs were more likely to have osteophytes than Caucasians. To determine the overall osteophyte score for each compartment, the higher score of tibial and femoral sides was used. TFJ sclerosis and cysts were scored as present or absent in each of the medial and lateral compartments; the presence of concomitant TFJ sclerosis in both the medial and lateral compartments was assessed as well.
PFJ radiographic features (Table I)
PFJ osteophyte severity was scored semi-quantitatively (0–3) in a similar fashion to the scoring of TFJ OA. Location of PFJ osteophytes and sclerosis was recorded for medial and lateral compartments, singly and together. The higher osteophyte score of the medial and lateral compartments was used to determine an overall PFJ osteophyte grade. PFJ OA was defined as the presence of an osteophyte of at least grade 112. Isolated PFJ OA was defined as the presence of PFJ OA in the absence of TFJ OA. The presence of tri-compartmental osteophytes was defined as the presence of PFJ osteophytes and medial and lateral TFJ osteophytes.
STATISTICAL ANALYSES
Descriptive statistics of TF-OA, and TFJ and PFJ individual radiographic features and their location were calculated for the four race and gender subgroups. Because OA is more prevalent in women than men14, we stratified our analyses by gender. Chi-square statistics were used to compare frequency and proportion by race within genders. The Hochberg method15 was used to control Type I error inflation due to multiple comparisons across outcomes within gender. Tests for interaction between race and the variables age, BMI, educational level, and history of previous knee injury were performed within gender sub-groups, and effect modification was determined to be present if the P-value of the interaction term was less than 0.10. Gender-specific multiple logistic regression was used to assess the magnitude of association between race and TFJ and PFJ outcomes, adjusting for age, BMI, education level, and history of previous knee injury. The test for proportional odds was conducted for multi-category outcomes in the logistic models to assess the odds of TF-OA, osteophytes and JSN across levels of severity; the proportional odds assumption was rejected if P-value < 0.05. Severity of TF-OA, TFJ OA and PFJ OA radiographic features was assessed only when the proportional odds assumption was supported and were reported as crude proportional odds ratios (PORs) and adjusted cumulative odds ratios (aPORs) and 95% CIs16,17. Generalized estimating equations (GEE) methodology was used to provide logistic models which adjusted for the correlation between pairs of knees for each individual using the GENMOD procedure in the Statistical Analysis System (SAS; version 8.2 software, SAS Institute, Cary, NC).
Results
Demographic and clinical characteristics of the sample stratified by gender are shown in Table II. Among the participants, 62% were female and one-third AA. There were no statistically significant race differences in mean age among men or women. AA women had a higher mean BMI than Caucasian women, while this was not the case in men. AA men and women were each more likely than their Caucasian counterparts to lack a high school diploma. There were no statistically significant race differences in self-reported previous history of right or left knee injury in men or women.
Table II.
Characteristics of the study sample (N = 3187), the Johnston County Osteoarthritis Project, 1991–1997
| Men | Women | |||||
|---|---|---|---|---|---|---|
| Caucasian (N = 883) |
African-American (N = 325) |
Caucasian (N = 1269) |
African-American (N = 710) |
|||
| Age | Mean (SD) | 61.3 (9.9) | 60.4 (11.1) | 62.0 (10.8) | 61.6 (11.0) | |
| BMI* | Mean (SD) | 27.3 (4.7) | 27.5 (5.3) | 28.6 (6.0) | 31.4 (7.2) | |
| Educational level, | <12-years** | N (%) | 297 (33.8) | 174 (66.2) | 457 (36.0) | 360 (50.8) |
| Previous | Right | N (%) | 103 (11.9) | 25 (8.1) | 121 (9.9) | 62 (9.0) |
| Injury | Left | N (%) | 79 (9.1) | 28 (9.0) | 122 (10.0) | 53 (7.7) |
P-values between races:
P < 0.01 in women;
P < 0.001 in men and women.
