Abstract
Activities of CD4+ regulatory (TREG) cells restore immune homeostasis during chronic inflammatory disorders. Roles for TREG cells in inflammation-associated cancers, however, are paradoxical. It is widely believed that TREG function in cancer mainly to suppress protective anticancer responses. However, we demonstrate here that TREG cells also function to reduce cancer risk throughout the body by efficiently downregulating inflammation arising from the gastrointestinal (GI) tract. Building on a “hygiene hypothesis” model in which GI infections lead to changes in TREG that reduce immune-mediated diseases, here we show that gut bacteria-triggered TREG may function to inhibit cancer even in extraintestinal sites. Ability of bacteria-stimulated TREG to suppress cancer depends on interleukin (IL)-10, which serves to maintain immune homeostasis within bowel and support a protective antiinflammatory TREG phenotype. However, under proinflammatory conditions, TREG may fail to provide antiinflammatory protection and instead contribute to a T helper (Th)-17-driven procarcinogenic process; a cancer state that is reversible by downregulation of inflammation. Consequently, hygienic individuals with a weakened IL-10 and TREG-mediated inhibitory loop are highly susceptible to the carcinogenic consequences of elevated IL-6 and IL-17 and show more frequent inflammation-associated cancers. Taken together, these data unify seemingly divergent disease processes such as autoimmunity and cancer and help explain the paradox of TREG and inflammation in cancer. Enhancing protective TREG functions may promote healthful longevity and significantly reduce risk of cancer.
Keywords: gut bacteria, regulatory T cells, IL-6, IL-17, colon cancer, breast cancer
Although improvements in cleanliness have reduced the incidence of many serious infections, with increased hygiene there has also been a concomitant increase in the incidence of several life-threatening ailments including allergies, autoimmune diseases and some types of cancer.1-3 The “hygiene hypothesis” suggests beneficial effects of infections. This is supported by the observations that early childhood infections are associated with reduced frequency of aberrant immune reactions such as allergies and asthma later in life.1 The observation that childhood infections suppress diseases associated with inflammation raises the possibility that intestinal bacterial infections may also protect against inflammation-associated cancers.
Risk of developing inflammation-associated cancer, such as colorectal cancer (CRC), is increased in societies with rigorous hygiene practices.2 In the context of cancer, inflammation is widely believed to represent the body’s fight against the tumor cells.4,5 Recent data, however, suggest just the opposite; inflammation may be a cause of cancer and is a powerful stimulus for tumor growth and invasion.6-8 It would follow logically that measures aimed at decreasing inflammation would be beneficial for cancer. Indeed, systemic nonsteroidal antiinflammatory drug (NSAID) use has been linked with a significant decrease in both sporadic and familial colon cancer9-11 and a trend toward decreased cancer of the breast.12 These opposing observations are not easily reconciled, and this is the basis for the paradox of inflammation and cancer.
Prior work in our laboratory13-15 and others16,17 supports a model in which enteric infections suppress inflammatory bowel disease (IBD) and cancer by modulating regulatory T cell (TREG) responses, consistent with the observations of Belkaid and Rouse.3 Specifically, we showed that the beneficial cancer-suppressing effects of microbial infections are dependent on Interleukin (IL)-10,13,15,18,19 a cytokine that also provides suppressive and feedback inhibitory effects on allergies and autoimmune responses.20 Early life exposures to microbial products have been well studied regarding the etiologies of allergies and asthma. It follows that reduced or delayed exposures to microbiota or their products in childhood might hinder normal immune functions in adult life. Although the hygiene hypothesis has been considered in depth for etiology of autoimmune diseases, few studies other than our own13-15 have addressed these concepts as they may relate to cancer in bowel or extraintestinal sites such as the breast.
Recent studies by Kuchroo and coworkers21,22 show that ability of TREG cells to inhibit autoimmune diseases depends on levels of inflammation and IL-6. In this paradigm, elevated levels of T helper type (Th)-17 cytokines contribute to autoimmune diseases. Taken together, these observations link the immune system, gastrointestinal infections and seemingly divergent downstream phenotypes: allergies, autoimmune disease and cancer. These observations suggest that TREG may do more to modulate cancer than block constructive anticancer responses.4,5 Using a model in which childhood infections protect from inflammatory diseases later in life, here we test whether TREG cell biology may help explain unanswered questions of cancer risk and modern lifestyle.
Material and Methods
Experimental animals
All animals were housed in AAALAC accredited facilities and maintained according to protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Massachusetts Institute of Technology. The 129 strain wild type and Rag2-deficient mice were obtained from Taconic Farms and then bred in-house to provide cell donors and experimental animals. IL-10-deficient mice on a C57BL/6 background were originally purchased from Jackson Labs, then backcrossed for 12 generations onto a 129 strain background and subsequently bred in-house for experiments. ApcMin/+ mice on a C57BL/6J background were originally obtained from Jackson Labs and bred in-house as (heterozygous × wt) crosses to provide ApcMin/+ mice and wt littermates for experimental mice, recipients and donors. FVB strain MMTV-HER2/neu mice were purchased from Jackson Labs. FVB wt mice were purchased from Jackson Labs and then bred in-house to produce experimental mice and cell donors.
Mice used in these experiments were specific pathogen free for pathogenic gut bacteria including Salmonella species, Citrobacter rodentium and Helicobacter sp., and also confirmed to be negative for murine viral pathogens and ecto- and endoparasites. Importantly, mice were Helicobacter hepaticus-free unless specifically experimentally infected with H. hepaticus.
Experimental design
A total of 298 mice were used for these experiments. One hundred twenty-four 129-strain mice were included in various treatment regimens or as experimental controls or as cell donors. The 129 strain mice were infected with H. hepaticus and then euthanized at 8–12 weeks postinfection (14–16 weeks of age). Eighty-eight C57BL/6 wt or ApcMin/+ mice were included in various treatment regimens or as experimental controls as outlined in Table 1. For the survival curve (Fig. 2d), mice were humanely euthanized according to institutional criteria (i.e., poor body condition score and large tumor size) or when exhibiting other signs of distress. An additional cohort of Treg-treated ApcMin/+ mice (N = 6) and wt (N = 6) counterparts were permitted to age normally for this survival curve comparison. Finally, 86 FVB strain wt or MMTV-HER2/neu mice were included in various treatment regimens as recipients of cells or anti-TNF or bacterial sonicates or used as experimental controls. For cell transfer experiments, FVB strain wt donor mice were infected with H. hepaticus (N = 8 mice per group) at 4–6 weeks of age and then underwent tissue collections for cell transfer 8 weeks later. Experiments were conducted using 5–8 mice per group as noted throughout the text.
Table 1.
Frequency of bowel and mammary cancer by treatment group
| Strain | Murine model | Sex | Infection (recipient) |
Bowel cancer (No. of mice with carcinoma/total) |
Mammary AdenoCA (No. of mice with adenoCA/total mice) |
|---|---|---|---|---|---|
| 129/SvEv | WT | M | - | 0/8 | 0/8 |
| IL10−/− | M | - | 0/8 | 0/8 | |
| Rag2−/− | M | - | 0/8 | 0/8 | |
| WT | M | Hh | 0/8 | 0/8 | |
| IL10−/− | M | Hh | 0/8 | 0/8 | |
| Rag2−/− | M | Hh | 1/8 | 0/8 | |
| IL10−/−Rag2−/− | M | Hh | 1/8 | 0/8 | |
| Rag2−/− + 300K Teff | M | Hh | 2/8 | 0/8 | |
| Rag2−/− + 300K Teff + 300K Treg | M | Hh | 0/8 | 0/8 | |
| Rag2−/− + 300K Treg | M | Hh | 0/8 | 0/8 | |
| Rag2−/− + 300K IL10−/−Treg | M | Hh | 8/81 | 0/8 | |
| Rag2−/− + 300K Hh+ IL10−/−Treg | M | Hh | 8/8 | 0/8 | |
| Rag2−/− + 100K Hh+ Treg | M | Hh | 1/81 | 0/8 | |
| Rag2−/− + 100K hygienic Treg | M | Hh | 7/8 | 0/8 | |
| C57BL/6 | WT | F | - | 0/8 | 0/8 |
| Min | F | - | 0/8 | 0/8 | |
| WT | F | Hh | 0/8 | 0/8 | |
| Min | F | Hh | 4/8 | 5/8 | |
| Min + Teff | F | - | 3/6 | 4/6 | |
| Min + Teff + 300KTreg | F | - | 0/6 | 0/6 | |
| Min + Teff + 100K Hh+ Treg | F | - | 0/6 | 0/6 | |
| Min + Teff + 100K hygienic Treg | F | - | 4/6 | 6/6 | |
| FVB | WT | F | - | 0/8 | 0/8 |
| HER2/neu | F | - | 0/8 | 8/8 | |
| WT+ Hh sterile sonicate | F | - | 0/8 | 0/8 | |
| HER2/neu+Hh (sterile) sonicate | F | - | 0/10 | 4/101 | |
| HER2/neu + 100K Hh+ Treg | F | - | 0/6 | 2/6 1 | |
| HER2/neu + 100K hygienic Treg | F | - | 0/6 | 4/6 |
p < 0.05.
p < 0.01.
Figure 2.
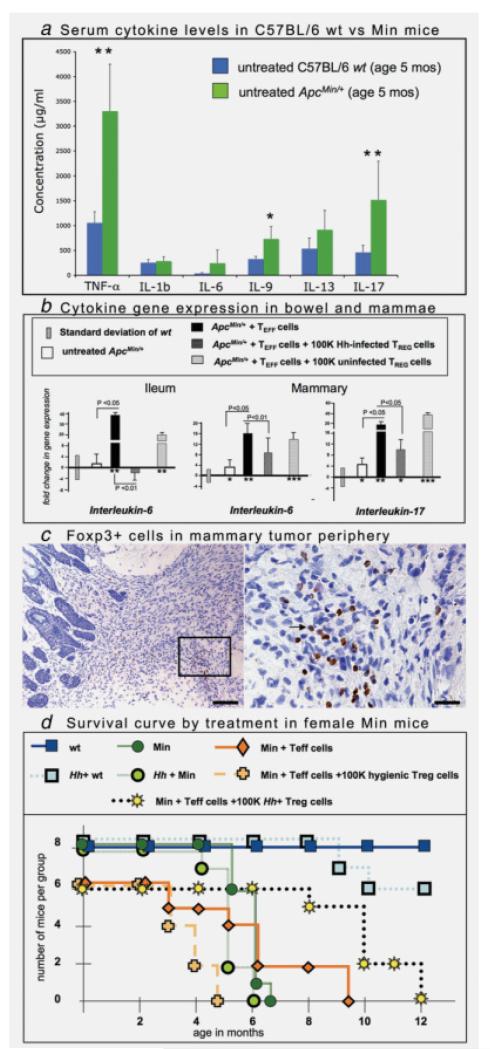
Gut bacteria-triggered TREG cells protect against intestinal and mammary neoplasia and increase survival. (a) Levels of TNF-α and IL-17 were significantly (p < 0.01) elevated in female Min mice. Serum cytokine levels of 5 months old C57Bl/6 wt (blue bars) and APCMin/+ mice (green bars) were measured using the Bioplex assay system. Statistically significant higher levels of TNF-α, IL-9 and IL-17 were detected in APCMin/+ mice. *p < 0.05; **p < 0.01. (b) TREG cells from “hygienic” Treg cell donors failed to reduce gene expression of IL-6 and IL-17. Levels of IL-6 and IL-17 were elevated (p < 0.05) within ileal and mammary tissues of recipients of TEFF cells. (c) Adoptive transfer of purified TEFF cells cause highly infiltrative mammary adenosquamous carcinoma in female APCMin/+ mice. Foxp3+ cells (right panel arrow) locate within tumor-associated inflammatory cell foci at the margins of tumors. The right panel provides a higher magnification of the same field. 3,3-Diaminobenzidine, hematoxylin counterstain. Bars: left panel: 100 μm; right panel: 25 μm. (d) Survival curve illustrating increased lifespan in APCMin/+ mouse recipients of TREG cells from H. hepaticus-infected syngeneic wt donor mice. For the survival curve, mice were humanely euthanized using institutional criteria (i.e., poor body condition score, large tumor size) or when exhibiting other signs of distress.
Experimental Helicobacter hepaticus infection
A total of 142 experimental mice were dosed at 2–3 months of age with H. hepaticus and housed separately in a biocontainment area within the same animal facility. H. hepaticus (strain 3B1, ATCC no. 51449) was grown under microaerobic conditions, prepared and confirmed pure as described elsewhere.13 Experimental mice received 0.2 ml of fresh inoculum by gastric gavage every other day for a total of 3 doses. Cecae and coli were collected 3–4 weeks postinfection at necropsy and analyzed by PCR using H. hepaticus-specific primers to confirm experimental infection.13
Adoptive transfer of T cells into recipient mice
CD4+ lymphocytes isolated from wt or IL-10-deficient mice using magnetic beads (Dynal) are sorted by hi-speed flow cytometry (MoFlow2) to obtain purified populations of CD4+CD45RBhi (TEFF) or CD4+CD45RBlo CD25+ (TREG) lymphocytes and determined to be ~96% pure as previously described elsewhere.13 Anesthetized recipient mice are injected intravenously in the retro-orbital sinus with 3 × 105 T cells as previously described.
Mice aged 3 months were injected with 3 × 105 TEFF cells (N = 40 mice), 3 × 105 TREG cells (N = 22 mice) or 1 × 105 TREG cells (N = 34 mice). Twenty-six of these mice received cotransfer of TEFF cells and TREG cells, as noted. For TREG cell assays, the donor mice for TEFF or TREG cells included both male and female syngeneic mice. Infected cell donors were dosed with H. hepaticus at least 8 weeks earlier.
TREG cell titration assay
To test whether antitumor potency of TREG cells can be enhanced by prior microbial challenge, we first established a suboptimal dose of CD45RBloCD25+ wt TREG cells (i.e., 1 × 105 cells/recipient) that reduces tumor burden approximately to 50% levels of the untreated controls within 4 weeks.13 Later, we used this lower dose regimen with either uninfected or infected cell donors as shown in Table 1.
Flow cytometry
Cells from the mesenteric lymph node or spleen were harvested after euthanasia and stimulated with PMA and Ionomycin for 4 hr. Golgiplug was added for the last 2 hr. Cells were fixed and permeabilized and then stained with anti-CD4, anti-Foxp3, anti-IL-10 and anti-IL-17 as described elsewhere.
TNF-α neutralization
Six MMTV-HER2/neu mice at age 6 months were treated with anti-TNFα antibody (clone XT-3; BioExpress, Lebanon, NH) at 100 μg per mouse 3 times weekly for 10 days. Mice were then euthanized and compared with age-matched mice that received sham IgG antibody alone (N = 6) or untreated controls (N = 8).
Feeding of H hepaticus sonicate
To investigate in vivo whether sterile preparations of H. hepaticus antigens alone are sufficient to generate protective host responses that prevent cancer, we treated 10 HER2/neu mice with sonicate of H hepaticus daily in drinking water and then examined mammary tumor frequency and burden. Bacterial sonicate was confirmed to be sterile by aerobic and anaerobic cultures and again on necropsy of mice. To achieve daily dosing, the equivalent of 109 live organisms that underwent sonication was suspended in drinking water.
Detection of systemic cytokine protein expression
Serum cytokine levels of 6 animals per group were analyzed using the Bioplex assay system (BioRad, Hercules, CA) according to the manufacturers protocol. Samples were analyzed in duplicate on a Bio-Plex 200 system (BioRad, Hercules, CA). Statistical analysis was performed using 2-tailed Student’s t test; a p value < 0.05 was considered statistically significant.
Histologic evaluation and immunohistochemistry
As described previously,13,19,23 the formalin-fixed tissues were processed, and tissue-sections were stained by H&E, Toluidine-Blue or IHC- evaluated by a veterinary pathologist blinded to sample identity. The frequency of mice with lesions was recorded for the various experimental groups.
Primary antibodies used for IHC included rat anti-Foxp3 (eBioscience, Inc., San Diego, CA), rabbit anti-IL-17 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), goat anti-IL6 (Santa Cruz Biotechnology) and rabbit-anti CD3 (Cell Marque, Rocklin, CA). Heat-induced antigen retrieval was performed with EDTA buffer pH8 for Foxp3 and IL-17 or with CC1 epitope retrieval solution (Ventana Medical Systems, Inc., Tucson, AZ) for IL-6 and CD3 detection. Anti-IL-17 and CD3 antibody binding was detected with goat anti-rabbit IgG Poly-HRP (Chemicon International, Temecula, CA). Anti-foxp3 and anti-IL-6 antibody binding was detected with species-appropriate biotinylated secondary antibodies (Serotec, Oxford, UK) and streptavidin peroxidase (Novocastra Laboratories Ltd, Newcastle upon Tyne, UK). Color was developed with DAB substrate-chromogen system (DakoCytomation, Glostrup, Denmark) and tissues were counterstained with hematoxylin.
Foxp3+ or IL-17+ cells were quantitatively assessed as previously described.15 Multiple representative 40× high-power fields were captured using a Nikon eclipse 50i microscope and a Nikon DS-5 M-L1 digital camera. Ten images were randomly selected per treatment group. Cell counts were recorded as the number of positive cells counted per image.
Mammary tumor counts were based on grossly evident tumor nodules in mammary tissue, and then compared between groups by unpaired Student’s t test. Mammary tumor volumes (cm3) were estimated based on dimensions of solid tumor tissue (excluding fluid-filled cysts) on necropsy, and then were compared between groups using Mann–Whitney U analyses.
Detection of cytokine mRNA expression
Total RNA from mammary or bowel tissue was prepared using Trizol reagent according to the supplier’s instruction (Invitrogen, Carlsbad, CA). Five micrograms of the respective RNA were used to generate cDNA using the High Capacity Archive Kit from A/B Applied Biosystems according to the supplier’s procedure. Levels of IL-6 and IL-17 transcripts in the cDNA samples were quantified with the corresponding commercial primers and probes in the ABI Prism Sequence Detection system 7700 (A/B Applied Biosystems). The levels of these cytokines among the samples were normalized by the level of the GAPDH transcripts and compared between the TREG cells-treated and untreated mice by the DDCt method described by A/B Applied Biosystems (User Bulletin no. 2).