Frequencies and proportions of TF-OA, TFJ JSN, sclerosis and cysts, and PFJ JSN and sclerosis are displayed in Figs. 1 (men) and 2 (women). Table III shows similar frequencies for osteophyte location. Results from multivariable analyses are displayed in Table IV. In the multivariable analyses, all associations were strengthened in men and weakened in women after adjustment.
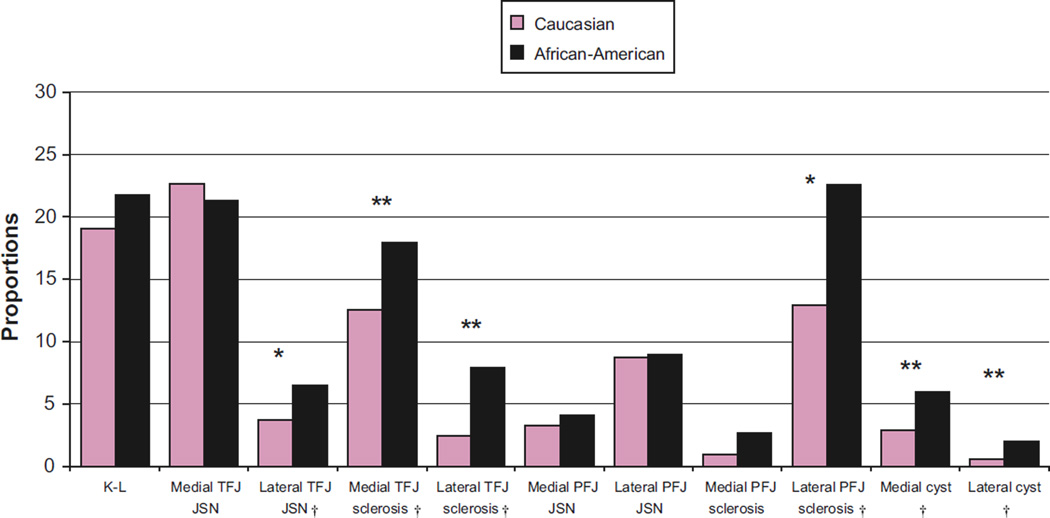
Fig. 1.
Proportion of tibiofemoral osteoarthritis (TF-OA), and TFJ and PFJ radiographic individual features in Caucasian and AA men. *P-value < 0.01, **P-value< 0.001. †Statistically significant after Hochberg correction for multiple comparisons.
Table III.
Frequencies and proportions of TFJ and PFJ osteophytes
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Caucasian (N = 1766) N† (%) |
African-American (N = 650) N† (%) |
P-value† | Caucasian (N= 2538) N† (%) |
African-American (N = 1420) N† (%) |
P-value‡ | |||
| TFJ | Medial | Yes | 513 (29.3) | 238 (37.1) | 0.001* | 933 (37.4) | 669 (48.1) | <0.001* |
| No | 1238 (70.7) | 403 (62.9) | 1561 (62.6) | 721 (51.9) | ||||
| Lateral | Yes | 357 (20.4) | 147 (23.0) | 0.177 | 526 (21.1) | 373 (27.1) | <0.001* | |
| No | 1391 (79.6) | 493 (77.0) | 1965 (78.9) | 1004 (72.9) | ||||
| Medial and lateral | Yes | 631 (36.1) | 264 (41.4) | 0.018* | 1036 (41.6) | 710 (51.6) | <0.001* | |
| No | 1117 (63.9) | 374 (58.6) | 1453 (58.4) | 667 (48.4) | ||||
| Isolated | Yes | 55 (16.5) | 15 (25.9) | 0.085 | 80 (17.2) | 38 (29.5) | 0.002* | |
| No | 279 (83.5) | 43 (74.1) | 386 (82.8) | 91 (70.5) | ||||
| PFJ | Medial | Yes | 219 (30.1) | 38 (25.8) | 0.304 | 148 (18.5) | 96 (33.9) | <0.001* |
| No | 509 (69.9) | 109 (74.2) | 651 (81.5) | 187 (66.1) | ||||
| Lateral | Yes | 322 (44.4) | 78 (53.4) | 0.045* | 275 (34.4) | 132 (47.1) | 0.001* | |
| No | 404 (55.7) | 68 (46.6) | 525 (65.6) | 148 (52.9) | ||||
| Medial and Lateral | Yes | 392 (54.0) | 88 (60.3) | 0.164 | 2071 (81.6) | 1289 (90.8) | <0.001* | |
| No | 334 (46.0) | 58 (39.7) | 467 (18.4) | 131 (9.2) | ||||
| Isolated | Yes | 223 (44.4) | 42 (49.4) | 0.393 | 142 (17.8) | 50 (17.9) | 0.97 | |
| No | 279 (55.6) | 43 (50.6) | 657 (82.3) | 230 (82.1) | ||||
| TFJ | Tri-compartmental | Yes | 1469 (84.0) | 595 (93.3) | ≤0.001* | 2103 (84.5) | 1286 (93.4) | <0.001* |
| and PFJ | No | 279 (16.0) | 43 (6.7) | 386 (15.5) | 91 (6.6) | |||
Statistically significant after Hochberg correction for multiple comparisons.
Numbers may not add up because of rounding and/or missing values.
Unadjusted chi-square test.
Table IV.
ORs and aORs and 95% CI for TFJ, PFJ and tri-compartmental TFJ–PFJ osteophytes for African American compared to Caucasians stratified by gender
| Men | Women | ||||||
|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted* OR (95% CI) | Crude OR (95% CI) | Adjusted* OR (95% CI) | ||||
| Osteophytes | TFJ | Medial | Femoral | 1.76 (1.21–2.56) | 2.08 (1.39–3.12) | 1.85 (1.50–2.29) | 1.53 (1.22–1.92) |
| Tibial | 1.46 (1.15–1.86) | 1.66 (1.28–2.14) | 1.54 (1.30–1.82) | 1.24 (1.03–1.49) | |||
| Femoral and tibial | 1.99 (1.35–2.94) | 2.37 (1.56–3.60) | 1.90 (1.53–2.35) | 1.57 (1.24–1.98) | |||
| Lateral | Femoral | 1.39 (0.92–2.10) | 1.58 (1.03–2.43) | 2.23 (1.72–2.90) | 1.79 (1.33–2.40) | ||
| Tibial | 1.15 (0.87–1.52) | 1.34 (0.99–1.79) | 1.31 (1.08–1.60) | 0.95 (0.76–1.19) | |||
| Femoral and tibial | 1.40 (0.87–2.26) | 1.65 (1.00–2.72) | 2.17 (1.63–2.88) | 1.68 (1.22–2.32) | |||
| Medial and lateral | 1.25 (0.99–1.58) | 1.45 (1.13–1.87) | 1.50 (1.27–1.77) | 1.19 (0.98–1.43) | |||
| Isolated | 1.77 (0.84–3.74) | 1.99 (0.90–4.38) | 1.97 (1.17–3.32) | 1.53 (0.88–2.66) | |||
| PFJ | Medial | 0.80 (0.48–1.33) | 0.97 (0.57–1.63) | 2.25 (1.55–3.27) | 1.42 (0.93–2.17) | ||
| Lateral | 1.46 (0.94–2.26) | 2.08 (1.27–3.42) | 1.71 (1.21–2.41) | 1.24 (0.83–1.84) | |||
| Medial and lateral | 1.31 (0.84–2.41) | 1.92 (1.16–3.18) | 1.61 (1.15–2.25) | 1.12 (0.75–1.66) | |||
| Isolated | 1.23 (0.72–2.09) | 1.83 (1.01–3.31) | 1.38 (0.86–2.19) | 1.18 (0.71–1.97) | |||
| TFJ and PFJ | Tri-compartmental | 2.64 (1.73–4.03) | 3.06 (1.96–4.78) | 2.61 (1.91–3.56) | 2.13 (1. –2.94) | ||
| JSN | TFJ | Medial | 0.93 (0.71–1.22) | 1.01 (0.75–1.35) | 0.95 (0.79–1.14) | 0.76 (0.63–0.93) | |
| Lateral | 1.79 (1.10–2.90) | 2.19 (1.32–3.65) | 1.77 (1.27–2.46) | 1.48 (1.02–2.16) | |||
| PFJ | Medial | 1.24 (0.44–3.51) | 1.40 (0.48–4.04) | 1.18 (0.50–2.78) | 1.28 (0.50–3.28) | ||
| Lateral | 1.03 (0.49–2.15) | 1.22 (0.59–2.54) | 1.42 (0.88–2.28) | 1.78 (1.05–3.02) | |||
Adjusted for age, gender, BMI, educational level and history of previous injury; statistically significant adjusted results in bold.