Statistical analyses
Total lesion counts were analyzed by unpaired Student’s t test. Cell counts between groups were compared using Mann–Whitney U analyses. Mammary tumor volume (cm3) was estimated based on dimensions of solid tumor tissue. For all statistical analyses Graphpad Prism version 4.0 for windows, GraphPad software, San Diego, CA, was used.
Results
Bacteria, IL-10 and TREG cells in IBD-associated colon cancer
To test whether the paradigm of TREG, IL-6 and Th-17 described in autoimmunity may help explain protective relationships between gut bacteria and increased cancer risks in developed countries, we first used mice lacking antiinflammatory cytokine Interleukin (IL)-10. An essential role for IL-10 in immune homeostasis has been amply demonstrated in animals devoid of IL-10 (IL10−/−), which are highly susceptible to bacteria-triggered inflammatory bowel disease (IBD).16,24 After infection with H. hepaticus, 129 strain mice without IL-10 (N = 8 mice per group) developed IBD and mucinous carcinoma resembling CRC seen in humans with colitis25; however, their wt counterparts infected with H. hepaticus had only minimal bowel pathology (N = 8 mice) (Table 1). Thus, mice required IL-10, a cytokine that inhibits allergies and autoimmune responses,20 to reduce likelihood of IBD and cancer.
On the basis of the earlier data,13,18,26,27 we postulated that protection from cancer resided in activities of IL-10 competent lymphocytes. We found that adoptive transfer of CD4+ lymphocytes from wt donor mice inhibited H. hepaticus-induced IBD and cancer in Rag2−/− mice; in contrast, IL-10-deficient CD4+ lymphocytes were not at all protective from IBD and CRC.27 Subdivision of wt CD4+ subsets revealed that purified CD4+CD45RBhi (TEFF) cells from wt donor mice did not protect from H. hepaticus gut bacteria-triggered cancer, whereas CD4+CD45RBlo (TREG) cells completely eliminated IBD and carcinoma in recipient mice (Table 1). This showed that protection from cancer resided in the CD4+CD45RBlo TREG subset. Indeed, TREG cells are widely recognized as pivotal in immune homeostasis and overall health due, at least in part, to coordinated antiinflammatory activities of cytokines IL-10 and transforming growth factor (TGF)-β.28
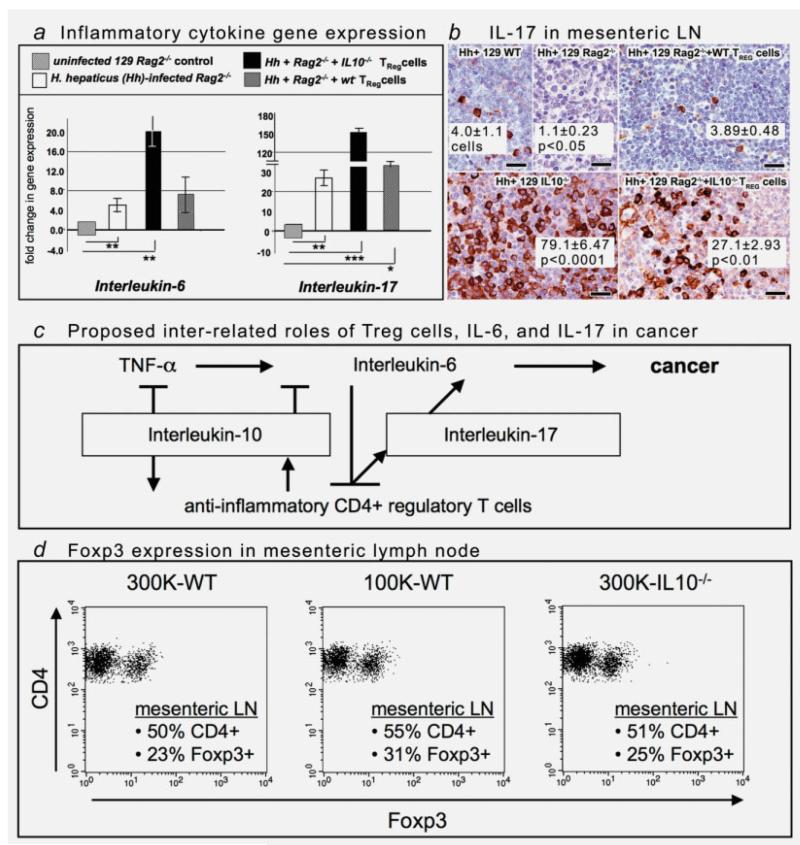
Intruigingly, transfer of TREG from IL-10−/− donor mice into 129 Rag2−/− mice not only failed to inhibit pathology but also significantly (p < 0.05) increased risk of bacteria-triggered neoplastic invasion (N = 8; Table 1). Absence of IL-10 in TREG also led to bacteria-triggered overexpression of IL-6 and IL-17 (Fig. 1a) that was not detected in matched recipients of wt TREG cells. Overexpression of IL-17 was evident at the site of H. hepaticus infection in lower bowel but most prominent within enlarged lymph nodes throughout the body (Fig. 1b). This demonstrated that lack of antiinflammatory IL-10 raised risk for a gut bacteria-triggered inflammatory host response, and this effect extended beyond the bowel.27,29 These findings supported a simplistic model with a GI bacteria-triggered systemic immune response that shifted CD4+ cells toward a Th-17 phenotype in the absence of IL-10.
Figure 1.
Interleukin-10-dependent functions of regulatory T cells restore immune homeostasis in 129 strain Rag2-deficient mice. (a) Gene expression of IL-6 and IL-17 were elevated (p < 0.05) within colonic tissues of infected recipients of IL10-deficient Treg cells. For comparison of mRNA levels, the target mRNA was normalized to that of the housekeeping gene GAPDH. Numbers on the y-axis represent mean fold change of target mRNA levels in reference to the control levels (B6 wt, defined as 0, standard deviation represented by solid bars). mos = age in months on necropsy. *p values were compared with the control. (b) Immunohistochemical staining illustrates overexpression of IL-17 in the mesenteric lymph nodes of mice lacking IL-10. The aberrant immunostaining pattern observed in IL-10−/− animals after infection with H. hepaticus can be appreciated by comparison with tissues of wt mice (top row). The carcinogenic effect was isolated to IL-10-deficient lymphocytes and was correlated with overexpression of IL-17. 3,3-Diaminobenzidine, hematoxylin counterstain. Bars: 25 μm. (c) Overview of interrelated pathways of inflammation involving Treg cells, IL-6 and IL-17 contributing to cancer development and growth. (d) Purified TREG cells of differing dosages or genotypes functioned differently, but these differences are not the result of differential expansion or recruitment of cells within lymph nodes. Each plot indicates % of MLN cells in the live cell gate that express CD4. The second number represents the % of CD4+ cells that expressed Foxp3.
In this 129 mouse model, however, not only lymphocytes but also antigen presenting cells (APCs) were a major source of IL-17 after transfer of IL-10−/− CD4+ lymphocytes into Rag2−/− mice (Fig. 1b). Adoptive transfer of IL-10-deficient TREG was also associated with increased frequency of IL-6- and Tgfβ-1-bearing neutrophils within the developing carcinoma (Fig. S1). Thus, although wt TREG completely inhibited cancer and maintained bowel homeostasis, transfer of IL-10−/− CD4+ cells led to just the opposite: increased innate immune inflammatory cell infiltrates, IL-6, IL-17 and cancer.
We have previously shown that supplementation with exogenous IL-10-Ig fusion protein was sufficient to normalize IL-6 and Tgfβ signaling within bowel and induce regression of colonic carcinoma and neoplastic invasion.15 Taken together, these data led us to postulate that individuals with insufficient IL-10 are more susceptible to uncontrollable elevations in IL-6 and IL-17 and may show more frequent inflammation-associated cancers in response to proinflammatory challenges such as with pathogenic enteric bacteria (Fig. 1c).
Gut bacteria trigger protective IL-10-dependent TREG cells
To test directly in IL-10-competent mice whether infections earlier in life - such as childhood exposure to bacterial products in humans - may protect from immune-mediated diseases later in life, we used a TREG cell titration assay as in Rao et al.13 Various concentrations of 129 strain wt donor cells, starting at 300K and progressing incrementally downward to 100K per mouse, were injected into H. hepaticus-infected Rag2−/− recipient animals. Importantly, Rag-deficient mice otherwise lack functional lymphocytes,26,30 providing a model animal to display isolated activities of purified lymphocytes. We found, when examining recipients of the lowest dosages (1 × 105 cells) of cells, that potency to protect from H hepaticus-induced IBD-associated CRC was increased when TREG came from wt donor mice that were previously exposed to H. hepaticus (Table 1). Importantly, TREG from H. hepaticus-infected donors required IL-10 to protect from IBD or cancer, even when added at the highest dosages (Table 1). These data matched earlier findings of Kullberg et al.16 showing in vivo and in vitro that prior H. hepaticus infection in TREG cell donors conveyed disease protection—not achievable with naïve TREG—and specifically attributable to production of IL-10 by TREG following challenge.
Interestingly, the lowest dosages (1 × 105 cells) of TREG cells from uninfected donor mice (e.g., “hygienic” donors) did not inhibit cancer and instead actually led to increased neoplastic epithelial invasion, similar to the outcome observed in Rag2−/− recipients of IL10−/− TREG cells (Table 1). We found that adoptive transfer of “hygienic” TREG into Rag2−/− led to increased frequency of IL-6-bearing neutrophils and macrophages within inflamed mucosa, a feature of inflammation that is likely to contribute to cancer and dysregulated TREG functions.21,31 Although TREG cells derived under these varying health status conditions performed differently, these differences were not the result of differential expansion or recruitment of cells within spleen or lymph nodes (Fig. 1d), as the percentage of Foxp3+ CD4+ cells in lymph node (25% in protected mice and 31% in mice with carcinoma) were similar or slightly increased in animals at greatest risk for carcinoma. Taken together, cancer risk was lowered in these animals by protective roles of bacteria-triggered TREG. Exposures to bacteria earlier in life served to increase efficiency of IL-10-dependent TREG-mediated resolution of inflammatory challenges arising later in life.
TREG cells, IL-6 and IL-17 in polyposis
To test whether roles for TREG in sporadic CRC also conform to the aforementioned paradigm of IL-6 and IL-17 in autoimmunity, we examined C57BL/6 mice heterozygous for a mutation in the Apc gene (ApcMin/+), which are genetically prone to intestinal polyps that mimic early stages of sporadic CRC in humans.32,33 Although risk for sporadic CRC is reduced by NSAID usage in humans, intestinal polyps are less clearly associated with inflammation than is IBD-CRC. We find, that despite a lack of overt inflammation, there were higher systemic levels of TNF-α, IL-6 and IL-17 in ApcMin/+ mice with intestinal polyposis (Fig. 2a). Importantly, this matches findings in colon cancer in humans.34
To test whether rigorous hygiene practices may also impact cancer risk less clearly linked with inflammation, as in this ApcMin/+ model, we again applied adoptive transfer of lymphocytes. We used mice that were maintained in a helicobacter-free facility. To exacerbate carcinogenesis without use of H hepaticus infection, we instead applied adoptive transfer of proinflammatory CD4+CD45RBhi (TEFF) cells, a mouse model used elsewhere to emulate autoimmune pathology in humans.28 In this model, ApcMin/+ mice developed CRC without IBD,23 and H. hepaticus infection was not required for CRC. Interestingly, female ApcMin/+ recipients of TEFF also have increased risk for mammary carcinoma.23 These mice developed thyroiditis and splenic extramedullary hematopoeisis (data not shown) as in women with autoimmune disorders.35 We found that expression levels of IL-6 and IL-17 were upregulated in bowel tissue (Fig. 2b) after TEFF cells, when compared with untreated ApcMin/+, and this was even in the absence of H. hepaticus infection.
In humans, the risk of developing CRC is lower in countries that have less stringent hygiene practices.2,36 To test this concept of whether TREG cells may fail to protect from pathology under more “hygienic” conditions in the adenoma-prone ApcMin/+ model, we again used a TREG cell titration assay. TREG were isolated from H. hepaticus-exposed or uninfected wt C57BL/6 littermate mice and then injected at different dosages into uninfected “hygienic” ApcMin/+ recipient animals. We found that at lower dosages (1 × 105 cells per mouse), TREG cells collected from the uninfected “hygienic” donor mice failed to provide significant protection against intestinal tumor development (Table 1). However, TREG cells from H. hepaticus-infected donors consistently provided complete protection, even at lowest dosages, when transferred into helicobacter-free “hygienic” ApcMin/+ recipient mice (Table 1). Furthermore, we found that TREG cells collected from H. hepaticus-exposed donor animals were more effective at suppressing both IL-6 and IL-17 levels in tumor-prone tissues when compared with suppressive ability of uninfected “hygienic” TREG cells (Fig. 2b). Therefore, bacteria-primed TREG cells did indeed suppress inflammation under hygienic recipient host conditions, and their ability to do so was more dependent on the prior conditions of the donor TREG, rather than that of the recipient animals.
“Hygienic” TREG cells led to increased mammary tumor burden
Surprisingly, ApcMin/+ recipients of TREG from the “hygienic” uninfected donor animals had a significantly (p < 0.05) greater mammary tumor burden (Table 2; N = 8 per group; 4.65 ± 1.15 cm3 in hygienic TREG cell corecipients of TEFF cells compared with 1.63 ± 0.47 cm3) than in matched TEFF cell recipients. In contrast, TREG cells collected from previously H. hepaticus-infected wt donors were completely protective against mammary tumor development (no tumors observed, Table 1). Importantly, expression of IL-17 was elevated in the mammary tissue from animals that received the TREG of uninfected “hygienic” donors (Fig. 2b), when compared with recipients of TREG from infected donor animals. The observation that adoptive transfer of TREG from “hygienic” donors actually increased rather than suppressed carcinoma was coincident with overexpression of IL-17 and matched our findings with TREG from “hygienic” donors in IBD-CRC in 129 strain Rag2−/− mice.
Table 2.
Mammary tumor burden in mice
| Mouse model | Treatment | Cell donor health status |
Recipient health status |
Sample size (n) |
Tumor volume (mammary) |
|---|---|---|---|---|---|
| B6 strain Min | - | - | Hh-free | 8 mice | None found |
| Min+TEFF | +TEFF | Hh-free | Hh-free | 8 | 1. 63 ± 0.47 cm3 |
| Min+TEFF+naive TREG | +TEFF+ naiveTREG | Hh-free | Hh-free | 8 | 4.65 ± 1.15 cm31 |
| Min+TEFF+HhTREG | +TEFF+Hh infected TREG | Hh -infected | Hh-free | 8 | None found |
| FVB strain HER2/neu | - | - | Hh-free | 8 | 3.61 ± 1.04 cm3 |
| FVB strain HER2/neu | sham IgG | - | Hh-free | 6 | 4.04 ± 2.11 cm3 |
| HER2/neu+antiTNF | Anti-TNF | - | Hh-free | 6 | 0.047 ± 0.03 cm31 |
| HER2/neu+HhTREG | Hh infectedTREG | Hh -infected | Hh-free | 6 | 1 |
| HER2/neu+Hh sonicate | Hh sonicate | - | Hh-free | 10 | 0.31 ± 0.18 cm31 |
p < 0.05.
Using IHC, tissues surrounding the mammary tumors of ApcMin/+ recipients were infiltrated by large numbers of cells expressing Foxp3 (Fig 2c), which is a widely accepted marker of TREG cells.21 Accumulation of Foxp3+ cells was detected only in recipients of TREG from “hygienic” donors at high risk for mammary tumors and not in recipients of TREG from “infected” donors. Importantly, TREG collected from donor mice infected with H. hepaticus provided complete protection from mammary tumors and did not demonstrate accumulations of Foxp3+ cells in mammary tissue. This bacteria-endowed TREG-mediated protection from mammary tumors emulated bacteria-endowed benefit of TREG in IBD.15-17 All these findings conformed with the “hygiene hypothesis” model in humans.
Gut bacteria-triggered TREG cells not only inhibited cancer but also increased longevity in recipient mice
Examination of additional ApcMin/+ animals, which were not euthanized at age 6 months but instead allowed to age normally, revealed that longevity was increased in recipients of TREG when compared with untreated ApcMin/+ animals. It was not determined whether increased survival (Fig. 2d) was directly attributable to lowered tumor burden alone or also the result of reduced systemic sequelae as related with IL-6 in humans.37 Only recipients of TREG cells from H. hepaticus-infected donors had benefit of increased longevity. Longevity-promoting effects of bacterial infection in ApcMin/+ mice were isolated to functions of bacteria-triggered TREG cells, rather than chronic infection with H. hepaticus, per se, which instead decreased lifespan of ApcMin/+ mice (Fig. 2d). This raised the possibility that microbial or synthetic antigen-triggered CD4+ cells may ultimately be applied to protect against a wide variety of chronic inflammatory disorders to promote healthful longevity in humans living under modernized “hygienic” conditions.