KELLGREN–LAWRENCE GRADE
AA men and women were approximately 35% more likely than Caucasians to have radiographic TF-OA (aOR = 1.36; 95% CI, 1.00–1.86 for men and crude odds ratios [OR] = 1.34; 95% CI, 1.11–1.62 for women), but this racial difference in women was no longer statistically significant after adjustment (data not shown). In addition, AA men were twice as likely to have more severe TF-OA as Caucasian men (aPOR = 2.08; 95% CI, 1.19–3.64), while AA women had odds almost 60% higher than Caucasian women across levels of severity (aPOR = 1.56; 95% CI, 1.06–2.29).
OSTEOPHYTES
AA men were more likely to have medial and the combination of medial and lateral TFJ osteophytes; lateral PFJ and combined medial and lateral PFJ osteophytes; and tri-compartmental TFJ–PFJ osteophytes than Caucasian men. Similar racial differences in osteophyte patterns were seen in the women, although only the associations with medial TFJ osteophytes and tri-compartmental TFJ–PFJ osteophytes remained statistically significant after adjustment. AA women were significantly more likely than Caucasian women to have lateral TFJ osteophytes as well (data not shown).
Regarding severity, AA men were 2.5 times, and AA women twice, as likely as their Caucasian counterparts to have more severe osteophytes at the medial TFJ compartment (aPOR = 2.50; 95% CI, 1.42–4.38 for men and aPOR = 1.98; 95% CI, 1.41–2.79 for women). AA women were also more likely than Caucasian women to have more severe osteophytes in the medial and lateral PFJ compartments (aPOR = 4.70; 95% CI, 1.81–12.24 for medial PFJ and aPOR = 2.57; 95% CI, 1.31–5.04 for lateral PFJ). In addition, AA men and women were both more likely than their counterparts to have medial osteophytes on both the femoral and tibial sides of the joint and on the femoral side of the lateral compartment (Table IV).
JSN
AA men and women were more likely to have lateral TFJ JSN as their Caucasian counterparts (Table IV). There was no racial difference in the frequency of medial TFJ JSN in women (Fig. 2), but both AA men and women were 80% more likely to have more severe JSN at the medial compartment (aPOR = 1.71; 95% CI, 1.00–2.19 for men and POR = 1.84; 95% CI, 1.28–2.64 for women before adjustment). However, among women, the racial difference was of borderline statistical significance after adjustment (aPOR = 1.77; 95% CI, 0.99–3.17). At the PFJ, there was no racial difference in JSN in the men, while AA women were 80% more likely to have lateral PFJ JSN than Caucasian women (Table IV).
Fig. 2.
Proportion of tibiofemoral osteoarthritis (TF-OA), and TFJ and PFJ radiographic individual features in Caucasian and AA women. *P-value< 0.01, **P-value< 0.001. †Statistically significant after Hochberg correction for multiple comparisons.