Mammary tumor growth requires sustained inflammation
We previously showed that mammary tumorigenesis in ApcMin/+ mice required TNF-α-dependent inflammation.13,23 In those earlier studies, intraperitoneal injection with anti-TNF-α antibody abrogated mammary tumor development mediated by CD4+ lymphocytes in ApcMin/+ mice.23 We postulated that initial failure to control bowel and systemic levels of TNF-α and inflammation may result in sustained subversion of TREG antiinflammatory functions leading to a downward immunological spiral and culminating in cancer growth. This possibility was best tested directly in immunologically intact animals without overt intestinal diseases such as IBD or intestinal polyposis. For this reason, we used female FVB strain MMTV-HER-2/neu (ErbB2) transgenic mice with a genetic predilection to mammary tumors resembling those seen in 30% of humans with breast cancer.12,38-41
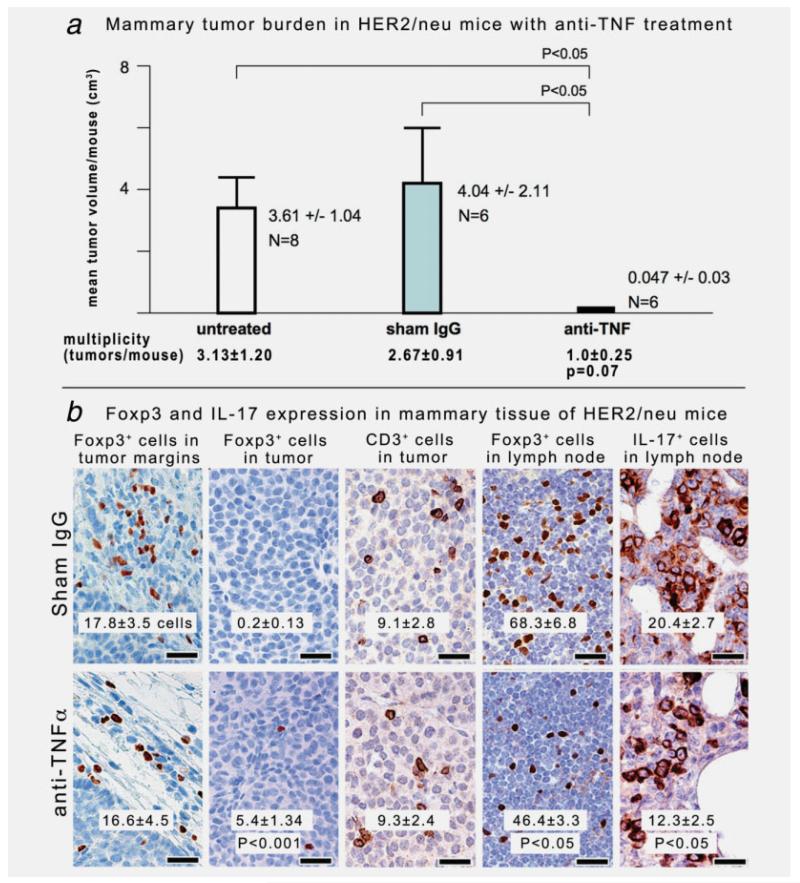
To directly test the requirement for inflammation and TREG in mammary cancer, we used anti-TNF-α treatment in HER2/neu mice. We selected 6-month-old HER2/neu mice with an established tumor burden. Anti-TNF-α was delivered by intraperitoneal injection 3× weekly for 10 days, and then tumors were evaluated upon necropsy. We found that neutralization of inflammation with anti-TNF-α led to a significantly (p < 0.05) reduced mammary tumor burden (anti-TNF-α = 0.047 ± 0.03 cm3/mouse vs. sham = 4.04 ± 2.11 vs. untreated 3.61 ± 1.04)(Fig. 3a). In addition, a trend emerged with lower mammary tumor multiplicity (p = 0.07) in mice treated with anti-TNF-α (μ = 1.0 ± 0.25 tumors/mouse) when compared with control animals (sham IgG μ = 2.7 ± 0.91 tumors/mouse and untreated μ = 3.1 ± 1.2) after only 10 days of antiinflammatory treatment.
Figure 3.
Neutralization of TNF-α restores epithelial and immune homeostasis in FVB strain HER2/neu mice. To directly test requirements for inflammation in mammary cancer, we used anti-TNF-α treatment in HER2/neu mice. We selected 6-month-old HER2/neu mice with a small but established tumor burden. Anti-TNF-α was delivered by intraperitoneal injection ×3 weekly for 10 days and then underwent necropsy immediately afterward. Mammary tumor counts were based on grossly evident tumor nodules in mammary tissue on necropsy and then compared between groups by unpaired Student’s t test. Mammary tumor volumes (cm3) were estimated based on dimensions of solid tumor tissue (excluding fluid-filled cysts) on necropsy and then were compared between groups using Mann–Whitney U analyses. (a) Blockade of TNF-α led to decreased tumor burden with significantly (p < 0.05) reduced volume and a trend (p = 0.07) toward fewer tumors in 6-month-old female HER2/neu mice. (b) Foxp3+ cells in untreated or sham mice were located within lymph nodes and inflammatory foci adjacent to, but not inside, mammary tumors. Treatment with anti-TNF-α significantly increased frequency of Foxp3+ cells within tumors. Similarly, anti-TNF-α treatment reduced (p < 0.05) the numbers of IL-17+ cells in mammary tumors (shown in right panels). Cell counts were performed as described in Material and Methods section. 3,3-Diaminobenzidine, hematoxylin counterstain. Bars: 25 μm.
Anti-TNF-α therapy also significantly (p < 0.001) increased frequency of intratumoral Foxp3+ cells coincident with decreased (p < 0.05) expression of IL-17 within tumors (Table 3 and Fig. 3b). In untreated mice, however, we found that intratumoral Foxp3+ cells were entirely absent from the mammary tumors (untreated or sham IgG-treated HER2/neu mice in Table 3; Fig. 3b). However, during cancer growth, Foxp3+ cells—while absent from within tumors—did accumulate in large numbers within lymph nodes and the desmoplastic tissue surrounding the mammary cancer in untreated and sham-treated mice (Fig. 3b), as they had in ApcMin/+ recipients of “hygienic” TREG (Fig. 2b). Underlying commonalities in these diverse mouse models are summarized in Figure 4. Taken together, these data helped explain how uncontrolled inflammation in the mammary tumor microenvironment may disable TREG, and then lead to increased IL17 and increased tumor growth. Neutralization of inflammation rapidly restored homeostasis and normalized TREG cell distribution.
Table 3.
Localization of Foxp3+ cells (mammary)
| Model | Treatment | Sample size (n) |
Health status |
Foxp3+ cells in lymph node |
Foxp3+ cells in tumor periphery |
Foxp3+ cells within tumor |
|---|---|---|---|---|---|---|
| FVB HER2/neu | - | 8 | Hh-free | 68.3 ± 6.8 cells | 17.8 ± 3.45 cells | 0.2 ± 0.13 cells |
| HER2/neu sham | Sham IgG | 6 | Hh-free | nd | 16.5 ± 2.75 | 0 |
| HER2/neu +anti-TNF | Anti-TNF | 6 | Hh-free | 46.4 ± 3.27 | 16.6 ± 4.47 | 5.4 ± 1.34 |
| HER2/neu + sonicate | Hh sonicate (sterile) | 10 | Hh-free | 39.5 ± 3.51 | 5.4 ± 1.18 | 1.10 ± 0.48 |
Cell counts between groups were compared using Mann–Whitney U analyses.
Foxp3+ lymph node (LN): p < 0.01; untreated (68.3 ± 6.8 cells) vs. anti-TNF-α (46.4 ± 3.27).
Foxp3+ in LN: p < 0.001; untreated (68.3 ± 6.8) vs. sterile Hh sonicate (39.5 ± 3.51).
Foxp3+ in tumor periphery: p < 0.001; untreated (17.8 ± 3.45) vs. Hh sonicate (5.4 ± 1.18).
Foxp3+ within tumor: p < 0.001; sham IgG (0 cells) vs. anti-TNF (5.4 ± 1.34).
Figure 4.
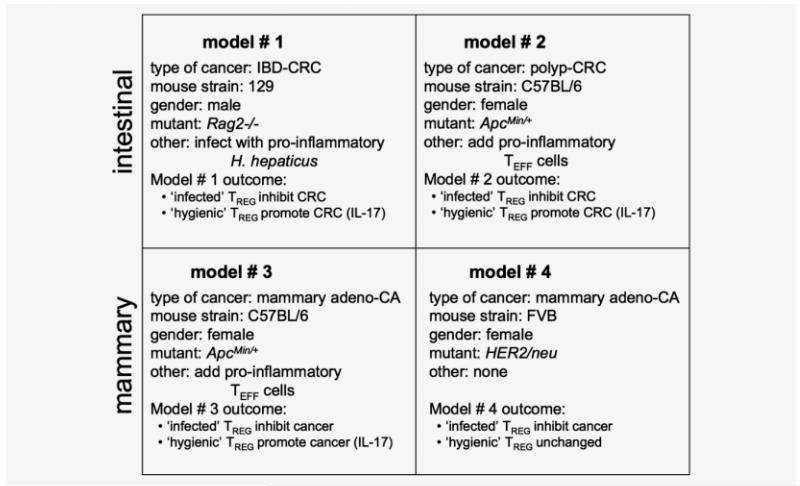
Summary of tumor outcomes in different mouse models of cancer. Mouse models that mimic cancer in humans were consistent with the “hygiene hypothesis” and connected seemingly divergent disease phenotypes including autoimmunity and cancer. Bacteria-triggered IL-10-dependent functions of TREG cells protect from inflammation-associated cancer.