SCLEROSIS
AA men were twice to almost 4 times as likely as Caucasian men to have sclerosis in multiple locations: medial TFJ sclerosis (aOR = 1.82; 95% CI, 1.30–2.54); lateral TFJ sclerosis (aOR = 3.86; 95% CI, 2.28–6.52); combined medial and lateral TFJ sclerosis (aOR = 2.10; 95% CI, 1.52–2.90); lateral PFJ sclerosis (aOR = 2.17; 95% CI = 1.19–3.96), combined medial and lateral PFJ sclerosis (aOR = 2.25; 95% CI, 1.24–4.08), and isolated PFJ sclerosis (aOR = 2.72; 95% CI, 1.34–5.52). AA women were over twice as likely to have lateral TFJ sclerosis (aOR = 2.63; 95% CI, 1.85–3.74) than Caucasian women; however, for all other sclerosis outcomes, racial associations in women were not statistically significant after adjustment.
CYSTS
AA men and women were more likely to have medial and lateral TFJ cysts than Caucasians as well, (aOR = 2.65; 95% CI, 1.64–4.28 for men and aOR = 1.66; 95% CI, 1.10–2.52 for women at the medial TFJ and aOR = 3.58; 95% CI, 1.20–10.69 for men and aOR = 4.37; 95% CI, 2.28–8.36 for women at the lateral TFJ).
There were no interactions between race and any of the co-variates tested in any models.
Discussion
Our study demonstrated racial differences in radiographic knee OA, not limited to TF-OA defined by overall KL grade, but also in the presence, severity, and location of the majority of the individual radiographic features of TFJ OA and PFJ OA. AAs were more likely to have TFJ osteophytes, and more likely for those osteophytes to be more severe and to involve multiple compartments, and both sides of the joint within compartments, than Caucasians. In the PFJ, AA men were more likely to have both medial and lateral osteophytes than Caucasians, and AA women had more severe PFJ osteophytes at the medial and lateral compartments. AA men were more likely than Caucasian men to have TFJ and PFJ sclerosis; and AA men and women were more likely to have cysts than Caucasians. Importantly, racial differences were not limited to these features, but also involved JSN. AAs were more likely to have lateral TFJ JSN and more severe medial TFJ JSN. AAs were more likely to have the lateral TFJ compartment affected in some radiographic features than Caucasians.
The results concerning the frequency of TFJ OA from our study differed somewhat from previous ones that examined racial differences in TF OA1–3. Both the NHANES-I analysis and the studies of pre- and perimenopausal women in Michigan found AA women to have radiographic TF-OA much more frequently than Caucasian women even after adjusting for BMI and other factors1,2. In addition, NHANES III data demonstrated that non-Hispanic blacks 60 years of age or older had a higher prevalence of radiographic TFJOA than non-Hispanic whites3. In our study, the racial differences in women were mostly explained by discrepancies in BMI, but this was not the case in men. Despite their having a similar, or even lower (difference not statistically significant), mean BMI, AA men were more likely than Caucasian men to have radiographic TF-OA, and to have it be more severe, suggesting another factor or factors powerful enough to overcome the strong effect of BMI upon knee OA. Possibilities include genetic differences, bone mineral density (BMD), occupational physical demands, diet and other lifestyle factors which can vary between racial and ethnic groups and are risk factors for knee OA.
How can we reconcile our finding that AA women did not appear to have more frequent radiographic knee OA, once BMI differences were controlled, with other studies? One possible reason for these discordant results may be analytic variation among the studies. Importantly, each of these other studies examined BMI in different ways than in our analysis. Anderson and Felson1 categorized BMI based upon Metropolitan Life table of ideal weights, while Sowers and colleagues2 defined their BMI variable for analysis as above or below the median. NHANES III study categorized BMI in three levels (<25; 25–29 and ≥30 kg/m2)3. Our study used BMI as a continuous variable. We suspect differences in control of confounding by BMI, study samples, acquisition and interpretation of X-rays, and the selected methodology to perform data analysis might explain discrepancies among the results of the three studies and ours. Variables adjusted in each analysis were also different, with Anderson and Felson1 accounting for age, smoking, uric acid, income, education, and marital status1; Sowers and colleagues2 included age, injury and smoking behavior. Dillon et al.3 adjusted for sex, age, race, BMI, occupation, smoking status, education, and income. NHANES-I and NHANES-III studies1,3 only included nonweight bearing X-rays, and as pointed out by the NHANES-III authors, their analysis might be biased due to an underestimation of the presence and/or severity of TF-OA. In addition, it was previously addressed that NHANES I study showed an underestimation of the prevalence of radiographic TF-OA reflected by under-read exams18. Lastly, the statistical data analysis in our study was done using GEE which evaluated the presence of TF-OA in each limb independently; in contrast, the other studies considered the overall presence of radiographic TF-OA regardless of the affected limb1–3.
Crucial results from our study concern the individual radiographic features of TFJ and PFJ OA. Here, AA men and women were more likely to have TFJ and PFJ osteophytes; and AA men were more likely to have TFJ and PFJ sclerosis than Caucasian men. In addition, AA men had more severe grades of TFJ osteophytes than Caucasian men; while AA women had more severe grades of both TFJ and PFJ osteophytes than Caucasian women. Further, AAs were more likely to have osteophytes at both femoral and tibial sides of the medial TFJ compartment. Lastly, AA men were almost twice as likely to have tri-compartmental TFJ and PFJ osteophytes as Caucasian men. Concordant with this racial difference in osteophytes, AAs were also more likely to have sclerosis, another feature suggesting the importance of bone in OA in this group. We can speculate, but not prove at this time, that this exuberance of osteophytosis in AAs may be related to higher BMD in this group, earlier onset of disease among AAs, or a higher likelihood of progression through other processes related to the pathogenesis of OA, such as possible differences in transforming growth factor-β(TG-β). This factor is known to be over-expressed in AAs19–22, and experimental studies have shown that TGF-βis highly involved in the formation and growth of osteophytes23–26. The biosynthesis of cartilage oligometric matrix protein (COMP), a non-collagenous matrix protein, by synovial cells and articular chondrocytes is also strongly induced by TGF-β27. We have previously reported that AA women in our study were more likely to have higher levels of serum COMP than Caucasian women, and that serum levels of COMP were associated with radiographic knee OA severity and multiple large joint involvement with OA28,29.
Importantly, these racial differences in radiographic features were not limited to osteophytes, cysts, and sclerosis, but also included JSN, a strong predictor of joint pain and the need for joint replacement30,31. AA men and women were more likely to have lateral TFJ JSN, and more likely to have more severe medial TFJ JSN than Caucasians, although the latter finding was not statistically significant after adjustment in women. These results may be limited by small numbers in higher grade categories.
We find it intriguing that some of the individual radiographic OA features were more likely to occur at the lateral TFJ compartment in AAs compared to Caucasians. For instance, AA men and women were more likely to have lateral TFJ JSN than Caucasians, and AA women were more likely to have lateral TFJ sclerosis than Caucasian women. Also, the strength of race-cysts association was substantially higher at the lateral TFJ compartment than medial TFJ compartment. Racial differences in hip anatomy32 or alignment might be potential factors responsible for discrepancies in the affected compartments, although results from our group did not show racial differences in static knee alignment33. Further research into racial differences in dynamic alignment or other processes, such as synovial inflammation or rate of cartilage degradation, and with magnetic resonance imaging, may elucidate potential explanatory factors for these observations.