Feeding of sterile gut bacteria protects from Erb2-associated mammary tumors
We hypothesized that optimally functioning TREG routinely inhibit inflammation arising from bowel to maintain systemic immune homeostasis and reduce tumor risk. Indeed, we found that adoptive transfer of purified TREG from H. hepaticus-infected wt FVB donor mice was sufficient to reduce risk of mammary cancer (p < 0.05; compared with untreated MMTV-HER-2/neu mice; Table 1), even when HER2/neu recipients were not themselves infected with H. hepaticus. Thus, anticancer protection imparted by gut bacteria via TREG was crossprotective and broad spectrum, involving more than protection from allergies or asthma, and extending to inflammation-associated cancers arising in distant tissues later in life.
To test practically whether exposure to gut bacterial antigens alone may protect from cancers in distant sites, HER2/neu mice were treated for 4 weeks with sterile (sonicated) equivalent of 109 H. hepaticus organisms added to drinking water. Treatment was initiated at age 20 weeks to precede overt tumor development. We found that HER-2/neu mice had a significantly (p < 0.05) reduced tumor burden when examined 4-6 weeks later upon euthanasia at 24-26 weeks (Table 2). Strikingly, Foxp3+ cells were significantly (p < 0.001; Table 3) reduced in the desmoplastic region surrounding the tumors after only 4 weeks of feeding of sonicates (N = 10; 5.4 ± 1.18 cells/field) when compared with untreated HER2/neu mice (N = 6; 17.8 ± 3.45 cells). Similarly, Foxp3+ cells that accumulated in large numbers within lymph nodes of untreated mice (68.3 ± 6.8 cells) were significantly (p < 0.01) reduced after feeding of sterile H. hepaticus sonicates (39.5 ± 3.51 cells).
Taken together, these data show that although pathogenic bacterial infections may trigger chronic inflammation leading to cancer, sterile enteric bacterial products may be utilized to stabilize immunity and offer protection from cancer. This raises the possibility that feeding of sterile bacterial products or crossreactive glycoproteins may be developed to provide protection from a wide variety of inflammatory disorders including cancers in humans.
Discussion
Data presented here, using mouse models that mimic cancer in humans, were consistent with the “hygiene hypothesis” and unified seemingly divergent disease phenotypes including autoimmunity and cancer. Findings matched the paradigm presented by Kuchroo and coworkers21,22 involving elevated levels of TNF-α and IL-6 that disabled antiinflammatory TREG and upregulated a T helper type (Th)-17 host response. Ability of bacteria-triggered TREG to protect from inflammation-associated cancer matched findings of Kullberg et al., Powrie and Maloy, and others28,42 showing IL-10-dependent ability of CD4+ cells to efficiently and constructively modulate host inflammatory responses and health. Under normal physiological conditions TREG responses are beneficial to the host to ignore or reinforce protective acute inflammation. Afterwards, TREG regain protection from pathologic sequellae of chronic inflammation.14 These normal protective processes that efficiently restore homeostasis seem to be disabled during chronic inflammatory diseases. Taken together, these observations connect the immune system, gastrointestinal infections and diverse immune-mediated diseases including allergies, autoimmune disease and cancer.
It has been well established in humans and in mice that chronic inflammation increases the risk of CRC.13,15,23,26,27,34,43,44 It is paradoxical, then, that the risk for developing CRC is actually lower in countries that have less stringent hygiene practices.2,36 This paradox was explained here using titration assays of CD4+ cells in adoptive transfer experiments. Increased likelihood of cancer due to infectious agents was offset by enhanced antiinflammatory protection from bacteria-triggered TREG. Thus, exposure to bacteria earlier in life serve to increase efficiency of TREG-mediated resolution of future inflammatory challenges. Titration assays revealed that “hygienic” TREG may under some circumstances serve to promote carcinogenesis and increase cancer risk. This may be due to inability of “hygienic” TREG to suppress uncommitted CD4+ cells recruited to Th-17, or this may also be due to Foxp3+ “hygienic” TREG redirected to Th-17 phenotype. In either event, we postulate this carcinogenic effect arises from dysregulation of protective inflammatory responses. In an aging or genetically susceptible host, elevated levels of inflammation, namely cytokines TNF-α and IL-6, serve to disable antiinflammatory TREG functions, which then leads to aberrant wound healing and contributes to cancer growth. CD4+ cell functions Foxp3+ redirected to Th-17 identity under these sustained are proinflammatory conditions, as described previously in humans and in mice.45,46 In contrast, TREG from a host with prior bacterial challenges (i.e., “infected” TREG) effectively suppressed inflammatory challenges, rapidly restored immune homeostasis and prevented cancer growth. TREG are widely recognized as pivotal in immune homeostasis and overall health.3,28 Requirement for IL-10 has been amply demonstrated in murine models at high risk for IBD.16,24,47,48 Extrapolating these results to humans, individuals with a naïve immune system and weakened IL-10- and TREG-mediated inhibitory loop would be more susceptible to uncontrollable inflammation and more frequent inflammation-associated cancers throughout the body later in life.
Ability of gut bacteria to inhibit mammary cancer was isolated to functions of CD4+ TREG cells. We hypothesize that benefit of TREG is a manifestation of CD4+ cell-mediated oral tolerance to gut bacterial antigens. Following this line of reasoning, host ability to regulate inflammation is critical. A robust TNF-α-triggered inflammatory response benefits the host to eliminate a pathogen.21,22,49-53 However, ability of TREG to regain homeostasis is disrupted when the bowel is chronically inflamed. This results in a downward spiral of sustained systemic inflammation with further disabling effects on TREG protective functions. Faubion et al.54 and others have demonstrated that TNF-α dependent inflammation arising from bowel leads to impaired thymic and peripheral TREG functions. This downward spiral is preventable with microbial challenges earlier in life that may serve to recruit IL-10-dependent CD4+ cells that protect from uncontrolled GI inflammation (shown in Fig. 5). Kullberg et al.16 showed that prior exposures to gut bacteria increase efficiency of IL-10-dependent TREG to downregulate inflammation and subsequently tip the systemic balance toward antiinflammatory activities and homeostasis. That these events rely on systemic interactions was displayed in an autoimmune condition such as multiple sclerosis (MS). Korn et al.49 showed that during MS the TREG relied on normalized levels of TNF-α and educated TREG to efficiently restore homeostasis. In support of this, immune homeostasis was restored in the present study using anti-TNF-α which yielded tumor remission in our mice.
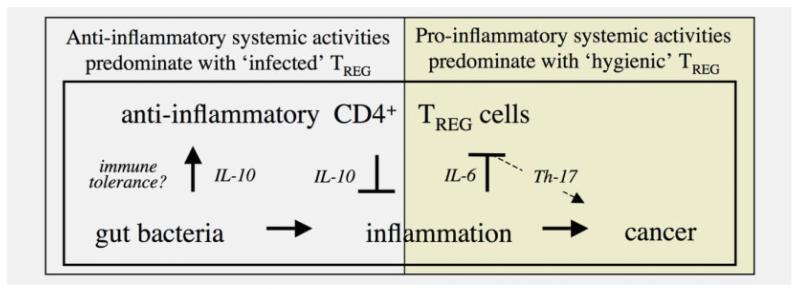
Figure 5.
Conceptual overview of immune factors contributing to cancer development and growth under hygienic host conditions. Under normal physiological conditions, TREG responses are beneficial to the host by reinforcing protective acute inflammation and then afterward regain protection from pathologic sequellae of chronic inflammation. During a proinflammatory microbial challenge, elevated levels of IL-6 upregulate a T helper type (Th)-17 host response that contributes to cancer growth and invasion. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Ability of properly functioning TREG to downregulate TNF-α and IL-6 and suppress cancer transcended gender differences in the animals examined here. In humans, men are up to 70% more likely to suffer dysregulated levels of IL-6 and develop colon cancer than are women.55,56 Any anti-cancer protective effect of being female is lost after ovary removal or menopause, implicating steroidal hormones such as estrogen in protecting against cancer. A pivotal role for steroidal hormones in cancer is also supported by epidemiological studies in women showing that57 hormone replacement therapy (HRT) with estrogen reduces risk of CRC.58-60 One possible explanation is that estrogen drives expansion of IL-10-dependent CD4+CD25+ TREG cells,61-64 a feature of ovulation and pregnancy that minimizes maternal rejection.65 This tolerance-based effect toward gut bacteria may serve to downregulate carcinogenic inflammation in the bowel in female animal; levels of cytokine IL-6 are generally lower than in males.34,66,67 It was surprising that gut bacteria-primed TREG were sufficient to inhibit mammary tumors. Perhaps, overexpression of IL-6 and IL-17 as shown here contribute to malignancy in breast and ovarian tissue.4 Important pathogenic roles for inflammatory cytokines TNF-α and IL-6 have also emerged in social stress, bereavement and aging in humans and animal models,68-71 perhaps providing clues as to why individuals living under these conditions may be at increased risk for cancer.