There are some potential limitations in our study. First, the information about radiographic PFJ OA was present only on a sub-sample of participants. Second, in the assessment of severity of TF-OA, and higher grades of osteophytes and JSN, some of the most extreme categories had relatively small numbers; nevertheless, the test of proportional odds was supported, suggesting the appropriateness of these analyses and interpretation. Lastly, our study was conducted in a relatively rural community where participants may have a higher predisposition to develop arthritis, than participants from urban studies10. However, our study sample had similar proportions of overweight or obese individuals as the nation as a whole does currently34, suggesting that our sample may indeed be generalizable beyond the strict confines of the time-frame and areas sampled4.
In conclusion, racial differences in radiographic features of knee OA extend beyond the mere presence at the TFJ by crude radiographic grades, but include increased prevalence of PFJ OA and lateral and tricompartmental disease. Racial differences in radiographic knee OA features might be due to a constellation of pathophysiologic processes and hopefully future research can explain this observed discrepancy.
Acknowledgment
Julius Atashili, MD, MPH, PhD for assistance on study design and statistical analyses.
Funding sources: Association of Schools of Public Health and the Centers for Disease Control and Prevention S043, S1733, S3486; National Institute of Arthritis, Musculoskeletal and Skin Diseases P-60-AR30701 and P-60-AR49465.
Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Conflict of interest
The authors have no conflicts of interest to disclose in relation to this manuscript.
References
- 1.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–89. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 2.Sowers M, Lachance L, Hochberg M, Jamada D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African-American and Caucasian women. Osteoarthritis Cartilage. 2000;8:69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 3.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the third national health and nutrition survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 4.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodward J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African-Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–180. [PubMed] [Google Scholar]
- 5.Wood PHN. Osteoarthritis in the community. Clin Rheum Dis. 1976;2:495–507. [Google Scholar]
- 6.Spector TD, Hart DJ, Byrne J, Harris PA, Dacre JE, Doyle DV. Definition of osteoarthritis of the knee for epidemiological studies. Ann Rheum Dis. 1993;52:790–794. doi: 10.1136/ard.52.11.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altman RD, Fries JF, Bloch DA, Carstens J, Cooke TD, Genant H, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30:1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 8.Lane NE, Nevitt MC, Genant HK, Hochberg MC. Reliability of new indices of radiographic osteoarthritis of the hand and hip and lumbar disc degeneration. J Rheumatol. 1993;20:1911–1918. [PubMed] [Google Scholar]
- 9.Scott WW, Jr, Lethbridge-Cejku M, Reichle R, Wigley FM, Tobin JD, Hochberg MC. Reliability of grading scales for individual radiographic features of osteoarthritis of the knee. The Baltimore longitudinal study of aging atlas of knee osteoarthritis. Invest Radiol. 1993;28:497–501. [PubMed] [Google Scholar]
- 10.Jordan JM, Linder GF, Renner JB, Fryer JG. The impact of arthritis in rural populations. Arthritis Care Res. 1995;8:242–250. doi: 10.1002/art.1790080407. [DOI] [PubMed] [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthritis. Ann Rheum Dis. 1957;16:494–501. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnett S, Hart DJ, Cooper C, Spector TD. London: Springer; 1994. A Radiographic Atlas of Osteoarthritis. [Google Scholar]
- 13.Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 14.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. doi: 10.1016/j.rdc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–803. [Google Scholar]
- 16.Zeger SL, Liang KY, Albert PS. Models for longitudinal data: a generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- 17.McCullagh P. Regression models for ordinal data. J R Stat Soc Series B. 1980;42:109–142. [Google Scholar]