Foxp3 is a widely accepted marker of TREG cells.21 Interestingly, in this study large numbers of Foxp3+ cells accumulated in tumor periphery and lymph nodes during development of cancer. However, Foxp3+ cells were seen intratumorally only during restorative treatments with 1) anti-TNF-α or 2) gut bacteria-educated “infected” TREG or 3) sterile gut bacterial sonicates. Within CRC lesions in bowel, intratumoral Foxp3+ cells were recently correlated with a favorable clinical outcome in human patients.72 This dearth of Foxp3+ cells within the tumor parenchyma during malignancy was most clearly demonstrated in Figure 3b using HER2/neu transgenic mice that have well-circumscribed mammary tumor tissue. These findings contrasted with those of Ambrosino et al.73 One possible explanation for this polarized distribution of Foxp3+ cells is that TREG may accumulate in the tumor vicinity and then may differentiate into IL-17-producing cells31,45,46 within the inflammatory milieu of the tumor. In any event, these effects are reversible using anti-TNF-α. Neutralization of systemic inflammation by treatment with anti-TNF-α resulted in fewer tumors, decreased Foxp3+ cell accumulations in surrounding tissues and also led to increased Foxp3+ cell counts within the tumors (Fig. 3b).
We postulate that host ability to rapidly restore immune homeostasis explains the “hygiene hypothesis” paradox and provide a basis for healthful longevity. Recent works of Mazmanian et al.17,42 involving protective cross-reactivity of gut bacterial antigens in IBD support this concept, along with our earlier findings in mouse models.13-15 In this study, exposure to gut bacterial antigens - either in the form of H. hepaticus sonicates fed to a host or H. hepaticus-educated TREG transferred into a host, served to protect from pathology whether or not cancer was induced by H. hepaticus. Immediate benefits of this type could be achieved in humans using in vitro stimulation with bacterial antigens to educate and tolerize CD4+ cells74 that may afterward be returned to a host. Similarly, feeding of bacterial antigens may lower cancer risk with population-based prophylactic and therapeutic potential. This approach would address the underlying “cause” of susceptibility to cancer, rather than targeting a “symptom” of accumulation of TREG during antitumor host responses.4,5 Many unanswered questions remain to optimize this process, especially regarding TREG identity and TREG recruitment during younger years rather than in later life.
Taken together, these data help explain the paradox of gut bacteria, inflammation and TREG cells in cancer. We discovered here that TREG triggered by gut bacterial antigens promote healthful longevity. TREG arising under hygienic conditions seem to be captive to the paradigm of IL-6 and IL-17, as in autoimmunity elucidated by Kuchroo and co-workers.21,22,49-53 Proinflammatory stimuli arising in a “hygienic” host may induce uncommitted CD4+ cells and possibly also Foxp3+ TREG themselves to fuel carcinogenic events by favoring a Th-17 host response. Thus, insufficient microbial exposures in modern societies may undermine protective immunity arising from bowel. This results in lower thresholds for future carcinogenic events in distant tissues such as prostate, breast or lung. Two faces of TREG have emerged here that highlight the importance of better understanding TREG biology. The apparent dichotomy of TREG in promoting healthful longevity, or, alternatively, exacerbating carcinogenesis, highlights the importance of exploiting TREG biology as a powerful tool in cancer therapy.
Acknowledgements
The authors thank Ms. Kathy Cormier and Mr. Chakib Boussahamain for help with histology and immunohistochemistry and Mr. Glenn Paradis and Ms. Michele Perry for assistance with cell sorting. This work was supported by R01CA108854 (S.E.E.), Department of Defense (DOD) Contract W81XWH-05-01-0460 (S.E.E.), P01CA26731 (S.E.E. and J.G.F.), and T32RR07036 (J.G.F. and S.E.E.). W.O. is recipient of an APART fellowship of the Austrian Academy of Sciences. This material was presented at the DOD Era of Hope meeting in Baltimore, MD, June 27, 2008.
Grant sponsor: National Institutes of Health (NIH); Grant number: R01CA108854, P01CA26731, T32RR07036; Grant sponsor: Department of Defense (DOD); Grant number: W81XWH-05-01-0460
Footnotes
Additional Supporting Information may be found in the online version of this article
References
- 1.Weiss ST. Eat dirt—the hygiene hypothesis and allergic diseases. N Engl J Med. 2002;347:930–1. doi: 10.1056/NEJMe020092. [DOI] [PubMed] [Google Scholar]
- 2.Fox JG, Beck P, Dangler CA, Whary MT, Wang TC, Shi HN, Nagler-Anderson C. Concurrent enteric helminth infection modulates inflammation and gastric immune responses and reduces helicobacter-induced gastric atrophy. Nat Med. 2000;6:536–42. doi: 10.1038/75015. [DOI] [PubMed] [Google Scholar]
- 3.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–60. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 6.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7:211–7. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–6. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labayle D, Fischer D, Vielh P, Drouhin F, Pariente A, Bories C, Duhamel O, Trousset M, Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991;101:635–9. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- 10.Marnett LJ, DuBois RN. COX-2: a target for colon cancer prevention. Annu Rev Pharmacol Toxicol. 2002;42:55–80. doi: 10.1146/annurev.pharmtox.42.082301.164620. [DOI] [PubMed] [Google Scholar]
- 11.Waddell WR, Gerner RE, Reich MP. Nonsteroid antiinflammatory drugs and tamoxifen for desmoid tumors and carcinoma of the stomach. J Surg Oncol. 1983;22:197–211. doi: 10.1002/jso.2930220314. [DOI] [PubMed] [Google Scholar]
- 12.Harris RE, Chlebowski RT, Jackson RD, Frid DJ, Ascenseo JL, Anderson G, Loar A, Rodabough RJ, White E, McTiernan A. Breast cancer and nonsteroidal anti-inflammatory drugs: prospective results from the Women’s Health Initiative. Cancer Res. 2003;63:6096–101. [PubMed] [Google Scholar]
- 13.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 14.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–50. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 15.Poutahidis T, Haigis KM, Rao VP, Nambiar PR, Taylor CL, Ge Z, Watanabe K, Davidson A, Horwitz BH, Fox JG, Erdman SE. Rapid reversal of interleukin-6-dependent epithelial invasion in a mouse model of microbially induced colon carcinoma. Carcinogenesis. 2007;28:2614–23. doi: 10.1093/carcin/bgm180. [DOI] [PubMed] [Google Scholar]
- 16.Kullberg MC, Jankovic D, Gorelick PL, Caspar P, Letterio JJ, Cheever AW, Sher A. Bacteria-triggered CD4(+) T regulatory cells suppress Helicobacter hepaticus-induced colitis. J Exp Med. 2002;196:505–15. doi: 10.1084/jem.20020556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 18.Erdman SE, Sohn JJ, Rao VP, Nambiar PR, Ge Z, Fox JG, Schauer DB. CD4+ CD25+ regulatory lymphocytes induce regression of intestinal tumors in ApcMin/+ mice. Cancer Res. 2005;65:3998–4004. doi: 10.1158/0008-5472.CAN-04-3104. [DOI] [PubMed] [Google Scholar]
- 19.Erdman SE, Rao VP, Poutahidis T, Ihrig MM, Ge Z, Feng Y, Tomczak M, Rogers AB, Horwitz BH, Fox JG. CD4(+)CD25(+) regulatory lymphocytes require interleukin 10 to interrupt colon carcinogenesis in mice. Cancer Res. 2003;63:6042–50. [PubMed] [Google Scholar]
- 20.Wills-Karp M, Santeliz J, Karp CL. The germless theory of allergic disease: revisiting the hygiene hypothesis. Nat Rev. 2001;1:69–75. doi: 10.1038/35095579. [DOI] [PubMed] [Google Scholar]
- 21.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 22.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 23.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 24.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boivin GP, Washington K, Yang K, Ward JM, Pretlow TP, Russell R, Besselsen DG, Godfrey VL, Doetschman T, Dove WF, Pitot HC, Halberg RB, et al. Pathology of mouse models of intestinal cancer: consensus report and recommendations. Gastroenterology. 2003;124:762–77. doi: 10.1053/gast.2003.50094. [DOI] [PubMed] [Google Scholar]
- 26.Erdman SE, Poutahidis T, Tomczak M, Rogers AB, Cormier K, Plank B, Horwitz BH, Fox JG. CD4+ CD25+ inhibit regulatory T lymphocytes microbially induced colon cancer in Rag2-deficient mice. Am J Pathol. 2003;162:691–702. doi: 10.1016/S0002-9440(10)63863-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-{alpha} trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci USA. 2009 Jan 27;106:1027–32. doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powrie F, Maloy KJ. Immunology. Regulating the regulators. Science. 2003;299:1030–1. doi: 10.1126/science.1082031. [DOI] [PubMed] [Google Scholar]
- 29.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+ CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J Exp med. 2003;197:111–9. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, Mendelsohn M, Charron J, Datta M, Young F, Stall AM, et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992;68:855–67. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- 31.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3-T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 32.Moser AR, Pitot HC, Dove WF. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–4. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 33.Powell SM, Zilz N, Beazer-Barclay Y, Bryan TM, Hamilton SR, Thibodeau SN, Vogelstein B, Kinzler KW. APC mutations occur early during colorectal tumorigenesis. Nature. 1992;359:235–7. doi: 10.1038/359235a0. [DOI] [PubMed] [Google Scholar]
- 34.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giani C, Fierabracci P, Bonacci R, Gigliotti A, Campani D, De Negri F, Cecchetti D, Martino E, Pinchera A. Relationship between breast cancer and thyroid disease: relevance of autoimmune thyroid disorders in breast malignancy. J Clin Endocrinol Metabol. 1996;81:990–4. doi: 10.1210/jcem.81.3.8772562. [DOI] [PubMed] [Google Scholar]
- 36.ACS Cancer Facts and Figures. US cancer fact and figures. 2004 American Cancer Society.
- 37.Maggio M, Guralnik JM, Longo DL, Ferrucci L. Interleukin-6 in aging and chronic disease: a magnificent pathway. J Gerontol. 2006;61:575–84. doi: 10.1093/gerona/61.6.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe LR, Chang SH, Tolle KC, Dillon R, Young LJ, Cardiff RD, Newman RA, Yang P, Thaler HT, Muller WJ, Hudis C, Brown AM, et al. HER2/neu-induced mammary tumorigenesis and angiogenesis are reduced in cyclooxygenase-2 knockout mice. Cancer Res. 2005;65:10113–19. doi: 10.1158/0008-5472.CAN-05-1524. [DOI] [PubMed] [Google Scholar]
- 39.Subbaramaiah K, Howe LR, Port ER, Brogi E, Fishman J, Liu CH, Hla T, Hudis C, Dannenberg AJ. HER-2/neu status is a determinant of mammary aromatase activity in vivo: evidence for a cyclooxygenase-2-dependent mechanism. Cancer Res. 2006;66:5504–11. doi: 10.1158/0008-5472.CAN-05-4076. [DOI] [PubMed] [Google Scholar]
- 40.Hosokawa Y, Papanikolaou A, Cardiff RD, Yoshimoto K, Bernstein M, Wang TC, Schmidt EV, Arnold A. In vivo analysis of mammary and non-mammary tumorigenesis in MMTV-cyclin D1 transgenic mice deficient in p53. Transgen Res. 2001;10:471–8. doi: 10.1023/a:1012064911751. [DOI] [PubMed] [Google Scholar]
- 41.Cardiff RD, Anver MR, Gusterson BA, Hennighausen L, Jensen RA, Merino MJ, Rehm S, Russo J, Tavassoli FA, Wakefield LM, Ward JM, Green JE. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene. 2000;19:968–88. doi: 10.1038/sj.onc.1203277. [DOI] [PubMed] [Google Scholar]
- 42.Chow J, Mazmanian SK. Getting the bugs out of the immune system: do bacterial microbiota “fix” intestinal T cell responses? Cell Host Microbe. 2009;5:8–12. doi: 10.1016/j.chom.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 43.Poutahidis T, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Fox JG, Ge Z, Erdman SE. CD4+ lymphocytes modulate prostate cancer progression in mice. Int J Cancer. 2009 Aug 15;125:868–78. doi: 10.1002/ijc.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gounaris E, Erdman SE, Restaino C, Gurish MF, Friend DS, Gounari F, Lee DM, Zhang G, Glickman JN, Shin K, Rao VP, Poutahidis T, et al. Mast cells are an essential hematopoietic component for polyp development. Proc Natl Acad Sci USA. 2007;104:19977–82. doi: 10.1073/pnas.0704620104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Radhakrishnan S, Cabrera R, Schenk EL, Nava-Parada P, Bell MP, Van Keulen VP, Marler RJ, Felts SJ, Pease LR. Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J Immunol. 2008;181:3137–47. doi: 10.4049/jimmunol.181.5.3137. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340–52. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 47.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kullberg MC, Ward JM, Gorelick PL, Caspar P, Hieny S, Cheever A, Jankovic D, Sher A. Helicobacter hepaticus triggers colitis in specific-pathogen-free interleukin-10 (IL-10)-deficient mice through an IL-12- and gamma interferon-dependent mechanism. Infect Immun. 1998;66:5157–66. doi: 10.1128/iai.66.11.5157-5166.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Backstrom BT, Sobel RA, Wucherpfennig KW, Strom TB, Oukka M, Kuchroo VK. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–31. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bettelli E, Korn T, Kuchroo VK. Th17: the third member of the effector T cell trilogy. Curr Opin Immunol. 2007;19:652–7. doi: 10.1016/j.coi.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Korn T, Oukka M, Kuchroo V, Bettelli E. Th17 cells: effector T cells with inflammatory properties. Sem Immunol. 2007;19:362–71. doi: 10.1016/j.smim.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–9. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- 53.Bettelli E, Kuchroo VK. IL-12- and IL-23-induced T helper cell subsets: birds of the same feather flock together. J Exp Med. 2005;201:169–71. doi: 10.1084/jem.20042279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Faubion WA, De Jong YP, Molina AA, Ji H, Clarke K, Wang B, Mizoguchi E, Simpson SJ, Bhan AK, Terhorst C. Colitis is associated with thymic destruction attenuating CD4+25+ regulatory T cells in theperiphery. Gastroenterology. 2004;126:1759–70. doi: 10.1053/j.gastro.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 55.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, Rosenberg CA, Taylor VM, Harris R, Chen C, Adams-Campbell LL, White E. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 56.Teitelbaum SL. Postmenopausal osteoporosis, T cells, and immune dysfunction. Proc Natl Acad Sci USA. 2004;101:16711–2. doi: 10.1073/pnas.0407335101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin J, Zhang SM, Cook NR, Manson JE, Buring JE, Lee IM. Oral contraceptives, reproductive factors, and risk of colorectal cancer among women in a prospective cohort study. Am J Epidemiol. 2007;165:794–801. doi: 10.1093/aje/kwk068. [DOI] [PubMed] [Google Scholar]
- 58.Burch JC, Byrd BF, Vaughn WK. The effects of long-term estrogen administration to women following hysterectomy. Frontiers Hormone Res. 1975;3:208–14. doi: 10.1159/000398277. [DOI] [PubMed] [Google Scholar]
- 59.Furner SE, Davis FG, Nelson RL, Haenszel W. A case-control study of large bowel cancer and hormone exposure in women. Cancer Res. 1989;49:4936–40. [PubMed] [Google Scholar]
- 60.Chen MJ, Longnecker MP, Morgenstern H, Lee ER, Frankl HD, Haile RW. Recent use of hormone replacement therapy and the prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:227–30. [PubMed] [Google Scholar]
- 61.Polanczyk MJ, Carson BD, Subramanian S, Afentoulis M, Vandenbark AA, Ziegler SF, Offner H. Cutting edge: estrogen drives expansion of the CD4+ CD25+ regulatory T cell compartment. J Immunol. 2004;173:2227–30. doi: 10.4049/jimmunol.173.4.2227. [DOI] [PubMed] [Google Scholar]
- 62.Matejuk A, Bakke AC, Hopke C, Dwyer J, Vandenbark AA, Offner H. Estrogen treatment induces a novel population of regulatory cells, which suppresses experimental autoimmune encephalomyelitis. J Neurosci Res. 2004;77:119–26. doi: 10.1002/jnr.20145. [DOI] [PubMed] [Google Scholar]
- 63.Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, et al. Induction of regulatory T cells by physiological level estrogen. J Cellular Physiol. 2008;214:456–64. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- 64.Prieto GA, Rosenstein Y. Oestradiol potentiates the suppressive function of human CD4 CD25 regulatory T cells by promoting their proliferation. Immunology. 2006;118:58–65. doi: 10.1111/j.1365-2567.2006.02339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. N Engl J Med. 2001;345:1400–8. doi: 10.1056/NEJMra000763. [DOI] [PubMed] [Google Scholar]
- 66.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, Karin M. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 67.Wang Y, Rickman BH, Poutahidis T, Schlieper K, Jackson EA, Erdman SE, Fox JG, Horwitz BH. c-Rel is essential for the development of innate and T cell-induced colitis. J Immunol. 2008;180:8118–25. doi: 10.4049/jimmunol.180.12.8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 69.Meagher MW, Johnson RR, Vichaya EG, Young EE, Lunt S, Welsh CJ. Social conflict exacerbates an animal model of multiple sclerosis. Trauma Violence Abuse. 2007;8:314–30. doi: 10.1177/1524838007303506. [DOI] [PubMed] [Google Scholar]
- 70.Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA. 2003;100:9090–5. doi: 10.1073/pnas.1531903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Baltgalvis KA, Berger FG, Pena MM, Davis JM, Muga SJ, Carson JA. Interleukin-6 and cachexia in ApcMin/+ mice. Am J Physiol. 2008;294:R393–401. doi: 10.1152/ajpregu.00716.2007. [DOI] [PubMed] [Google Scholar]
- 72.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J Clin Oncol. 2009;27:186–92. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 73.Ambrosino E, Spadaro M, Iezzi M, Curcio C, Forni G, Musiani P, Wei WZ, Cavallo F. Immunosurveillance of Erbb2 carcinogenesis in transgenic mice is concealed by a dominant regulatory T-cell self-tolerance. Cancer Res. 2006;66:7734–40. doi: 10.1158/0008-5472.CAN-06-1432. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann P, Eder R, Kunz-Schughart LA, Andreesen R, Edinger M. Large-scale in vitro expansion of polyclonal human CD4(+)CD25 high regulatory T cells. Blood. 2004;104:895–903. doi: 10.1182/blood-2004-01-0086. [DOI] [PubMed] [Google Scholar]