- 18.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41(5):778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 19.August P, Leventhal B, Suthanthiran M. Hypertension-induced organ damage in African Americans: transforming growth factor-beta (1) excess as a mechanism for increased prevalence. Curr Hypertens Rep. 2000;2:184–191. doi: 10.1007/s11906-000-0080-5. [DOI] [PubMed] [Google Scholar]
- 20.August P, Suthanthiran M. Transforming growth factor beta and progression of renal disease. Kidney Int Suppl. 2003:S99–S104. doi: 10.1046/j.1523-1755.64.s87.15.x. [DOI] [PubMed] [Google Scholar]
- 21.Suthanthiran M, Khanna A, Cukran D, Adhikarla R, Sharma VK, Sigh T, et al. Transforming growth factor-beta 1 hyperexpression in African American end-stage renal disease patients. Kidney Int. 1998;53:639–644. doi: 10.1046/j.1523-1755.1998.00858.x. [DOI] [PubMed] [Google Scholar]
- 22.Suthanthiran M, Li B, Song JO, Ding R, Sharma VK, Schwartz JE, et al. Transforming growth factor-beta 1 hyperexpression in African-American hypertensives: a novel mediator of hypertension and/or target organ damage. Proc Natl Acad Sci USA. 2000;97:3479–3484. doi: 10.1073/pnas.050420897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scharstuhl A, Glansbeek HL, van Beuningen HM, Vitters EL, van der Kraan PM, van den Berg WB. Inhibition of endogenous TGF-beta during experimental osteoarthritis prevents osteophyte formation and impairs cartilage repair. J Immunol. 2002;169:507–514. doi: 10.4049/jimmunol.169.1.507. [DOI] [PubMed] [Google Scholar]
- 24.Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduction of osteophyte formation and synovial thickening by adenoviral overexpression of transforming growth factor beta/bone morphogenetic protein inhibitors during experimental osteoarthritis. Arthritis Rheum. 2003;48:3442–3451. doi: 10.1002/art.11328. [DOI] [PubMed] [Google Scholar]
- 25.Uchino M, Izumi T, Tominaga T, Wakita R, Minehara H, Sekiguchi M, et al. Growth factor expression in the osteophytes of the human femoral head in osteoarthritis. Clin Orthop Relat Res. 2000:119–125. doi: 10.1097/00003086-200008000-00017. [DOI] [PubMed] [Google Scholar]
- 26.Verdier MP, Seite S, Guntzer K, Pujol JP, Boumediene K. Immunohistochemical analysis of transforming growth factor beta isoforms and their receptors in human cartilage from normal and osteoarthritic femoral heads. Rheumatol Int. 2005;25:118–124. doi: 10.1007/s00296-003-0409-x. [DOI] [PubMed] [Google Scholar]
- 27.Recklies AD, Baillargeon L, White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41:997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 28.Clark AG, Jordan JM, Vilim V, Renner JB, Dragomir AD, Luta G, et al. Serum cartilage oligometric matrix protein reflects osteoarthritis presence and severity: the Johnston County Osteoarthritis Project. Arthritis Rheum. 1999;42:2356–2364. doi: 10.1002/1529-0131(199911)42:11<2356::AID-ANR14>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 29.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48:675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 30.Gossec L, Hawker G, Davis AM, Millefert JF, Lohmander LS, Altman R, et al. OMERACT/OARSI initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. An OMERACT 8 Special Interest Group. J Rheumatol. 2007;34(6):1432–1435. [PubMed] [Google Scholar]
- 31.Gossec L, Jordan JM, Mazzuca SA, Lam MA, Fang F, Renner JB, et al. Comparative evaluation of 3 semi-quantitative radiographic grading techniques for knee osteoarthritis in terms of validity and reproducibility in 1, 759 X-rays: OARSI-OMERACT Task Force. Osteoarthritis Cartilage. 2008;16(7):742–748. doi: 10.1016/j.joca.2008.02.021. [DOI] [PubMed] [Google Scholar]
- 32.Weidow J, Mars I, Karrholm J. Medial and lateral osteoarthritis of the knee is related to variations of hip and pelvic anatomy. Osteoarthritis Cartilage. 2005;13(6):411–417. doi: 10.1016/j.joca.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 33.Nelson AE, Braga L, Braga-Baiak A, Atashili J, Schwartz TA, Renner JB, et al. Static knee alignment measurements among Caucasians and African-Americans: the Johnston County Osteoarthritis Project. J Rheum. 2009;36(9):1987–1990. doi: 10.3899/jrheum.081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]